Abstract
The selective serotonin reuptake inhibitor fluoxetine induces hippocampal neurogenesis, stimulates maturation and synaptic plasticity of adult hippocampal neurons, and reduces motor/sensory and memory impairments in several CNS disorders. In the setting of traumatic brain injury (TBI), its effects on neuroplasticity and function have yet to be thoroughly investigated. Here we examined the efficacy of fluoxetine after a moderate to severe TBI, produced by a controlled cortical impact. Three days after TBI or sham surgery, mice were treated with fluoxetine (10 mg/kg/d) or vehicle for 4 weeks. To evaluate the effects of fluoxetine on neuroplasticity, hippocampal neurogenesis and epigenetic modification were studied. Stereologic analysis of the dentate gyrus revealed a significant increase in doublecortin-positive cells in brain-injured animals treated with fluoxetine relative to controls, a finding consistent with enhanced hippocampal neurogenesis. Epigenetic modifications, including an increase in histone 3 acetylation and induction of methyl-CpG-binding protein, a transcription factor involved in DNA methylation, were likewise seen by immunohistochemistry and quantitative Western immunoblots, respectively, in brain-injured animals treated with fluoxetine. To determine if fluoxetine improves neurological outcomes after TBI, gait function and spatial learning and memory were assessed by the CatWalk-assisted gait test and Barnes maze test, respectively. No differences in these parameters were seen between fluoxetine- and vehicle-treated animals. Thus while fluoxetine enhanced neuroplasticity in the hippocampus after TBI, its chronic administration did not restore locomotor function or ameliorate memory deficits.
Key words: controlled cortical impact, histone acetylation, memory, methyl-CpG binding domain 1, selective serotonin reuptake inhibitor
Introduction
The selective serotonin reuptake inhibitor (SSRI) fluoxetine targets a class of amplifying neural progenitors by increasing the rate of symmetric divisions (Encinas et al., 2006). Chronic administration of fluoxetine not only leads to increased proliferation and an increased number of hippocampal neurons, but also stimulates maturation and synaptic plasticity of adult hippocampal granule cells (Wang et al., 2008). Beyond the normal brain, chronic fluoxetine boosts hippocampal neurogenesis in a spontaneous model of type I diabetic mice (Beauquis et al., 2009). Fluoxetine also modulates plasticity in other non-neurogenic brain regions, such that it promotes the recovery of visual function and reinstates ocular dominance plasticity in the adult visual cortex in animals with amblyopia (Maya Vetencourt et al., 2008).
There is emerging evidence that fluoxetine is involved in epigenetic/chromatin remodeling. DNA methylation is an important epigenetic mechanism that regulates many cellular events during development and memory consolidation (Miller et al., 2010). Histone acetylation is also an important mechanism for the regulation of gene expression and neuroplasticity (Crosio et al., 2003; Guan et al., 2002; Levenson and Sweatt, 2006; Miller et al., 2010). Increased histone acetylation underlies environmental enrichment-induced improvement of memory in CK-p25 transgenic mice with neurodegeneration (Fischer et al., 2007). The methyl-CpG binding domain 1 (MBD1) protein, a transcription factor involved in DNA methylation, is induced following fluoxetine administration in the adult rat dentate gyrus to which the serotonergic projection terminates (Cassel et al., 2006).
Fluoxetine improves outcomes in models of neurologic-based impairments. Chronic fluoxetine treatment reduces depression via both neurogenesis-dependent and neurogenesis-independent mechanisms (David et al., 2009; Dulawa et al., 2004; Holick et al., 2008; Santarelli et al., 2003). Beneficial effects of fluoxetine have been noted in animal models of stroke, multiple sclerosis, and epilepsy (Mostert et al., 2008), as well as in patients with Alzheimer's disease, stroke, Huntington's disease, multiple sclerosis, traumatic brain injury (TBI), and epilepsy (Mostert et al., 2008). In the setting of stroke, fluoxetine not only improves the quality of life in patients with emotional disturbances, but also increases motor output (Berends et al., 2009), leading to improved motor skills on the affected side (Pariente et al., 2001). In addition to changes in motor function, fluoxetine fosters long-term improvement in executive function (Narushima et al., 2007), and enhances memory and cognition in patients with mild cognitive impairment (Mowla et al., 2007).
Although SSRIs are used for the treatment of depression and sleep dysfunction in traumatic brain-injured patients (Patterson et al., 1997), their specific effects on post-injury neuroplasticity and function are not well understood. Here our objective was to further address the functional efficacy and underlying mechanisms of chronic fluoxetine treatment in an experimental murine model of TBI. Our findings demonstrate that although chronic administration of fluoxetine increases hippocampal neurogenesis and induces epigenetic modifications in the injured brain, it does not alter gait-related deficits in paw placement or memory impairment.
Methods
Animals and housing
This study was conducted in accordance with the animal care guidelines issued by the National Institutes of Health and by the San Francisco Veterans Affairs Medical Center Animal Care and Use Committee. Adult male C57BL/6 mice 2.5 months of age, weighing 24–30 g, purchased from Charles River Laboratories, Inc. (Wilmington, MA), were housed in institutional standard cages (4 mice per cage) on a 12-h light/12-h dark cycle with ad libitum access to water and food before and during experimental procedures.
Surgical procedure and drug administration
The animals were randomly assigned to two procedure groups for receiving TBI (total n = 78) or sham surgery (total n = 70). The animals were anesthetized with isoflurane/O2/N2O (1.5/30/68.5%) during surgery, and the core temperature was maintained at 37 ± 0.5°C with a heating blanket and rectal thermistor servo-loop during both the surgical and the postoperative recovery period.
After being secured in a stereotaxic frame (Kopf Instruments, Tujunga, CA), followed by a midline skin incision, a 3-mm-diameter circular craniotomy was performed with a dental drill, lateral (right side) to the mid-sagittal suture, centered at –2.0 mm AP and 2.0 mm ML relative to the bregma. The animal was then subjected to a controlled cortical impact as previously described (Bilgen, 2005). The impactor, operated by a linear motor and microprocessor controller (Linmot, Zurich, Switzerland), was equipped with a polished stainless steel tip 2.0 mm in diameter. The impactor tip was first centered over the craniotomy, and was slowly lowered until the tip just contacted the dura (as confirmed by an operating microscope). The impact injury was generated using the following parameters: 1.5 m/sec strike velocity, 1.25 mm depth of penetration, and 155 msec contact time. These parameters produce a moderate to severe level of injury in mice, causing significant motor impairment (Neumann et al., 2009). The scalp was then closed with sutures and each animal was given 1.0 mL of isotonic saline subcutaneously after the operation to prevent dehydration. Sham animals received craniotomy but no impact. Four days following TBI or sham surgery, each group of mice was randomly divided into two subgroups for saline or fluoxetine administration. In order to avoid learning-induced neural plasticity and epigenetic changes from repeated handling/training during behavioral assessment, immunohistochemistry and Western blot analysis were conducted in a separate cohort of mice from the behavioral study. Following 4 weeks of daily IP injection at 10 mg/kg, one group of animals was euthanized for neurogenesis and epigenetic studies. The remaining mice were subjected to behavioral tests. The group assignment of each animal with respect to treatment was concealed from the experimenters who conducted the procedures and analysis.
