Abstract
Resistance to thyrotropin (TSH) (RTSH; defined by elevated TSH and a normal or hypoplastic thyroid gland) can be caused by mutations in genes encoding the TSH receptor and PAX8, and it has been linked to a locus on chromosome 15. In two nonconsanguineous families with nongoitrous euthyroid hyperthyrotropinemia, typical of the RTSH phenotype, exome analysis identified five rare DUOX2 gene variants (p.A649E, p.P1391A, p.R885L, p.G488R, and p.SF965-6SfsX29) found to be pathogenic. This form of nongoitrous dyshormonogenesis masquerades both clinically and biochemically as RTSH. Accordingly, mutations in DUOX2 should be added to those of SLC26A4 as causes of RTSH.
Keywords: : DUOX2, RTSH, elevated TSH, hyperthyrotropinemia
Introduction
Resistance to TSH (RTSH) denotes reduced sensitivity to a biologically active thyrotropin (TSH). Affected individuals have elevated TSH levels but no goiter (normal or hypoplastic thyroid glands) and normal or low serum thyroid hormone concentrations (depending on the degree of hyposensitivity to TSH). This is common in newborns with congenital hypothyroidism based on blood TSH. RTSH is commonly caused by mutations in the TSH receptor (TSHR) (1).
Over the last 11 years we identified 77 families with RTSH. Loss of function mutations in TSHR were found in 11 families, mutations in PAX8 in 4 families and dominantly inherited RTSH was linked to a locus on chromosome 15 in 11 families (1). To identify genetic causes of RTSH, we conducted whole exome (WES) or whole genome sequencing (WGS) on 12 families in which Sanger sequencing failed to identify TSHR and PAX8 mutations. Herein, we describe two families that were found to harbor DUOX2 mutations.
Patients
Family 1
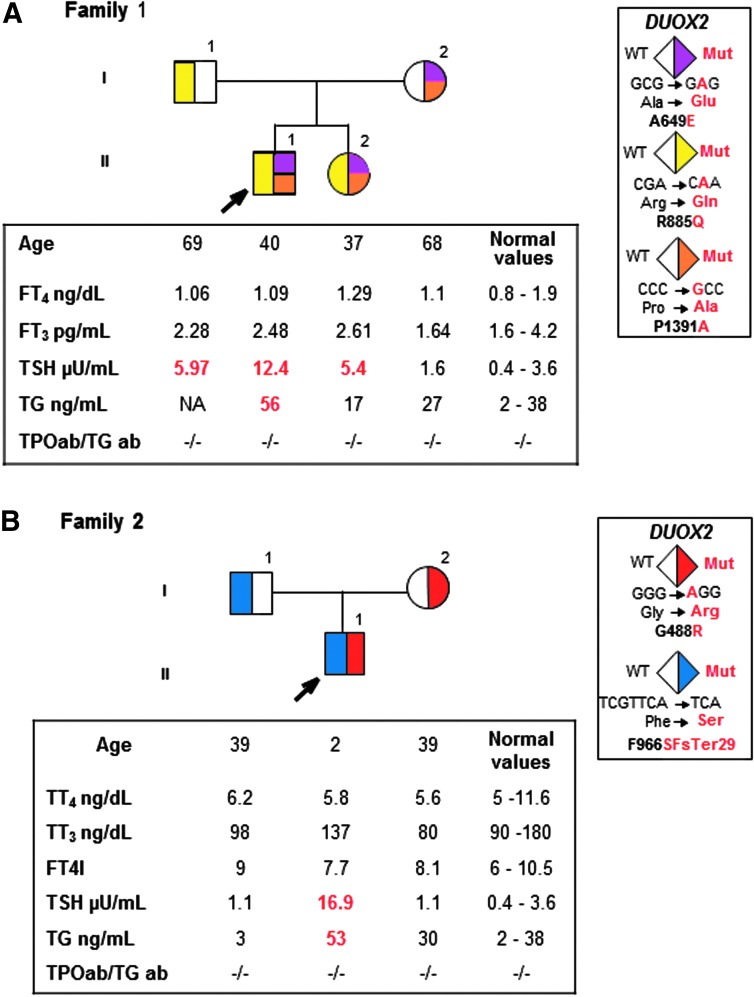
The proband and his sister had congenital hypothyroidism based on elevated neonatally determined blood TSH of 58 and 89 mu/L, respectively. Both had small, eutrophic thyroid glands as determined by ultrasound. Their father was hypothyroid, and their mother had not undergone thyroid testing. Both parents self-identified as Japanese. The siblings and father were placed on levothyroxine (LT4). When tested at 55 years of age, the mother had mild hypothyroidism, with free thyroxine index 5.9 (6–10.5), TSH 39 mU/L (0.4–3.6 mU/L), and thyroglobulin 79 ng/mL (2–38 ng/mL) (Fig. 1), and was placed on LT4. Testing 13 years later, after LT4 replacement was discontinued in all family members for 6 weeks, showed that everyone except the mother had mildly elevated TSH but normal iodothyronine levels (Fig. 1A).
