Abstract
Background: In addition to its role in adaptive thermogenesis, brown adipose tissue (BAT) may protect from weight gain, insulin resistance/diabetes, and metabolic syndrome. Prior studies have shown contradictory results regarding the influence of thyroid hormone (TH) levels on BAT volume and activity. The aim of this pilot study was to gain further insights regarding the effect of TH treatment on BAT function in adult humans by evaluating the BAT mass and activity prospectively in six patients, first in the hypothyroid and then in the thyrotoxic phase.
Methods: The study subjects underwent 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) scanning after cold exposure to measure BAT mass and activity while undergoing treatment for differentiated thyroid cancer, first while hypothyroid following TH withdrawal at the time of the radioactive iodine treatment and then three to six months after starting TH suppressive treatment when they were iatrogenically thyrotoxic. Thermogenic and metabolic parameters were measured in both phases.
Results: All study subjects had detectable BAT under cold stimulation in both the hypothyroid and thyrotoxic state. The majority but not all (4/6) subjects showed an increase in detectable BAT volume and activity under cold stimulation between the hypothyroid and thyrotoxic phase (total BAT volume: 72.0 ± 21.0 vs. 87.7 ± 16.5 mL, p = 0.25; total BAT activity 158.1 ± 72.8 vs. 189.0 ± 55.5 SUV*g/mL, p = 0.34). Importantly, circulating triiodothyronine was a stronger predictor of energy expenditure changes compared with cold-induced BAT activity.
Conclusions: Iatrogenic hypothyroidism lasting two to four weeks does not prevent cold-induced BAT activation, while the use of TH to induce thyrotoxicosis does not consistently increase cold-induced BAT activity. It remains to be determined which physiological factors besides TH play a role in regulating BAT function.
Keywords: : brown adipose tissue, thyroid hormone, hypothyroidism, thyrotoxicosis, thermogenesis
Introduction
In the midst of worldwide obesity and diabetes epidemics, it is crucial to find new avenues to gain insight into the pathophysiology and treatment of these disorders. Brown adipose tissue (BAT) is of particular interest, since in addition to its role in adaptive thermogenesis, it may also protect against weight gain, insulin resistance/diabetes, and metabolic syndrome (1–3). Indeed, because of its very high metabolic activity, very little functional BAT can have a profound metabolic impact (4,5).
Studies using positron emission tomography/computed tomography (PET/CT) scanning have shown metabolically active BAT in adult humans whose activity correlated inversely with body fat (5–8). Significant amounts of functional BAT have been detected in >95% of lean subjects in response to acute and chronic cold exposure in controlled prospective studies (5,8–10). Since BAT activity is induced by cold exposure, BAT might play an important role in human thermogenesis and energy balance. This is in accordance with studies in small rodents, which showed that cold exposure stimulates BAT activity by increasing sympathetic nerve activity and by affecting local thyroid hormone (TH) metabolism (11,12). Cold exposure increases the expression and activity of tissue deiodinase 2 (DIO2), which stimulates the conversion of thyroxine (T4) to active triiodothyronine (T3), resulting in increased local T3 production from T4 and TH receptor saturation (13). Both catecholamines and local T3 act synergistically to stimulate uncoupling protein 1 (UCP-1) expression and through this thermogenesis in BAT. UCP-1 uncouples oxidative phosphorylation, thus representing the key protein in BAT thermogenesis (12). TH also has direct effects on oxidative phosphorylation and regulates the mitochondrial proliferation, differentiation, and maturation through genomic and non-genomic mechanisms (14,15). In addition, TH is known to play an important role in brown adipocyte differentiation (16,17). Thus, TH has been shown to have an important role in cold adaptation in rodents, and it may be important for temperature adaptation in humans (18). Understanding cold adaptation in adult humans is important, since factors that increase energy dissipation through facultative thermogenesis could potentially be used as anti-obesity agents (18,19). If TH can influence BAT mass and activity and through this energy expenditure, then TH or its analogues might be useful in the treatment of obesity and metabolic syndrome.
A few published human studies have shown contradictory results reporting increased BAT volume and activity in either hypothyroid or thyrotoxic subjects (20–23). To gain further insight regarding the effect of TH status on BAT in adult humans and whether it could be a feasible treatment strategy for activating BAT thermogenesis, this study evaluated the BAT mass and activity prospectively in six patients who were undergoing treatment for thyroid cancer, first in the hypothyroid and then in the thyrotoxic phase. The study patients underwent thyroidectomy followed by TH withdrawal to induce hypothyroidism prior to radioactive iodine (RAI) treatment and then started TH suppressive treatment with the goal of maintaining mild thyrotoxicosis long-term to prevent recurrence. The study patients underwent two PET-CT scans to measure BAT mass and activity, first while profoundly hypothyroid at the time of the RAI treatment and then three to six months after starting TH suppressive treatment when they were thyrotoxic. The BAT mass and activity measured on these two scans were compared. The relationship between BAT, TH status, and calorimetric and metabolic parameters were also evaluated.
Materials and Methods
Study participants
Eight patients diagnosed with papillary thyroid cancer who underwent thyroidectomy and were scheduled to receive RAI treatment after TH withdrawal were recruited for the study. The study was approved by the Institutional Review Board of Beth Israel Deaconess Medical Center (BIDMC) and Joslin Diabetes Center, and patients provided written informed consent. Exclusion criteria included use of medications that can affect BAT, such as beta blockers, adrenergic agonists, benzodiazepines; use of medications that can affect thyroid function test interpretation, including estrogen, aspirin, phenytoin; or use of systemic, oral, or intravenous corticosteroids or other medications known to cause insulin resistance in the previous six weeks. Patients with a history of any local or systemic infectious disease with fever or requiring antibiotic within four weeks of the PET/CT scans were not enrolled in the study.
