Abstract
Although graphene oxide (GO) has been used in multidisciplinary areas due to its excellent physicochemical properties, its environmental behavior and fate are still largely unclear. In this study, batch experiments, spectroscopy analysis and theoretical calculations were addressed to promote a more comprehensive understanding toward the coagulation behavior of GO onto TiO2 under various environmental conditions (pH, co-existing ions, temperature, etc.). The results indicated that neutral pH was beneficial to the removal of GO due to the electrostatic interaction. The presence of cations accelerated GO coagulation significantly owing to the influence of electrical double layer compression. On the contrary, the presence of anions improved the stability of GO primarily because of electrostatic repulsion and steric hindrance. Results of XRD, FTIR and XPS analysis indicated that the coagulation of GO on TiO2 was mainly dominated by electrostatic interactions and hydrogen bonds, which were further evidenced by DFT calculations. The high binding energy further indicated the stability of GO + TiO2 system, suggesting that TiO2 can be used as an effective coagulant for the efficient elimination and coagulation of GO from aqueous solutions. These findings might likely lead to a better understanding of the migration and transformation of carbon nanomaterials in the natural environment.
With the fast development of technology and dramatic enhancement in the production of manufactured nanomaterials, the carbon-based nanomaterials (e.g., carbon nanotubes, carbon nanofibers, carbon dots and graphene) have obtained intriguing attention for their commercial, electronic, and medical applications1,2,3,4. Graphene has been extensively termed as the “most exciting nanomaterial” due to its remarkable properties (e.g., mechanical strength, electrical and thermal conductivities etc.) ref. 5. Graphene oxide (GO), one of the most important graphene derivatives, has abundant epoxy and hydroxyl functional groups at its basal plane, as well as carbonyl and carboxyl groups situated at the edges6. The existences of these functional groups make GO highly hydrophilic, which permits GO easily swell and disperse in water7. The unusual structure and superior physicochemical properties of GO maintain its great promises in multidisciplinary areas, such as water treatment8, nanosensors9, supercapacitors10 and nanomedicine11. However, on the other hand, the released GO is unavoidable to be toxic pollutant during manufacturing, developing and disposing processes12.
The GO in the natural environment may directly or indirectly affect human health and ecosystems. Recent studies have demonstrated the toxicity of GO to different kinds of organisms. Ren et al.13 found that GO at ultra-trace concentration (0.01 μg/L) in water could trigger Parkinson’s disease-like symptoms and metabolic disturbance in zebrafish larvae. Tu et al.14 demonstrated that GO could induce cell damage in E. coli by extracting phospholipid molecules from the outer cell membrane. Liao et al.15 reported that GO showed the greater hemolytic activity of hemoglobin from suspended red blood cells as compared to the aggregated GO. Hence, the understanding of the physical and chemical behavior of GO in the natural environment is significantly to evaluate its toxicity to human beings. From the literature survey, the efficient way to lower the GO concentration in water is the coagulation of GO, which forms large agglomerates that can be easily separated by aggregation, filtration or centrifugation12,15,16. Thereby, the coagulation kinetics and stability of GO have been extensively investigated in the literatures4,17,18,19, and the results showed that the stability of GO in aqueous solution was strongly influenced by ionic strength and pH values. In our group, the aggregation behaviors of GO on Al2O3 and layered double hydroxides (LDHs) indicated that the stability of GO was strongly dependent on solution conditions and the coagulation was mainly dominated by the electrostatic interaction and hydrogen bonds12,16,20. Similarly, some works also reported that the solution conditions affected the fate and transport of GO in porous media4,21,22,23. However, to the best of our knowledge, the coagulation behavior of GO on titanium dioxide (TiO2) under complicated aqueous environmental conditions have not been reported yet, especially the study from theoretical calculations.
As a multifunctional material, TiO2 has already been proved to have outstanding performances in photocatalytic24,25,26 and energy areas27,28,29. It has also been widely served as an environmental friendly model mineral for the removal of environmental contaminants due to its well-known properties such as high chemical and structural stability, abundant sources, low solubility at the whole pH range and close to neutral pH for point of zero charge30. Particularly, the composites of TiO2 and carbon-based nanomaterials are currently recognized as highly effective adsorbent and catalyst in the purification of wastewater24,31,32,33. Moon et al.34 prepared the rGO-TiO2 composites based on pH-induced aggregation for the efficient photocatalytic oxidation of arsenite. Wang et al.35 observed the accelerated sedimentation of TiO2-GO and attributed it to the aggregation of TiO2-GO resulted from the electrostatic attraction between TiO2 and GO. These studies indicated that TiO2 may be suitable materials for GO coagulation from solution to solid particles. However, systematic studies on the interaction mechanism between TiO2 and GO over a broad range of conditions have not been reported heretofore. In addition, spectroscopic evidence and theoretical calculations are crucial to understand the interaction mechanism at molecular level, which is significant to evaluate the migration behavior of GO in natural environment. Such knowledge is significant to better assessment the environmental behavior of toxic GO nanomaterials and to provide basis for further investigation of carbon-based nanomaterials.
The objectives of the current study are: (1) to study the influence of pH, co-existing ions, contact time, temperature and TiO2 contents on GO coagulation onto TiO2; (2) to characterize the microscopic surface changes before and after GO deposition on TiO2 using scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transformed infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS); (3) to further confirm the interaction mechanisms of GO with TiO2 by density functional theory (DFT) calculations. It is a highlight to understand the coagulation behavior of GO onto TiO2 through the investigation of macroscopic experiments, spectroscopy analysis and theoretical calculations. Furthermore, the coagulation behavior offers new insight into the interaction of GO with TiO2, which can further understand the behavior of carbon-based materials at solid/water interfaces in natural environment.
