Abstract
Background
Papillary thyroid carcinoma (PTC) patients presenting with cervical lymph nodes (LN) metastases (M) have a variable outcome. The objective of this study is to assess the value of meticulous histopathologic examination and genotyping in stratifying these patients into clinically relevant prognostic subgroups.
Methods
This was a retrospective clinical and histopathological review of PTC patients with lymph node metastases at presentation identified between 1980 and 2002 in a single institution. Primary tumors from patients who later recurred were matched to a group of patients who did not recur and subjected to mass spectrometry genotyping encompassing the most significant oncogenes in thyroid carcinomas.
Results
There were 246 patients who satisfied the inclusion criteria. The median follow-up was 10.8 years. The presence of >3 metastatic nodes was an independent predictor of decreased recurrence free survival (p=0.03). In patients <45 years, none of 45 with 1–2 metastatic LN recurred, including 26 patients followed for a median of 13 years without radioactive iodine (RAI) therapy. BRAF mutations were found in 28 (78%) of 36 genotyped tumors. Combined positivity for BRAF and extra-nodal extension was much stronger in predicting disease specific survival (DSS) (p=0.004) than the single analysis of BRAF (p=0.12) or extra-nodal extension (p=0.02).
Conclusions
(i) The number of metastatic LN is an independent predictor of recurrence in all age groups and identifies a subset of young patients with excellent prognosis who may not benefit from RAI therapy. (ii) Combined positivity for BRAF and extra-nodal extension has additive prognostic value in predicting DSS. (iii) Classification systems that assign the same magnitude of risk for recurrence or death to all patients with N1 disease should be revisited.
Introduction
The presence of any cervical lymph node metastasis (LNM), whether microscopic or single, upgrades a patient with papillary thyroid carcinoma (PTC) from a low to an intermediate risk category according to the American Thyroid Association (ATA) guidelines (1). Using the tumor node metastasis American Joint Committee on Cancer staging system (AJCC), an older individual (>45 years) with papillary microcarcinoma will move from AJCC stage I (T1N0Mx) to stage III (T1N1aMx) if a metastatic LN is identified in the central neck and to stage IVa (T1N1bMx) if the LN is identified in the lateral neck (2). This upstaging often leads to more aggressive therapy (3,4), as the risk of persistent/recurrent disease is significantly higher in ATA intermediate risk (21%) than ATA low risk patients (3%) (5), and the risk of death is higher in stage III and IV patients (12.2% and 59.3% respectively) than in Stage I patients (0.2%) (6). However, the most recently updated guidelines from the ATA acknowledge the need to stratify patients with LNM (N1disease) into high and low risk groups, as additional therapy such as radioactive iodine (RAI) remnant ablation may be indicated in one but not the other (1). Several studies have shown that patients with N1 disease have variable outcomes. Indeed, the presence of palpable LNM (7), a higher number of metastatic nodes, large metastatic nodes, and extranodal extension have been shown to correlate with poorer outcome (8–10). Although the histopathologic and clinical analysis were detailed in some of these studies, none have accounted for the size of the metastatic foci within the lymph nodes (LN), the subtyping of the primary tumor, and of the metastatic foci into histologic variants of PTC. In addition, the prognostic value of the molecular alterations in these tumors has not been assessed. The aims of this study were to characterize PTC patients with LNM at the histopathologic and molecular levels, and to identify predictors of outcome in N1 disease.
Materials and Methods
Patient population and inclusion criteria
The institutional database was searched for all cases with a diagnosis of thyroid carcinoma treated at Memorial Sloan-Kettering Cancer Center (MSKCC) between January 1980 and December 2002. The slides from the cases included in the study were examined by two head and neck pathologists with special interest in thyroid neoplasia (R.A.G. and M.R.). The pathologists were blinded to the clinical outcome of all patients studied. The thyroid carcinomas were classified according to the last World Health Organization classification of endocrine tumors, except for PTC tall cell variant and poorly differentiated thyroid carcinomas (11). The latter tumor was defined as a carcinoma displaying high mitotic activity (≥5 mitosis/10 high power fields, 400×) and/or tumor necrosis, and showing follicular cell differentiation at the morphologic or immunohistochemical level (12). Tall cell variant was characterized as a papillary carcinoma composed of >50% of tall cells. The latter cell type was defined as having a height at least twice its width with an oncocytic cytoplasm.
Patients were included in the study if they had (i) PTC as their primary tumor with (ii) LNM at presentation. The study was approved by the Institutional Review Board of MSKCC.
Clinical parameters
The patient's electronic medical records were reviewed for the age at diagnosis, type of surgery, and adjuvant treatment including RAI therapy. The disease status at recurrence or follow-up was based on a combination of clinical and imaging assessments, including history, physical examination, RAI dosimetry, cross sectional imaging, and/or positron emission tomography scanning. Since many cases from the 1980s did not have adequate biochemical data, biochemical recurrence was not assessed. The type of cervical LN dissection was recorded as Central; Lateral; Central and Lateral; Other. The latter category included mostly peri-thyroidal LNM removed with the thyroid specimen, or patients with mediastinal LNM or LN biopsies. The date of initial surgery, first recurrence, and last date of follow-up were documented. The status at last follow-up was recorded as follows: no evidence of disease; alive with disease; dead of other causes; and dead of disease.