Study protocol
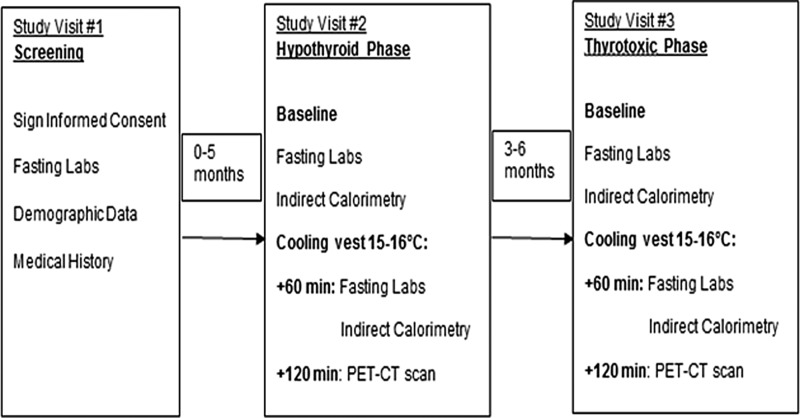
The study protocol is shown in Figure 1. The patients participated in a short screening visit to evaluate eligibility prior to RAI treatment followed by two longer study visits when the whole-body BAT volume and activity were measured by 18F-fluorodeoxyglucose (18F-FDG) PET/CT under cold stimulation at 15–16°C, first in the hypothyroid state at the time of RAI treatment and then in the thyrotoxic state three to six months after starting TH suppressive treatment. Six out of the eight patients who started the study completed all study visits and were included in the analysis. Prior to participating in the study, one patient underwent completion thyroidectomy for a thyroid nodule positive for papillary thyroid carcinoma on fine-needle aspiration cytology, while five patients underwent hemithyroidectomy followed by completion thyroidectomy for thyroid nodules with fine-needle aspiration showing indeterminate cytology (two patients), for a large thyroid nodule with benign cytology (one patient), for a large thyroid nodule with non-diagnostic cytology (one patient), and for recurrent thyroid cancer after undergoing lobectomy in the remote past (one patient). Preparation for the hypothyroid phase of the evaluation occurred at least six weeks postoperatively for five patients and consisted of a six-week L-thyroxine (LT4) withdrawal with a four-week liothyronine bridging support (12.5 μg twice daily) to minimize clinical symptoms followed by two weeks of complete TH withdrawal and a low-iodine diet prior to the clinically indicated 131I administration. One study patient was already hypothyroid at the time of her initial endocrine clinic visit with a thyrotropin (TSH) level of 22 μIU/mL (reference range 0.27–4.2 μIU/mL). LT4 was discontinued at that time, and the patient received RAI treatment two weeks later without the liothyronine bridging support.
FIG. 1.
Study protocol.
The patients were prepared for the study visits, as previously described (24,25). Baseline vital signs and laboratory tests (thyroid function tests, insulin, HbA1c, and lipid panel) were obtained. Baseline indirect calorimetry was performed during both the hypothyroid and thyrotoxic phase after the patients were placed in a supine position and quiet environment for 30 minutes in the Clinical Research Center (CRC). The room temperature was maintained >23°C throughout the stay in the CRC. Core body temperature was monitored during the study visits.
To maximize the BAT activity/FDG uptake and PET-CT scan BAT visualization, the patients were then transported to a room set at 20°C and wore a surgeon's cooling vest (Polar Products, Inc.) set to 15–16°C, as described previously (24,25). Sixty minutes after wearing the vest, a second set of vital signs and laboratory tests (thyroid function tests, glucose, insulin, and free fatty acids [FFAs]) were obtained, and an intravenous bolus of 592 MBq (16 mCi) of 18F-FDG was administered. This dose was calculated based on the sensitivity of the scanner available at the time of the study. The patients remained in the semi-darkened quiet room for another 60 minutes while wearing the cooling vest, and at this time, a second measure of indirect calorimetry was performed. Following 120 minutes of cold exposure, the vest was removed, and BAT images were acquired using a 4-MDCT scanner (Discovery/LightSpeed PET/CT; GE Healthcare) following a previously described imaging protocol (24). BAT volume, metabolic activity, and maximum standardized uptake value (SUVmax) were measured in the cervical, supraclavicular, and anterior thoracic depots from vertebral level C3 to T7 using the PET-CT Viewer shareware, as described previously (24). The BAT metabolic activity represents the primary endpoint of this study and is calculated in a three-step process. First, in each axial slice on CT, a voxel is defined as containing adipose tissue if it has a HU density between −250 and −10. Second, the amount of retained FDG in each voxel is quantified by measuring the mean amount of positron-derived gamma radiation in the voxel compared to the injected dose per body mass (MBq/mL of voxel)/(MBq/g of body mass), giving the “meanSUV*g/mL.” Finally, the total-body metabolic activity is calculated by taking the sum of the volume of BAT from each voxel = “mL*meanSUV*g/mL.” As expected, according to the method used to quantify BAT parameters, there was a strong positive correlation between the cold-induced BAT volume and activity of the study subjects in the hypothyroid phase (ρ = >0.99; p = 0.01), as well as thyrotoxic phase (ρ = 0.94; p = 0.005). SUVmax reflects the highest amount of retained FDG anywhere within the tissue. This metric was used to compare BAT activity with more homogeneous tissues such as liver and skeletal muscle. The SUVmax of the FDG uptake in the right lobe of the liver and skeletal muscle (erector spinae) was also measured.
Physiological and laboratory parameters
Blood pressure (BP) and heart rate (HR) were measured using a SureSigns VS3 vital signs monitor (Philips Healthcare). Core body temperature was measured with a temporal artery thermometer (Exergen Co.). Indirect calorimetry measurements, including the resting metabolic rate (RMR), energy expenditure (EE), respiratory quotient (RQ), and substrate utilization, were calculated using Sensormedics VMAX Encore equipment (VIASYS Respiratory Care, Inc.), as well as techniques well established in the laboratory and standardized values. Insulin and TSH were measured according to standard procedures at the Harvard Catalyst CRC (http://catalyst.harvard.edu/services/hccrc-lab/). Total T4, total T3, free T3, T3 uptake, free T4 index, thyroxin binding globulin (TBG), FFAs, lipid panel, and HbA1c were measured using standard procedures at the Laboratory Corporation of America. Glucose was measure at the BIDMC clinical laboratory.
Statistical analysis
This pilot study was designed to evaluate whether the TH status had an effect on BAT mass and activity in adult humans, specifically whether treatment with TH increases BAT mass and activity as the subjects transitioned from hypothyroidism to thyrotoxicosis. It was anticipated that consenting and screening would take place for up to 10 patients, so that six to eight patients would complete all segments of the study (completion rate of 80%).
Data were analyzed using IBM SPSS Statistics for Windows v13.0 (IBM Corp.) and SAS (SAS Institute). All p-values presented are two-tailed, and values ≤0.05 were considered to indicate statistical significance, while values <0.10 were considered to represent a trend to achieve significance. Changes in BAT volume, activity, and maximum standardized uptake value (SUVmax), as well as hormonal and metabolic changes in the study patients between the hypothyroid and thyrotoxic state were evaluated using the non-parametric Wilcoxon signed-rank test. Associations between BAT volume and activity and TH and metabolic parameters were evaluated by Spearman correlation.
