Abstract
Background
Chromium is an essential trace element and nutritional supplement that has garnered interest for use as a weight loss aid.
Objective
This trial assesses the effects of chromium picolinate supplementation, alone and combined with nutritional education, on weight loss in apparently healthy overweight adults.
Design
This was a randomized, double-blind, placebo-controlled trial of 80 otherwise healthy, overweight adults assessed at baseline for central adiposity measured by computerized tomography. Subjects were randomly assigned to daily ingestion of 1000 μg of chromium picolinate or placebo for 24 weeks. All subjects received passive nutritional education at the 12-week point in both the intervention and control groups. Outcomes include weight, height, blood pressure, percent body fat, serum, and urinary biomarkers.
Results
At baseline, both the chromium and placebo groups had similar mean body mass index (BMI) (chromium = 36 ± 6.7 kg/m2 versus placebo = 36.1 ± 7.6 kg/m2; p = 0.98). After 12 weeks, no change was seen in BMI in the intervention as compared to placebo (chromium = 0.3 ± 0.8 kg/m2 versus placebo = 0.0 ± 0.4 kg/m2; p = 0.07). No change was seen in BMI after 24 weeks in the intervention as compared to placebo (chromium = 0.1 ± 0.2 kg/m2 versus placebo = 0.0 ± 0.5 kg/m2; p = 0.81). Variation in central adiposity did not affect any outcome measures.
Conclusions
Supplementation of 1000 μg of chromium picolinate alone, and in combination with nutritional education, did not affect weight loss in this population of overweight adults. Response to chromium did not vary with central adiposity.
Introduction
Over 65% of adults in the United States are overweight or obese, defined as a body mass index (BMI) at or above 25 or 30 kg/m2, respectively.1–3 The health consequences of obesity are well characterized.4 A strong relationship exists between BMI and all-cause mortality5,6; obesity contributes substantially to cardiovascular risk,7–9 and excess body weight is a potent risk factor for most cancers.10,11 Considering the health consequences of obesity, there is a growing need for safe and effective aids to weight loss.
The Nutrition Business Journal reported that supplement sales grew from $8.6 to $23.7 billion between 1994 and 2007.12 Sports nutrition and weight loss supplements accounted for approximately 27% of total sales.13–15
Despite the growing consumer market for use of dietary supplements, efficacy in weight loss remains unsubstantiated. A 2004 systematic review concluded that the evidence for most dietary supplements as aids in reducing body weight is inconclusive.16 A notable exception is ephedra, found to be an effective weight loss aid,17 though banned from the market by the U.S. Food and Drug Administration in 2004 due to safety concerns.12
Chromium is an essential trace element and nutritional supplement that has garnered interest for use as a weight loss aid.18 Purported benefits of supplementation include increased lean body mass, decreased body fat, and greater resting energy expenditure.19
Chromium has been thought to be the active ingredient in glucose tolerance factor, a complex of molecules that includes glycine, cysteine, glutamic acid, nicotinic acid, and chromium.20 This complex of molecules found in high amounts in brewer's yeast and other foods functions synergistically to potentiate the effects of insulin21–23 by increasing insulin binding to cells, upregulating receptors, and improving affinity.24 Some reports suggest that chromium could suppress appetite and stimulate thermogenesis through sensitization of insulin-sensitive glucoreceptors in the brain.25 Body fat distribution is related to insulin sensitivity; peripheral fat is more insulin sensitive than central fat found in the chest and abdomen.26
A meta-analysis of 10 double-blind, placebo-controlled trials provides evidence of a relatively small reduction in body weight (1.1–1.2 kg over 10–13 weeks) in overweight and obese individuals receiving chromium picolinate.27
This trial was designed to assess the effects of chromium picolinate supplementation alone and combined with a nutrition education intervention on weight loss in both men and women, and to assess any effects attributable to anthropometry (body fat distribution).
Methods
Participants
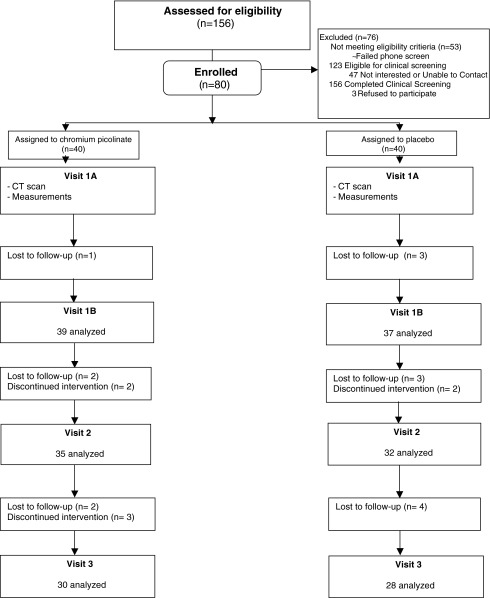
A total of 80 adults (40 female and 40 male) were recruited from the Lower Naugatuck Valley, CT, through newspaper advertisements and posters in medical offices affiliated with Griffin Hospital (Fig. 1). All participants were overweight (body–mass index [BMI] > 25 kg/m2) nonsmoking adults ages 25–75 with abdominal adiposity (waist circumference > 80 cm in females and >100 cm in males).28 Exclusion criteria included contraindication to abdominal computed tomography (CT) scans (weight > 375 pounds, claustrophobia, unstable vital signs, or radiation procedure in past 6 months), diagnosed diabetes, diagnosed eating disorder, uncontrolled hypertension, emphysema, intestinal or stomach disease, kidney disease (serum creatinine > 2), substance abuse, pregnancy, or intention to become pregnant during the study.
FIG. 1.
Study flow diagram. CT, computed tomography.