Tissue preparation, immunohistochemistry staining, and cell counting
The animals were anesthetized with ketamine (80 mg/kg; Parke-Davis, Morris Plains, NJ), and xylazine (20 mg/kg; Butler, Columbus, OH), and perfused transcardially with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4). The brains were removed, fixed overnight in 4% PFA-PB, and placed in 20% sucrose for 48 h. Coronal sections were cut at 40 μm on a microtome and collected serially. The sections were immunostained using the following reagents: goat anti-doublecortin (DCX, 1 μg/mL: Santa Cruz Biotechnology, Santa Cruz, CA); rabbit-anti-MBD1 (1 μg/mL; Millipore, Temecula, CA); biotinylated sheep anti-goat and anti-rabbit (5 μg/mL; Amersham, Cleveland, OH); ABC solution (Vector Laboratories, Burlingame, CA); and diaminobenzidine-tetrachloride (DAB Fast; Sigma-Aldrich, St Louis, MO). For unbiased stereological estimation of cell numbers (Stereo Investigator, MicroBrightField; MBF Bioscience, Williston, VT) in the dentate gyrus (DG) and striatum, every sixth coronal section spanning the entire region was used. To achieve a coefficient of error (CE) of less than 0.10, counting frames of 15 × 15 × 20 μm were used in a 40 × 40 μm matrix (for estimating DCX- and MBD1-expressing cells), that were randomly superimposed onto the region of interest by the program as previously described (Liu et al., 2007; Matsumori et al., 2006).
Western blot analysis
Histone acetylation was assessed by Western blotting because the immunohistochemistry with rabbit anti-acetyl-H3 antibody did not yield specific staining. Cell lysates were prepared by homogenizing dissected hippocampal tissue in lysis buffer (50 mM Tris-Cl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 2 mM EDTA, 10 mM NaF, 1 mM Na3VO4, and protease inhibitors complete; Roche, Mannheim, Germany) with a 23-gauge syringe. Lysates were cleared from cellular debris by centrifugation, and protein concentrations were determined using the DC protein assay (Bio-Rad, Hercules, CA). Proteins were denatured in Laemmli loading buffer at 95°C for 5 min, and 15–30 μg per lane were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% gels. The proteins were blotted onto polyvinylidene difluoride membranes (Millipore). The membranes were blocked with QuickBlocker (EMD, San Diego, CA) for 1 h at room temperature, and probed overnight at 4°C with goat anti-actin (Santa Cruz Biotechnology), rabbit anti-H3, and rabbit anti-acetyl-H3 (recognizing histone acetylation at the Lys9 and Lys14 residues). Binding was detected using secondary anti-goat or anti-rabbit horseradish peroxidase-conjugated antibodies (Sigma-Aldrich). Immunoreactive bands were visualized using chemiluminescent ECL plus substrate (Amersham Biosciences, Piscataway, NJ). Blot membranes were stained with Coomassie brilliant blue G-250 (Bio-Rad) to ensure that comparable protein amounts had been loaded and the transfer was proper.
Computer-assisted method for gait analysis
Mice were subjected to three consecutive runs of gait assessment at 31 days after TBI or sham surgery (or 28 days after fluoxetine or vehicle), using the CatWalk automated gait analysis system (Noldus Information Technology, Wageningen, The Netherlands), as previously described (Neumann et al., 2009). The apparatus is made of a 1.3-meter-long glass plate with dim fluorescent light beamed into the glass from the side. In a darkened environment (below 1 lux of illumination), the light is reflected downward and images of the footprints are recorded by the camera under the walkway when the animal's paws come in contact with the glass surface. The images from each trial were converted into digital signals and processed with a threshold set at 30 arbitrary units (a.u., ranging from 0–225, meaning all pixels brighter than 30 a.u. were used). The imaging setting used for this study defines 1 pixel to be 0.085 mm. Following the identification and labeling of each footprint, the two most relevant parameters in paw placement were analyzed and compared (intensity and maximum area). Trials containing uneven walking velocities or with significant stops on the walkway were excluded from the analysis.
Porsolt forced swimming test
Depression-like behavior was tested with the Porsolt forced swimming test, a standard test to determine the effect of antidepressants in laboratory rodents (Porsolt et al., 1977a, 1977b). The mice were placed in a narrow cylinder (25 cm high and 14 cm in diameter) of water (depth: 16 cm, 23–25°C) from which there is no escape, for 6 min. After an initial period of vigorous activity lasting for approximately 2 min, the animals adopted a characteristic immobile posture, which has come to be known as behavioral despair or learned helplessness. The last 4 min of the recorded sessions were evaluated by two blinded observers. The duration of the immobility time was recorded in seconds, the period when the animal was floating in the water in an upright position, and made only slight movements in order to keep its head above water. The durations for climbing and swimming behaviors were also summed accordingly. The values from two observers were averaged to obtain the final data.
Barnes maze test
The Barnes maze was used to measure spatial learning and memory, which depends on hippocampal function. A black acrylic escape tunnel (19 × 6 × 5 cm in size) was placed under one of the holes on a circular platform (120 cm in diameter) with 40 holes (each 5 cm in diameter) along the perimeter of the platform (Hamilton Kinder, Poway, CA) elevated 60 cm above the floor. To rule out any potential stress response following the forced swimming test, the mice were handled and acclimated in the behavioral testing room for 3 days prior to the Barnes maze test. Mice from each experimental group were randomly assigned to locate the escape tunnel from one of the four predetermined locations to rule out spatial preference. Blowing fans and 500 lux of bright light were used to discourage the mice from wandering aimlessly. The mice were trained to locate the escape tunnel from different counterbalanced starting positions in two successive daily sessions for 6 days (3 trials per session, 3 min per trial), with a 1-h inter-session interval. The trials were recorded and analyzed using the Noldus EthoVision video tracking system (Noldus, Leesburg, VA).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). All statistical analyses were carried out with Statview 5.0.1 software (SAS Institute Inc., Cary, NC). Statistical significance was evaluated using two-way analysis of variance (ANOVA), or two-way repeated-measures ANOVA (RANOVA), followed by post-hoc paired comparisons using Fisher's protected least significant difference test when appropriate. Values p < 0.05 were considered significant.
Results
Chronic fluoxetine treatment induces hippocampal neurogenesis
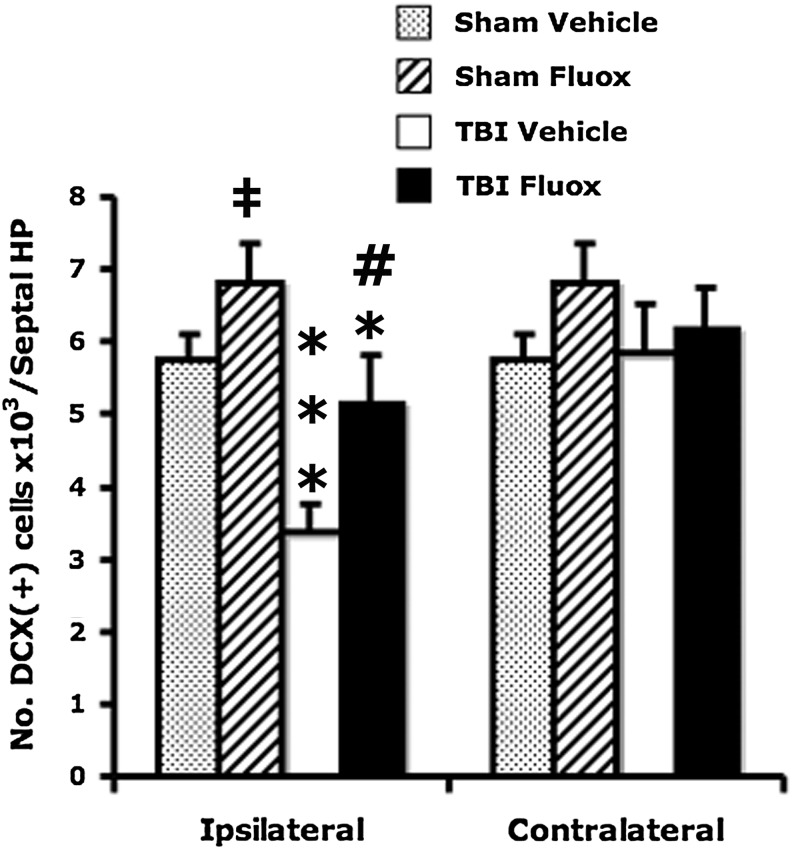
Fluoxetine enhances hippocampal neurogenesis and increases DCX cells in normal mice (Encinas et al., 2006). We determined whether fluoxetine also increases hippocampal DCX cells under conditions of TBI. Whereas TBI reduced DCX cells in the ipsilateral DG (TBI effect: F1,25 = 16.6, p < 0.0005), there was an increase in the number of these cells in the fluoxetine-treated groups, compared to their vehicle-treated counterparts (fluoxetine effect: F1,25 = 8.7, p < 0.01). Fluoxetine increased DCX cells in the ipsilateral DG of brain-injured mice (post-hoc: p < 0.05). No significant differences were seen in the number of DCX-expressing cells in the contralateral DG due to either TBI (F1,20 = 0.65, p > 0.42) or fluoxetine (F1,20 = 2.19, p > 0.15) (Fig. 1).