Results and Discussion
Influence of solution pH
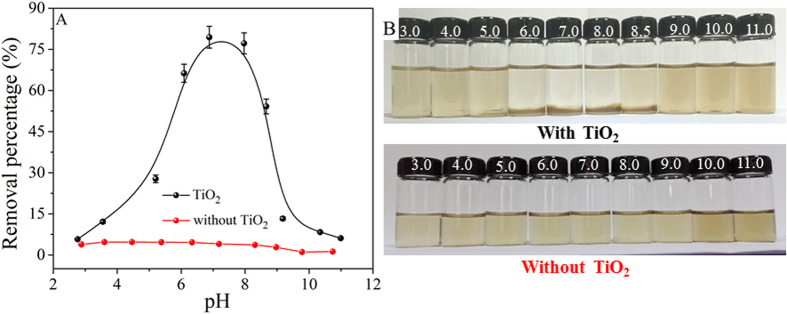
The coagulation behaviors of GO in the presence and absence of TiO2 at different pH values were shown in Fig. 1. The concentration of Ti ions in the solution was analyzed using an inductively coupled plasma mission spectroscopy (ICPE-9000, Shimadzu). It was found that Ti was not detected at 3.0 < pH < 11.0, indicating that TiO2 owned a good chemical and structural stability. In the absence of TiO2, it was clear that the deposition of GO was negligible (Fig. 1A), suggesting that GO was very stable in aquatic solution, which was also evidenced from the visually express in Fig. 1B. The results demonstrated that the GO suspension was very stable in a wide pH range, which was consistent with the literature17. However, after adding TiO2 to GO suspension, one can see that the deposition of GO increased sharply from 5% to 80% as the pH increased from 3.0 to 7.0, and then decreased greatly from 80% to 6% in the pH range of 7.0–11.0. To provide more intuitive evidence about the coagulation process of GO, the reaction photographs of GO with TiO2 as a function of pH were also presented in Fig. 1B. At low and high pH values, the suspension was homogeneous with a light claybank color, suggesting the good stability of GO suspension. Apparent precipitation was formed at neutral pH, suggesting the strongly deposition of GO onto TiO2 at neutral pH values. Similarly, the coagulation of GO onto Al2O3 and LDHs was also dependent on pH due to the electrostatic interaction, hydrogen bond and Lewis acid-base interaction12,16,20. According to the zeta potential analysis (Figure S2), the point of zero charge (pHPZC = 6.0) of TiO2 indicated that the surface of TiO2 was mainly positively charged at pH < 6.0, whereas the GO was negatively charged at the whole pH. Therefore, the enhanced coagulation of GO onto TiO2 at pH 2.0–6.0 can be attributed to electrostatic attraction between negatively charged GO and positively charged TiO2. The high level deposition of GO at pH 6.0–8.0 was ascribed to the strong chemical interaction and electrostatic interaction16,20. At pH > 8.0, deprotonation of carboxylic groups was found to play a vital effect on the stability of GO16,18,36. Furthermore, the concentration of OH− in aqueous solution increased with pH increasing, which can compete with GO for interaction with the functional groups on TiO2. In consequence, the negatively charged GO was difficult to be attached to the negatively charged TiO2 at high pH values due to the strong electrostatic repulsion, which resulted in the stable dispersion of GO in aqueous solutions.
Figure 1.
(A) Removal percentages of GO as a function of pH in the absence and presence of TiO2. (B) Photograph illustration of the influence of pH on GO coagulation after 24 h. C(GO)initial = 25 mg/L, m/V = 0.1 g/L, T = 25 °C.
Influence of cations
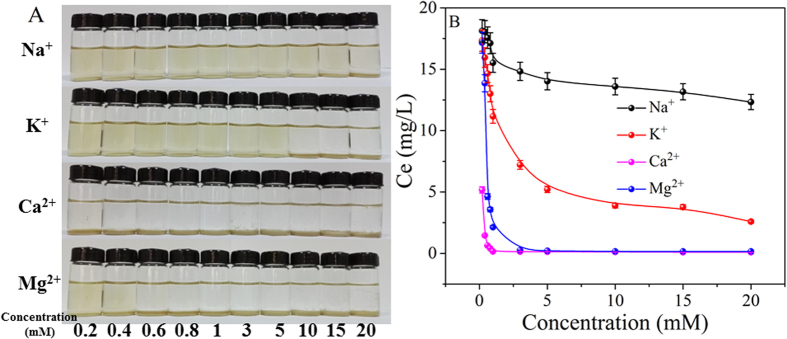
The common environmental cations (Na+, K+, Ca2+ and Mg2+) were introduced to investigate the coagulation behavior of GO onto TiO2. Figure 2A visually expressed the influence of cation types and concentrations on the coagulation of GO. From Fig. 2A, one can see that GO was homogeneously dispersed in low cation concentration and visible precipitation was formed as the cation concentration increased to a critical value (e.g. 20 mM Na+, 10 mM K+, 0.2 mM Ca2+ and 0.6 mM Mg2+). In order to supply more quantitative knowledge about the deposition behaviors of GO, the equilibrium concentration (Ce) of GO in supernatant at different electrolyte concentrations (NaCl, KCl, CaCl2, and MgCl2) were shown in Fig. 2B. GO was stable at relatively low concentrations (e.g. 0.01 to 1.0 mM) for monovalent cations and the difference between Na+ and K+ was negligible. Above 1.0 mM, the obvious differences among Na+ and K+ were observed, and the removal percentage of GO increased notably with increasing Na+ or K+ concentration. The maximum removal percentage of 51% in the presence of Na+ and that of 90% in the presence of K+ were found. Compared to monovalent cations, the significant deposition of GO was observed in the presence of Ca2+ and Mg2+ ions, i.e., as low as 0.2 mM for Ca2+ and 0.4 mM for Mg2+. The destabilization of GO in the presence of different kinds of cations were in the sequence of Ca2+ > Mg2+ >> K+ > Na+. However, Schulze-Hardy rule showed the similar deposition effect of the same valent cations18, which was a little different to the results of this work. This may be attributed to the different interaction properties of cations with GO, and thereby resulted in the more complicated coagulation properties of GO on TiO2 particles. Wu et al.18 also showed that the aggregating/destabilizing capacity of divalent cations were higher than monovalent cations and the reason was that divalent cations can interact with the functional groups on GO surfaces, particularly at the edges. The concentrations of Na+ and K+ in most natural water environment are less than 10 mM, therefore GO will be very stable and have strong migration potential in aqueous solution if no other cations are present in the natural water37. While, Ca2+ and Mg2+ concentrations in natural aquatic environments often over 0.1 mM, the common environmental divalent cations are more aggressive in GO destabilization, and then results in the deposition of GO in natural water conditions18,22. This behavior may be due to the strong binding capacity of divalent cations to functional groups of GO38.