Those meeting initial prescreening criteria (n = 156) underwent clinical screening examination consisting of height, weight, BMI, blood pressure, and waist–hip measurements and blood profiles inclusive of lipid panel (total cholesterol, high-density lipoprotein [HDL], low-density lipoprotein [LDL] and triglycerides), comprehensive metabolic panel, fasting plasma glucose, fasting insulin, C-reactive protein, and lipoprotein-associated phospholipase A2 (Lp-PLA2). In addition, percent body fat was recorded via bioelectrical impedance using the Bio Analogics ELGII Health Management System (HMS; www.bioanalogics.com). A urine pregnancy test for human chorionic gonadotropin was performed on female patients to ascertain nonpregnant status at baseline.
The study protocol and consent form were approved by the Griffin Hospital (Derby, CT) Institutional Review Board and the Yale University (New Haven, CT) Human Investigation Committee. Written informed consent was obtained, and all subjects received $150 for their participation. Subjects signed a written study commitment agreement explaining the number of visits, outcome measures, and focus on weight, percent body fat, and cardiac risk measures.
Interventions
Subjects were randomized to daily ingestion of 1000 μg of chromium picolinate or placebo (1630 mg of dicalcium phosphate). The 1000 μg dose was chosen because it has been shown to be safe and effective in modifying blood sugar and insulin levels18,29 and used in other clinical trials.30 Subjects randomized to chromium picolinate were instructed to ingest a 500-μg capsule twice per day during the intervention period for a total ingestion of 1000 μg per day for 6 months. Those randomized to placebo were instructed to ingest an 815-mg capsule twice per day during the intervention period for a total ingestion of 1630 mg of dicalcium phosphate. Subjects were instructed to consume these capsules with water with morning and evening meals and to continue with their usual dietary patterns and physical activity routines for the first 12 weeks of intervention.
A low-intensity nutrition education and weight loss program commenced at 12 weeks up to the 24-week point in both the intervention and control groups. This lifestyle intervention was reflective of the fact that in any real-world setting, a patient interested in weight loss would be unlikely to rely solely on a chromium supplement. In all probability, some effort at “dieting” would accompany use of the supplement. The program consisted of free access to a weight loss website (www.thewaytoeat.net) and a copy of a book on nutrition and weight management.31 The nutrition education intervention was implemented at week 12 in order to assess any differential effects between chromium alone or in conjunction with the nutrition education intervention, and to standardize the weight loss efforts of study participants. It was meant to substitute for independent weight loss efforts by the participants, and/or the basic weight loss advice patients would be likely to receive from a primary care provider.
Objectives
This trial assessed the effects of chromium picolinate supplementation, alone and combined with nutritional education on weight loss in apparently healthy overweight adults.
Outcomes
Weight, height, and blood pressure were measured at each visit. Prior to each assessment, subjects fasted for 8 hours for serum and bioimpedance measures. Body weight was measured to the nearest 0.5 pound using a balance-type medical scale. Height was measured in inches with instructions for the subject to stand on the middle of the scale with back against the measuring bar standing straight, without shoes, heels together. Two (2) readings of blood pressure were taken in a seated position 10 minutes apart using an electronic sphygmomanometer. Other outcome measures included waist–hip ratio, percent body fat, central adiposity, serology, and urine chromium.
Waist circumference was measured around the narrowest point between ribs and hips when viewed from the front after exhaling. Hip circumference was measured at the point where the buttocks extended the maximum, when viewed from the side. Recordings were made for each site to the nearest 1 cm using a cloth tape without compression of skin.32,33
Percent body fat was recorded via bioelectrical impedance. The imperceptible electrical current was passed through electrodes in the subject's foot and hand to compute body density and body-fat percentage. The primary outcome measure was to demonstrate a decrease in body fat from baseline in adults with BMI ≥ 25, due to sustained ingestion of chromium picolinate. Resistance and reactance were measured with the Bio Analogic ELG II and the percent body fat was determined with the use of Health Management System software (www.bioanalogics.com).
For bioelectrical impedance scans, subjects were instructed to fast and refrain from exercise 8 hours before the scan. Subjects were also instructed to refrain from drinking alcohol 24 hours before the scan. Additional instructions included removing metallic jewelry and maintaining adequate hydration the day before the scan.
Central adiposity was measured at baseline to determine the area of subcutaneous versus visceral adipose tissue. Central adiposity was measured on a 16-slice helical G scanner at Griffin Hospital, using standard procedures.34–36 Subjects lay supine with their arms over their heads. A CT scan was performed at the abdominal level (between L4 and L5 vertebrae), using a radiograph of the skeleton as a reference to establish the position of the scan to the nearest millimeter. Total abdominal adipose tissue area was calculated by delineating the surface with a graph pen and then computing the adipose tissue surface using an attenuation range of −190 to −30 Hounsfield units using sliceOmatic® image analysis software.34 The abdominal subcutaneous adipose tissue area was calculated by subtracting the visceral adipose tissue area from the total abdominal adipose tissue area using previously published criteria.36 The calculations for subcutaneous and visceral adipose tissue were performed at Hôpital Laval Research Center, Québec, Canada.
At each visit, lipid profile, fasting plasma glucose, fasting insulin, Lp-PLA2, and C-reactive protein (CRP) were assessed. All screening and serum laboratory assays with the exception of Lp-PLA2 and CRP were performed at Griffin Hospital. Lp-PLA2 and CRP analysis were performed at diaDexus, Inc. (www.diadexus.com). Liver and kidney function were monitored throughout the study by serum measurements of transaminases, blood urea nitrogen, and creatinine.
Subjects also provided a urine specimen for analysis of chromium output to corroborate self-report of regular use of treatment assignment. Urine chromium was collected at Griffin Hospital and was analyzed by Quest Laboratories.
