Abstract
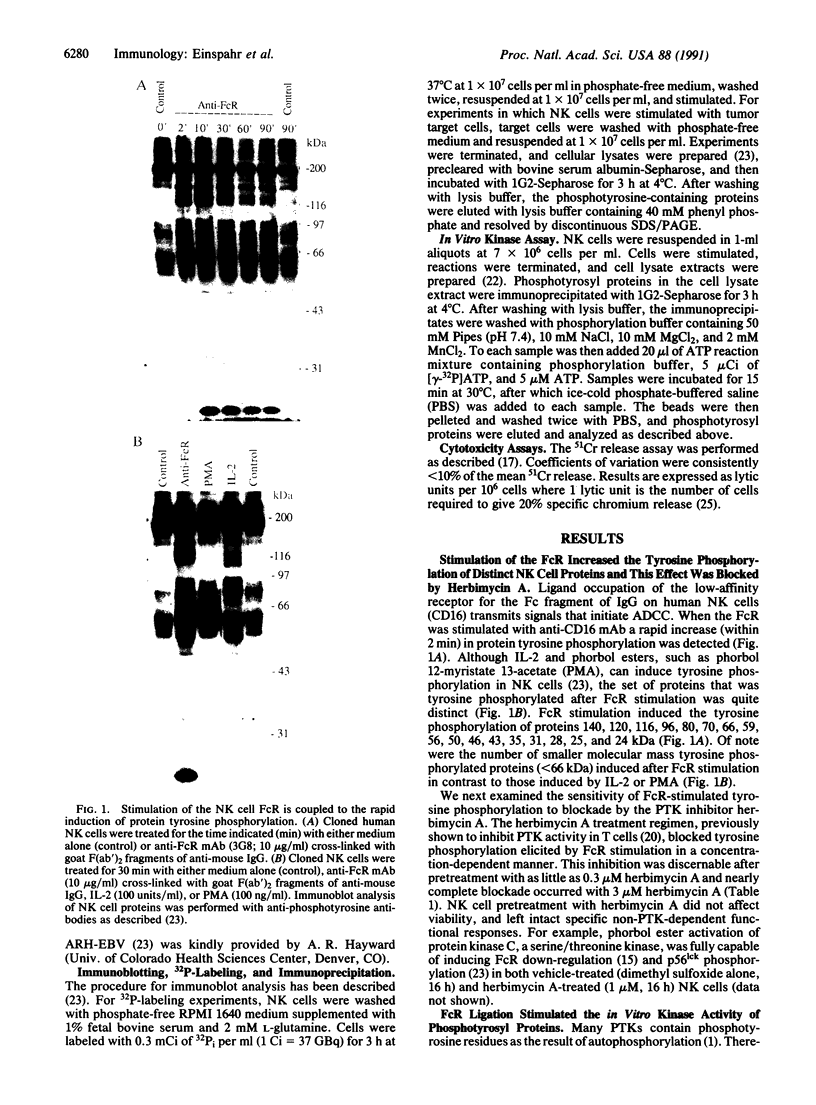
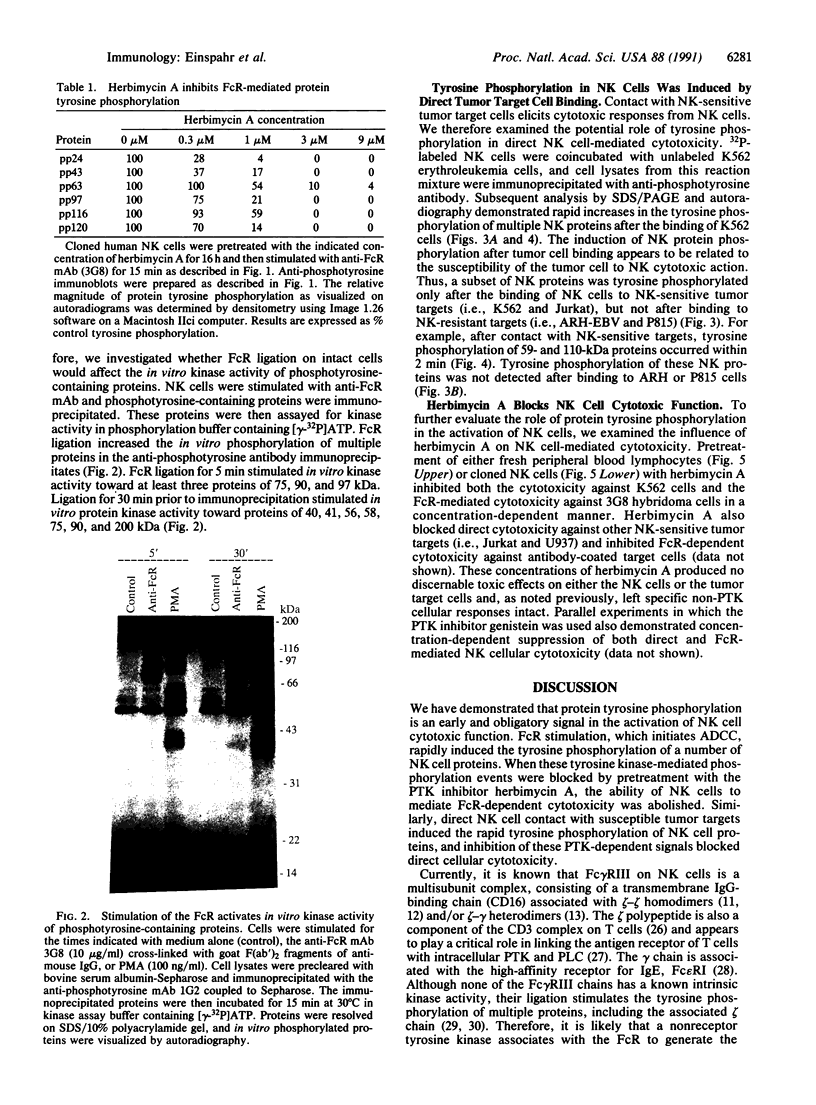
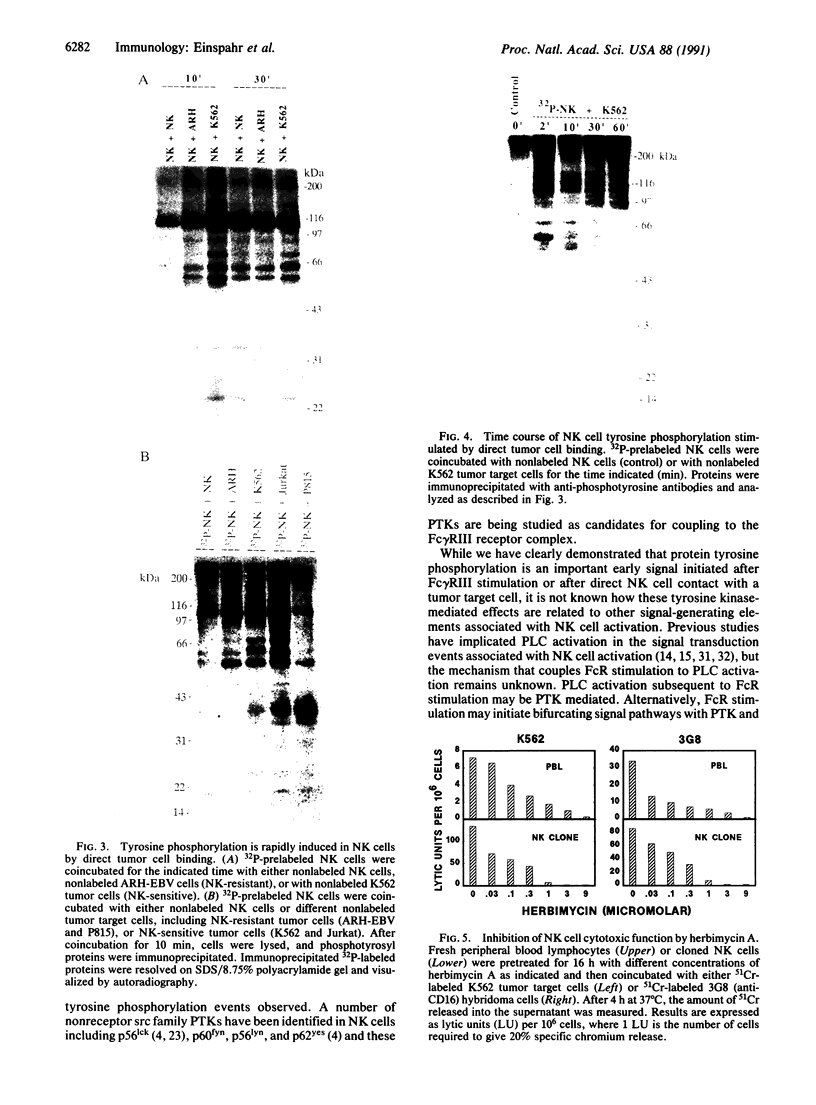
Natural killer (NK) cells are a unique subpopulation of lymphocytes with the capability to kill malignant cells via one of two alternative mechanisms: (i) Fc receptor-dependent cytotoxicity against antibody-coated targets or (ii) direct cell-mediated cytotoxicity. However, the molecular mechanisms that trigger and subsequently regulate NK cell cytotoxicity are incompletely understood. We have therefore investigated the role of protein tyrosine phosphorylation in the transmembrane signaling initiated after Fc receptor stimulation or direct tumor cell contact in clonal CD16+/CD3- human NK cells. We report that stimulation of the Fc receptor rapidly induced the tyrosine phosphorylation of a number of NK cell proteins. These effects occurred within 2 min, were maximal at 10 min, and declined toward baseline after 60 min. In addition, Fc receptor ligation increased the in vitro protein kinase activity of NK cell phosphotyrosyl proteins. We have also demonstrated that direct contact of NK cells with K562 tumor cells induced the rapid phosphorylation of distinct NK cell phosphotyrosyl proteins. Furthermore, the protein-tyrosine kinase inhibitor herbimycin A blocked NK cell cytotoxic function in a concentration-dependent manner. Our results suggest that protein tyrosine phosphorylation is an obligatory early proximal signal in activating the cytotoxic function of NK cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Caligiuri M., Ritz J., Schlossman S. F. CD3-negative natural killer cells express zeta TCR as part of a novel molecular complex. Nature. 1989 Sep 14;341(6238):159–162. doi: 10.1038/341159a0. [DOI] [PubMed] [Google Scholar]
- Augustine J. A., Schlager J. W., Abraham R. T. Differential effects of interleukin-2 and interleukin-4 on protein tyrosine phosphorylation in factor-dependent murine T cells. Biochim Biophys Acta. 1990 May 2;1052(2):313–322. doi: 10.1016/0167-4889(90)90227-5. [DOI] [PubMed] [Google Scholar]