Results
The baseline characteristics of the six patients who completed the study are summarized in Table 1. Five patients had a normal baseline TSH prior to being enrolled in the study. One patient who was enrolled at the time of her completion thyroidectomy had a low baseline TSH of 0.19 μIU/mL (reference range 0.27–4.2 μIU/mL) on TH treatment prior to starting the study. All patients were hypothyroid after TH withdrawal at the time of the first study PET/CT scan. In four out of the six study patients, the final TSH was in the thyrotoxic range, while the fifth patient had a TSH in the low reference range at 0.38 μIU/mL three to six months after starting TH suppressive treatment at the time of the second PET/CT scan. However, the sixth patient had a final TSH of 5.46 μIU/mL. Therefore, although the mean was in the euthyroid range, all patients but one were thyrotoxic at the time of the second PET/CT scan.
Table 1.
Baseline Characteristics of the Study Subjects
| Mean ± SEM | Range | |
|---|---|---|
| Sex | 1 M/5 F | |
| Age (years) | 43.5 ± 4.7 | 22-53 |
| Weight (kg) | 75.3 ± 5.7 | 60.6–98.8 |
| Body mass index (kg/m2) | 25.45 ± 2.5 | 19.6–35.85 |
| Baseline TSH (μIU/mL) | 1.36 ± 0.66 | 0.19–2.09 |
| Thyroid cancer TNM stage | I–IVA |
SEM, standard error of the mean; M, male; F, female; TSH, thyrotropin; TNM, tumor node metastasis.
Thyroid function tests and metabolic parameters
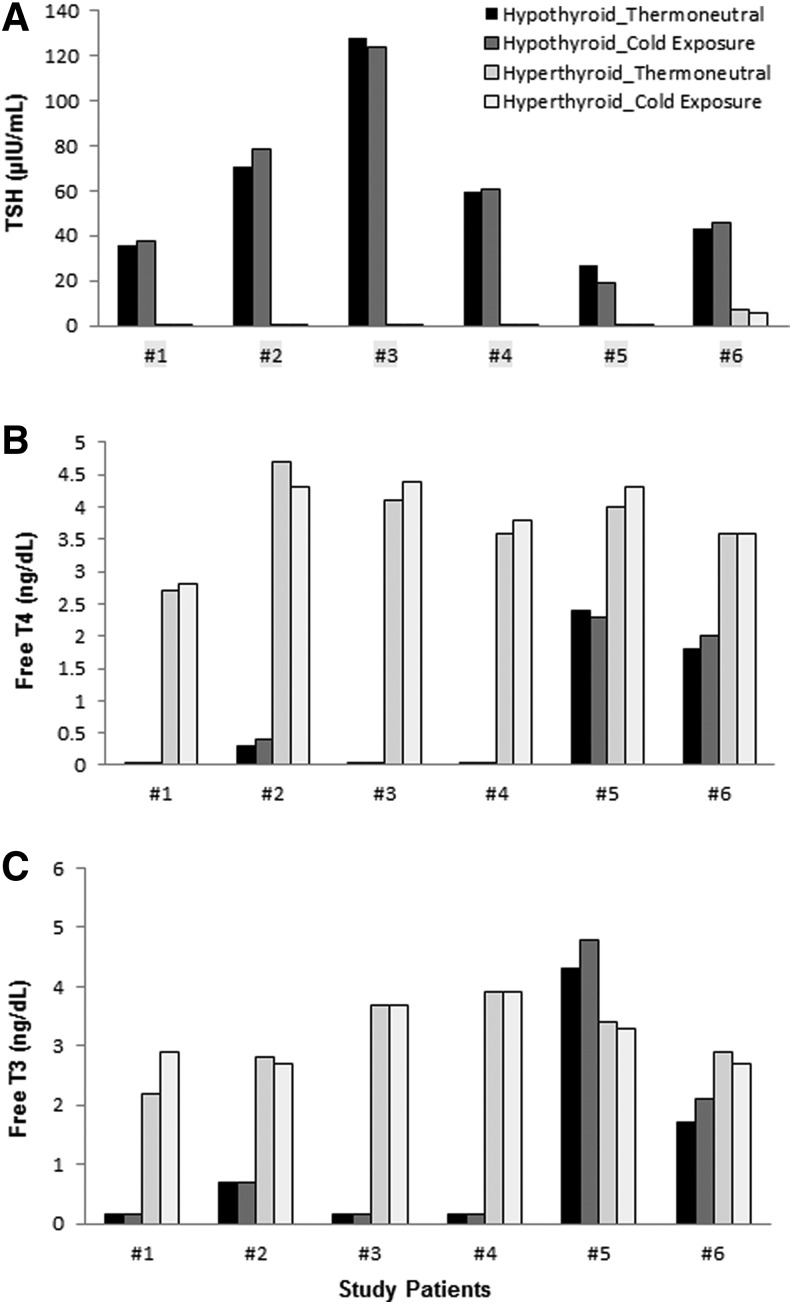
TSH was higher while total T4, free T4 index, free T3, and T3 uptake were lower in the hypothyroid compared with the thyrotoxic state (trend only for free T3; Fig. 2A–C and Table 2). There was no significant difference in the total T3 between the hypothyroid and thyrotoxic state. There was no significant change in any thyroid function test after 60 minutes of cold exposure during each state. TBG levels did not change between the hypothyroid and thyrotoxic states. Total and low-density lipoprotein (LDL) cholesterol levels were higher in the hypothyroid compared with the thyrotoxic state. There were no significant changes in fasting triglyceride, free fatty acids, glucose, insulin, and HbA1c between the hypothyroid and thyrotoxic states (Table 2).
FIG. 2.
Changes in thyroid function in individual subjects measured at room temperature and after 60 minutes of cold exposure (15–16°C) in the hypothyroid state and then in the thyrotoxic state. (A) Changes in serum thyrotropin (TSH). (B) Changes in serum free thyroxine. (C) Changes in serum free triiodothyronine (T3).
Table 2.