FIG. 1.
Chronic administration of fluoxetine increases the total number of immature neurons in the dentate gyrus. There was a significant reduction in total DCX-immunoreactive cells in the ipsilateral dentate gyrus following unilateral traumatic brain injury compared to sham injury in both vehicle-treated (post-hoc: ***p < 0.005), and in fluoxetine-treated (post-hoc: *p < 0.05) groups. However, chronic administration of fluoxetine increased the total number DCX-immunoreactive cells in the ipsilateral dentate gyrus of the sham (post-hoc: ‡p < 0.05) and TBI (post-hoc: #p < 0.05) groups (TBI, traumatic brain injury; Fluox, fluoxetine; DCX, doublecortin).
Chronic fluoxetine treatment alters epigenetic signaling
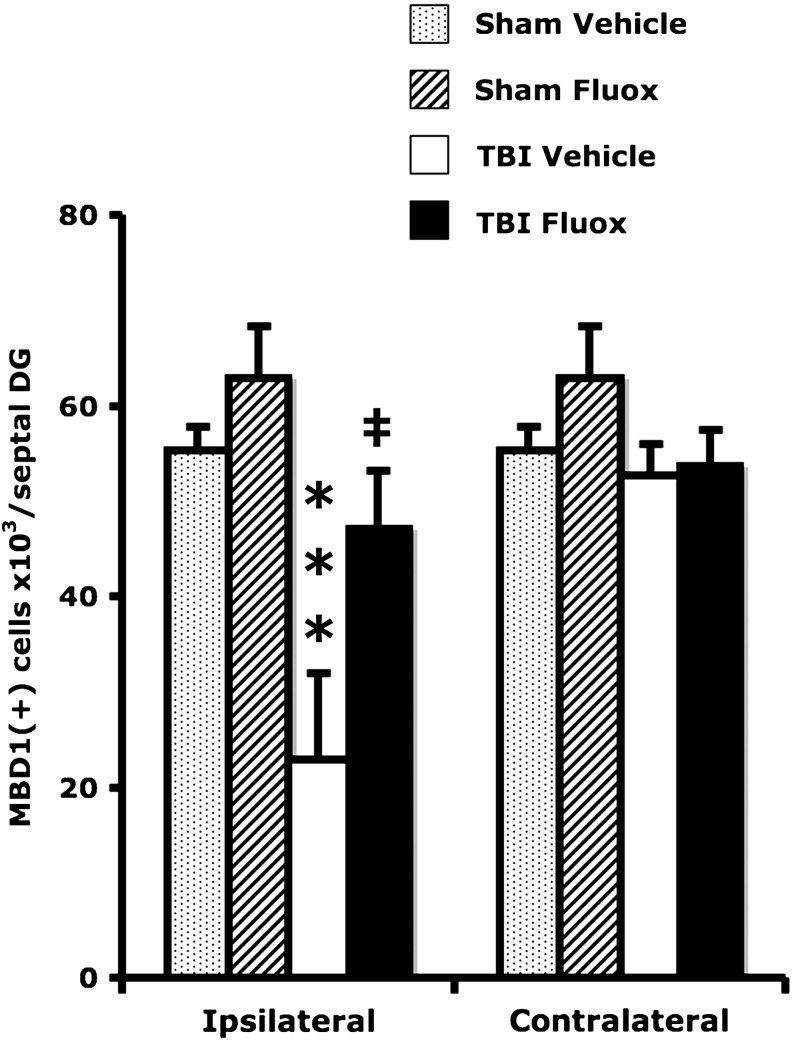
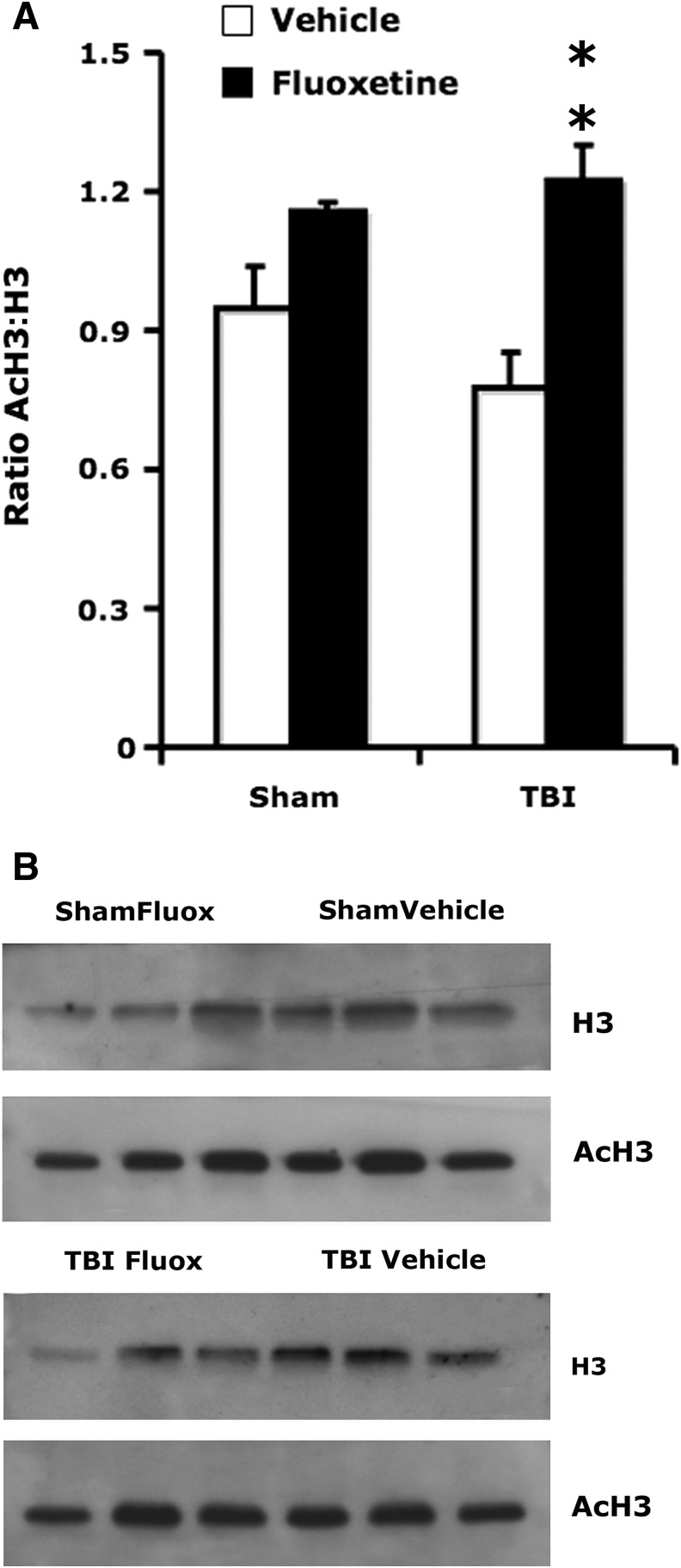
Epigenetic modifications are often associated with learning and memory (Levenson and Sweatt, 2006). Since DNA methylation was implicated in memory consolidation (Miller et al., 2010), we determined the expression of MBD1, one of the main transcription factors involved in DNA methylation. TBI significantly reduced MBD1 expression in the ipsilateral DG (F1,20 = 16.1, p < 0.001), while fluoxetine enhanced MBD1 expression (F1,20 = 6.9, p < 0.05). Chronic fluoxetine increased the number of granule cells expressing MBD1 in brain-injured animals (post-hoc: p < 0.05). Neither TBI (F1,20 = 2.16, p > 0.15), nor fluoxetine (F1,20 = 1.14, p > 0.29), affected MBD1 expression in the contralateral DG (Fig. 2). Fluoxetine had a strong effect on the level of acetylated histone 3 expression in the hippocampus (F1,11 = 16.3, p < 0.005). Most importantly, chronic treatment with fluoxetine enhanced the expression of acetylated histone 3 in brain-injured animals (post-hoc: p < 0.01; Fig. 3).