Sample size
The sample size was determined to allow for approximately 20% attrition and noncompliance per treatment arm and provide at least 80% power with maximum allowable type I error of 5%. The study was specifically powered to compare chromium to placebo and demonstrate a 5.1% decrease in percent body fat (the primary outcome) from baseline due to sustained (daily for 12 weeks) ingestion of 1000 μg of chromium picolinate (500 μg BID) compared to placebo.
Randomization
Subjects were enrolled and randomized using balanced allocation within gender to ensure that an equal number of males and females were randomized to receive chromium and placebo. (Fig. 1). Outcome assessments were made at baseline, 12 weeks, and 24 weeks to identify the singular effects of chromium and placebo on weight loss, as well as the combined effects of chromium in the context of a nutrition education intervention.
Blinding
Subjects and study personnel were blinded to the intervention. Chromium and placebo were prepackaged and shipped from the manufacturer to the study site. Bottles were labeled and coded by an unblinded individual unaffiliated with the study. Investigators thus only knew the treatment assignment (group A or B) of the subjects without knowledge of whether these contained chromium or placebo.
Statistical methods
Repeated-measures analysis of variance was used to determine change in percent body weight, BMI, and serology after intervention between the two treatment groups. Paired t tests were also used to evaluate the change from baseline (pretreatment) in percent body fat, weight, BMI, and serology following each treatment. The combined effects of independent variables (abdominal fat distribution and demographics) and treatment assignment on these outcomes were assessed with multivariable models using analysis of covariance.
Analysis was performed using the SAS for Windows version 9.1 (SAS Institute, Cary, NC) software. In all analyses, a two-tailed α of less than 0.05 was considered statistically significant. Results are expressed as means ± standard deviation (SD) in text and tables.
Results
Participant flow
The two treatment arms were comparable (p > 0.05) at baseline (Table 1) for all the outcome measures (i.e., anthropometric measures, blood pressure, serology, and urine chromium). The study participants in both treatment groups were overweight or obese at baseline (intervention group mean BMI = 36 kg/m2; control group BMI = 36.1 kg/m2).
Table 1.
Baseline Values
| Outcome measures | Chromium picolinate (n = 40) | Placebo (n = 40) | p-value |
|---|---|---|---|
| Anthropometric measures | |||
| BMI (kg/m2) | 36.0 ± 6.7 | 36.1 ± 7.6 | 0.98 |
| Waist–hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.94 |
| Total body fat (%) | 35.9 ± 8.6 | 37.2 ± 0.1 | 0.48 |
| Blood pressure | |||
| Systolic (mm Hg) | 132.5 ± 16.6 | 137.1 ± 17.6 | 0.23 |
| Diastolic (mm Hg) | 79.5 ± 9.7 | 80.9 ± 10.9 | 0.55 |
| Serum measures | |||
| Fasting plasma glucose (mg/dL) | 100.0 ± 9.9 | 100.3 ± 10.6 | 0.91 |
| Fasting serum insulin (μ/mL) | 8.7 ± 4.6 | 9.8 ± 6.7 | 0.43 |
| Lp-PLA2 (PLAC test) (ng/mL) | 222.9 ± 57.0 | 222.1 ± 67.0 | 0.96 |
| Cellular adhesion molecules (nmol/min/mL) | 149.9 ± 32.0 | 139.3 ± 42.2 | 0.29 |
| High-sensitivity CRP (mg/dL) | 223.2 ± 303.7 | 267.4 ± 267.1 | 0.53 |
| Lipid panel | |||
| Triglyceride (mg/dL) | 114.4 ± 60.1 | 119.7 ± 66.4 | 0.71 |
| Cholesterol (mg/dL) | 196.8 ± 35.1 | 191.2 ± 35.9 | 0.48 |
| HDL (mg/dL) | 50.5 ± 12.7 | 48.4 ± 11.6 | 0.43 |
| LDL (mg/dL) | 123.6 ± 31.0 | 120.4 ± 32.5 | 0.65 |
| Cholesterol/HDL ratio | 4.1 ± 1.2 | 4.1 ± 1.0 | 0.95 |
| Basic metabolic panel | |||
| BUN (mg/dL) | 14.9 ± 4.0 | 15.4 ± 3.4 | 0.49 |
| Creatinine (mg/dL) | 1.0 ± 0.2 | 1.0 ± 0.1 | 0.63 |
| Sodium (mEq/L) | 140.4 ± 1.4 | 140.2 ± 1.5 | 0.59 |