Figure 2.
(A) Photograph illustration of the influence of cations on GO coagulation onto TiO2 after 24 h. (B) Concentrations of the residual GO in the supernatant as a function of the cation concentrations. C(GO)initial = 25 mg/L, m/V = 0.1 g/L, pH = 5.0 ± 0.1, T = 25 °C.
Influence of anions
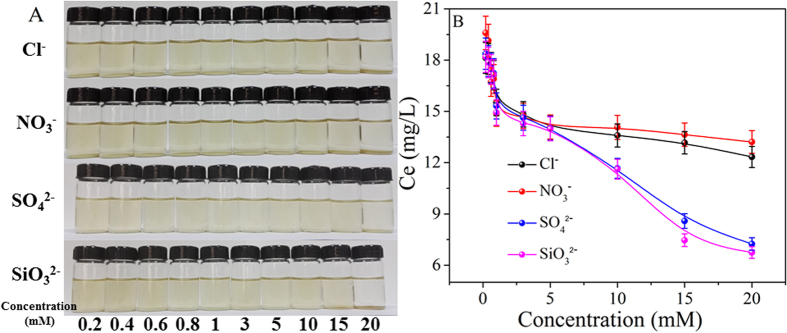
To further understand the interaction mechanisms of GO with TiO2 under different solution chemistry conditions, the coagulation process under different concentrations of co-existing anions were shown in Fig. 3. Figure 3A revealed a direct residual GO concentrations in the supernatant after reaction with TiO2 for 24 h in the presence of different anions and concentrations. It is clear to see that the anions (NO3−, Cl−, SO42− and SiO32−) had little influence on GO deposition, the minor difference was attributed to their negligible difference on Na loadings. The anion-π interactions between GO and anions occurred as GO can act as an electron acceptor for anion sorption39. Shi et al.40 demonstrated that the anion-π bond was unpredictable strong using a computational method on the basis of density functional theory. Furthermore, Hao et al.41 reported that 7,7,8,8-tetracyanoquinodimethane had strong π-π stacking interactions with graphene which can effectively prevent the inter- or intra-π-π stacking of graphene, leading to the dispersion of functionalized graphene. Therefore, the negligible effect of anions on GO deposition can be attributed to the strong anion-π interactions which prevented the agglomeration of GO and leading to the excellent water dispersibility. Besides, the anions may be adsorbed on the positively charged surface of TiO2 through electrostatic attraction, and the surface-adsorbed anions blocked parts of active sites on TiO2 surface and thus prevented the coagulation of GO due to the steric effects and enhanced electrostatic repulsion.
Figure 3.
(A) Photograph illustration of the influence of anions on GO coagulation onto TiO2 after 24 h. (B) Concentrations of the residual GO in the supernatant as a function of the anion concentrations. C(GO)initial = 25 mg/L, m/V = 0.1 g/L, pH = 5.0 ± 0.1, T = 25 °C.
In the presence of anions, the coagulation of GO can be attributed to a balance between the anions (NO3−, Cl−, SO42− and SiO32−) and Na+. The presence of anions resulted in the more negative surface charge of GO and stabilized GO in solution, while the increase of Na+ concentration leaded to the deposition of GO due to electrostatic attraction12. With the increase of electrolyte concentrations, the increased Na+ concentration had more pronounced influence on the stability of GO than the increased anions (NO3−, Cl−, SO42− and SiO32−). From Fig. 3B, one can see that the final values of Ce slowly decreased as the electrolyte concentration increased. Compared with NaCl and NaNO3, each mole of Na2SO4 and Na2SiO3 includes two moles of Na+, therefore, the coagulation of GO onto TiO2 in the presence of different anions are in the sequence of SO42− ≈ SiO32− > Cl− ≈ NO3−.
GO coagulation
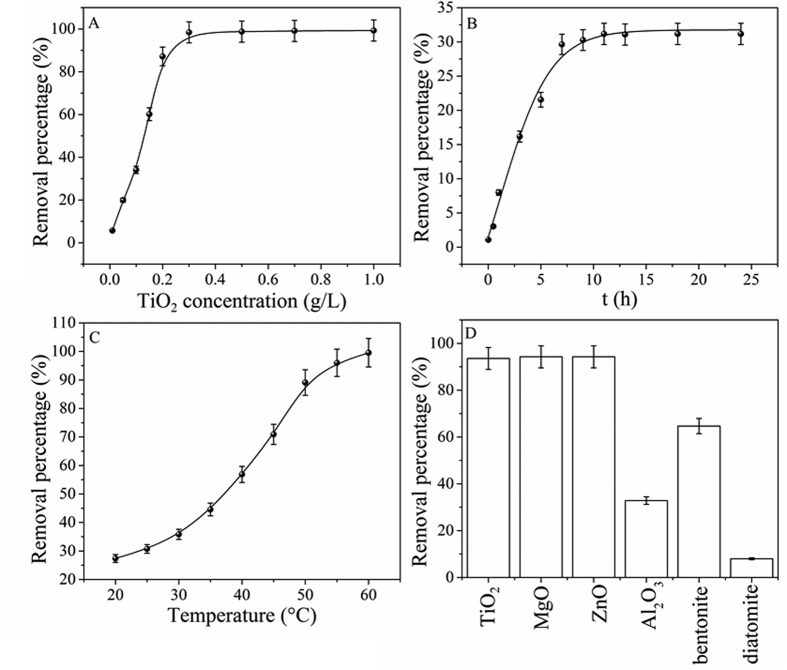
Solid content is an important parameter in the removal of pollutants due to the limited amounts of functional groups and binding sites available for pollutant uptake16,42. From Fig. 4A, one can see that the removal percentage of GO increased from 5% to 99% as the TiO2 content increased from 0.01 to 0.3 g/L. The removal percentage of GO increased sharply at C[TiO2] < 0.3 g/L, and then a flat curve increased slowly at C[TiO2] > 0.3 g/L. The effective precipitation and polymerization between TiO2 and GO led to the quickly increase of GO deposition with TiO2 content increasing at C[TiO2] < 0.3 g/L43. At C[TiO2] > 0.3 g/L, most of GO was attached to the surface of TiO2, thereby the removal of GO from solution to solid particles increased slowly with TiO2 concentration increasing.