FIG. 2.
Chronic administration of fluoxetine increases the number of MBD1-expressing cells in the dentate gyrus. There was a significant reduction in the total number of MBD1-immunoreactive cells in the ipsilateral dentate gyrus following unilateral TBI compared to sham animals only in the vehicle-treated groups (post-hoc: ***p < 0.005). Chronic fluoxetine treatment increased the total number of MBD1-immunoreactive cells in the ipsilateral dentate gyrus of the TBI (post-hoc: ‡p < 0.05) groups (TBI, traumatic brain injury; Fluox: fluoxetine; MBD1, methyl-CpG-binding domain-1).
FIG. 3.
Chronic administration of fluoxetine increases histone 3 acetylation in the hippocampus. (A) There was a significant increase in the ratio of acetylated histone 3 normalized by total histone 3 (AcH3:H3) in brain-injured mice treated with fluoxetine compared to brain-injured mice treated with saline (post-hoc: **p < 0.01). (B) Representative images of Western blots showing the expression of total histone 3 (H3) and acetylated histone 3 (AcH3; Fluox: fluoxetine; TBI, traumatic brain injury).
Chronic fluoxetine treatment does not improve gait function after TBI
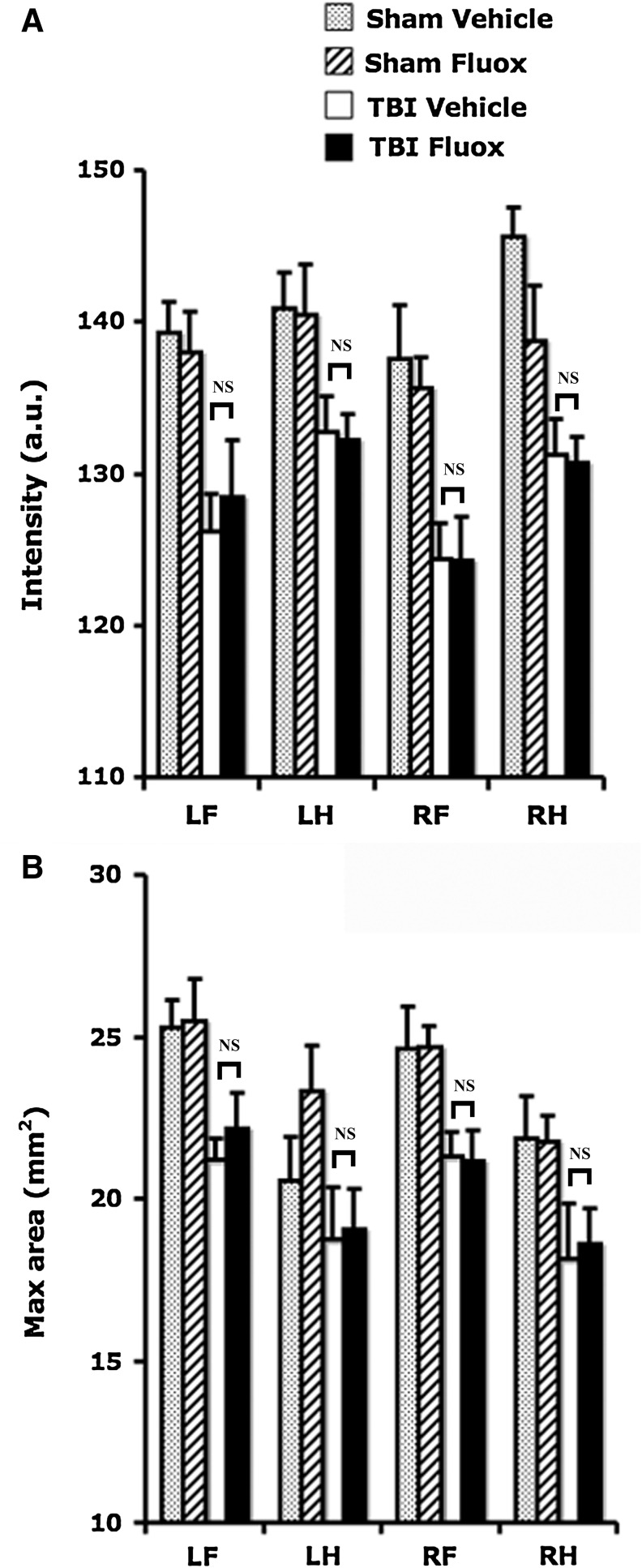
Previously we have shown that TBI induced changes in gait function at 4 days post-injury (Neumann et al., 2009). To determine whether TBI leads to long-lasting gait impairment and if fluoxetine influences this outcome, mice were subjected to the CatWalk-assisted gait function test following 4 weeks of fluoxetine or vehicle treatment, beginning at 4 days post-injury. Two-way ANOVA revealed a significant effect of TBI on paw intensity (TBI effect: F1,140 = 61.4, p < 0.0001; paw effect: F1,140 = 4.8, p < 0.005), and on maximal area of paws during floor contact (TBI effect: F1,140 = 31.1, p < 0.0001; paw effect: F1,140 = 8.2, p < 0.0001). However, fluoxetine did not have an effect on either intensity (F1,140 = 0.7, p > 0.39), or maximal area (F1,140 = 0.8, p > 0.36), in sham and brain-injured animals (interaction TBI × fluoxetine: p > 0.29 for intensity; p > 0.78 for maximal area; Fig. 4).
FIG. 4.
Chronic fluoxetine administration does not reverse TBI-induced impairment in gait spatial parameters. The intensity (A) and maximum areas (B) were reduced at all paws at 5 weeks following TBI. Fluoxetine did not have any effect on either intensity or maximum area in sham or brain-injured mice (NS, not significant between TBI/vehicle-treated and TBI/fluoxetine-treated groups; TBI, traumatic brain injury; Fluox, fluoxetine; RF, right fore; RH, right hind; LF, left fore; LH, left hind; a.u., arbitrary units).