Changes in the Subject Characteristics Measured at Room Temperature and After Cold Exposure (15–16°C) in the Hypothyroid State and then in the Thyrotoxic State
| Characteristic (units) | Hypothyroid room temperature | Hypothyroid cold exposure | Thyrotoxic room temperature | Thyrotoxic cold exposure |
|---|---|---|---|---|
| Temperature (°C) | 36.7 ± 0.1 | 36.2 ± 0.2 | 36.5 ± 0.1 | 36.5 ± 0.2 |
| Systolic BP (mmHg) | 118.3 ± 4.0 | 127.6 ± 3.2 | 118.5 ± 4.3 | 126.2 ± 5.8 |
| Diastolic BP (mmHg) | 75.5 ± 3.5 | 84.0 ± 2.9a | 71.2 ± 2.5 | 78.7 ± 2.5b,d |
| Heart rate (bpm) | 66.8 ± 3.5 | 60.0 ± 1.5a | 64.5 ± 4.4 | 65.2 ± 5.7 |
| Free fatty acids (mEq/L) | — | 0.63 ± 0.09 | — | 0.62 ± 0.07 |
| Triglycerides (mg/dL) | 99.8 ± 28.4 | — | 84.2 ± 20.0 | — |
| Total cholesterol (mg/dL) | 279.7 ± 46.3 | — | 201.3 ± 24.6c | — |
| LDL cholesterol (mg/dL) | 173.2 ± 39.6 | — | 110.7 ± 23.3c | — |
| HDL cholesterol (mg/dL) | 86.5 ± 6.8 | — | 74.2 ± 6.6 | — |
| Glucose (mg/dL) | — | 81.5 ± 4.8 | — | 84.7 ± 3.8 |
| Insulin (μIU/mL) | 6.5 ± 2.0 | 5.6 ± 1.9 | 6.4 ± 2.5 | 5.4 ± 1.6 |
| Hemoglobin A1c (%) | 5.6 ± 0.2 | — | 5.6 ± 0.03 | — |
| TSH (μIU/mL) | 60.7 ± 14.9 | 60.8 ± 15.0 | 1.2 ± 1.1c | 1.0 ± 0.9d |
| Total T4 (μg/dL) | 2.7 ± 1.4 | 2.9 ± 1.5 | 10.6 ± 0.8c | 11.0 ± 0.9d |
| Free T4 index | 0.77 ± 0.4 | 0.81 ± 0.43 | 3.8 ± 0.3c | 3.9 ± 0.25d |
| Total T3 (ng/dL) | 49.7 ± 24.3 | 53.3 ± 26.1 | 102.2 ± 14.5 | 106.0 ± 12.3 |
| Free T3 (pg/mL) | 1.2 ± 0.7 | 1.3 ± 0.8 | 3.2 ± 0.3c | 3.2 ± 0.2 |
| T3 uptake (%) | 25.8 ± 1.7 | 25.7 ± 1.5 | 36.2 ± 1.6c | 35.3 ± 1.5d |
| Thyroxin binding globulin (μg/mL) | — | 18.2 ± 2.7 | — | 14.2 ± 1.6 |
| Resting metabolic rate (kcal/day) | 1299.0 ± 57.7 | 1497.2 ± 106.7a | 1470.0 ± 81.9c | 1612.8 ± 46.9b |
| Respiratory quotient | 0.82 ± 0.02 | 0.80 ± 0.02 | 0.84 ± 0.03 | 0.81 ± 0.02 |
Values represent mean ± SEM. Vitals, laboratory tests, and indirect calorimetry were measured after 60 minutes of cold exposure, while BAT activity, volume, and SUVmax were measured after 120 minutes of cold exposure at 15–16°C.
p ≤ 0.05, hypothyroid state room temperature vs. hypothyroid state after cold exposure.
p ≤ 0.05, thyrotoxic state room temperature vs. thyrotoxic state after cold exposure.
p ≤ 0.05, hypothyroid state room temperature vs. thyrotoxic state room temperature.
p ≤ 0.05, hypothyroid state after cold exposure vs. thyrotoxic state after cold exposure.
BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; T4, thyroxine; T3, triiodothyronine; BAT, brown adipose tissue; SUVmax, maximum standardized uptake value.
Vital signs
Systolic pressure BP did not change significantly during the study. Diastolic BP increased after 60 minutes of cold exposure during both the hypothyroid and thyrotoxic states, the levels after cold exposure being higher in the hypothyroid compared with the thyrotoxic state. HR decreased significantly after 60 minutes of cold exposure in the hypothyroid but not the thyrotoxic phase. There were no other significant changes in vital signs measured at room temperature and after 60 minutes of cold exposure during the hypothyroid state as well as the thyrotoxic state or between the two states (Table 2).
Energy expenditure
The baseline RMR of the study subjects measured at room temperature was higher in the thyrotoxic state compared with the hypothyroid state. The RMR increased after 60 minutes of cold exposure during both the hypothyroid and thyrotoxic states. There was no difference between the RMR measured in the two states after cold exposure. There were no other significant changes in RMR or RQ measured at room temperature and after 60 minutes of cold exposure during the hypothyroid state as well as the thyrotoxic state or between the two states (Table 2). There was a positive correlation between the RQ measured at room temperature and after 60 minutes of cold exposure in the hypothyroid state (ρ = 0.83; p = 0.04). There was no correlation between the RMR measured at room temperature and after 60 minutes of cold exposure during each state.