Figure 4. Coagulation of GO onto TiO2.
(A) Effect of TiO2 concentrations, T = 25 °C; (B) Effect of contact time, m/V = 0.1 g/L and T = 25 °C; (C) Effect of temperature, m/V = 0.1 g/L; and (D) Comparison of GO coagulation capacities on different materials, m/V = 1 g/L, T = 25 °C; C(GO)initial = 25 mg/L and pH = 5.0 ± 0.1.
Figure 4B showed the removal of GO from aqueous solutions by TiO2 at pH 5.0 ± 0.1 as a function of contact time. The deposition of GO increased quickly with the increase of contact time and reached saturation after 10 hours of contact time. At initial contact time, large amount of functional groups on the surfaces of TiO2 were available for the binding of GO, which resulted in the quick uptake of GO to TiO2 surfaces. More aggregation sites and functional groups on TiO2 were available for the coagulation of GO at the initial contact time, and the deposition of GO on TiO2 was easily and quickly. With increasing contact time, the available sites were occupied by GO and thereby the uptake of GO became slow. The fast aggregation velocity suggested that strong chemisorption devoted to the deposition of GO onto TiO2, which was significant for the application of TiO2 to remove GO from aqueous solutions in natural environment.
To further gain insight into the coagulation behavior of GO in aqueous solution, the removal percentage of GO at different temperatures were shown in Fig. 4C. It was clear that the temperature played an important role on the deposition of GO and distinct precipitate was formed at T > 50 °C (Figure S3). According to Fig. 4C, the removal percentage of GO on TiO2 increased from 25% to 99% with the temperature increased from 20 to 60 °C, indicating that higher temperature was benefical for the aggregation of GO.
The removal percentage of GO on different coagulants were shown in Fig. 4D. One can see that TiO2 had similar deposition capacity with ZnO and MgO, however it was clearly to see that there were some suspended solids on the bottom of MgO and on the top of ZnO (Figure S4). The results indicated that the deposition of GO to TiO2 was more stable than to ZnO and MgO. In addition, it was interesting to notice that TiO2 has higher deposition capacity as compared with natural clay materials (e.g. bentonite and diatomite). On the basis of aforementioned analysis, it is clearly that TiO2 can be potentially used as a cost-effective coagulant for the efficient elimination of GO from aqueous solutions, which could efficiently decrease the potential toxicity of GO in the natural environment.
Spectroscopic analysis
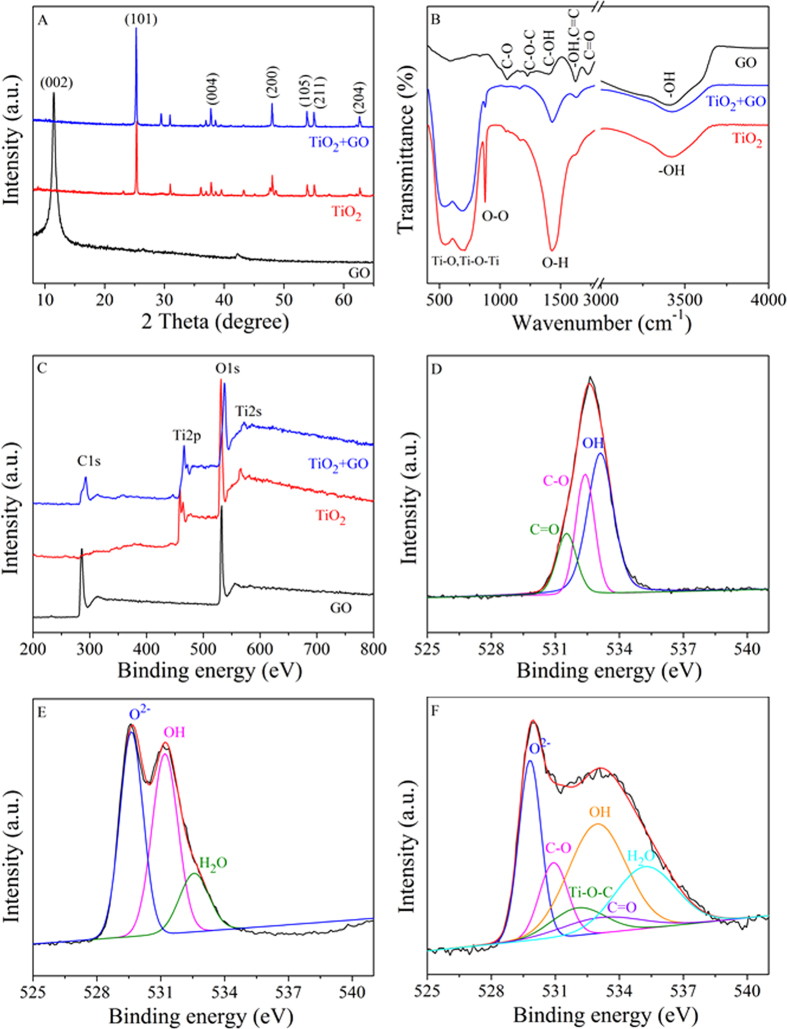
To help deduce the interaction mechanism of GO with TiO2, the XRD, FTIR and XPS analysis of TiO2, GO and the TiO2 after GO coagulation (TiO2 + GO) were collected and compared in detail. The XRD patterns were shown in Fig. 5A. In the XRD pattern of TiO2, the peaks at 25.3°, 37.8°, 48.0°, 53.9°, 55.1° and 62.7° were indexed to the typical representations of the anatase phase of (101), (004), (200), (105), (211) and (204) reflections (JCPDS card No. 21-1272), respectively24. The diffraction peaks of TiO2 shifted slightly after GO coagulation, indicating that small crystal sized TiO2 was formed, similar results were also obtained in hybrid TiO2@rGO28. However, the representative (002) reflection at 2θ = 11.4° of GO was imperceptible, implying a decreased layer-stacking regularity and a highly disordered overlay of individual GO nanosheets were formed in the TiO2 architecture after GO aggregation31,44. Furthermore, from the SEM images (Figure S5), it was obvious that the surface of TiO2 was composed of stacked GO nanosheets, revealing that GO had been deposited on the surface of TiO2.