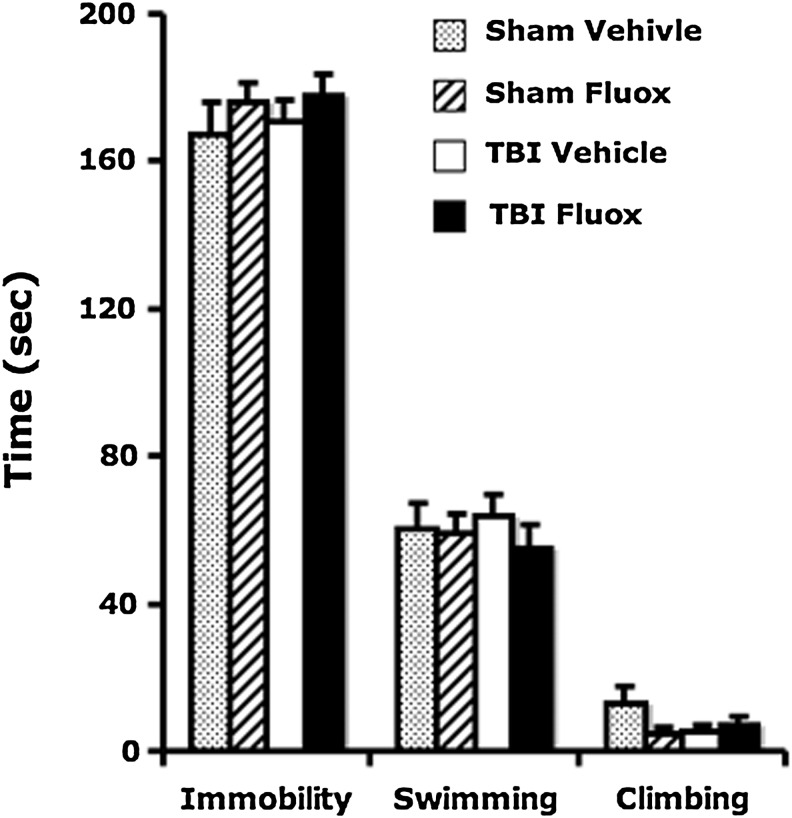
Neither TBI nor chronic fluoxetine treatment affects depression-like behavior
Similarly to human post-injury syndrome, a depression-like state has been observed following episodes of TBI in laboratory animals (Milman et al., 2005). To determine the effects of TBI and the antidepressant fluoxetine on depression-like behavior, mice were evaluated with the forced swimming test. Brain-injured animals did not display increased depression-like behavior during the forced swimming test compared to their sham counterparts (TBI effect: F1,110 = 0.34, p > 0.58). Chronic fluoxetine treatment also did not affect passive floating behavior in sham or brain-injured mice (fluoxetine effect: F1,110 = 1.72, p > 0.19; interaction: TBI × fluoxetine: F1,110 = 0.12, p > 0.74). There was no significant difference observed in swimming or climbing behavior between groups (Fig. 5).
FIG. 5.
Neither TBI nor fluoxetine administration affects depression-like behavior in the forced swimming test. Following the initial 2 min of adjustment in the cylinder, the total time spent in immobility, swimming, and climbing was summed for each behavior. There was no significant difference in time spent in each behavior due to either TBI or fluoxetine (TBI, traumatic brain injury; Fluox: fluoxetine).
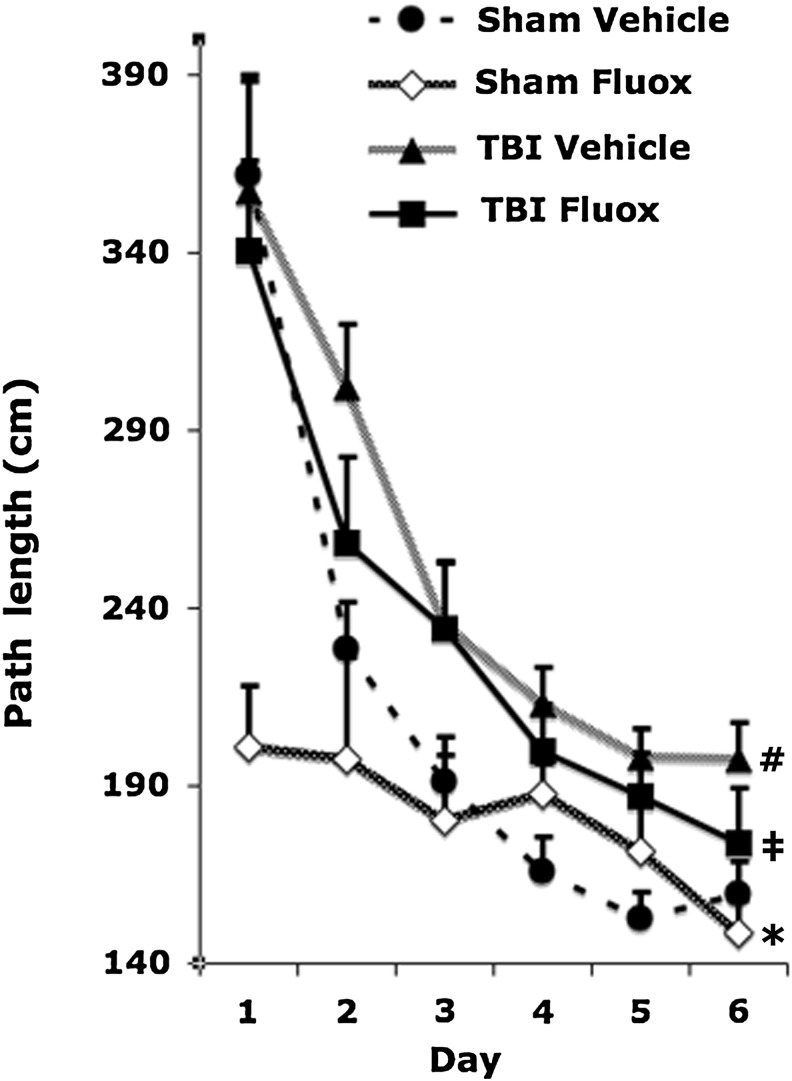
Chronic fluoxetine treatment does not reduce TBI-induced impairment in spatial learning and memory
Chronic fluoxetine administration was shown to be effective in reducing spatial memory deficits in animals with ischemic brain injury (Li et al., 2009). Here we determined whether fluoxetine would reduce cognitive deficits resulting from TBI. Mice were subjected to memory testing using spatial learning in the Barnes maze that relies heavily on hippocampal function. Over the 6 days of training, all mice learned the spatial task, as evidenced by progressively less distance traveled to reach the escape tunnel in the Barnes maze test (F5,290 = 67.6; p < 0.0001). TBI induced a significant spatial learning and memory impairment (TBI effect on path length: F1,58 = 58.6.0; p < 0.0001). Chronic fluoxetine administration reduced path length to find the target compared to vehicle treatment (drug effect on path length: F1,58 = 6.1; p < 0.05; Fig. 6). However, the effect of fluoxetine on reducing path length to find the target was not significant in the TBI subjects (post-hoc: p = 0.13).
FIG. 6.