| Potassium (mEq/L) | 4.1 ± 0.3 | 4.2 ± 0.3 | 0.22 |
| Chloride (mEq/L) | 102.4 ± 2.4 | 102.0 ± 2.0 | 0.45 |
| CO2 (mEq/L) | 28.3 ± 2.2 | 30.2 ± 11.5 | 0.30 |
| Calcium (mg/dL) | 9.2 ± 0.4 | 9.4 ± 0.3 | 0.13 |
| Anion gap | 9.8 ± 1.8 | 9.8 ± 1.8 | 0.90 |
| BUN/creatinine ratio | 15.6 ± 4.3 | 16.0 ± 3.7 | 0.65 |
| Total protein (g/dL) | 7.1 ± 1.1 | 7.3 ± 0.4 | 0.45 |
| Albumin (g/dL) | 4.1 ± 0.3 | 4.1 ± 0.3 | 0.97 |
| AST (IU/L) | 22.7 ± 4.9 | 24.8 ± 5.8 | 0.09 |
| ALT (IU/L) | 34.4 ± 10.6 | 35.8 ± 12.0 | 0.58 |
| Alkaline phosphate (IU/L) | 74.5 ± 16.7 | 76.9 ± 19.0 | 0.55 |
| Total bilirubin (mg/dL) | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.83 |
| Globulin (g/dL) | 3.2 ± 0.3 | 3.1 ± 0.3 | 0.91 |
| Albumin/globulin ratio | 1.7 ± 0.4 | 1.3 ± 0.2 | 0.37 |
| Urinary analysis | |||
| Chromium/creatinine ratio | 0.1 ± 0.3 | 0.1 ± 0.2 | 0.94 |
| Urine chromium (ng/mL) | 0.2 ± 0.1 | 0.3 ± 0.3 | 0.35 |
| Urine creatinine (mg/dL) | 138.4 ± 65.8 | 149.9 ± 59.2 | 0.57 |
| CT scan | |||
| Surface of total adipose tissue at L4–L5 level (cm2) | 586.1 ± 179.3 | 598.3 ± 175.2 | 0.81 |
| Attenuation of total adipose tissue at L4–L5 level (HU) | −96.6 ± 3.9 | −96.2 ± 3.6 | 0.75 |
| Surface of visceral adipose tissue at L4–L5 level (cm2) | 199.2 ± 85.9 | 207.0 ± 110.1 | 0.73 |
| Attenuation of visceral adipose tissue at L4–L5 level (HU) | −88.1 ± 5.7 | −88.4 ± 6.7 | 0.80 |
| Surface of subcutaneous adipose tissue at L4–L5 level (cm2) | 401.2 ± 120.4 | 399.1 ± 110.4 | 0.95 |
| Attenuation of subcutaneous adipose tissue at L4-L5 level (HU) | −99.4 ± 4.3 | −98.6 ± 3.9 | 0.49 |
| Sagittal diameter at L4–L5 level (cm) | 29.1 ± 4.6 | 29.0 ± 5.2 | 0.97 |
BMI, body–mass index; Lp-PLA2, lipoprotein-associated phospholipase A2; CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CT, computed tomography; HU, Hounsfield units.
Subjects randomized to chromium picolinate had comparable urinary chromium to subjects receiving placebo (p = 0.33). Of the subjects completing the trial (n = 58), 44 subjects (76%) had pill counts reflecting greater than 80% adherence.
Adverse effects
One subject in the chromium picolinate group experienced urticaria 35 days after initiating daily supplement intake. He was instructed to immediately cease taking the supplement, and the urticaria resolved within 4 days.
After 12 weeks (chromium alone) (Table 2)
Table 2.
Change in Outcome Measures from Baseline Values
| 12 weeks | 24 weeks | |||||
|---|---|---|---|---|---|---|
| Outcome measures | Chromium picolinate (n = 35) | Placebo (n = 32) | p-value | Chromium picolinate (n = 30) | Placebo (n = 28) | p-value |
| Anthropometric measures | ||||||
| BMI (kg/m2) | 0.3 ± 0.8 | 0.0 ± 0.4 | 0.07 | 0.1 ± 0.2 | 0.0 ± 0.5 | 0.81 |
| Waist–hip ratio | −0.0 ± 0.0 | 0.0 ± 0.0 | 0.18 | −0.0 ± 0.0 | 0.0 ± 0.0 | 0.16 |
| Total body fat (%) | 0.3 ± 1.2 | −0.8 ± 3.8 | 0.11 | 0.2 ± 1.0 | −0.9 ± 3.8 | 0.13 |
| Blood pressure | ||||||
| Systolic (mm Hg) | 1.3 ± 8.3 | −0.5 ± 6.3 | 0.30 | 1.5 ± 6.2 | −1.2 ± 6.7 | 0.07 |
| Diastolic (mm Hg) | 0.5 ± 6.9 | −0.4 ± 5.2 | 0.55 | 1.4 ± 6.7 | −0.3 ± 4.5 | 0.21 |
| Serum measures | ||||||
| Fasting plasma glucose (mg/dL) | 0.0 ± 3.1 | 1.2 ± 4.3 | 0.15 | 1.0 ± 4.5 | 1.0 ± 3.8 | 1.00 |
| Fasting serum insulin (μ/mL) | 0.8 ± 1.9 | 0.5 ± 1.6 | 0.50 | 0.2 ± 2.1 | −0.2 ± 3.0 | 0.54 |
| Lp-PLA2 (PLAC test) (ng/mL) | −3.8 ± 44.7 | 6.3 ± 44.3 | 0.36 | 7.9 ± 57.8 | −0.3 ± 56.3 | 0.56 |
| Cellular adhesion molecules (nmol/min/mL) | −1.4 ± 18.1 | −0.5 ± 19.8 | 0.86 | −4.0 ± 21.6 | −0.2 ± 16.8 | 0.44 |
| High-sensitivity CRP (mg/dL) | 36.4 ± 341.0 | 15.7 ± 202.2 | 0.78 | −25.0 ± 141.6 | −5.6 ± 154.5 | 0.60 |
| Lipid panel | ||||||
| Triglyceride (mg/dL) | −0.4 ± 21.5 | 3.3 ± 18.9 | 0.42 | 5.3 ± 25.2 | 5.0 ± 23.7 | 0.95 |
| Cholesterol (mg/dL) | 1.6 ± 10.7 | 3.4 ± 14.8 | 0.53 | 5.6 ± 10.7 | 2.7 ± 14.0 | 0.31 |