Histopathologic analysis
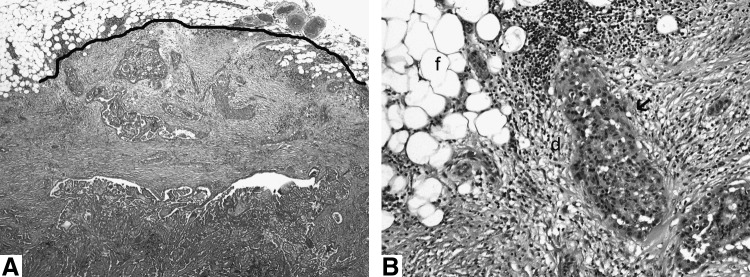
The largest dimension of the tumor was recorded as dictated during gross examination. The mitotic rate of the tumor was determined by counting 10 contiguous high power fields (400×) corresponding to 2.4 mm2. Tumor necrosis was defined by a “comedo-like” appearance composed of degenerating cytoplasm and punctate, karyorrhectic nuclear debris. The presence of vascular invasion was noted only when such foci were present within or beyond the capsule in accordance with criteria outlined by the Armed Forces Institute of Pathology fascicle (13). Areas of vascular invasion that were closely adjacent to one another were counted as separate foci. Angioinvasion was categorized as present or absent. The capsule status was recorded as complete, partial, or absent. A tumor was considered infiltrative if it lacked a complete capsule and invaded in between non-neoplastic follicles in an irregular fashion in the form of invasive islands. Extra-thyroid extension (ETE) was defined as tumor cells invading beyond the thyroid capsule into peri-thyroid soft tissue or organs. ETE was subdivided into (i) None (ii) Focal: Presence of 1–2 microscopic foci of ETE measuring ≤1 mm each. (iii) Extensive: Presence of >2 microscopic foci of ETE (≤1 mm in size each) or any foci >1 mm in size. The latter category includes patients with gross ETE into muscle and adjacent viscera. The status of the resection margins was reported as positive (tumor present at the surgical margin) or negative (no tumor at surgical margin). The number of LN examined microscopically as well as the number of nodes with metastatic carcinoma was recorded. For the survival analysis, the number of metastatic nodes was categorized using two alternative cutoffs: that is, <3 and >3, or <5 and >5 metastatic nodes. The greatest diameter of the largest metastatic node was measured as well as the largest size of the largest metastatic tumor focus. For the survival analysis, the size of the largest metastatic node and of the largest metastatic focus were categorized as <1 cm and >1 cm. The most predominant histologic type of tumor in the LN was recorded. Extra-nodal extension was defined as invasion of peri-nodal tissue (Fig. 1) and recorded as present or absent.
FIG. 1.
(A) Low-power view of a metastatic lymph node displaying an irregular contour (thick black line) due to extra-nodal extension in a 77-year-old man with multiple nodal metastases (>3) who recurred ∼3 years after diagnosis. (B) High-power view of the same lymph node. The extra-nodal tumor nest (arrow) has elicited a desmoplastic response (d) in the peri-nodal adipose tissue (f).
Genotyping
Four sections of 10 μm from each formalin-fixed paraffin-embedded tissue block were subjected to DNA extraction using the PUREGene Genomic DNA purification kit (Gentra, Inc., Minneapolis, MN). Mutation detection was performed as previously described (14). We used mass spectrometry Sequenom–based genotyping assay (Sequenom Mass array; Sequenom, San Diego CA), which consists of 116 assays, to interrogate mutations in 16 genes, including the most common thyroid oncogenes such as BRAF, NRAS, HRAS, KRAS, PIK3CA, and AKT1. Since the mass spectrometry genotyping assays for codons 12 and 13 of HRAS were not informative, we designed primers for this region and sequenced all the tumors that were wild type for BRAF or RAS mutations by mass spectrometry genotyping (14).
Screening for RET/PTC rearrangements
RET rearrangements were analyzed for tumors that were wild type for RAS and BRAF. We used tumor cDNA as template for quantitative PCR to analyze for unbalanced expression of exons 10–11 relative to 12–13 of RET, which flank the rearrangement site in intron 11. Samples with 12–13>10–11 expression were screened for specific RET recombination events using primers bracketing the fusion points of RET/PTC1, and RET/PTC3, respectively, as previously described (14,15).
Statistical analysis of survival outcomes
Recurrence free survival (RFS) was defined as the time interval between the date of initial surgery and date of 1st recurrence. Disease specific survival (DSS) was calculated from the date of initial surgery to last follow-up. DSS was defined as the presence or absence of death of disease at last follow-up. Two-tailed Fisher exact test was used to assess the relation between categorical variables. Survival outcomes were calculated using the Kaplan–Meier method and compared using the log-rank. A probability (p) value ≤0.05 was considered significant. Univariate analysis was carried out using the log rank test and multivariate analysis using a Cox proportional hazard model. Statistical analysis was carried out using commercially available software (SPSS for Windows, version 17.0 [SPSS Inc, Chicago, IL] and JMP version 4.0 [SAS Institute Inc, Cary, NC]).
Results
Clinico-pathologic data and survival analysis on the whole study population
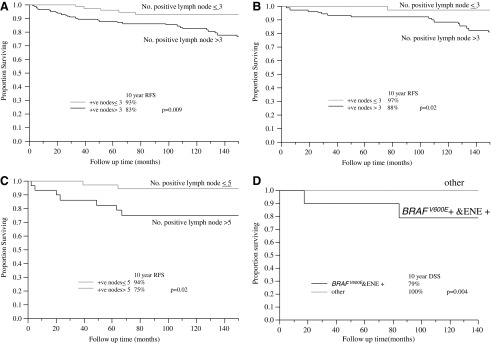
Out of 829 carcinomas of follicular cell origin in the database, 246 (30%) had PTC in their primary tumor with LNM at presentation and were, therefore, included in the study. Table 1 shows the clinico-pathologic features of the cases, including their histotype. Of note, 214 (87%) patients had no capsule or were partially encapsulated, and were infiltrative tumors. The number of LN removed and examined microscopically ranged from 1 to 119 with a median of 24 LN resected per patient at the initial surgery. Table 2 displays the prognostic value of the various histopathologic and clinical parameters in the whole patient cohort. At 10 years of follow-up, the DSS of the whole patient population was 98%, and the overall (any recurrence) RFS was 86%. The neck and distant RFS at 10 years were 91% and 94%, respectively. In regard to overall RFS, older age, extensive ETE and number of positives nodes (>3) (Fig. 2A) were independent predictors of outcome in multivariate analysis (Table 3). Older age, positive margins, and extra-nodal extension predicted for death of disease in univariate analysis. It was not possible to perform multivariate analysis for DSS since very few patients died.