Glucose uptake in BAT and other tissues
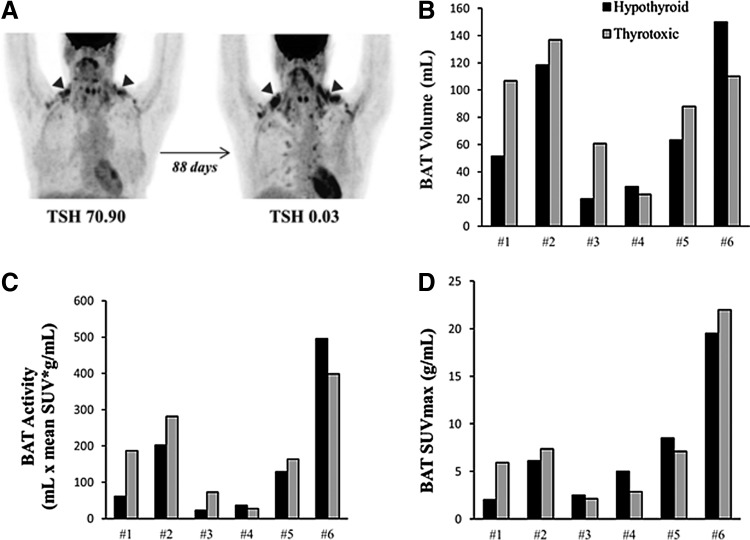
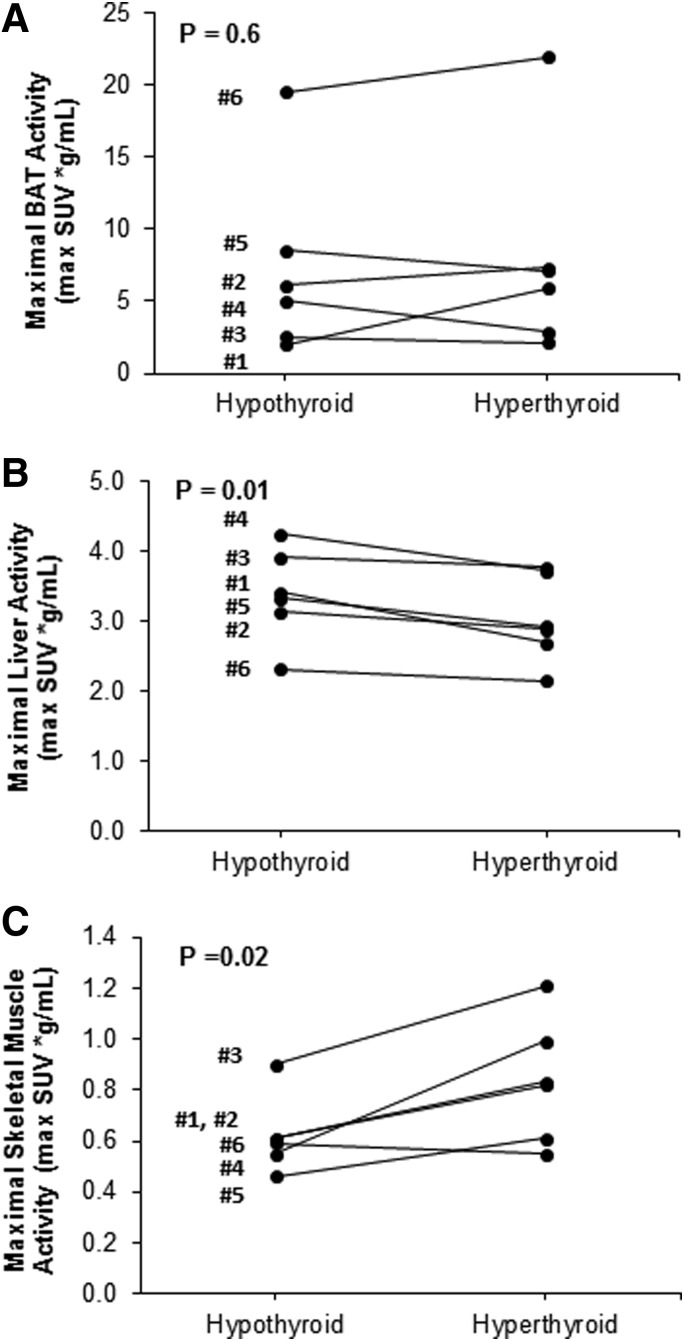
All patients had detectable BAT after acute cold exposure in both the hypothyroid state and the thyrotoxic state (Fig. 3A–D and Table 3). Other tissues showed significant but small changes in glucose uptake. The SUVmax of the liver decreased (3.39 ± 0.27 vs. 3.03 ± 0.25 g/mL; p = 0.01), while the SUVmax of the skeletal muscle increased (0.62 ± 0.06 vs. 0.84 ± 0.10 g/mL; p = 0.02), when going from the hypothyroid to the thyrotoxic phase (Fig. 4A–C).
FIG. 3.
Changes in brown adipose tissue (BAT) volume, activity, and maximum standardized uptake value (SUVmax) in individual subjects measured after 120 minutes of cold exposure (15–16°C) in the hypothyroid state and then in the thyrotoxic state. (A) Brown fat 18F-fluorodeoxyglucose (FDG) uptake in a study subject (53 years old, female, body mass index 19.6 kg/m2) illustrated using coronal representations of positron emission tomography in the hypothyroid state (TSH = 70.90 μIU/mL) and then in the thyrotoxic state (TSH = 0.03 μIU/mL), the black arrows pointing to the right and left supraclavicular BAT depots. (B) Changes in BAT volume. (C) Changes in BAT activity. (D) Changes in BAT SUVmax.
Table 3.
Changes in BAT Volume, Activity, and SUVmax Measured After 120 minutes of Cold Exposure (15–16°C) in the Hypothyroid State and in the Thyrotoxic State in the Six Study Subjects
| Mean ± SEM | Range | |
|---|---|---|
| BAT volume (mL) | ||
| Hypothyroid | 72.0 ± 21.0 | 20–150 |
| Thyrotoxic | 87.7 ± 16.5 | 23.5–136.9 |
| Change | 15.7 ± 13.9 | −39.8 to 55.3 |
| % Change | 53.35 ± 35.9% | −26.5% to 203.4% |
| BAT activity (mL × mean SUV* g/mL) | ||
| Hypothyroid | 158.1 ± 72.8 | 23.1–495.8 |
| Thyrotoxic | 189.0 ± 55.5 | 27.85–398.8 |
| Change | 30.9 ± 31.5 | −97 to 125.85 |
| % Change | 74.35 ± 44.5% | −23.8% to 218.2% |
| BAT SUVmax (g/mL) | ||
| Hypothyroid | 7.3 ± 2.6 | 2.0–19.5 |
| Thyrotoxic | 7.9 ± 2.95 | 2.1–22 |
| Change | 0.6 ± 0.95 | −2.15 to 3.9 |
| % Change | 25.6 ± 35.1% | −42.8% to 195% |
% Change from the baseline hypothyroid value.
FIG. 4.
Changes in 18F-FDG uptake between the hypothyroid and hyperthyroid state measured in different tissues in individual study subjects. (A) BAT. (B) Liver. (C) Skeletal muscle.
The majority but not all (4/6) patients showed an increase in detectable, acute, cold-induced BAT volume and activity between the hypothyroid and thyrotoxic states. The increases were not correlated with the seasonal trends in outdoor temperatures the patients experienced while they were taking TH. The change in BAT volume, activity, and SUVmax between the two states were not significant. In the two patients who did not show an increase in BAT parameters between the hypothyroid and thyrotoxic state, the RMR also did not change after 60 minutes of cold exposure in both the hypothyroid state and the thyrotoxic state. However, there was a positive correlation between the BAT volume as well as BAT activity measured after cold exposure in the hypothyroid and thyrotoxic states (BAT volume: ρ = 0.83, p = 0.04; BAT activity: ρ = 0.89, p = 0.02).
There was a negative correlation between the cold-induced BAT volume as well as activity and the subjects' weight and body mass index in both the hypothyroid and thyrotoxic states. There was no correlation between the BAT parameters and the subjects' age and core body temperature. The baseline systolic BP measured in the hypothyroid state correlated strongly and positively with the BAT mass and activity measured in both states.