Figure 5. The characterization of GO, TiO2 and TiO2 + GO.
(A) XRD patterns; (B) FTIR spectra; (C) XPS survey spectra. O 1s XPS spectra of GO (D), TiO2 (E) and TiO2 + GO (F).
As can be seen from the FTIR spectra in Fig. 5B, the GO showed various adsorption bands for water -OH stretching (3400 cm−1), carboxylates or ketones C = O stretching (1730 cm−1), water -OH bending and C = C stretching (1627 cm−1), alcoholic C-OH bending (1400 cm−1), epoxide C-O-C (1230 cm−1) and C-O stretching (1057 cm−1)26,45. The fundamental vibrations of TiO2 appeared at 400–900 cm−1 which were ascribed to the stretching vibrations of Ti-O and Ti-O-Ti bonds, the peak at 3420 cm−1 was due to the stretching of the hydroxyl group46,47. The sharp peak at 1432 cm−1 was a characteristic band of hydroxyl group deformation vibration45. The band at 877 cm−1 may be due to O-O vibration, indicating the existence of peroxide bond at TiO2 surface. For TiO2 + GO sample, the absence of C = O, C-O and C-O-C bands indicated that strong chemical bonds were formed between TiO2 and GO34. Compared with pure TiO2, the intensities of O-H (1432 cm−1) and O-O (877 cm−1) vibration bands decreased after GO aggregation, suggesting the formation of hydrogen bond (O-H…O) between GO and TiO225,48. Furthermore, the appearance of graphene skeleton peak at 1627 cm−1 (C = C stretching) and the slightly shift of Ti-O-Ti bond (400–900 cm−1) demonstrated the formation of Ti-O-C bonds. This behavior had also been reported for the anchoring of TiO2 nanoparticles on graphene nanosheets because of the strong chemically bond between GO and TiO249. The FTIR analysis provided indirect evidence that GO was deposited on the surface of TiO2 through chemisorption and hydrogen bond.
The interaction mechanisms of GO with TiO2 were further investigated by XPS. The chemical properties of different elements in TiO2, GO and TiO2 + GO were shown in Fig. 5C–F. The survey spectra (Fig. 5C) showed clearly the existence of C, O in GO, Ti, O in TiO2 and C, Ti, O in TiO2 + GO. In addition, the binding energy values of Ti2p and O1s of TiO2 + GO remarkably shifted to higher wavelength as compared with TiO2, which was presumably due to strong chemical interaction between TiO2 and GO16. Similarly, Razzaq et al.50 observed that the Ti2p and O1s peaks of rGO + TiO2 shifted to higher binding energies as compared to pure TiO2, and they proposed that the drainage of electrons from Ti to rGO and the formation of bonds between TiO2 with rGO. The high resolution of O 1s spectra were shown in Fig. 5D–F. The O1s spectrum of GO (Fig. 5D) can be divided into three components located at 533.1, 532.4 and 531.5 eV which corresponded to the -OH, C-O (epoxy and hydroxyl) and C = O (carbonyl and carboxyl) groups, respectively51. The O 1s spectrum of TiO2 (Fig. 5E) was assigned to lattice oxygen O2− (529.6 eV), terminal -OH (532.2 eV) and adsorbed H2O (532.6 eV), respectively36. As shown in Fig. 5F, the terminal -OH and lattice oxygen O2− shifted to higher binding energy compared to the O1s spectrum of TiO2 before GO aggregation, and the -OH and O2− appeared at the binding energies of 533.0 and 529.8 eV, respectively, suggesting that hydrogen bond was formed between TiO2 and oxygen functional groups on GO25. At the same time, the new peaks of C-O and C = O appeared in the O 1s spectrum of TiO2 + GO, which suggested that GO had been deposited on the surface of TiO216. Interestingly, the new peak appeared at 532.2 eV can be attributed to Ti-O-C bond formed by the TiO2 and carboxyl group of GO, confirming the presence of GO in TiO2 + GO50. A prominent decrease in the peak intensities of C-O and C = O were observed in case of TiO2 + GO as compared to GO, further suggesting the interaction between TiO2 and surface functional groups of GO.
DFT calculation
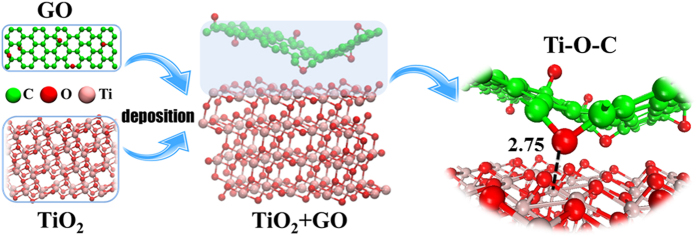
The interaction mechanism of GO onto TiO2 was further evidenced by the DFT calculations. The Vienna ab initio simulation package (VASP) (version 5.3.5) was implemented to perform the geometric optimization and static total energy calculations for the coagulation models52,53,54. Computational details were summarized in SI. The optimized structure of GO deposition on TiO2 was shown in Fig. 6. Owing to the oxygen-containing functional groups of GO plane, the minimum Ti(TiO2)-O(GO) distance was 2.75 Å. The binding energy (Eb) (Table S1) was calculated as the following: Eb = EGO + 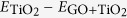 , where EGO,
, where EGO, 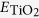 and
and 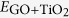 represent energies of the coagulation GO, TiO2 and the hybrid TiO2 + GO system, respectively. A high positive Eb between GO and TiO2 (5.79 eV) suggested that the TiO2 + GO system was stable and the chemisorption was probably the main coagulation mechanism of GO to TiO2, complementing to the physisorption behavior16, which indicated that strong interaction existed between GO and TiO2, and TiO2 was an effective coagulant for the elimination of GO from natural environment.
represent energies of the coagulation GO, TiO2 and the hybrid TiO2 + GO system, respectively. A high positive Eb between GO and TiO2 (5.79 eV) suggested that the TiO2 + GO system was stable and the chemisorption was probably the main coagulation mechanism of GO to TiO2, complementing to the physisorption behavior16, which indicated that strong interaction existed between GO and TiO2, and TiO2 was an effective coagulant for the elimination of GO from natural environment.