FIG. 1.
(A) Pedigrees of family 1 with thyroid function test results after discontinuation of levothyroxine for 6 weeks. These are aligned with each symbol representing a family member. Abnormal values are shown in bold numbers; high values are in red. See color legend identifying each mutation. (B) Pedigree of family 2, presented in the same way as in family 1. FT3, free triiodothyronine; FT4, free thyroxine; FT4I, free thyroxine index; TG, thyroglobulin; TGab, thyroglobulin antibodies; TPOab, thyroperoxidase antibodies; TSH, thyroid-stimulating hormone; TT4, total thyroxine; TT3, total triiodothyronine.
Family 2
The 2-year-old male proband had elevated TSH on neonatal screening. At 1 week, TSH was 64 and 70 mU/L (0.7–18 mU/L). No thyroid hormone treatment was initiated because four follow-up serum TSH values ranged from 5.2 to 11.0 mU/L with free T4 and T3 concentrations above the mid normal range. At 2 years of age iodothyronine levels continued to be normal and thyroglobulin mildly elevated. Radioiodide scan showed a thyroid gland in situ. His parents had normal thyroid function tests (Fig. 1B). At 2.5 years, treatment with 25 μg levothyroxine daily was started. Parents self-identified as English and were nonconsanguineous.
Results
Informed consent was obtained from all individuals prior to testing. We performed WGS of family 1 and WES of family 2 and analyzed the data under models of inheritance consistent with segregation of RTSH. Five different rare variants (i.e., global allele frequency <1%) in DUOX2 were identified in the two families and the presence of each variant was confirmed by Sanger sequencing. The siblings of family 1 were compound heterozygous for three missense DUOX2 mutations in exons 17, 20, and 31, resulting in p.A649E, p.R885L, and p.P1391A (Fig. 1A) (RefSeq transcript NM_014080). Each of these variants is predicted by PolyPhen-2, CADD, and SIFT scores (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy) to be deleterious and has been previously reported to be associated with congenital hypothyroidism (2,3). The father was heterozygous for one of the mutations (p.R885L), while the mother had two (p.A649E and p.P1391A) (Fig. 1A). The mother was also heterozygous for a common polymorphism (p.H678R) in exon 17 (mean allele frequency of ∼42% in individuals sampled from sub-Saharan African populations) (Fig. 1A).
The proband of family 2 was compound heterozygous, inheriting a missense mutation, p.G488R, in exon 13 from the mother and a four nucleotide frameshift deletion (c.2895-2898delGTTC) in exon 22 from the father (Fig. 1B) that is predicted to result in a premature stop codon at amino acid position 994 (p.SF965-6SfsX29). Both variants have been reported in patients with high TSH levels and they are predicted to be deleterious based on CADD, PolyPhen-2, and SIFT scores (Supplementary Table S1).