Table 1.
Clinico-Pathologic Features of All 246 Papillary Carcinomas with Lymph Node Metastasis
| Characteristic | Number of patients (%) | Characteristic | Number of patients (%) |
|---|---|---|---|
| Age, years | Predominant histologic type of tumor in lymph nodeg | ||
| Median | 36 | Classic PTC | 179 (75%) |
| <45 | 168 (68%) | FVPTC | 11 (4%) |
| >45 | 78 (32%) | Tall cell | 45 (19%) |
| Gender | Poorly differentiated | 3 (1%) | |
| Female | 155 (63%) | Psammoma bodies | 2 (1%) |
| Male | 91 (37%) | Most aggressive PTC subtype in lymph nodeg | |
| Tumor size (cm)a | Classic PTC | 179 (76%) | |
| Median | 1.9 | FVPTC | 11 (5%) |
| <1.5 | 100 (42%) | Tall cell | 45 (19%) |
| >1.5 | 139 (58%) | Extra-nodal extensionh | |
| PTC subtypes | No | 161 (68%) | |
| Classic PTC (87% infiltrative) | 136 (55.3%) | Yes | 75 (32%) |
| FVPTC (16 infiltrative, 7 encapsulated) | 23 (9.3%) | Multicentricityi | |
| Tall cell PTC | 44 (18%) | No | 136 (57%) |
| Microcarcinoma | 35 (14.2%) | Yes | 102 (43%) |
| Otherb | 8 (3.2%) | Thyroid surgery | |
| Tumor capsule | Less than TT | 69 (28%) | |
| Completely encapsulated | 28 (11%) | TT | 177 (72%) |
| Not/partially encapsulated | 218 (89%) | RAI ablation | |
| Vascular invasionc | Yes | 140 (57%) | |
| Absent | 222 (91%) | None | 106 (43%) |
| Present | 22 (9%) | Neck dissection | |
| Extra-thyroid extensiond | Central | 70 (28%) | |
| None | 87 (36%) | Lateral | 63 (26%) |
| Focal | 41 (17%) | Central and Lateral | 86 (35%) |
| Extensive | 115 (47%) | Other | 27 (11%) |
| Marginse | Recurrencej | ||
| Negative | 198 (81%) | Present | 34 (14%) |
| Positive | 46 (19%) | Absent | 202 (86%) |
| Number of metastatic nodes | Status at last FUj | ||
| Median | 6 | DOD | 5 (2%) |
| ≤3 | 95 (39%) | AWD | 16 (7%) |
| >3 | 151 (61%) | NED | 215 (91%) |
| Size of largest metastatic node (cm)f | Follow-up | ||
| Median (range) | 1.3 (0.1–4.5) | Median (range) | 10.8 (0.1–28.8) |
| ≤1 | 103 (44%) | ||
| >1 | 133 (56%) | ||
| Size of largest metastatic focus in lymph node (cm)f | |||
| Median (range) | 1.1 (0.1–4.5) | ||
| ≤1 | 113 (48%) | ||
| >1 | 123 (52%) | ||
Tumor size could not be accurately assessed in seven cases.
Other includes diffuse sclerosing variant and solid variant PTC.
In two cases, angioinvasion could not be accurately assessed.
Extra-thyroid extension could not be evaluated in three patients.
Margin status was not assessable in two cases.
In 10 cases, the size of the largest metastatic node and the largest metastatic foci in lymph node (LN) could not be accurately assessed.
In six cases, the PTC subtype could not be evaluated in the LN because of inadequate LN material (two patients had only psammoma bodies in LN, two cases displayed poorly differentiated thyroid carcinoma [PDTC], and one case was considered as PTC progressing toward PDTC in the LN).
In 10 cases, extra-nodal extension was equivocal or could not be assessed because of inadequate material.
Multicentricity defined as >2 foci of carcinoma could not be accurately evaluated in eight cases.
Ten patients were lost for follow-up (FU).
RAI, radioactive iodine; PTC, papillary thyroid carcinoma; FVPTC, follicular variant of papillary thyroid carcinoma; NED, no evidence of disease; AWD, alive with disease; DOD, death of disease; TT, total thyroidectomy.
Table 2.
Prognostic Factors of Survival by Univariate Analysis in Papillary Thyroid Carcinoma with Nodal Metastasis
| Overall RFS | Neck RFS | Distant RFS | DSS | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | 10 years RFS (%) | p | 10 years RFS (%) | p | 10 years RFS (%) | p | 10 years DSS (%) | p |
| Age, years | 0.008 | 0.23 | 0.0008 | 0.0002 | ||||
| <45 | 91 | 93 | 98 | 100 | ||||
| >45 | 76 | 86 | 86 | 95 | ||||
| Gender | 0.66 | 0.9 | 0.68 | 0.93 | ||||
| Female | 85 | 89 | 95 | 100 | ||||
| Male | 87 | 92 | 94 | 97 | ||||
| Tumor size (cm) | 0.2 | 0.35 | 0.49 | 0.89 | ||||
| ≤1.5 | 89 | 92 | 95 | 99 | ||||
| >1.5 | 84 | 90 | 93 | 98 | ||||
| PTC subtypes | 0.06 | 0.17 | 0.39 | 0.08 | ||||
| FVPTC | 100 | 100 | 100 | 100 | ||||
| Microcarcinoma | 93 | 96 | 97 | 100 | ||||
| Classic PTC | 86 | 90 | 95 | 99 | ||||
| Tall cell PTC | 79 | 87 | 89 | 94 | ||||
| DFS and solid variant | 75 | 88 | 88 | 100 | ||||
| Vascular invasion | 0.52 | 0.81 | 0.32 | 0.46 | ||||
| Absent | 87 | 91 | 95 | 98 | ||||
| Present | 79 | 89 | 90 | 100 | ||||
| Extra-thyroid extension | 0.007 | 0.1 | 0.37 | 0.32 | ||||
| None | 94 | 96 | 96 | 98 | ||||
| Focal | 95 | 97 | 97 | 100 | ||||
| Extensive | 77 | 85 | 92 | 98 | ||||
| Margins | 0.59 | 0.25 | 0.03 | 0.0001 | ||||
| Negative | 87 | 90 | 96 | 99 | ||||