Figure 6. The optimized structures for the system of TiO2 + GO.
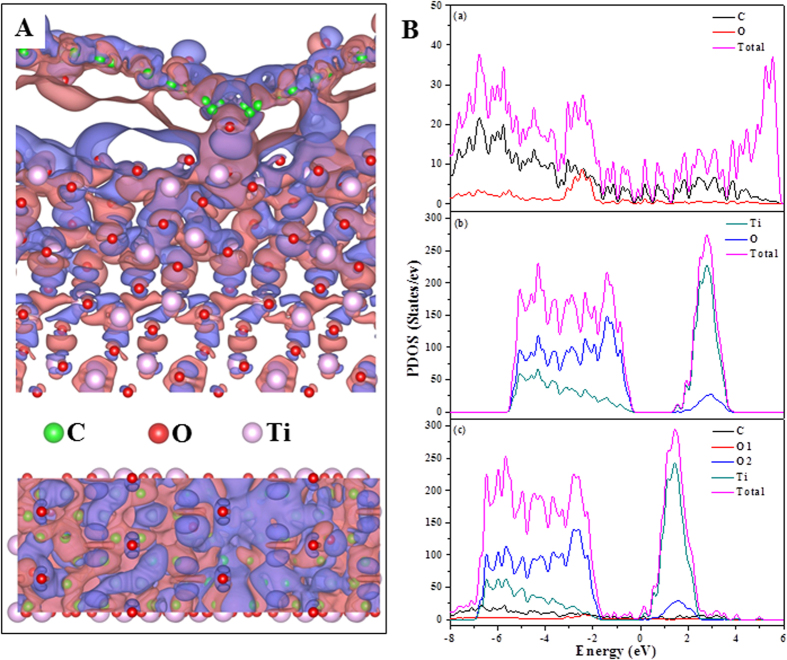
The calculated charge density distribution and the projected density of states (PDOS) of the hybrid TiO2 + GO system were shown in Fig. 7. As shown in Fig. 7A, the three dimensional charge density difference plot with an isosurface value of 10−5 e Å3 was obtained by subtracting the calculated electronic charges of the individual GO and TiO2 (101) from that of TiO2 + GO. The purple and blue bubbles represented positive and negative charges, respectively. It can be clearly seen that the electrostatic interaction was formed between GO and TiO2. With further Bader charge analysis55, we discovered that there was an average charge transfer of around 0.06 e from GO to TiO2 (101) facets. Other significant evidence of the notable charge transference between GO and the TiO2 (101) facets was the comparison between the PDOS plots for GO, TiO2 and TiO2 + GO. As shown in Fig. 7B(c), from −7 to −2 eV range, there was an obvious hybridization between C, O and Ti orbitals. Because of the interaction and redistribution, the peaks of O1 and C atoms in TiO2 + GO system almost disappeared when compared with GO. Meanwhile, the peaks of O2 and Ti atoms in TiO2 + GO system shifted to lower energy levels compared to pure TiO2. The theoretical calculation suggested the presence of strong interactions between TiO2 and GO. Comprehensive consideration of the results of XRD, FTIR, XPS and theoretical calculations, it proved that chemical bonds, hydrogen bonds and electrostatic interactions dominated the coagulation of GO on the surface of TiO2 from aqueous solutions.
Figure 7.
(A) The distribution of differential charge density between TiO2 and GO with the isovalue of 10−5 e Å−3. Purple and blue represent positive and negative charges, respectively. Above is the side view and below is the top view. (B) Partial density of states (PDOS) of (a) GO, (b) TiO2 and (c) TiO2 + GO system. O1 is the oxygen of GO and O2 represents the oxygen in TiO2.
In conclusion, this paper is the first study to investigate the coagulation behavior of GO onto TiO2 under different environmental solution conditions. Electrostatic interaction is found to play a key role in GO removal by TiO2 when pH changes. Divalent cations are more effective than monovalent cations in aggregating/destabilizing GO suspensions through the interaction with oxygen-containing functional groups on GO surfaces. GO remains highly stable under different anions due to electrostatic repulsion and steric hindrance. The spectroscopy analysis and DFT calculations further evidence the electrostatic interactions and hydrogen bonds between GO and TiO2. The abovementioned insights into the coagulation of GO onto TiO2 under different solution chemistry conditions are crucial toward understanding the long-term transport and fate of GO.
Methods and Materials
Materials and characterization
GO was synthesized from flake graphite (48 μm, 99.95% purity) by using the modified Hummers’ method56. More detailed procedures about the preparation of GO were supplied in Supporting Information (SI). Milli-Q water was used in all experiments.
The TiO2 before and after GO coagulation were characterized by the scanning electron microscopic (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS). More detailed characterization processes were described in SI.
GO Coagulation Experiments
The coagulation experiments were accomplished in a series of 20 mL vials equipped with Teflon-lined screw caps at 25 ± 1 °C by using batch technique. A certain amount of TiO2 (0.1 g/L), GO stock suspension (25 mg/L) and the background electrolytes (NaCl, KCl, MgCl2, CaCl2, NaNO3, Na2SiO3 or Na2SO4) (0–20 mM) were added to the vials to obtain the required concentrations of different components, and the samples were left undisturbed on a flat surface for 24 h to allow for the complete settlement of TiO2 and the large GO aggregates. The desired pH was adjusted by adding negligible volumes of 0.01 or 0.1 M HCl and NaOH. The detail experimental process was described in SI. The concentration of GO was analyzed by ultraviolet-visible spectrophotometer (UV-2550, PerkinElmer) at wavelength of 230 nm (Figure S1). All experimental data were obtained by the average values of triple parallel samples and the error bars were within ±5%.