- Benhamou M., Gutkind J. S., Robbins K. C., Siraganian R. P. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspahr K. J., Abraham R. T., Dick C. J., Leibson P. J. Protein tyrosine phosphorylation and p56lck modification in IL-2 or phorbol ester-activated human natural killer cells. J Immunol. 1990 Sep 1;145(5):1490–1497. [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. src-related tyrosine protein kinases as signaling components in hematopoietic cells. Cancer Cells. 1990 Oct;2(10):303–310. [PubMed] [Google Scholar]
- Golden A., Brugge J. S. Thrombin treatment induces rapid changes in tyrosine phosphorylation in platelets. Proc Natl Acad Sci U S A. 1989 Feb;86(3):901–905. doi: 10.1073/pnas.86.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind J. S., Robbins K. C. Translocation of the FGR protein-tyrosine kinase as a consequence of neutrophil activation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8783–8787. doi: 10.1073/pnas.86.22.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma Y., Okabe-Kado J., Hozumi M., Uehara Y., Mizuno S. Induction of erythroid differentiation of K562 human leukemic cells by herbimycin A, an inhibitor of tyrosine kinase activity. Cancer Res. 1989 Jan 15;49(2):331–334. [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991 Mar 8;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Samelson L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990 Mar 1;144(5):1591–1599. [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Schieven G. L., Siegel J. N., Phillips A. F., Samelson L. E. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7722–7726. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Yu G., Phillips J. H. Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature. 1989 Dec 14;342(6251):803–805. doi: 10.1038/342803a0. [DOI] [PubMed] [Google Scholar]