| Positive | 82 | 95 | 87 | 94 | ||||
| Number of metastatic nodes | 0.009 | 0.02 | 0.8 | 0.68 | ||||
| ≤3 | 93 | 96 | 94 | 98 | ||||
| >3 | 82 | 88 | 94 | 98 | ||||
| Size of largest metastatic node (cm) | 0.05 | 0.28 | 025 | 0.48 | ||||
| ≤1 | 90 | 93 | 96 | 100 | ||||
| >1 | 83 | 90 | 93 | 98 | ||||
| Size of largest metastatic focus in lymph node (cm) | 0.1 | 0.54 | 0.14 | 0.38 | ||||
| ≤1 | 89 | 91 | 97 | 100 | ||||
| >1 | 84 | 91 | 92 | 98 | ||||
| Most predominant PTC subtype in lymph nodea | 0.16 | 0.3 | 0.66 | 0.62 | ||||
| FVPTC | 100 | 100 | 100 | 100 | ||||
| Classic PTC | 87 | 92 | 95 | 99 | ||||
| Tall cell | 78 | 85 | 93 | 96 | ||||
| Most aggressive PTC subtype in lymph nodea | 0.05 | 0.09 | 0.64 | 0.19 | ||||
| FVPTC | 100 | 100 | 100 | 100 | ||||
| Classic PTC | 87 | 92 | 95 | 100 | ||||
| Tall cell | 80 | 87 | 93 | 95 | ||||
| Extra-nodal extension | 0.009 | 0.19 | 0.02 | 0.004 | ||||
| No | 92 | 94 | 97 | 100 | ||||
| Yes | 75 | 86 | 92 | 97 | ||||
| Multicentricity | 0.12 | 0.06 | 0.56 | 0.32 | ||||
| No | 88 | 94 | 95 | 98 | ||||
| Yes | 82 | 86 | 93 | 98 | ||||
| Thyroid surgery | 0.6 | 0.64 | 0.24 | 0.23 | ||||
| TT | 84 | 90 | 92 | 97 | ||||
| Sub TT | 100 | 100 | 100 | 100 | ||||
| Lobectomy | 91 | 93 | 98 | 100 | ||||
| Neck dissection | 0.1 | 0.44 | 0.002 | 0.01 | ||||
| Central | 87 | 88 | 98 | 100 | ||||
| Lateral | 93 | 93 | 100 | 100 | ||||
| Central and lateral | 78 | 90 | 86 | 95 | ||||
| Other | 93 | 93 | 93 | 100 | ||||
| RAI therapy | 0.65 | 0.96 | 0.19 | 0.12 | ||||
| Yes | 89 | 92 | 97 | 100 | ||||
| None | 84 | 89 | 93 | 97 | ||||
Only classic PTC, tall cell PTC, and follicular variant PTC were included in the survival analysis.
FIG. 2.
(A) Overall recurrence free survival (RFS) stratified by number of positive nodes in the whole patient cohort. Patients with >3 metastatic nodes have a significantly lower RFS than those with <3 metastatic nodes. (B) Overall RFS stratified by number of positive nodes in patients <45 years old. Patients with >3 metastatic nodes have a significantly lower RFS than those with <3 metastatic nodes. (C) Neck RFS stratified by number of positive nodes in patients >45 years old. Patients with >5 metastatic nodes have a significantly lower RFS than those with ≤5 metastatic nodes. (D) Disease specific survival (DSS) stratified by combined BRAFV600E mutational and extra-nodal extension (ENE) status in 36 genotyped patients (15 with recurrence and 25 without) matched for various clinico-histopathologic parameters. Patients with extra-nodal extension who are BRAFV600E positive have a lower DSS than the remaining individuals. +ve, positive nodes.
Table 3.
Independent Prognostic Factors for Recurrence in Multivariate Analysis According to Age
| Whole patient population | RR | [95% CI] | p |
|---|---|---|---|
| Overall RFS | |||
| Age | 2.5 | [1.2–5.1] | 0.01 |
| Extra-thyroid extension | 2.6 | [1–6.5] | 0.05 |
| No of metastatic nodes (>3) | 3.2 | [1.1–8.8] | 0.03 |
| Neck RFS | |||
| No of metastatic nodes (>3) | 4.3 | [1.2–15.2] | 0.03 |
| Distant RFS | |||
| Age | 7.6 | [2.0–28.8] | 0.003 |
| Patients <45 years old | |||
| Overall RFS | |||
| No of metastatic nodes (>3) | 8.2 | [0.9–72.2] | 0.05 |
| Neck RFS | |||
| None found | |||
| Distant RFS | |||
| NA | |||
| Patients ≥45 years old | |||
| Overall RFS | |||
| None found | |||
| Neck RFS | |||
| No of metastatic nodes (>5) | 9.6 | [1.1–83.3] | 0.04 |
| Distant RFS | |||
| Margins | 4.7 | [1–21.6] | 0.04 |
RR, risk ratio; CI, confidence interval; NA, not applicable (multivariate was not possible in view of the low number of events).
Survival analysis in young patients
In the 168 patients under the age of 45, there was no death of disease with a median follow-up of 12 years. Eighteen patients recurred (15 in the neck and 3 at distant sites), with a 10 year overall RFS of 91%. The 10 year neck and distant RFS were 93% and 98%, respectively. By univariate analysis, the presence of multicentricity and number of metastatic nodes (>3) correlated with decreased overall RFS (p=0.02). In multivariate analysis, the number of positive nodes (>3) was the only independent predictor of overall RFS (Fig. 2B) (Table 3). In regard to neck RFS, only the number of positive nodes (>3) predicted worse outcome (p=0.04). This factor was, however, not significant in multivariate analysis. Large tumor size was the only factor that correlated with decreased distant RFS (p=0.04). In patients <45 years, none of 45 cases with 1–2 metastatic nodes recurred including 26 patients followed for a median of 13 years without RAI therapy (Table 4).