Chronic fluoxetine treatment does not reduce TBI-induced impairments in spatial learning and memory. Mice subjected to TBI and treated with vehicle on average traveled longer distances to find the escape tunnel than sham-vehicle treated animals (post-hoc: sham-vehicle versus TBI-vehicle: #p < 0.0001). Although brain-injured mice treated with fluoxetine had a tendency to perform better on the Barnes maze test than brain-injured mice treated with vehicle, the difference was not statistically significant (post-hoc: TBI-vehicle versus TBI-fluoxetine: p = 0.13). Shams receiving chronic fluoxetine treatment performed better than sham mice receiving saline or brain-injured mice receiving chronic fluoxetine (post-hoc: sham-vehicle versus sham-fluoxetine: *p < 0.05; sham-fluoxetine versus TBI-fluoxetine: ‡p < 0.0001; TBI, traumatic brain injury; Fluox, fluoxetine).
Discussion
Due to the widely recognized therapeutic efficacy of fluoxetine for a number of neurodegenerative disorders and brain injuries, and its potential to enhance neuroplasticity, we determined whether chronic treatment with fluoxetine increased hippocampal neurogenesis and epigenetic modifications, and whether it reduced functional impairment in an experimental model of TBI. We found that a delayed and chronic regimen of fluoxetine treatment not only increased numbers of immature neurons, but also enhanced the expression of MBD1 and histone 3 acetylation in the ipsilateral hippocampus following TBI. However, deficits in gait and memory function, characteristic of the brain-injured group, remained unchanged in brain-injured animals treated with fluoxetine.
A plethora of cellular and biochemical changes induced by fluoxetine are thought to be important for neuronal survival and neuroplasticity, two interrelated processes. Fluoxetine enhances glycogenolysis in astrocytes, which could improve the energy supply to axons and neurons (Chen et al., 1995; Kong et al., 2002; Zhang et al., 1993). Fluoxetine also modulates ion channels and receptors. It blocks voltage-gated calcium and sodium channels, and decreases conductance of mitochondrial voltage-dependent anion channels (VDACs; Nahon et al., 2005). TREK channels, family members of the two-pore domain potassium (K2P) channels, are also inhibited by fluoxetine (Kennard et al., 2005). Of interest, inhibition of VDAC or Na+/Ca2+ channels by fluoxetine is neuroprotective (Nahon et al., 2005; Ouardouz et al., 2005; Shimizu et al., 1999). Furthermore, fluoxetine attenuates kainate-induced neuronal cell death in the mouse hippocampus (Jin et al., 2009). Fluoxetine activates the dopamine- and cAMP-regulated phosphoprotein of MW 32 kD (DARPP-32), and increases the phosphorylation state and efficacy of several ion channels and ionotropic receptors, such as AMPA receptors. The DARPP-32-induced increase in AMPA receptor phosphorylation and conductance might underlie the antidepressant actions of fluoxetine (Svenningsson et al., 2005).
Fluoxetine upregulates the expression and phosphorylation of cAMP-responsive element binding protein, and increases the production of brain-derived neurotrophic factor, both of which are crucial for neuroplasticity. Chronic fluoxetine treatment increases adult neurogenesis in the DG, and stimulates maturation and synaptic plasticity of dentate granule cells (Wang et al., 2008). Furthermore, the effect of fluoxetine on long-term potentiation in the DG depends on neurogenesis (Wang et al., 2008). Although TBI activates and stimulates type-I quiescent progenitor cells to proliferate, it also eliminates DCX-expressing late progenitor cells in the DG (Yu et al., 2008). Here we show a significant reduction in neurogenesis and immature neurons in the brain-injured ipsilateral DG, consistent with a previous report (Rola et al., 2006). Thus, despite the proliferation of type I progenitor cells, the local environment in the DG may not be favorable to the survival of newborn neurons, thus causing a net decrease in the total DCX population in the long run. Chronic fluoxetine administration was able to partially restore the ipsilateral DCX cells lost to TBI. Whether the restoration of type II progenitor cells by fluoxetine requires an increase in the proliferation of early progenitor cells, and/or improves the survival of existing DCX-expressing type II cells, warrants further investigation.
Chromatin remodeling plays an important role in neuroplasticity, learning, and memory (Levenson and Sweatt, 2006). Environmental enrichment (EE) induces hippocampal and cortical acetylation and methylation of histones 3 and 4, leading to increased dendritic sprouting, increased numbers of synapses, and recovery of long-term memory, in mice with neurodegeneration (Fischer et al., 2007). Most interestingly, the effects of EE on histone acetylation and memory is mimicked by inhibiting histone deacetylase (HDAC; Fischer et al., 2007). Following moderate TBI in immature rats, there is a decrease in histone H3 acetylation and methylation in the CA3 region of the hippocampus (Gao et al., 2006). Consistent with this finding, our results in adult brain-injured mice showed decreased hippocampal histone H3 acetylation and reduced MBD1 expression in the dentate gyrus. Not surprisingly, an HDAC inhibitor attenuates TBI-induced reductions in hippocampal acetyl-histone H3 (Zhang et al., 2008), and enhances learning and memory when combined with behavioral therapy following TBI (Dash et al., 2009). Repeated exposure to fluoxetine for 10 days induces MBD1 and methyl-CpG-binding protein 2 (MeCP2) in the normal adult dentate gyrus (Cassel et al., 2006). Following neural stem cell differentiation in vitro, there is an increase in the amount of MBD1 expression (Singh et al., 2009); however, we are not certain whether the fluoxetine-induced increase in the number of DCX-expressing cells is attributed to increased hippocampal progenitor cell differentiation, or enhanced survival of immature neurons. To better understand chronic fluoxetine-induced neuroplasticity in the injured brain, it is necessary to identify the target genes specifically affected by the increase in MBD1 expression and H3 acetylation.
Although fluoxetine increases motor output and improves motor skills in chronic stroke patients (Berends et al., 2009; Pariente et al., 2001), it does not reduce the gait impairments seen in our brain-injured mice. A lack of improvement in functional recovery has also been reported in a number of brain-injury models including TBI following fluoxetine administration (Boyeson et al., 1994; Li et al., 2009; Wilson and Hamm, 2002), suggesting that enhanced signaling via the 5-HT system is not sufficient to restore motor function after brain injury.
Fluoxetine increases extracellular serotonin, leading to the activation of 5-HT receptors. The effects of manipulating the 5-HT1A receptor on learning and memory are well studied, but the results are contradictory due to differences in behavioral paradigms, doses, and specificity of drugs. 5-HT1A agonists such as buspirone and 8-OH-DPAT impair water maze performance (Carli and Samanin, 1992; Carli et al., 1992; Liang et al., 1998), while the selective 5-HT1A antagonist WAY-100635 reduces memory impairment (Boast et al., 1999; Carli et al., 1997). Post-training, but not pre-training, administration of 5-HT uptake inhibitors facilitates memory consolidation (Meneses, 2002, 2007). A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after TBI facilitates motor recovery and acquisition of spatial learning (Cheng et al., 2008). A recent study also suggests that a delayed 4-week, rather than a 2-week, fluoxetine regimen is required to reverse spatial cognitive deficits caused by cerebral ischemia (Li et al., 2009). Moreover, the observed benefit of fluoxetine in memory function is related to an enhancement of hippocampal neurogenesis. Although the 5-week chronic fluoxetine intervention used in our study had a positive effect on spatial learning, it was not sufficient to reverse TBI-induced deficits in learning and memory. A TBI-induced increase in the expression of 5-HT1A receptor might contribute to its cognitive sequelae, but fluoxetine was not able to reduce 5-HT1A receptor expression or memory impairment following lateral fluid percussion injury (Wilson and Hamm, 2002). Consistent with previous findings, our study suggests that fluoxetine is not therapeutic in treating motor or cognitive deficits in moderate to severe TBI.