Additional Information
How to cite this article: Yu, S. et al. Complex Roles of Solution Chemistry on Graphene Oxide Coagulation onto Titanium Dioxide: Batch Experiments, Spectroscopy Analysis and Theoretical Calculation. Sci. Rep. 7, 39625; doi: 10.1038/srep39625 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (91326202, 21577032, 21225730 and 21403064), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions, and the Fundamental Research Funds for the Central Universities (JB2015001).
Footnotes
Author Contributions S.Y. and X.-K.W. designed the experiments. S.Y. and X.-X.W. analyzed the data and wrote the paper. S.Y. and R.Z. performed the experiments and characterized materials. S.Y., T.Y. and Y.A. performed the theoretical calculation. S.Y., T.W. and W.H. participated in the discussions. T.H., A.A. and X.-K.W. discussed the results and provided valuable suggestions.
References
- Nel A., Xia T., Mädler L. & Li N. Toxic potential of materials at the nanolevel. Science 311, 622–627 (2006). [DOI] [PubMed] [Google Scholar]
- Brumfiel G. Britain’s big bet on graphene: manchester institute will focus on commercial applications of atom-thick carbon sheets. Nature 488, 140–141 (2012). [DOI] [PubMed] [Google Scholar]
- Petersen E. J. et al. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ. Sci. Technol. 45, 9837–9856 (2011). [DOI] [PubMed] [Google Scholar]
- Lanphere J. D., Luth C. J. & Walker S. L. Effects of solution chemistry on the transport of graphene oxide in saturated porous media. Environ. Sci. Technol. 47, 4255–4261 (2013). [DOI] [PubMed] [Google Scholar]
- Lotya M. et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 131, 3611–3620 (2009). [DOI] [PubMed] [Google Scholar]
- Chen D., Feng H. B. & Li J. H. Graphene oxide: preparation, functionalization, and electrochemical applications. Chem. Rev. 112, 6027–6053 (2012). [DOI] [PubMed] [Google Scholar]
- Stankovich S. et al. Graphene-based composite materials. Nature 442, 282–286 (2006). [DOI] [PubMed] [Google Scholar]
- Zhao G. X. et al. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 45, 10454–10462 (2011). [DOI] [PubMed] [Google Scholar]
- Yu S. J. et al. Label-free immunosensor for the detection of kanamycin using Ag@Fe3O4 nanoparticles and thionine mixed graphene sheet. Biosens. Bioelectron. 48, 224–229 (2013). [DOI] [PubMed] [Google Scholar]
- Ogata C. et al. All-graphene oxide device with tunable supercapacitor and battery behaviour by the working voltage. Chem. Commun. 52, 3919–3922 (2016). [DOI] [PubMed] [Google Scholar]
- del Mercato L. L. et al. Biocompatible multilayer capsules engineered with a graphene oxide derivative: synthesis, characterization and cellular uptake. Nanoscale 8, 7501–7512 (2016). [DOI] [PubMed] [Google Scholar]
- Ren X. M. et al. Impact of Al2O3 on the aggregation and deposition of graphene oxide. Environ. Sci. Technol. 48, 5493–5500 (2014). [DOI] [PubMed] [Google Scholar]
- Ren C. X., Hu X. G., Li X. Y. & Zhou Q. X. Ultra-trace graphene oxide in a water environment triggers Parkinson’s disease-like symptoms and metabolic disturbance in zebrafish larvae. Biomaterials 93, 83–94 (2016). [DOI] [PubMed] [Google Scholar]
- Tu Y. S. et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nano. 8, 594–601 (2013). [DOI] [PubMed] [Google Scholar]
- Liao K. H., Lin Y. S., Macosko C. W. & Haynes C. L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 3, 2607–2615 (2011). [DOI] [PubMed] [Google Scholar]
- Zou Y. D. et al. Coagulation behavior of graphene oxide on nanocrystallined Mg/Al layered double hydroxides: batch experimental and theoretical calculation study. Environ. Sci. Technol. 50, 3658–3667 (2016). [DOI] [PubMed] [Google Scholar]
- Chowdhury I. et al. Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment. Environ. Sci. Technol. 47, 6288–6296 (2013). [DOI] [PubMed] [Google Scholar]
- Wu L. et al. Aggregation kinetics of graphene oxides in aqueous solutions: experiments, mechanisms, and modeling. Langmuir 29, 15174–15181 (2013). [DOI] [PubMed] [Google Scholar]
- Wang X. S. et al. Rolling up graphene oxide sheets into micro/nanoscrolls by nanoparticle aggregation. J. Mater. Chem. 22, 17441–17444 (2012). [Google Scholar]
- Wang J. et al. Performances and mechanisms of Mg/Al and Ca/Al layered double hydroxides for graphene oxide removal from aqueous solution. Chem. Eng. J. 297, 106–115 (2016). [Google Scholar]
- Feriancikova L. & Xu S. P. Deposition and remobilization of graphene oxide within saturated sand packs. J. Hazard. Mater. 235–236, 194–200 (2012). [DOI] [PubMed] [Google Scholar]
- Sun Y. Y. et al. Transport, retention, and size perturbation of graphene oxide in saturated porous media: effects of input concentration and grain size. Water Res. 68, 24–33 (2015). [DOI] [PubMed] [Google Scholar]
- Liu L. et al. Deposition and transport of graphene oxide in saturated and unsaturated porous media. Chem. Eng. J. 229, 444–449 (2013). [Google Scholar]
- Liang Y. Y. et al. TiO2 nanocrystals grown on graphene as advanced photocatalytic hybrid materials. Nano Res. 3, 701–705 (2010). [Google Scholar]
- Gao Y., Hu M. & Mi B. X. Membrane surface modification with TiO2-graphene oxide for enhanced photocatalytic performance. J. Membrane Sci. 455, 349–356 (2014). [Google Scholar]
- Wang P. et al. One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl. Catal. B: Environ. 132–133, 452–459 (2013). [Google Scholar]