Table 4.
Outcome of 26 Patients <45 Years Old with 1–2 Metastatic Nodes and No Radioactive Iodine Therapy
| Recurrence | 0/26 |
| Age (median) | 33.5 years |
| Primary tumor size (median) | 1.8 cm |
| Positive margins | 2/26 (8%) |
| Gross ETEa | 3/25 (12%) |
| Follow-up (median) | 12.99 years |
Gross ETE was recorded on the basis of the operating room report.
ETE, Extra-thyroid extension.
Survival analysis in older patients
There were 5 deaths of disease in the 78 patients >45 years of age, with a 10 year DSS of 95% and a median follow-up of 9.8 years. Sixteen patients recurred (9 neck and 9 distant recurrences) with a 10-year overall RFS of 76%. The 10 year neck and distant RFS was each 86%. In regard to neck RFS, the number of positive nodes (>5) was the only independent predictor of outcome (p=0.02) (Fig. 2C) (Table 3). On multivariate analysis, the presence of positive margins was the only independent factor for decreased distant RFS (Table 3). By univariate analysis, the presence of positive margins (p<0.0001), a combined central and lateral neck dissection (p=0.02) and the presence of extra-nodal extension (p=0.008) correlated with decreased DSS.
Survival analysis in patients without RAI therapy
In the 106 patients who did not receive RAI therapy, there was no death of disease with a median follow-up of 15.4 years. Fifteen patients recurred (12 in the neck and 3 at distant sites) with a 10 year overall RFS of 89%. The 10 year neck and distant RFS were 92 and 97%, respectively. By univariate analysis, several variables correlated with outcome, but none was an independent predictor in multivariate analysis (Table 5).
Table 5.
Prognostic Factors of Recurrence by Univariate Analysis in Papillary Thyroid Carcinoma with Nodal Metastasis Not Treated with Radioactive Iodine Therapy
| Overall RFS | Neck RFS | Distant RFS | ||||
|---|---|---|---|---|---|---|
| Characteristic | 10 years RFS (%) | p | 10 years RFS (%) | p | 10 years RFS (%) | p |
| Age, years | 0.06 | 0.44 | 0.03 | |||
| <45 | 93 | 95 | 99 | |||
| ≥45 | 71 | 83 | 88 | |||
| PTC subtypes | 0.003 | 0.49 | 0.009 | |||
| FVPTC | 100 | 100 | 100 | |||
| Microcarcinoma | 95 | 95 | 100 | |||
| Classic PTC | 91 | 93 | 98 | |||
| Tall cell PTC | 76 | 89 | 88 | |||
| DFS and solid variant PTC | 33 | 67 | 67 | |||
| Extra-thyroid extension | 0.06 | 0.08 | 0.59 | |||
| None | 97 | 100 | 97 | |||
| Focal | 95 | 95 | 100 | |||
| Extensive | 79 | 84 | 95 | |||
| Number of metastatic nodes | 0.05 | 0.04 | 0.93 | |||
| ≤3 | 94 | 97 | 97 | |||
| >3 | 86 | 90 | 96 | |||
| Most aggressive PTC subtype in lymph nodea | 0.05 | 0.005 | 0.66 | |||
| FVPTC | 100 | 100 | 100 | |||
| Classic PTC | 90 | 95 | 96 | |||
| Tall cell | 81 | 81 | 100 | |||
| Extra-nodal extension | 0.05 | 0.09 | 0.52 | |||
| No | 93 | 96 | 97 | |||
| Yes | 69 | 76 | 93 | |||
Only classic PTC, tall cell PTC, and follicular variant PTC were included in the survival analysis.
Survival analysis in patients treated with RAI
In the 140 patients treated with RAI, there were 4 deaths of disease with a 10 years DSS of 97% and a median follow-up of 9.6 years. Nineteen patients recurred (12 neck and 9 distant recurrences) with a 10 year overall RFS of 84%. The 10 year neck and distant RFS was 89% and 93%, respectively. By multivariate analysis, only age was an independent predictor of overall RFS with a risk ratio (RR) [95% confidence interval (CI)] of 3.2 [1.3–8.2]; p=0.01. In regard to neck RFS, none of the variables studied were significant in univariate analysis. Both age and extra-nodal extension were independent factors in predicting distant RFS in multivariate analysis with RR [95% CI] of 5.9 [1.2–28.6]; p=0.03 and 5.2 [1–26.1]; p=0.04, respectively. By univariate analysis, older age and positive margins correlated with decreased DSS (p=0.008 and 0.02 respectively), while there was a trend toward worse DSS with extra-nodal extension (p=0.07).
Effect of RAI therapy on survival
There was no statistical difference within each clinico-pathologic category (e.g., within cases with <3 metastatic nodes) between patients who were treated with RAI and those who were not. It is noteworthy that RAI treated patients as a group harbored more aggressive clinico-pathologic features than those who did not receive RAI therapy. Indeed, patients treated with RAI were significantly older (p<0.001), had larger primary tumor (p=0.02), harbored more aggressive PTC subtype in the primary (p=0.004), were more multicentric (p=0.02), displayed a higher rate of positive margins (p=0.002), and extra-nodal extension (p=0.0001) than their RAI naïve counterparts.