The prevalence of depression following TBI in humans has been reported to be as high as 77% (Jorge and Starkstein, 2005), yet diagnosing depression is often challenging due to the overlapping symptoms seen after TBI. The forced swimming test (FST) was designed as a primary screening test for antidepressants, but the sensitivity of the FST in detecting TBI-induced depression has not been consistent. Although depression-like behavior during the FST has been reported previously in a mild TBI model using weight drop (Milman et al., 2005; Shapira et al., 2007), it was not detected in our current mouse TBI model, or in the fluid-percussion injury model (Jones et al., 2008). Potential contributing factors to the differential detection of depression-like behavior by FST after brain injury may lie in differences in the models and severity of experimental TBI, the timing of the affective measures used following injury, and/or other methodological differences. Mouse strain also plays a role in the immobile behavior seen during FST, both at baseline and following fluoxetine treatment (Dulawa et al., 2004). Unlike the highly anxiogenic BALB/cJ strain, C57BL/6 mice do not exhibit reduced depression-like behavior during FST following fluoxetine treatment, consistent with our current results. A recent study suggests that an increased level of 5-HT1A receptor exacerbates behavioral despair, and reduces behavioral response to antidepressants (Richardson-Jones et al., 2010). However, it is unlikely that the strain difference in depressive behavior or sensitivity to antidepressant treatment is related to the endogenous level of 5-HT1A receptor, as the behavioral effects of chronic fluoxetine in BALB/cJ mice do not seem to require the 5-HT1A receptor (Holick et al., 2008).
SSRIs including fluoxetine have been reported to improve neurological manifestations clinically, although good trials with sufficient sample size and follow-up time in patients with TBI are lacking. The current preclinical study provides additional insight into fluoxetine-induced neuroplasticity, particularly in the hippocampus. However, the neurobiological effects of fluoxetine, even when applied chronically, are not effective in restoring function in moderate to severe TBI.
Acknowledgments
This work was supported by a VA Merit award (to J.L.), and a Department of Defense collaborative in Neuroscience Center of Excellence (to J.L.).
Author Disclosure Statement
No competing financial interests exist.
References
- Beauquis J. Roig P. De Nicola A.F. Saravia F. Neuronal plasticity and antidepressants in the diabetic brain. Ann. NY Acad. Sci. 2009;1153:203–208. doi: 10.1111/j.1749-6632.2008.03983.x. [DOI] [PubMed] [Google Scholar]
- Berends H.I. Nijlant J. van Putten M. Movig K.L. Single dose of fluoxetine increases muscle activation in chronic stroke patients. Clin. Neuropharmacol. 2009;32:1–5. [PubMed] [Google Scholar]
- Bilgen M. A new device for experimental modeling of central nervous system injuries. Neurorehabil. Neural Repair. 2005;19:219–226. doi: 10.1177/1545968305278635. [DOI] [PubMed] [Google Scholar]
- Boast C. Bartolomeo A.C. Morris H. Moyer J.A. 5HT antagonists attenuate MK801-impaired radial arm maze performance in rats. Neurobiol. Learn. Mem. 1999;71:259–271. doi: 10.1006/nlme.1998.3886. [DOI] [PubMed] [Google Scholar]
- Boyeson M.G. Harmon R.L. Jones J.L. Comparative effects of fluoxetine, amitriptyline and serotonin on functional motor recovery after sensorimotor cortex injury. Am. J. Phys. Med. Rehabil. 1994;73:76–83. doi: 10.1097/00002060-199404000-00002. [DOI] [PubMed] [Google Scholar]
- Carli M. Bonalumi P. Samanin R. WAY 100635, a 5-HT1A receptor antagonist, prevents the impairment of spatial learning caused by intrahippocampal administration of scopolamine or 7-chloro-kynurenic acid. Brain Res. 1997;774:167–174. doi: 10.1016/s0006-8993(97)81700-3. [DOI] [PubMed] [Google Scholar]
- Carli M. Lazarova M. Tatarczynska E. Samanin R. Stimulation of 5-HT1A receptors in the dorsal hippocampus impairs acquisition and performance of a spatial task in a water maze. Brain Res. 1992;595:50–56. doi: 10.1016/0006-8993(92)91451-j. [DOI] [PubMed] [Google Scholar]
- Carli M. Samanin R. 8-Hydroxy-2-(di-n-propylamino)tetralin impairs spatial learning in a water maze: role of postsynaptic 5-HT1A receptors. Br. J. Pharmacol. 1992;105:720–726. doi: 10.1111/j.1476-5381.1992.tb09045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel S. Carouge D. Gensburger C. Anglard P. Burgun C. Dietrich J.B. Aunis D. Zwiller J. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol. Pharmacol. 2006;70:487–492. doi: 10.1124/mol.106.022301. [DOI] [PubMed] [Google Scholar]
- Cheng J.P. Hoffman A.N. Zafonte R.D. Kline A.E. A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav. Brain Res. 2008;194:79–85. doi: 10.1016/j.bbr.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Peng L. Zhang X. Stolzenburg J.U. Hertz L. Further evidence that fluoxetine interacts with a 5-HT2C receptor in glial cells. Brain Res. Bull. 1995;38:153–159. doi: 10.1016/0361-9230(95)00082-p. [DOI] [PubMed] [Google Scholar]
- Crosio C. Heitz E. Allis C.D. Borrelli E. Sassone-Corsi P. Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J. Cell Sci. 2003;116:4905–4914. doi: 10.1242/jcs.00804. [DOI] [PubMed] [Google Scholar]
- Dash P.K. Orsi S.A. Moore A.N. Histone deactylase inhibition combined with behavioral therapy enhances learning and memory following traumatic brain injury. Neuroscience. 2009;163:1–8. doi: 10.1016/j.neuroscience.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D.J. Samuels B.A. Rainer Q. Wang J.W. Marsteller D. Mendez I. Drew M. Craig D.A. Guiard B.P. Guilloux J.P. Artymyshyn R.P. Gardier A.M. Gerald C. Antonijevic I.A. Leonardo E.D. Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa S.C. Holick K.A. Gundersen B. Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Encinas J.M. Vaahtokari A. Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. USA. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A. Sananbenesi F. Wang X. Dobbin M. Tsai L.H. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Gao W.M. Chadha M.S. Kline A.E. Clark R.S. Kochanek P.M. Dixon C.E. Jenkins L.W. Immunohistochemical analysis of histone H3 acetylation and methylation—evidence for altered epigenetic signaling following traumatic brain injury in immature rats. Brain Res. 2006;1070:31–34. doi: 10.1016/j.brainres.2005.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z. Giustetto M. Lomvardas S. Kim J.H. Miniaci M.C. Schwartz J.H. Thanos D. Kandel E.R. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- Holick K.A. Lee D.C. Hen R. Dulawa S.C. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Jin Y. Lim C.M. Kim S.W. Park J.Y. Seo J.S. Han P.L. Yoon S.H. Lee J.K. Fluoxetine attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Brain Res. 2009;1281:108–116. doi: 10.1016/j.brainres.2009.04.053. [DOI] [PubMed] [Google Scholar]
- Jones N.C. Salzberg M.R. Kumar G. Couper A. Morris M.J. O'Brien T.J. Elevated anxiety and depressive-like behavior in a rat model of genetic generalized epilepsy suggesting common causation. Exp. Neurol. 2008;209:254–260. doi: 10.1016/j.expneurol.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Jorge R.E. Starkstein S.E. Pathophysiologic aspects of major depression following traumatic brain injury. J. Head Trauma Rehabil. 2005;20:475–487. doi: 10.1097/00001199-200511000-00001. [DOI] [PubMed] [Google Scholar]