| HDL (mg/dL) | 0.3 ± 2.8 | 0.4 ± 3.5 | 0.81 | 1.3 ± 5.4 | 0.3 ± 3.7 | 0.34 |
| LDL (mg/dL) | 1.5 ± 10.6 | 1.3 ± 12.7 | 0.96 | 3.3 ± 10.3 | 0.5 ± 11.8 | 0.25 |
| Cholesterol/HDL ratio | 0.0 ± 0.3 | 0.0 ± 0.2 | 0.93 | 0.1 ± 0.4 | 0.0 ± 0.3 | 0.73 |
| Basic metabolic panel | ||||||
| BUN (mg/dL) | 0.2 ± 1.6 | 0.4 ± 1.7 | 0.74 | 0.2 ± 1.2 | −0.1 ± 1.7 | 0.41 |
| Creatinine (mg/dL) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.63 | −0.0 ± 0.0 | 0.0 ± 0.1 | 0.20 |
| Sodium (mEq/L) | −0.1 ± 1.0 | 0.1 ± 0.7 | 0.34 | −0.0 ± 1.0 | −0.1 ± 0.7 | 0.70 |
| Potassium (mEq/L) | 0.0 ± 0.2 | −0.1 ± 0.2 | 0.047 | 0.0 ± 0.2 | −0.0 ± 0.2 | 0.30 |
| Chloride (mEq/L) | 0.0 ± 0.7 | 0.0 ± 1.1 | 1.00 | 0.1 ± 0.8 | 0.2 ± 1.3 | 0.83 |
| CO2 (mEq/L) | 0.6 ± 1.1 | 0.3 ± 0.9 | 0.12 | 0.4 ± 1.1 | 0.1 ± 1.0 | 0.12 |
| Calcium (mg/dL) | −0.0 ± 0.2 | −0.0 ± 0.2 | 0.95 | 0.1 ± 0.3 | 0.0 ± 0.2 | 0.13 |
| Anion gap | −0.7 ± 1.1 | −0.2 ± 0.8 | 0.05 | −0.5 ± 1.0 | −0.4 ± 0.8 | 0.62 |
| BUN/creatinine ratio | 0.0 ± 1.9 | 0.2 ± 2.3 | 0.70 | 0.2 ± 1.2 | −0.3 ± 1.7 | 0.21 |
| Total protein (g/dl) | −0.3 ± 1.1 | −0.1 ± 0.2 | 0.24 | −0.0 ± 0.2 | −0.0 ± 0.2 | 0.81 |
| Albumin (g/dL) | 0.0 ± 0.2 | 0.1 ± 0.2 | 0.89 | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.28 |
| AST (IU/L) | 0.8 ± 2.6 | −0.1 ± 1.0 | 0.04 | 8.7 ± 47.8 | 0.2 ± 2.5 | 0.27 |
| ALT (IU/L) | 1.2 ± 4.2 | 0.4 ± 2.8 | 0.29 | 10.0 ± 49.7 | 1.3 ± 4.6 | 0.28 |
| Alkaline phosphate (IU/L) | 1.4 ± 4.7 | 0.9 ± 3.6 | 0.61 | 3.8 ± 10.8 | 1.3 ± 4.2 | 0.18 |
| Total bilirubin (mg/dL) | 0.0 ± 0.1 | 0.0 ± 0.1 | 0.90 | 0.0 ± 0.1 | 0.0 ± 0.1 | 0.82 |
| Globulin (g/dL) | −0.1 ± 0.2 | −0.1 ± 0.2 | 0.34 | −0.1 ± 0.2 | −0.1 ± 0.2 | 0.33 |
| Albumin/globulin ratio | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.81 | 0.1 ± 0.2 | 0.1 ± 0.1 | 0.31 |
| Urinary analysis | ||||||
| Chromium/creatinine ratio | 6.8 ± 6.2 | 0.0 ± 0.2 | 0.07 | |||
| Urine chromium (ng/mL) | 0.4 ± 1.4 | −0.0 ± 0.1 | 0.33 | |||
| Urine creatinine (mg/dL) | −2.6 ± 13.4 | −12.6 ± 37.0 | 0.29 | |||
BMI, body–mass index; Lp-PLA2, lipoprotein-associated phospholipase A2; CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Anthropometric measures
After intervention for 12 weeks, there was no change in BMI in the chromium group as compared to the placebo group (chromium = 0.3 ± 0.8 kg/m2 versus placebo = 0.0 ± 0.4 kg/m2; p = 0.07). Similarly, there was no change in percent body fat as compared to placebo (chromium = 0.3 ± 1.2 versus placebo = −0.8 ± 3.8; p = 0.11).
Serology
No change was seen in fasting plasma glucose (FPG) and fasting serum insulin (FSI) levels from baseline (FPG: chromium = 0.0 ± 3.1 mg/dL versus placebo = 1.2 ± 4.3 mg/dL; p = 0.15; insulin: chromium = 0.8 ± 1.9 μ/mL versus placebo = 0.5 ± 1.6 μ/mL; p = 0.50). Lp-PLA2 and cell adhesion molecules (CAM) decreased nonsignificantly in the chromium group, as compared to placebo (Lp-PLA2: chromium = −3.8 ± 44.7 ng/mL versus placebo = 6.3 ± 44.3ng/mL; p = 0.36; CAM: chromium = −1.4 ± 18.1 nmol/min/mL versus placebo = −0.5 ± 19.8 nmol/min/mL; p = 0.86). CRP increased nonsignificantly in the chromium group, as compared to placebo (chromium = −36.4 ± 341.0 mg/dL versus placebo = 15.7 ± 202.2 mg/dL; p = 0.78).
Lipids
The total cholesterol/HDL ratio did not change in the chromium group as compared to the placebo group (chromium = 0.0 ± 0.3 versus placebo = 0.0 ± 0.2; p = 0.78).
After 24 weeks (chromium in the context of lifestyle intervention)
Anthropometric measures
After intervention for 24 weeks, there was no change in BMI in the chromium group as compared to the placebo group (chromium = 0.1 ± 0.2 kg/m2 versus placebo = 0.0 ± 0.5 kg/m2; p = 0.81). Similarly, no improvement was observed in percent body fat as compared to placebo (chromium = 0.2 ± 1.0 versus placebo = −0.9 ± 3.8; p = 0.13).