Genotyping of primary tumors
Of the 34 patients who recurred, 15 had available paraffin embedded tissue for genomic analysis. These 15 recurring patients were matched with 21 patients who did not relapse for the following: age, gender, primary tumor size, frequency of tall cell variant in primary, presence and extent of ETE, number of metastatic nodes, and presence of extra-nodal extension (Table 6). We could not match patients for RAI therapy. BRAFV600E mutations were found in 28 (78%) of 36 primary tumors. RET-PTC fusion was found in 3 patients, who all had nonencapsulated classical PTC. These three patients had RET/PTC recombination events not detected by the specific reverse transcriptase PCR reactions for RET/PTC 1 and 3. Five patients were wild type for all the genetic aberrations tested. The clinico-pathologic features of the cases according to BRAFV600E status are displayed in Table 7. Of note, BRAF positivity correlated with the presence of tall cell variant of papillary carcinoma (p=0.01). There was no significant correlation between BRAF positivity and RFS. In regard to DSS, there was a trend toward a decrease in DSS in BRAF positive compared with BRAF negative patients by univariate analysis (p=0.12). Within this group of genotyped tumors, extra-nodal extension correlated with decreased DSS (p=0.02). The combined presence of BRAF and extra-nodal extension provided additive prognostic value. Positivity for both BRAFV600E and extra-nodal extension imparted a lower DSS (79% at 10 years) than negativity for both or either variable (100% at 10 years) (p=0.004) (Fig. 2D).
Table 6.
Clinico-Pathologic Features of 15 Recurring and 21 Nonrecurring Matched Papillary Thyroid Cancer Cases with Nodal Metastasis Subjected to Genotyping
| Recurring (n=15) | Not recurring (n=21) | p-valuea | |
|---|---|---|---|
| Median age, years | 52 | 51 | |
| Female:male ratio | 11/15 (73%) | 13/21 (61%) | 0.7 |
| Median size of primary (cm) | 1.8 | 1.8 | |
| Tall cell frequency in primary tumor | 6/15 (40%) | 8/21 (38%) | 1 |
| ETE present | 12/15 (80%) | 15/21 (71%) | 0.7 |
| ETE extensive | 10/15 (66%) | 12/21 (57%) | 0.7 |
| Median number of metastatic nodes | 6 | 6 | |
| >3 metastatic nodes | 11/15 (73%) | 13/21 (61%) | 0.7 |
| Extra-nodal extension present | 7/14 (50%) | 5/20 (25%) | 0.16 |
| RAI therapy | 7/15 (47%) | 21/21 (100%) | 0.0002 |
Fisher's exact test.
Table 7.
Clinico-Pathologic Features According to BRAFV600E Status in 36 Patients Whose Primary Tumor was Genotyped
| BRAF positive | BRAF negative | pa | |
|---|---|---|---|
| Age | |||
| <45 | 7 (25%) | 5 (62.5%) | 0.086 |
| >45 | 21 (75%) | 3 (37.5%) | |
| Gender | |||
| Male | 11 (39%) | 1 (12.5%) | 0.2 |
| Female | 17 (61%) | 7 (87.5%) | |
| Primary tumor size | |||
| ≤1.5 cm | 12 (43%) | 4 (50%) | 1 |
| >1.5 cm | 16 (57%) | 4 (50%) | |
| PTC subtypes | |||
| Tall cell (n=14) | 14 (50%) | 0 | 0.01b |
| Classical (n=19) | 13 (46%) | 6 (75%) | |
| Infiltrative FVPTC (n=2) | 0 | 2 (25%) | |
| Microcarcinoma, infiltrative (n=1) | 1 (4%) | 0 | |
| No. of positive nodes | |||
| ≤3 | 10 (36%) | 1 (12.5%) | 0.388 |
| >3 | 18 (64%) | 7 (87.5%) | |
| Extra-nodal extension (n=34)c | |||
| Yes | 10 (37%) | 2 (40%) | 1 |
| No | 17 (63%) | 5 (60%) | |
| Size of largest positive node (n=35)d | |||
| ≤1 cm | 14 (51%) | 1 (14%) | 0.1 |
| >1 cm | 13 (49%) | 7 (86%) | |
| Size of largest metastatic focus (n=35)d | |||
| ≤1 cm | 15 (56%) | 2 (25%) | 0.2 |
| >1 cm | 12 (44%) | 6 (75%) | |
| Vascular invasion | |||
| Present | 0 | 1 (12.5%) | 0.22 |
| Absent | 28 (100%) | 7 (87.5%) | |
| ETE | |||
| Extensive | 18 (64%) | 4 (50%) | 0.6 |
| Focal | 2 (7%) | 3 (37.5%) | |
| None | 8 (29%) | 1 (12.5%) | |
Fisher's exact test.
Tall cell vs. nontall cell.
In 2 cases, extra nodal extension could not be evaluated.
In one individual, the size of the metastatic node and largest metastatic focus could not be assessed.
Discussion
The vast majority of PTCs in this study (87%) were infiltrative tumors lacking complete encapsulation. This high frequency is expected in view of the fact that infiltrative variants of PTC, such as tall cell and classical PTC, are known to have a high nodal metastatic rate (16), and constituted almost 75% of our study cohort. Infiltrative follicular variant of papillary thyroid carcinoma (FVPTC) compose a minor fraction of all FVPTC (∼18%), and yet they were overrepresented in this patient population, confirming our previous findings that infiltrative FVPTC correlates with a high rate of metastatic nodes, whereas encapsulated FVPTC rarely metastasize to LN (17). Most of the genotyped tumors in the current series harbored BRAF mutations. This is easily explained by the fact that classical, tall cell, and infiltrative follicular variant tumors, which have a high BRAF mutation rate (16,17), accounted for 97% of our genotyped cases. As shown in previous publications (16), we found a very strong correlation between BRAFV600E positivity and the identification of tall cell variant PTC (p=0.01). The latter subtype of PTC is known to be almost always infiltrative. While BRAF mutation was very common, none of the genotyped cases displayed RAS mutations. The strong correlation between the presence of BRAF mutation, infiltrative variants of PTC, and nodal metastases confirms that BRAFV600E is a marker of invasiveness in thyroid carcinomas, whereas RAS mutation correlates with tumor encapsulation (17). Although the exact mechanism for tumor infiltration in BRAF mutated carcinoma is not known, BRAFV600E seems to promote tumor invasiveness via an increase in metalloproteinases, secreted enzymes that cause extracellular matrix degradation favoring tumor invasion (18,19).