- Wang X. Y., Sun L. D., Zhang S. & Wang X. Ultralong, small-diameter TiO2 nanotubes achieved by an optimized two-step anodization for efficient dye-sensitized solar cells. ACS Appl. Mater. Interfaces 6, 1361–1365 (2014). [DOI] [PubMed] [Google Scholar]
- Liu H. Q. et al. Ultrasmall TiO2 nanoparticles in situ growth on graphene hybrid as superior anode material for sodium/lithium ion batteries. ACS Appl. Mater. Interfaces 7, 11239–11245 (2015). [DOI] [PubMed] [Google Scholar]
- Liu H. Q. et al. Improved dehydrogenation performance of LiBH4 by confinement into porous TiO2 micro-tubes. J. Mater. Chem. A 2, 9244–9250 (2014). [Google Scholar]
- Comarmond M. J. et al. Uranium sorption on various forms of titanium dioxide-influence of surface area, surface charge, and impurities. Environ. Sci. Technol. 45, 5536–5542 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang Z. Y. et al. One-pot self-assembled three-dimensional TiO2-graphene hydrogel with improved adsorption capacities and photocatalytic and electrochemical activities. ACS Appl. Mater. Interfaces 5, 2227–2233 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. P25-graphene composite as a high performance photocatalyst. ACS Nano 4, 380–386 (2010). [DOI] [PubMed] [Google Scholar]
- Chen X. B. & Mao S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007). [DOI] [PubMed] [Google Scholar]
- Moon G. H. et al. Platinum-like behavior of reduced graphene oxide as a cocatalyst on TiO2 for the efficient photocatalytic oxidation of arsenite. Environ. Sci. Technol. Lett. 1, 185–190 (2014). [Google Scholar]
- Wang G. et al. Highly recoverable TiO2-GO nanocomposites for stormwater disinfection. Water Res. 94, 363–370 (2016). [DOI] [PubMed] [Google Scholar]
- Tan X. L., Fan Q. H., Wang X. K. & Grambow B. Eu(III) sorption to TiO2 (anatase and rutile): batch, XPS, and EXAFS studies. Environ. Sci. Technol. 43, 3115–3121 (2009). [DOI] [PubMed] [Google Scholar]
- Yang K. J., Chen B. L., Zhu X. Y. & Xing B. S. Aggregation, adsorption and morphological transformation of graphene oxide in aqueous solutions containing different metal cations. Environ. Sci. Technol. 50, 11066–11075 (2016). [DOI] [PubMed] [Google Scholar]
- Chowdhury I. et al. Aggregation and stability of reduced graphene oxide: complex roles of divalent cations, pH, and natural organic matter. Environ. Sci. Technol. 49, 10886–10893 (2015). [DOI] [PubMed] [Google Scholar]
- Zhao J., Wang Z. Y., White J. C. & Xing B. S. Graphene in the aquatic environment: adsorption, dispersion, toxicity and transformation. Environ. Sci. Technol. 48, 9995–10009 (2014). [DOI] [PubMed] [Google Scholar]
- Shi G. S., Ding Y. H. & Fang H. P. Unexpectedly strong anion-π interactions on the graphene flakes. J. Comput. Chem. 33, 1328–1337 (2012). [DOI] [PubMed] [Google Scholar]
- Hao R., Qian W., Zhang L. H. & Hou Y. L. Aqueous dispersions of TCNQ-anion-stabilized graphene sheets. Chem. Commun. 48, 6576–6578 (2008). [DOI] [PubMed] [Google Scholar]
- Hu R., Shao D. D. & Wang X. K. Graphene oxide/polypyrrole composites for highly selective enrichment of U(VI) from aqueous solutions. Polym. Chem. 5, 6207–6215 (2014). [Google Scholar]
- Wang L. F. et al. Coagulation kinetics of humic aggregates in mono- and di-valent electrolyte solutions. Environ. Sci. Technol. 47, 5042–5049 (2013). [DOI] [PubMed] [Google Scholar]
- Li W. et al. Sol-gel design strategy for ultradispersed TiO2 nanoparticles on graphene for high-performance lithium ion batteries. J. Am. Chem. Soc. 135, 18300–18303 (2013). [DOI] [PubMed] [Google Scholar]
- Sun Y. B. et al. Adsorption and desorption of U(VI) on functionalized graphene oxides: a combined experimental and theoretical study. Environ. Sci. Technol. 49, 4255–4262 (2015). [DOI] [PubMed] [Google Scholar]
- Nakamura R. & Nakato Y. Primary intermediates of oxygen photoevolution reaction on TiO2 (rutile) particles, revealed by in situ FTIR absorption and photoluminescence measurements. J. Am. Chem. Soc. 126, 1290–1298 (2004). [DOI] [PubMed] [Google Scholar]
- Lucarelli L., Nadtochenko V. & Kiwi J. Environmental photochemistry: quantitative adsorption and FTIR studies during the TiO2-photocatalyzed degradation of orange II. Langmuir 16, 1102–1108 (2000). [Google Scholar]
- Medhekar N. V., Ramasubramaniam A., Ruoff R. S. & Shenoy V. B. Hydrogen bond networks in graphene oxide composite paper: structure and mechanical properties. ACS Nano 4, 2300–2306 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang K., Kemp K. C. & Chandra V. Homogeneous anchoring of TiO2 nanoparticles on graphene sheets for waste water treatment. Mater. Lett. 81, 127–130 (2012). [Google Scholar]
- Razzaq A., Grimes C. A. & In S. I. Facile fabrication of a noble metal-free photocatalyst: TiO2 nanotube arrays covered with reduced graphene oxide. Carbon 98, 537–544 (2016). [Google Scholar]
- Chen C. M. et al. Annealing a graphene oxide film to produce a free standing high conductive graphene film. Carbon 50, 659–667 (2012). [Google Scholar]
- Kresse G. & Hafner J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994). [DOI] [PubMed] [Google Scholar]
- Kresse G. & Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996). [DOI] [PubMed] [Google Scholar]
- Kresse G. & Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- Tang W., Sanville E. & Henkelman G. A grid-based Bader analysis algorithm without lattice bias. J. Phys.: Condens. Matter 21, 084204 (2009). [DOI] [PubMed] [Google Scholar]
- Hummers W. S. & Offeman R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339–1339 (1958). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.