Serology
Fasting plasma glucose and fasting serum insulin levels in the chromium group did not improve as compared to the placebo group (FPG: chromium = 1.0 ± 4.5 mg/dL versus placebo = 1.0 ± 3.8 mg/dL; p = 1.00; FSI: chromium = 0.2 ± 2.1 μ/mL versus placebo = −0.2 ± 3.0 μ/mL; p = 0.54). CRP and CAM decreased nonsignificantly in the chromium group as compared to placebo (CRP: chromium = −25.0 ± 141.6 mg/dL versus placebo = −5.6 ± 154.5 mg/dL; p = 0.60; CAM: chromium = −4.0 ± 21.6 nmol/min/mL versus placebo = −0.2 ± 16.8 nmol/min/mL; p = 0.44). Lp-PLA2 increased nonsignificantly in the chromium group as compared to placebo (chromium = 7.9 ± 57.8 ng/mL versus placebo = −0.3 ± 56.3 ng/mL; p = 0.56).
Lipids
The total cholesterol/HDL ratio did not improve in the chromium group as compared to the placebo (chromium = 0.1 ± 0.4 versus placebo = 0.0 ± 0.3; p = 0.73).
Analysis using multivariable models controlling for visceral fat distribution and demographics did not significantly alter results.
Discussion
In this study of 80 overweight or obese adult men and women with elevated waist circumference, chromium supplementation did not improve weight, blood glucose, percent body fat, or lipid measures. To our knowledge, this is the first study to examine the effects of the ingestion of 1000 μg of chromium picolinate combined with a nutrition education intervention on weight loss.
Previous studies suggest that the primary factor for a clinical response to chromium is insulin resistance.25,37–39 In subjects who have type 2 diabetes and who use sulfonylurea agents, Martin39 demonstrated that chromium picolinate improves insulin sensitivity, glucose control, and attenuates body weight and visceral fat compared with placebo. Baseline insulin sensitivity was found to account for nearly 40% of the variance in the clinical response to chromium.25 In contrast, in this study, the baseline FPG levels in both the chromium and placebo groups were normal (Table 1). Our results are consistent with a meta-analysis finding no association between chromium and glucose or insulin concentrations among nondiabetic subjects.40 A 2007 trial using a lower dose of chromium picolinate (200 μg) in nondiabetic women demonstrated no effect on body weight, composition, or iron status.41 In 1995, a study conducted using 400 μg of chromium picolinate had no effects in reducing body fat percentage.42 It is unknown whether chromium supplementation modifies energy intake or expenditure.39
Other studies have shown modest weight loss with chromium supplementation. A meta-analysis conducted on 10 randomized controlled trials (RCTs) showed that the observed effect with chromium picolinate was a small reduction of 1.1–1.2 kg (0.08–0.2 kg/week) compared with placebo in overweight and obese subjects.16 An RCT in 42 overweight women receiving 1000 μg of chromium picolinate demonstrated 0.5 kg weight loss over 8 weeks, while subjects receiving placebo gained 0.5 kg during this same time period, although the difference was not statistically significant.43
In a small randomized trial, Cefalu et al.44 found an increase in insulin sensitivity in insulin-resistant individuals when supplemented with 1000 μg of chromium picolinate. These results, however, have not been replicated.45 As exercise-related weight loss is associated with increased insulin sensitivity, it is plausible that chromium supplementation can aid in this process. Our trial, however, did not assess insulin sensitivity. In a recent trial of 60 obese subjects, Iqbal et al. found that 500 μg of chromium picolinate did not improve insulin sensitivity. A statistically significant increase in acute insulin response to glucose was found, though no effects were seen on other measures of glucose metabolism, lipids, body weight, and inflammatory markers.46 High-intensity aerobic exercise is known to increase insulin sensitivity47; the combination with chromium may confer synergistic benefits. This may be the mechanism in Kaats' finding of statistically significant reductions in weight, body fat, and fat mass in a randomized trial of 130 subjects recruited from fitness and athletic clubs. All subjects (chromium and placebo arms) lost weight during the intervention, though the subjects in the chromium groups demonstrated greater weight loss and improvement in body composition than those on placebo.48
The nutrition education intervention incorporated after 12 weeks did not demonstrate a significant effect on any outcome measure. This may be due to the passive nature of the intervention without rigorous follow-up and caloric assessments. This approach was intentionally designed to mimic real-world scenarios where patients interested in weight loss would be likely to combine use of any supplement with a lifestyle change. In general, various approaches to “dieting” have been demonstrated to work in the short term, while very few, if any, demonstrate efficacy in the long term.49 The nutrition program selected is healthful and balanced, and provided a standardized approach for study participants.
Our trial aimed to assess the effects of anthropometry on chromium-mediated weight loss. We found no variation by visceral fat distribution nor changes in BMI, and percent body fat. Variable results in prior studies may have been due to obesity characterized by differential fat distribution. Obesity, in general, is associated with insulin resistance, although insulin sensitivity varies significantly in nonobese persons due to body fat distribution.26 Persons with more peripheral fat distribution are more insulin sensitive than those who have body fat primarily distributed centrally in the chest and abdomen. Furthermore, abdominal fat tends to be more lipolytic than subcutaneous fat and not as sensitive to the counterlipolytic effect of insulin.26
We hypothesized that chromium might be most supportive of weight loss when excess weight was centrally distributed, and thus most associated with insulin resistance. We thus recorded baseline measures of central adiposity using CT. No association was found, however, between variation in central fat volume and response to chromium supplementation. Other researchers have found intriguing results with chromium on central adiposity. In 37 subjects with type 2 diabetes, Martin et al. demonstrated that supplementation of 1000 μg chromium picolinate added to sulfonylurea use significantly attenuated body weight gain and visceral fat accumulation compared with subjects receiving sulfonylurea alone.39 Perhaps chromium supplementation alone does not confer specific benefits on obesity, though it may potentiate the action of other agents that increase absolute insulin levels or enhance insulin sensitivity. The sample size of this pilot trial limited the opportunity for subgroup analysis, and thus type II error is a possibility.