Interrelationships among metabolic measures
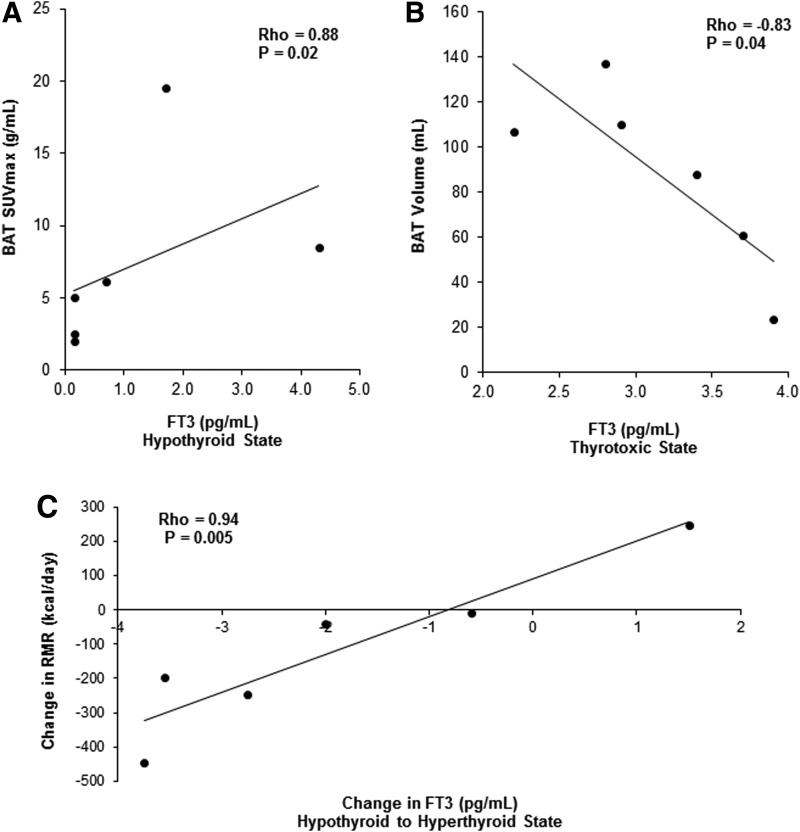
Total T4, free T4 index, total T3, and free T3 correlated positively with the cold-induced BAT SUVmax in the hypothyroid state (all variables in hypothyroid state: ρ = 0.88, p = 0.02; Fig. 5A). Free T3 correlated negatively with the cold-induced BAT volume and activity in the thyrotoxic state (free T3 at room temperature/BAT volume: ρ = –0.83, p = 0.04; free T3 at room temperature/BAT activity: ρ = –0.77, p = 0.07; freeT3 after cold exposure/BAT volume: ρ = –0.99, p = 0.01; free T3 after cold exposure/BAT activity: ρ = –0.99, p = 0.01; Fig. 5B). Total T3 also correlated negatively with the cold-induced BAT volume and tended to correlate negatively with the BAT activity in the thyrotoxic state (total T3 at room temperature/BAT volume: ρ = –0.83, p = 0.04; total T3 after cold exposure/BAT volume: ρ = –0.83, p = 0.04; total T3 at room temperature/BAT activity: ρ = –0.77, p = 0.07; total T3 after cold exposure/BAT activity: ρ = –0.77, p = 0.07).
FIG. 5.
Correlations of circulating T3 with BAT and with resting metabolic rate (RMR). (A) Positive correlation between free T3 and BAT in the hypothyroid state. (B) Negative correlation between free T3 and BAT in the hyperthyroid state. (C) Positive correlation between the change in RMR and the change in free T3 between the hypothyroid and hyperthyroid state.
Several facets of thyroid status correlated with RMR. There was a significant inverse relationship between the baseline TSH and RMR in the hypothyroid state (ρ = –0.89; p = 0.02). In addition, there was a significant positive correlation between the change in RMR after 60 minutes of cold exposure and the change in total T3 as well as free T3 between the hypothyroid and thyrotoxic state (change in total T3 after 60 minutes of cold exposure: ρ = 0.94, p = 0.005; change in free T3 after 60 minutes of cold exposure: ρ = 0.94, p = 0.005; Fig. 5C).
For exploratory analyses, the relationships between detectable cold-induced BAT metabolism and other metabolic parameters were considered. Serum thyroglobulin (Tg) levels correlated negatively with BAT mass and activity in each state (hypothyroid state, BAT volume and activity: Tg ρ = –0.89, p = 0.02; hyperthyroid state, BAT volume: ρ = –0.83, p = 0.04; and BAT activity: ρ = –0.94, p = 0.005), and HbA1c correlated negatively with BAT volume and activity in the hypothyroid state (ρ = –0.90; p = 0.015). Total and LDL cholesterol correlated negatively with BAT activity (ρ = –0.83; p = 0.04), while high-density lipoprotein cholesterol correlated positively with BAT mass and activity (BAT volume: ρ = +0.90, p = 0.015; BAT activity: ρ = +0.84, p = 0.04) in the thyrotoxic state.
Discussion
Given the central role that TH plays in regulating whole-body energy expenditure and BAT function, this pilot study was designed to evaluate how promising treatment with TH would be toward increasing BAT thermogenesis. However, because of the numerous adverse effects of TH supplementation in healthy humans, a novel study population is required in order to evaluate the full capacity of dosing with TH. A series of patients with thyroid cancer was therefore chosen as the model because their standard course of treatment leads to iatrogenic hypothyroidism followed by TH treatment to the thyrotoxic state. All six study patients demonstrated detectable BAT under cold stimulation in both the hypothyroid and thyrotoxic state. The majority but not all (4/6) patients showed an increase in BAT volume and activity under cold stimulation between the hypothyroid and thyrotoxic phase. In addition, there was a positive correlation between the cold-induced BAT volume as well as BAT activity in the hypothyroid and thyrotoxic phase for each patient.