- Leibson P. J., Midthun D. E., Windebank K. P., Abraham R. T. Transmembrane signaling during natural killer cell-mediated cytotoxicity. Regulation by protein kinase C activation. J Immunol. 1990 Sep 1;145(5):1498–1504. [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- O'Shea J. J., Weissman A. M., Kennedy I. C., Ortaldo J. R. Engagement of the natural killer cell IgG Fc receptor results in tyrosine phosphorylation of the zeta chain. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):350–354. doi: 10.1073/pnas.88.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff D. G., Ra C. S., Frank S. J., Klausner R. D., Kinet J. P. Family of disulphide-linked dimers containing the zeta and eta chains of the T-cell receptor and the gamma chain of Fc receptors. Nature. 1990 Sep 13;347(6289):189–191. doi: 10.1038/347189a0. [DOI] [PubMed] [Google Scholar]
- Paya C. V., Schoon R. A., Leibson P. J. Alternative mechanisms of natural killer cell activation during herpes simplex virus infection. J Immunol. 1990 Jun 1;144(11):4370–4375. [PubMed] [Google Scholar]
- Pierce J. H. Oncogenes, growth factors and hematopoietic cell transformation. Biochim Biophys Acta. 1989 Dec 17;989(2):179–208. doi: 10.1016/0304-419x(89)90042-5. [DOI] [PubMed] [Google Scholar]
- Pross H. F., Baines M. G., Rubin P., Shragge P., Patterson M. S. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981 Jan;1(1):51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Patel M. D., Weissman A. M., Harford J. B., Klausner R. D. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986 Sep 26;46(7):1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- Seaman W. E., Eriksson E., Dobrow R., Imboden J. B. Inositol trisphosphate is generated by a rat natural killer cell tumor in response to target cells or to crosslinked monoclonal antibody OX-34: possible signaling role for the OX-34 determinant during activation by target cells. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4239–4243. doi: 10.1073/pnas.84.12.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele T. A., Brahmi Z. Phosphatidylinositol metabolism accompanies early activation events in tumor target cell-stimulated human natural killer cells. Cell Immunol. 