- Kennard L.E. Chumbley J.R. Ranatunga K.M. Armstrong S.J. Veale E.L. Mathie A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br. J. Pharmacol. 2005;144:821–829. doi: 10.1038/sj.bjp.0706068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong E.K. Peng L. Chen Y. Yu A.C. Hertz L. Up-regulation of 5-HT2B receptor density and receptor-mediated glycogenolysis in mouse astrocytes by long-term fluoxetine administration. Neurochem. Res. 2002;27:113–120. doi: 10.1023/a:1014862808126. [DOI] [PubMed] [Google Scholar]
- Levenson J.M. Sweatt J.D. Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cell Mol. Life Sci. 2006;63:1009–1016. doi: 10.1007/s00018-006-6026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K.C. Tsui K.Y. Tyan Y.M. Chiang T.C. Buspirone impaired acquisition and retention in avoidance tasks: involvement of the hippocampus. Chin. J. Physiol. 1998;41:33–44. [PubMed] [Google Scholar]
- Liu Z. Fan Y. Won S.J. Neumann M. Hu D. Zhou L. Weinstein P.R. Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Li W.L. Cai H.H. Wang B. Chen L. Zhou Q.G. Luo C.X. Liu N. Ding X.S. Zhu D.Y. Chronic fluoxetine treatment improves ischemia-induced spatial cognitive deficits through increasing hippocampal neurogenesis after stroke. J. Neurosci. Res. 2009;87:112–122. doi: 10.1002/jnr.21829. [DOI] [PubMed] [Google Scholar]
- Matsumori Y. Hong S.M. Fan Y. Kayama T. Hsu C.Y. Weinstein P.R. Liu J. Enriched environment and spatial learning enhance hippocampal neurogenesis and salvages ischemic penumbra after focal cerebral ischemia. Neurobiol. Dis. 2006;22:187–198. doi: 10.1016/j.nbd.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt J.F. Sale A. Viegi A. Baroncelli L. De Pasquale R. O'Leary O.F. Castren E. Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Meneses A. Involvement of 5-HT(2A/2B/2C) receptors on memory formation: simple agonism, antagonism, or inverse agonism? Cell Mol. Neurobiol. 2002;22:675–688. doi: 10.1023/A:1021800822997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A. Stimulation of 5-HT1A, 5-HT1B, 5-HT2A/2C, 5-HT3 and 5-HT4 receptors or 5-HT uptake inhibition: short- and long-term memory. Behav. Brain Res. 2007;184:81–90. doi: 10.1016/j.bbr.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Miller C.A. Gavin C.F. White J.A. Parrish R.R. Honasoge A. Yancey C.R. Rivera I.M. Rubio M.D. Rumbaugh G. Sweatt J.D. Cortical DNA methylation maintains remote memory. Nat. Neurosci. 2010;13:664–666. doi: 10.1038/nn.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman A. Rosenberg A. Weizman R. Pick C.G. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J. Neurotrauma. 2005;22:1003–1010. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- Mostert J.P. Koch M.W. Heerings M. Heersema D.J. De Keyser J. Therapeutic potential of fluoxetine in neurological disorders. CNS Neurosci. Ther. 2008;14:153–164. doi: 10.1111/j.1527-3458.2008.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla A. Mosavinasab M. Pani A. Does fluoxetine have any effect on the cognition of patients with mild cognitive impairment? A double-blind, placebo-controlled, clinical trial. J. Clin. Psychopharmacol. 2007;27:67–70. doi: 10.1097/JCP.0b013e31802e0002. [DOI] [PubMed] [Google Scholar]
- Nahon E. Israelson A. Abu-Hamad S. Varda S.B. Fluoxetine (Prozac) interaction with the mitochondrial voltage-dependent anion channel and protection against apoptotic cell death. FEBS Lett. 2005;579:5105–5110. doi: 10.1016/j.febslet.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Narushima K. Paradiso S. Moser D.J. Jorge R. Robinson R.G. Effect of antidepressant therapy on executive function after stroke. Br. J. Psychiatry. 2007;190:260–265. doi: 10.1192/bjp.bp.106.025064. [DOI] [PubMed] [Google Scholar]
- Neumann M. Wang Y. Kim S. Hong S.M. Jeng L. Bilgen M. Liu J. Assessing gait impairment following experimental traumatic brain injury in mice. J. Neurosci. Methods. 2009;176:34–44. doi: 10.1016/j.jneumeth.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M. Zamponi G.W. Barr W. Kiedrowski L. Stys P.K. Protection of ischemic rat spinal cord white matter: Dual action of KB-R7943 on Na+/Ca2+ exchange and L-type Ca2+ channels. Neuropharmacology. 2005;48:566–575. doi: 10.1016/j.neuropharm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Pariente J. Loubinoux I. Carel C. Albucher J.F. Leger A. Manelfe C. Rascol O. Chollet F. Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann. Neurol. 2001;50:718–729. doi: 10.1002/ana.1257. [DOI] [PubMed] [Google Scholar]
- Patterson D.E. Braverman S.E. Belandres P.V. Speech dysfunction due to trazodone–fluoxetine combination in traumatic brain injury. Brain Inj. 1997;11:287–291. doi: 10.1080/026990597123593. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D. Bertin A. Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977b;229:327–336. [PubMed] [Google Scholar]
- Porsolt R.D. Le Pichon M. Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977a;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones J.W. Craige C.P. Guiard B.P. Stephen A. Metzger K.L. Kung H.F. Gardier A.M. Dranovsky A. David D.J. Beck S.G. Hen R. Leonardo E.D. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R. Mizumatsu S. Otsuka S. Morhardt D.R. Noble-Haeusslein L.J. Fishman K. Potts M.B. Fike J.R. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp. Neurol. 2006;202:189–199. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Santarelli L. Saxe M. Gross C. Surget A. Battaglia F. Dulawa S. Weisstaub N. Lee J. Duman R. Arancio O. Belzung C. Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Shapira M. Licht A. Milman A. Pick C.G. Shohami E. Eldar-Finkelman H. Role of glycogen synthase kinase-3beta in early depressive behavior induced by mild traumatic brain injury. Mol. Cell Neurosci. 2007;34:571–577. doi: 10.1016/j.mcn.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Shimizu S. Narita M. Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Singh R.P. Shiue K. Schomberg D. Zhou F.C. Cellular epigenetic modifications of neural stem cell differentiation. Cell Transplant. 2009;18:1197–1211. doi: 10.3727/096368909X12483162197204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P. Nairn A.C. Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. Aaps J. 2005;7:E353–E360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.W. David D.J. Monckton J.E. Battaglia F. Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J. Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.S. Hamm R.J. Effects of fluoxetine on the 5-HT1A receptor and recovery of cognitive function after traumatic brain injury in rats. Am. J. Phys. Med. Rehabil. 2002;81:364–372. doi: 10.1097/00002060-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Yu T.S. Zhang G. Liebl D.J. Kernie S.G. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J. Neurosci. 2008;28:12901–12912. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. West E.J. Van K.C. Gurkoff G.G. Zhou J. Zhang X.M. Kozikowski A.P. Lyeth B.G. HDAC inhibitor increases histone H3 acetylation and reduces microglia inflammatory response following traumatic brain injury in rats. Brain Res. 2008;1226:181–191. doi: 10.1016/j.brainres.2008.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Peng L. Chen Y. Hertz L. Stimulation of glycogenolysis in astrocytes by fluoxetine, an antidepressant acting like 5-HT. Neuroreport. 1993;4:1235–1238. doi: 10.1097/00001756-199309000-00006. [DOI] [PubMed] [Google Scholar]