It is important to consider that all study patients showed detectable cold-activated BAT in the hypothyroid state, even at TSH levels >100 μIU/mL, which indicates that even low levels of TH are adequate for BAT activation in adult humans. In this study, serum T3 levels correlated negatively with cold-induced BAT mass and activity in the thyrotoxic state in adult humans. These apparently counterintuitive findings suggest that circulating T3 may be a marker of thyroid function without direct effects on BAT. Studies in rodents have shown that the BAT DIO2 is stimulated by the sympathetic nervous system (SNS) and inhibited by its substrate T4 (11,12,26). Excessive circulating T4 levels could result in inhibition of the DIO2 and proportionally lower local T3 concentrations and UCP1 activation in hyperthyroid subjects (12), while low circulating T4 levels could activate DIO2, increase the local T3 production, and activate the UCP1 in hypothyroid patients (27,28). In addition, it should be taken into consideration that the SNS and TH act synergistically to respond and adjust the body to different environmental factors, such as cold exposure. Studies in small rodents have shown that cold-exposure stimulates BAT activity by increasing central sympathetic nerve activity and by affecting local TH metabolism (11,12). Both catecholamines and local T3 act synergistically to stimulate UCP-1 expression and through this thermogenesis in BAT. In hypothyroidism, there is an increase, while in thyrotoxicosis there is a decrease in the central sympathetic outflow, as measured by plasma and urinary norepinephrine (NE) levels as well as NE turnover in different tissues such as BAT. However, there is also a reduced/exaggerated response to catecholamines in different tissues because of decreased/increased beta-adrenergic receptor density as well as postreceptor mechanisms, which usually overrides the effect of TH on the central SNS outflow and explains the clinical manifestations in hypothyroidism and thyrotoxicosis, respectively (12,29). Thus, the regulatory mechanisms involved in regulating BAT are complex, and one of these mechanisms may be predominant, which could explain the different BAT responses to cold exposure noted in different patients.
No correlation was found between the change in thyroid function or the change in RMR and the change in cold-induced BAT volume and activity between the hypothyroid and thyrotoxic states. This may be explained by the fact that TH influences both the basal/obligatory and facultative thermogenesis through different mechanisms (11,30). A treatment that would selectively stimulate facultative thermogenesis could be beneficial for weight loss and improvement in metabolic parameters without the side effects of elevated circulating TH levels.
This study showed that circulating T3 is a stronger predictor of the change in energy expenditure compared with cold-induced BAT activity. However, the response to TH treatment led to opposite responses among two of the principal thermogenic organs: glucose uptake in the liver was lower, but skeletal muscle uptake was higher. Therefore, it cannot be assumed that all tissues respond the same to chronic treatment with TH. The findings also support the hypothesis that TH contributes significantly to cold-induced thermogenesis by affecting multiple organs, including skeletal muscle, in addition to BAT (21,22,31,32). In this study, there was a more consistent increase in the skeletal muscle activity compared with the BAT activity between the hypothyroid and thyrotoxic states. This may be explained by the fact that the skeletal muscle activity is mainly regulated by TH, while the synergistic activity with the SNS may be less important in this tissue (33). In addition, the difference between the two tissues could be potentially explained by the fact that the study patients took high-dose LT4 at the time of the second PET-CT scan, which can decrease DIO2 activity; this enzyme has more important effects in BAT than in skeletal muscle (34).
The few published human studies looking at the relationship between thyroid status and BAT function have shown contradictory results (20–23). Skarulis et al. reported detectable BAT measured by PET-CT in a hypothyroid patient and an increase in BAT parameters when the patient became thyrotoxic upon resuming TH treatment as part of follow-up for thyroid cancer (20). In contrast, a severely hypothyroid child with Hashimoto's thyroiditis was found with a significant supraclavicular BAT volume measured by MRI and BAT activity measured by infrared thermal imaging, which both decreased after two months of TH replacement (23). Lahesmaa et al. reported a threefold increase in glucose uptake measured by FDG-PET in 10 hyperthyroid patients compared with euthyroid patients (21), while Zhang et al. reported no detectable BAT in 10 patients with newly diagnosed Graves' hyperthyroidism before starting treatment (22). Additional human studies are needed to gain insight into the factors that moderate the effect of TH on BAT volume and activity. It may be that TH directly stimulates BAT, but the general increase in the resting metabolic rate may obviate the need for additional BAT in some patients. The end result of these two simultaneous but opposite effects of TH on BAT may explain why some patients increase their BAT while others have less. In addition, as described above, the complex interaction between TH and SNS in regulating BAT may play a role.
Thyroid cancer patients were recruited for this pilot study. This represents an excellent study model for BAT evaluation, since treatment for thyroid cancer involves several steps, including thyroidectomy, followed by induction of short-term hypothyroidism prior to RAI treatment and then long-term suppressive TH treatment to prevent recurrence. Therefore, the changes in BAT mass and activity can be evaluated between the hypothyroid and thyrotoxic states in this patient population.
This is a pilot study designed to obtain preliminary data in a small but physiologically informative group of patients with thyroid cancer whose clinical course involves treatment with high-dose LT4 to change their thyroid status from severe hypothyroidism to mild thyrotoxicosis. The signal from this cohort would be a best-case scenario for any studies in healthy subjects or patients with milder hypothyroidism. The data show that based on the mean change in BAT metabolic activity, >50 pairs of subjects would have to be studied to demonstrate a significant effect of TH treatment on detectable BAT. Therefore, this pilot study has achieved one of its essential goals and demonstrated that is not very feasible to use LT4 treatment in most populations as a way of increasing BAT mass or metabolic activity.
As known from prior animal as well as human studies, chronic cold exposure is probably the strongest determinant of BAT volume and activity (9,10). Based on the study design, it was not possible to distinguish the effects of chronic cold from the chronic administration of high doses of TH easily. However, the change in BAT activity was not correlated with the season of TH treatment, so the effects of cold exposure were not likely to have substantially confounded the modest effect seen from chronic exposure to high doses of TH. This study measured cold-induced BAT, since significant amounts of functional BAT have been detected in >95% of lean subjects in response to acute cold exposure (5,8), while significantly fewer subjects showed detectable BAT via PET-CT scanning when studied at room temperature. With the current study design, it was possible to evaluate the effect of TH on cold-induced BAT by studying the same patients after cold exposure in both the hypothyroid and thyrotoxic state. Therefore, it can be inferred that the change in the BAT mass and activity between the two phases is related to the TH status change.
In conclusion, this pilot study found that all six study patients had detectable BAT after two hours of cold exposure in both the hypothyroid and thyrotoxic states, and the majority but not all (4/6) patients showed an increase in cold-induced BAT volume and activity between the hypothyroid and thyrotoxic states. Therefore, iatrogenic hypothyroidism lasting two to four weeks does not prevent cold-induced BAT activation. At the same time, the use of TH to induce thyrotoxicosis does not consistently increase cold-induced BAT activity. Although the use of LT4 is not feasible, using a BAT-specific TH analog may be still feasible to increase BAT thermogenesis as a treatment for obesity and metabolic disease. Further studies are needed to evaluate this and to determine which physiological factors besides TH are involved in stimulating BAT growth and thermogenesis.