The data just mentioned demonstrate that PTC patients with LNM can be stratified into prognostically relevant categories using traditional clinico-pathologic features. Indeed, in the current study, a simple count of the number of metastatic nodes was an independent and powerful predictor of RFS in patients with PTC. Leboulleux et al. have found that the presence of >3 metastatic nodes correlates with higher recurrence in PTC, but this finding was restricted to those nodes with extra-nodal extension (8). In the papers by Sugitani et al. and Ito et al., the presence of five or more metastatic nodes was an independent predictor of recurrence but only in young patients (9,20). Our results confirm the importance of the number of metastatic nodes in predicting recurrence, and extend the findings of previous publications. Indeed, we found that the number of metastatic nodes, irrespective of whether they also had extra-nodal extension, is an independent variable for recurrence not only in young patients but also in older individuals. Although the presence of a large size metastatic node correlated with worse RFS in our series, the size of the largest metastatic tumor focus was unable to predict outcome in patients with PTC. This lack of correlation could be due to the difficulty in assessing metastatic tumor burden in a LN using a unidimensional measurement of the largest tumor focus. The latter may not be an accurate representation of metastatic tumor volume. While extra-nodal extension was a predictor of recurrence and more importantly death of disease, one should acknowledge that in practice the microscopic analysis of extra-nodal extension is subject to significant inter-observer variability due to lack of stringent criteria for its recognition.
Perhaps, one of the most important and interesting finding in this study is the lack of recurrence in any of the 45 young patients with 1–2 metastatic nodes including 26 patients followed for a median of 13 years without RAI therapy. While these results provide reassuring data that the simple presence of LNM in young patients does not convey a high risk of recurrence, these findings, however, cannot be used to study the effect of RAI therapy on survival, as RAI-treated patients as a group harbored much more aggressive clinico-pathologic features than those who did not receive RAI therapy.
While many papers have analyzed the prognostic value of BRAF and its relationship with nodal metastasis (21–25), none have assessed its value in stratifying patients with N1 disease. In that regard, we found a trend toward worse DSS in those patients with BRAFV600E mutations. The prognostic value of BRAFV600E was markedly increased when combined with the presence of extra-nodal extension. Indeed, the correlation between positivity for both parameters and death of disease (p=0.004) is much stronger than the one predicted by the single analysis of BRAF (p=0.12) or extra-nodal extension (p=0.02).This could be due to the fact that tumor initiation by oncogenic BRAF is a required but insufficient event for progression of BRAF positive PTC. Indeed, Knauf et al. have shown in a preclinical model that progression of BRAF-induced thyroid cancer to a more aggressive phenotype is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFβ signaling transition (26). Similarly, an oncogenic event responsible or associated with extra-nodal extension may have increased the metastatic potential of the BRAF positive tumors in this study leading to higher death rate. Alternatively, the presence of extra-nodal extension may be a marker of tumor cell dissemination. Patients with BRAFV600E tumor without extra-nodal extension may have locoregional confined disease that is cured by surgery, while those with BRAF positive tumor with extra-nodal extension have already disseminated and are difficult to treat in view of the propensity of BRAF tumor cells to be resistant to RAI therapy (27). Since almost all the genotyped cases in this study were infiltrative PTC and the vast majority were BRAFV600E positive, this survival data suggest that BRAF mutation when combined with extra-nodal extension has prognostic value for DSS even within infiltrative PTC.
In conclusion, simple histologic parameters such as the number of metastatic nodes are able to stratify patients with N1 disease and may have important clinical implications. It may help identify a subset of patients at low risk (similar to the one with N0 or Nx disease) that may not require RAI administration sparing numerous young patients the side effects and costs of such therapy. Although the data are conflicting as to whether adjuvant therapy improves outcome in N1 disease (1), the results of this study will help restrict the use of adjuvant RAI therapy to those patients with N1 disease at highest risk of recurrence. The presence of BRAFV600E in the primary tumor when combined with extra-nodal extension seems to have some additive prognostic value in predicting death. The findings just mentioned should prompt re-evaluation of classification systems that assign the same magnitude of risk of recurrence or death to all patients with N1 disease.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. Mclver B. Pacini F. Schlumberger M. Sherman SI. Stewart DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Edge SB, editor; Byrd DR, editor; Compton CC, editor; Fritz AG, editor; Greene FL, editor; Trotti A, editor. AJCC Cancer Staging Manual. 7th. Springer; New York, NY: 2010. pp. 67–74. [Google Scholar]
- 3.Hughes DT. White ML. Miller BS. Gauger PG. Burney RE. Doherty GM. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. 2010;148:1100–1106. doi: 10.1016/j.surg.2010.09.019. discussion 1006–1007. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet S. Hartl D. Leboulleux S. Baudin E. Lumbroso JD. Al Ghuzlan A. Chami L. Schlumberger M. Travagli JP. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab. 2009;94:1162–1167. doi: 10.1210/jc.2008-1931. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle RM. Tala H. Shah J. Leboeuf R. Ghossein R. Gonen M. Brokhin M. Omry G. Fagin JA. Shaha A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American thyroid association staging system. Thyroid. 2010;20:1341–1349. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SEER Survival Monograph: Cancer Survival Among Adults: U.S., SEER Program, 1988–2001, Patient and Tumor Characteristics. In: Ries LAG, editor; Young JL, editor; Keel GE, editor; Eisner MP, editor; Lin YD, editor; Horner M-J, editor. National Cancer Institute, SEER Program; Bethesda, MD: 2007. NIH Pub. No. 07–6215. [Google Scholar]