A limitation of this trial is a relatively small sample that was largely homogeneous in demographics and socioeconomic status. Furthermore, chromium viability was not assessed; it may be possible that samples degraded in potency over time, especially since urine chromium values were not significantly different between intervention and control subjects. We did not track intake of other sources of chromium—multivitamins and fortified foods—which may result in differential intakes among subjects.
Conclusions
In conclusion, chromium picolinate did not affect weight loss in the apparently healthy overweight adults enrolled in this trial. Variable efficacy of chromium was not seen with variation in baseline levels of abdominal adiposity. Our findings as consistent with other recent studies examining the relationship between chromium supplementation and weight loss,16,41–43 and reduce enthusiasm for the use of chromium as a nutritional supplement for controlling weight. Benefit of chromium supplementation in subgroups of overweight patients, such as those with demonstrable insulin resistance and those on intense exercise regimens, remains a possibility warranting further research.
Acknowledgments
Funding for and products used in this study were provided by Nutrition 21, Inc.
Disclosure Statement
No competing financial interests exist.
References
- 1.Mokdad AH. Ford ES. Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.Mascie-Taylor CG. Karim E. The burden of chronic disease. Science. 2003;302:1921–1922. doi: 10.1126/science.1092488. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL. Carroll MD. Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Thompson D. Edelsberg J. Colditz GA, et al. Lifetime health and economic consequences of obesity. Arch Intern Med. 1999;159:2177–2183. doi: 10.1001/archinte.159.18.2177. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE. Thun MJ. Petrelli JM, et al. Body–mass index and mortality in a prospective cohort of U.S. adults. NEJM. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 6.Katz D. Obesity … be dammed! What it will take to turn the tide. Harvard Health Policy Rev. 2006:135–151. [Google Scholar]
- 7.Reaven G. Abbasi F. McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res. 2004;59:207–223. doi: 10.1210/rp.59.1.207. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay RS. Howard BV. Cardiovascular risk associated with the metabolic syndrome. Curr Diab Rep. 2004;4:63–68. doi: 10.1007/s11892-004-0013-9. [DOI] [PubMed] [Google Scholar]
- 9.Sowers JR. Frohlich ED. Insulin and insulin resistance: Impact on blood pressure and cardiovascular disease. Med Clin North Am. 2004;88:63–82. doi: 10.1016/s0025-7125(03)00128-7. [DOI] [PubMed] [Google Scholar]
- 10.Pischon T. Nothlings U. Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67:128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 11.Calle EE. Rodriguez C. Walker-Thurmond K. Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. NEJM. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 12.US Government Accountability Office. Dietary Supplements FDA Should Take Further Actions to Improve Oversight and Consumer Understanding. Washington, DC: Jan, 2009. [Google Scholar]
- 13.Industry overview. Nutr Business J. 1999;4:1–5. [Google Scholar]
- 14.Sarubin A. The Health Professional Guide to Popular dietary supplements. Chicago, IL: American Diabetes Association; 2000. [Google Scholar]
- 15.NBJ's Sports Nutrition. Weight Loss Report 2007–2008. Boulder: Jan, 2008. [Google Scholar]
- 16.Pittler MH. Ernst E. Dietary supplements for body-weight reduction: A systematic review. Am J Clin Nutr. 2004;79:529–536. doi: 10.1093/ajcn/79.4.529. [DOI] [PubMed] [Google Scholar]
- 17.Pittler MH. Ernst E. Complementary therapies for reducing body weight: A systematic review. Int J Obes (Lond) 2005;29:1030–1038. doi: 10.1038/sj.ijo.0803008. [DOI] [PubMed] [Google Scholar]
- 18.Cefalu WT. Hu FB. Role of chromium in human health and in diabetes. Diabetes Care. 2004;27:2741–2751. doi: 10.2337/diacare.27.11.2741. [DOI] [PubMed] [Google Scholar]
- 19.Diaz ML. Watkins BA. Li Y, et al. Chromium picolinate and conjugated linoleic acid do not synergistically influence diet- and exercise-induced changes in body composition and health indexes in overweight women. J Nutr Biochem. 2008;19:61–68. doi: 10.1016/j.jnutbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Mertz W. Chromium in human nutrition: A review. J Nutr. 1993;123:626–633. doi: 10.1093/jn/123.4.626. [DOI] [PubMed] [Google Scholar]
- 21.Toepfer EW. Mertz W. Polansky MM, et al. Preparation of chromium-containing material of glucose tolerance factor activity from brewer's yeast extracts and by synthesis. J Agric Food Chem. 1976;25:162–166. doi: 10.1021/jf60209a056. [DOI] [PubMed] [Google Scholar]
- 22.Mirsky N. Berdicevsky I. Effects of insulin and glucose tolerance factor on glucose uptake by yeast cells. Biol Signals. 1994;3:271–277. doi: 10.1159/000109554. [DOI] [PubMed] [Google Scholar]
- 23.Frauchiger MT. Wenk C. Colombani PC. Effects of acute chromium supplementation on postprandial metabolism in healthy young men. J Am Coll Nutr. 2004;23:351–357. doi: 10.1080/07315724.2004.10719378. [DOI] [PubMed] [Google Scholar]
- 24.Anderson RA. Cheng N. Bryden NA, et al. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZQ. Qin J. Martin J, et al. Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metabolism. 2007;56:1652–1655. doi: 10.1016/j.metabol.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn SE. Hull RL. Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 27.Pittler MH. Stevinson C. Ernst E. Chromium picolinate for body weight reduction: Meta-analysis of randomized trials. Int J Obes Relat Metab Disord. 2003;27:522–529. doi: 10.1038/sj.ijo.0802262. [DOI] [PubMed] [Google Scholar]
- 28.NCEP. ATP III Guidelines At-A-Glance Quick Desk Reference. NIH Publication No. 01-3305. 2001.