Acknowledgments
This work was supported by the 2012 Harvard Catalyst Clinical Research Center (HCCRC) Resources and Services Funding Award; the 2013 Harvard Catalyst Clinical Research Center (HCCRC) Laboratory Support and Genotyping Award; National Institutes of Health (NIH) grants K23 DK081604, the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); and the Clinical Translational Science Awards UL1RR025758, UL1TR000170 and UL1TR001102 to Harvard University and its affiliated academic healthcare centers from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
We thank the Beth Israel Deaconess Medical Center (BIDMC) Clinical Research Center nursing team, Bionutrition Core, and nuclear medicine technologists for the excellent support they provided; Michelle Beck for supervising the study development and progression; Anthony Hollenberg for reviewing the manuscript; Matthew R Palmer for reviewing the PET-CT study protocol; Lauren Weiner for her assistance in coordinating the clinical studies; and our volunteers for their commitment to the studies.
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Schulz TJ, Tseng YH. 2013. Brown adipose tissue: development, metabolism and beyond. Biochem J 453:167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. 2007. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci U S A 104:2366–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowell BB, Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. 1993. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366:740–742 [DOI] [PubMed] [Google Scholar]
- 4.Foster DO, Frydman ML. 1979. Tissue distribution of cold-induced thermogenesis in conscious warm or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol 57:257–270 [DOI] [PubMed] [Google Scholar]
- 5.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. 2009. Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525. Erratum in N Engl J Med 361:1123. [DOI] [PubMed] [Google Scholar]
- 6.Lean ME, James WP, Jennings G, Trayhurn P. 1986. Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin Sci (Lond) 71:291–297 [DOI] [PubMed] [Google Scholar]
- 7.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. 2009. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. 2009. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508 [DOI] [PubMed] [Google Scholar]
- 9.van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jörgensen JA, Wu J, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. 2013. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 123:3395–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. 2013. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 123:3404–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva JE. 2006. Thermogenic mechanisms and their hormonal regulation. Physiol Rev 86:435–464 [DOI] [PubMed] [Google Scholar]
- 12.Silva JE, Bianco SD. 2008. Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid 18:157–165 [DOI] [PubMed] [Google Scholar]
- 13.Bianco AC, Silva JE. 1988. Cold exposure rapidly induces virtual saturation of brown adipose tissue nuclear T3 receptors. Am J Physiol 255:E496–503 [DOI] [PubMed] [Google Scholar]
- 14.Harper ME, Seifert EL. 2008. Thyroid hormone effects on mitochondrial energetics. Thyroid 18:145–156 [DOI] [PubMed] [Google Scholar]
- 15.Lee JY, Takahashi N, Yasubuchi M, Kim YI, Hashizaki H, Kim MJ, Sakamoto T, Goto T, Kawada T. 2012. Triiodothyronine induces UCP-1 expression and mitochondrial biogenesis in human adipocytes. Am J Physiol Cell Physiol 302:C463–472 [DOI] [PubMed] [Google Scholar]
- 16.Obregon MJ. 2008. Thyroid hormone and adipocyte differentiation. Thyroid 18:185–195 [DOI] [PubMed] [Google Scholar]
- 17.Obregon MJ. 2014. Adipose tissues and thyroid hormones. Front Physiol 5:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurberg P, Andersen S, Karmisholt J. 2005. Cold adaptation and thyroid hormone metabolism. Horm Metab Res 37:545–549 [DOI] [PubMed] [Google Scholar]
- 19.Wijers SL, Saris WH, van Marken Lichtenbelt WD. 2009. Recent advances in adaptive thermogenesis: potential implications for the treatment of obesity. Obes Rev 10:218–226 [DOI] [PubMed] [Google Scholar]
- 20.Skarulis MC, Celi FS, Mueller E, Zemskova M, Malek R, Hugendubler L, Cochran C, Solomon J, Chen C, Gorden P. 2010. Thyroid hormone induced brown adipose tissue and amelioration of diabetes in a patient with extreme insulin resistance. J Clin Endocrinol Metab 95:256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahesmaa M, Orava J, Schalin-Jäntti C, Soinio M, Hannukainen JC, Noponen T, Kirjavainen A, Iida H, Kudomi N, Enerbäck S, Virtanen KA, Nuutila P. 2014. Hyperthyroidism increases brown fat metabolism in humans. J Clin Endocrinol Metab 99:E28–35 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Miao Q, Ye H, Zhang Z, Zuo C, Hua F, Guan Y, Li Y. 2014. The effects of thyroid hormones on brown adipose tissue in humans: a PET-CT study. Diabetes Metab Res Rev 30:513–520 [DOI] [PubMed] [Google Scholar]
- 23.Kim MS, Hu HH, Aggabao PC, Geffner ME, Gilsanz V. 2014. Presence of brown adipose tissue in an adolescent with severe primary hypothyroidism. J Clin Endocrinol Metab 99:E1686–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, Holman AR, Tal I, Palmer MR, Kolodny GM, Kahn CR. 2012. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A 109:10001–10005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cypess AM, Weiner LS, Roberts-Toler C, Elía EF, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, Kolodny GM. 2015. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullur R, Liu YY, Brent GA. 2014. Thyroid hormone regulation of metabolism. Physiol Rev 94:355–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bianco AC, McAninch EA. 2013. The role of thyroid hormone and brown adipose tissue in energy homoeostasis. Lancet Diabetes Endocrinol 1:250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianco AC, Maia AL, da Silva WS, Christoffolete MA. 2005. Adaptive activation of thyroid hormone and energy expenditure. Biosci Rep 25:191–208 [DOI] [PubMed] [Google Scholar]
- 29.Young JB, Bürgi-Saville ME, Bürgi U, Landsberg L. 2005. Sympathetic nervous system activity in rat thyroid: potential role in goitrogenesis. Am J Physiol Endocrinol Metab 288:E861–867 [DOI] [PubMed] [Google Scholar]
- 30.Cannon B, Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359 [DOI] [PubMed] [Google Scholar]
- 31.Silva JE, Bianco SD. 2008. Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid 18:157–165 [DOI] [PubMed] [Google Scholar]
- 32.Laurberg P, Andersen S, Karmisholt J. 2005. Cold adaptation and thyroid hormone metabolism. Horm Metab Res 37:545–549 [DOI] [PubMed] [Google Scholar]
- 33.Solmonson A, Mills EM. 2016. Uncoupling proteins and the molecular mechanisms of thyroid thermogenesis. Endocrinology 157:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werneck-de-Castro JP, Fonseca TL, Ignacio DL, Fernandes GW, Andrade-Feraud CM, Lartey LJ, Ribeiro MB, Ribeiro MO, Gereben B, Bianco AC. 2015. Thyroid hormone signaling in male mouse skeletal muscle is largely independent of D2 in myocytes. Endocrinology 156:3842–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]