- 7.Bardet S. Malville E. Rame JP. Babin E. Samama G. De Raucourt D. Michels JJ. Reznik Y. Henry-Amar M. Macroscopic lymph-node involvement and neck dissection predict lymph-node recurrence in papillary thyroid carcinoma. Eur J Endocrinol. 2008;158:551–560. doi: 10.1530/EJE-07-0603. [DOI] [PubMed] [Google Scholar]
- 8.Leboulleux S. Rubino C. Baudin E. Caillou B. Hartl DM. Bidart JM. Travagli JP. Schlumberger M. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005;90:5723–5729. doi: 10.1210/jc.2005-0285. [DOI] [PubMed] [Google Scholar]
- 9.Sugitani I. Kasai N. Fujimoto Y. Yanagisawa A. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004;135:139–148. doi: 10.1016/s0039-6060(03)00384-2. [DOI] [PubMed] [Google Scholar]
- 10.Cranshaw IM. Carnaille B. Micrometastases in thyroid cancer. An important finding? Surg Oncol. 2008;17:253–258. doi: 10.1016/j.suronc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 11.DeLellis RA, editor; Lloyd RV, editor; Heitz PU, editor; Eng C, editor. World Health Organization Classification of Tumours., Pathology & Genetics Tumours of the Endocrine Organs. IARC Press; Lyon: 2004. [Google Scholar]
- 12.Hiltzik D. Carlson DL. Tuttle RM. Chuai S. Ishill N. Shaha A. Shah JP. Singh B. Ghossein RA. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106:1286–1295. doi: 10.1002/cncr.21739. [DOI] [PubMed] [Google Scholar]
- 13.Rosai J. Carcangiu ML. Delellis RA. Tumors of the thyroid gland. In: Rosai J, editor; Sobin LH, editor. Atlas of Tumor Pathology. Vol. 5. Armed Forces Institute of Pathology; New York: 1992. pp. 161–182. [Google Scholar]
- 14.Ricarte-Filho JCM. Ryder M. Chitale DA. Rivera M. Heguy A. Ladanyi M. Janakiraman M. Solit D. Knauf JA. Tuttle RM. Ghossein RA. Fagin JA. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA and AKT1. Cancer Res. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imkamp F. von Wasielewski R. Musholt TJ. Musholt PB. Rearrangement analysis in archival thyroid tissues: punching microdissection and artificial RET/PTC 1–12 transcripts. J Surg Res. 2007;143:350–363. doi: 10.1016/j.jss.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Adeniran AJ. Zhu Z. Gandhi M. Steward DL. Fidler JP. Giordano TJ. Biddinger PW. Nikiforov YE. Correlation between genetic alterations and microscopic features. Clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–222. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 17.Rivera M. Ricarte-Filho J. Knauf J. Shaha A. Tuttle RM. Fagin JA. Ghossein RA. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histologic subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23:1191–1200. doi: 10.1038/modpathol.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesa C., Jr. Mirza M. Mitsutake N. Sartor M. Medvedovic M. Tomlinson C. Knauf JA. Weber GF. Fagin JA. Conditional activation of RET/PTC3 and BRAFV600E in thyroid cells is associated with gene expression profiles that predict a preferential role of BRAF in extracellular matrix remodeling. Cancer Res. 2006;66:6521–6529. doi: 10.1158/0008-5472.CAN-06-0739. [DOI] [PubMed] [Google Scholar]
- 19.Frasca F. Nucera C. Pellegriti G. Gangemi P. Attard M. Stella M. Loda M. Vella V. Giordano C. Trimarchi F. Mazzon E. Belfiore A. Vigneri R. BRAFV600E mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008;15:191–205. doi: 10.1677/ERC-07-0212. [DOI] [PubMed] [Google Scholar]
- 20.Ito Y. Fukushima M. Tomoda C. Inoue H. Kihara M. Higashiyama T. Uruno T. Takamura Y. Miya A. Kobayashi K. Matsuzuka F. Miyauchi A. Prognosis of patients with papillary thyroidcarcinoma having clinically apparent metastasis to the lateral compartment. Endocr J. 2009;56:759–766. doi: 10.1507/endocrj.k09e-025. [DOI] [PubMed] [Google Scholar]
- 21.Kim TY. Kim WB. Rhee YS. Song JY. Kim JM. Gong G. Lee S. Kim SY. Kim SC. Hong SJ. Shong YK. The BRAF mutation is useful for prediction of clinicalrecurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol. 2006;65:364–368. doi: 10.1111/j.1365-2265.2006.02605.x. [DOI] [PubMed] [Google Scholar]
- 22.Jung CK. Kang YG. Bae JS. Lim DJ. Choi YJ. Lee KY. Unique patterns of tumor growth related with the risk of lymph node metastasis in papillary thyroid carcinoma. Mod Pathol. 2010;23:1201–1208. doi: 10.1038/modpathol.2010.116. [DOI] [PubMed] [Google Scholar]
- 23.Kebebew E. Weng J. Bauer J. Ranvier G. Clark OH. Duh QY. Shibru D. Bastian B. Griffin A. The Prevalence and Prognostic Value of BRAF Mutation in Thyroid Cancer. Ann Surg. 2007;246:466–471. doi: 10.1097/SLA.0b013e318148563d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frasca F. Nucera C. Pellegriti G. Gangemi P. Attard M. Stella M. Loda M. Vella V. Giordano C. Trimarchi F. Mazzon E. Belfiore A. Vigneri R. BRAFV600E mutation and the biology of papillary thyroid cancer. Endocr-Relat Cancer. 2008;15:191–205. doi: 10.1677/ERC-07-0212. [DOI] [PubMed] [Google Scholar]
- 25.Sykorova V. Dvorakova S. Ryska A. Vcelak J. Vaclavikova E. Laco J. Kodetova D. Kodet R. Cibula A. Duskova J. Hlobilkova A. Astl J. Vesely D. Betka J. Hoch J. Smutny S. Cap J. Vlcek P. Novak Z. Bendlova B. BRAFV600E mutation in the pathogenesis of a large series of papillary thyroid carcinoma in Czech Republic. J Endocrinol Invest. 2010;33:318–324. doi: 10.1007/BF03346593. [DOI] [PubMed] [Google Scholar]
- 26.Knauf JA. Sartor MA. Medvedovic M. Lundsmith E. Ryder M. Salzano M. Nikiforov YE. Giordano TJ. Ghossein RA. Fagin JA. Progression of BRAF-induced thyroid cancer is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFβ signaling. Oncogene. 2011;30:3154–3162. doi: 10.1038/onc.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321:86–93. doi: 10.1016/j.mce.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]