- 29.Anderson RA. Effects of chromium on body composition and weight loss. Nutr Rev. 1998;56:266–270. doi: 10.1111/j.1753-4887.1998.tb01763.x. [DOI] [PubMed] [Google Scholar]
- 30.Iqbal N. Cardillo S. Volger S, et al. Chromium picolinate does not improve key features of metabolic syndrome in obese nondiabetic adults. Metab Syndr Relat Disord. 2009;7:143–150. doi: 10.1089/met.2008.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz D. Gonzalez M. The Way to Eat: A Six-Step Path to Lifelong Weight Control. Naperville, IL: Sourcebooks, Inc.; 2004. [Google Scholar]
- 32.Welborn TA. Dhaliwal SS. Bennett SA. Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust. 2003;179:580–585. doi: 10.5694/j.1326-5377.2003.tb05704.x. [DOI] [PubMed] [Google Scholar]
- 33.Pare A. Dumont M. Lemieux I, et al. Is the relationship between adipose tissue and waist girth altered by weight loss in obese men? Obes Res. 2001;9:526–534. doi: 10.1038/oby.2001.69. [DOI] [PubMed] [Google Scholar]
- 34.Sjostrom L. Kvist H. Cederblad A. Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol. 1986;250(6 pt 1):E736–E745. doi: 10.1152/ajpendo.1986.250.6.E736. [DOI] [PubMed] [Google Scholar]
- 35.Ferland M. Despres JP. Tremblay A, et al. Assessment of adipose tissue distribution by computed axial tomography in obese women: Association with body density and anthropometric measurements. Br J Nutr. 1989;61:139–148. doi: 10.1079/bjn19890104. [DOI] [PubMed] [Google Scholar]
- 36.Imbeault P. Lemieux S. Prud'homme D, et al. Relationship of visceral adipose tissue to metabolic risk factors for coronary heart disease: Is there a contribution of subcutaneous fat cell hypertrophy? Metabolism. 1999;48:355–362. doi: 10.1016/s0026-0495(99)90085-9. [DOI] [PubMed] [Google Scholar]
- 37.Broadhurst CL. Domenico P. Clinical studies on chromium picolinate supplementation in diabetes mellitus: A review. Diabetes Technol Ther. 2006;8:677–687. doi: 10.1089/dia.2006.8.677. [DOI] [PubMed] [Google Scholar]
- 38.Anderson RA. Kozlovsky AS. Chromium intake, absorption and excretion of subjects consuming self-selected diets. Am J Clin Nutr. 1985;41:1177–1183. doi: 10.1093/ajcn/41.6.1177. [DOI] [PubMed] [Google Scholar]
- 39.Martin J. Wang ZQ. Zhang XH, et al. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Diabetes Care. 2006;29:1826–1832. doi: 10.2337/dc06-0254. [DOI] [PubMed] [Google Scholar]
- 40.Althuis MD. Jordan NE. Ludington EA. Wittes JT. Glucose and insulin responses to dietary chromium supplements: A meta-analysis. Am J Clin Nutr. 2002;76:148–155. doi: 10.1093/ajcn/76.1.148. [DOI] [PubMed] [Google Scholar]
- 41.Lukaski HC. Siders WA. Penland JG. Chromium picolinate supplementation in women: Effects on body weight, composition, and iron status. Nutrition. 2007;23:187–195. doi: 10.1016/j.nut.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Trent LK. Thieding-Cancel D. Effects of chromium picolinate on body composition. J Sports Med Phys Fitness. 1995;35:273–280. [PubMed] [Google Scholar]
- 43.Anton SD. Morrison CD. Cefalu WT, et al. Effects of chromium picolinate on food intake and satiety. Diabetes Technol Ther. 2008;10:405–412. doi: 10.1089/dia.2007.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cefalu WT. Bell-Farrow AD. Stegner J, et al. Effect of chromium picolinate on insulin sensitivity in vivo. J Trace Elements Exp Med. 1999;12:17–83. [Google Scholar]
- 45.Trumbo PR. Ellwood KC. Chromium picolinate intake and risk of type 2 diabetes: An evidence-based review by the United States Food and Drug Administration. Nutr Rev. 2006;64:357–363. doi: 10.1111/j.1753-4887.2006.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 46.Iqbal N. Cardillo S. Volger S, et al. Chromium picolinate does not improve key features of metabolic syndrome in obese nondiabetic adults. Metab Syndr Relat Disord. 2009;7:143–150. doi: 10.1089/met.2008.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DiPietro L. Dziura J. Yeckel CW. Neufer PD. Exercise and improved insulin sensitivity in older women: Evidence of the enduring benefits of higher intensity training. J Appl Physiol. 2006;100:142–149. doi: 10.1152/japplphysiol.00474.2005. [DOI] [PubMed] [Google Scholar]
- 48.Kaats G. Blum K. Pullin D, et al. A randomized, double-masked, placebo-controlled study of the effects of chromium picolinate supplementation on body composition: A replication and extension of a previous study. Curr Ther Res. 1998;59:379–388. [Google Scholar]
- 49.Dansinger ML. Gleason JA. Griffith JL, et al. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]