1988 Apr 1;112(2):402–413. doi: 10.1016/0008-8749(88)90309-7. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Hori M., Takeuchi T., Umezawa H. Screening of agents which convert 'transformed morphology' of Rous sarcoma virus-infected rat kidney cells to 'normal morphology': identification of an active agent as herbimycin and its inhibition of intracellular src kinase. Jpn J Cancer Res. 1985 Aug;76(8):672–675. [PubMed] [Google Scholar]
- Unkeless J. C., Scigliano E., Freedman V. H. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- Vivier E., Morin P., O'Brien C., Druker B., Schlossman S. F., Anderson P. Tyrosine phosphorylation of the Fc gamma RIII(CD16): zeta complex in human natural killer cells. Induction by antibody-dependent cytotoxicity but not by natural killing. J Immunol. 1991 Jan 1;146(1):206–210. [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Localization of tyrosine kinase-coding region in v-abl oncogene by the expression of v-abl-encoded proteins in bacteria. J Biol Chem. 1985 Jan 10;260(1):64–71. [PubMed] [Google Scholar]
- Weissman A. M., Hou D., Orloff D. G., Modi W. S., Seuanez H., O'Brien S. J., Klausner R. D. Molecular cloning and chromosomal localization of the human T-cell receptor zeta chain: distinction from the molecular CD3 complex. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9709–9713. doi: 10.1073/pnas.85.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windebank K. P., Abraham R. T., Powis G., Olsen R. A., Barna T. J., Leibson P. J. Signal transduction during human natural killer cell activation: inositol phosphate generation and regulation by cyclic AMP. J Immunol. 1988 Dec 1;141(11):3951–3957. [PubMed] [Google Scholar]
- Zanovello P., Rosato A., Bronte V., Cerundolo V., Treves S., Di Virgilio F., Pozzan T., Biasi G., Collavo D. Interaction of lymphokine-activated killer cells with susceptible targets does not induce second messenger generation and cytolytic granule exocytosis. J Exp Med. 1989 Sep 1;170(3):665–677. doi: 10.1084/jem.170.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]