Discussion
Families 1 and 2 share both pathogenic DUOX2 variants and an elevated serum TSH with normal thyroid hormone concentrations without goiter, consistent with RTSH, rather than dyshormonogenesis. DUOX2 is a nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) expressed at the apical membrane of follicular thyroid cells. It generates H2O2, the essential electron acceptor for the thyroperoxidase (TPO)-catalyzed iodination and coupling reactions (4). Despite its involvement in hormonogenesis, DUOX2 gene mutations cause variable phenotypes, with most persons having no goiter and 5% having a small thyroid gland (5). The hypothyroidism is not permanent and can fluctuate over time, especially in individuals with a monoallelic pathogenic variant, as in patient I-2 of family 1 described in this study.
The findings in family 1 were unanticipated as, based on the apparent dominant mode of inheritance, we originally predicted that RTSH was caused by a variant in non-coding sequences that regulate TSH receptor expression (1). However, the presence of a pathogenic noncoding variant was excluded by WGS. Family 2 was part of a cohort on which we performed WES looking explicitly for novel genes underlying RTSH. Together, the finding of pathogenic variants in DUOX2 in both families with RTSH suggests that mutations in DUOX2 can cause at least two different phenotypes, RTSH and dyshormonogenesis, as recently shown for mutations in the SLC26A4 gene (6).
Supplementary Material
Contributor Information
Collaborators: University of Washington Center for Mendelian Genomics
Acknowledgments
This work was supported in part by grant R37DK15070 from the National Institutes of Health to S.R. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. Sequencing was provided by the University of Washington Center for Mendelian Genomics (UW-CMG) and was funded by the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute grant 2UM1HG006493 to Drs. Debbie Nickerson, Michael Bamshad, and Suzanne Leal. We are grateful to Dr. Charles G.D. Brook from the The Middlesex and Great Ormond Street Hospital, London, UK, for referral of family 2.
Author Disclosure Statement
No competing financial interests exist.
Web Resources
The URLs for data presented herein are as follows:
Exome Variant Server, NHLBI Exome Sequencing Project (ESP6500), Seattle, WA: http://evs.gs.washington.edu/EVS (accessed August 2016).
ExAC Browser (Beta), Exome Aggregation Consortium, Cambridge, MA: http://exac.broadinstitute.org (accessed April 2015).
References
- 1.Refetoff S, Wiess RE, Grasberger H. 2016. Resistance to thyrotropin and thyrotropin-releasing hormone. In: Cooper DS. (ed) UpToDate. Waltham, MA. Available at: https://www.uptodate.com/contents/resistance-to-thyrotropin-and-thyrotropin-releasing-hormone (accessed August5, 2016)
- 2.Park KJ, Park HK, Kim YJ, Lee KR, Park JH, Park JH, Park HD, Lee SY, Kim JW. 2016. DUOX2 Mutations are frequently associated with congenital hypothyroidism in the Korean population. Ann Lab Med 36:145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin HY, Heo SH, Kim YM, Kim GH, Choi JH, Lee BH, Yoo HW. 2014. High frequency of DUOX2 mutations in transient or permanent congenital hypothyroidism with eutopic thyroid glands. Horm Res Paediatr 82:252–260 [DOI] [PubMed] [Google Scholar]
- 4.Grasberger H, Refetoff S. 2016. Congenital defects of thyroid hormone synthesis. In: Weiss RE, Refetoff S. (eds) Genetic Diagnosis of Endocrine Disorders, 2 ed. Academic Press, London, pp 117–125 [Google Scholar]
- 5.Fu C, Luo S, Zhang S, Wang J, Zheng H, Yang Q, Xie B, Hu X, Fan X, Luo J, Chen R, Su J, Shen Y, Gu X, Chen S. 2016. Next-generation sequencing analysis of DUOX2 in 192 Chinese subclinical congenital hypothyroidism (SCH) and CH patients. Clin Chim Acta 458:30–34 [DOI] [PubMed] [Google Scholar]
- 6.Kuhnen P, Turan S, Frohler S, Guran T, Abali S, Biebermann H, Bereket A, Gruters A, Chen W, Krude H. 2014. Identification of PENDRIN (SLC26A4) mutations in patients with congenital hypothyroidism and “apparent” thyroid dysgenesis. J Clin Endocrinol Metab 99:E169–176 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.