Abstract
Salmonella enterica cause diarrheal and systemic diseases and are of considerable concern worldwide. Vaccines that are cross-protective against multiple serovars could provide effective control of Salmonella-mediated diseases. Bacteria-derived outer membrane vesicles (OMVs) are highly immunogenic and are capable of eliciting protective immune responses. Alterations in lipopolysaccharide (LPS) length can result in outer membrane remodeling and composition of outer membrane proteins (OMPs) changing. In this study, we investigated the impact of truncated LPS on both the production and immunogenicity of Salmonella OMVs, including the ability of OMVs to elicit cross-protection against challenge by heterologous Salmonella strains. We found that mutations in waaJ and rfbP enhanced vesiculation, while mutations in waaC, waaF and waaG inhibited this process. Animal experiments indicated that OMVs from waaC, rfaH and rfbP mutants induced stronger serum immune responses compared to OMVs from the parent strain, while all elicited protective responses against the wild-type S. Typhimurium challenge. Furthermore, intranasal or intraperitoneal immunization with OMVs derived from the waaC and rfbP mutants elicited significantly higher cross-reactive IgG responses and provided enhanced cross-protection against S. Choleraesuis and S. Enteritidis challenge than the wild-type OMVs. These results indicate that truncated-LPS OMVs are capable of conferring cross protection against multiple serotypes of Salmonella infection.
Keywords: Lipopolysaccharide (LPS), Outer membrane vesicles (OMVs), Truncated LPS, Cross-protection, Mouse model
1. Introduction
Enteric pathogens typically cause gastrointestinal diseases originating from infections that are contacted through the contaminated foods or water (Kozak et al., 2013). Among all enteric pathogens, Salmonella enterica is of particular clinical prevalence in humans and animals (Eng et al., 2015; Majowicz et al., 2010). Non-typhoidal Salmonella (NTS) has been estimated to cause over 93.8 million cases of foodborne illness and gastroenteritis worldwide, resulting in 155,000 deaths annually (Eng et al., 2015; Majowicz et al., 2010). Moreover, death predominantly occurs among children younger than 3 years and among immune-compromised patients, such as human immunodeficiency virus (HIV)-infected adults in developing countries (Feasey et al., 2012).
Due to the widespread distribution and diversity of pathogenic Salmonella serotypes, cross-protective vaccines are a good option for the control of Salmonella diseases (Mahan et al., 2012). Currently, there are Salmonella enterica vaccines targeted against S. Typhi for typhoid fever in human use. And several efforts have been made to develop vaccines against non-typhoidal Salmonella (Ferreira et al., 2015; Tennant et al., 2011). Subunit vaccines have historically provided the effective, but short-term immunity (Girard et al., 2006). Polysaccharide-protein conjugates are being investigated (Simon et al., 2013), but they are likely to provide, at best, only limited protection against heterologous Salmonella serotypes (MacLennan et al., 2014), consequently, a multiple-antigen vaccine is needed for broad protection (Singh, 2009). Live attenuated Salmonella vaccines provide strong protection, but the potential for insufficient attenuation suffers the risks of reversion to virulence in immune-compromised or elderly individuals (Feasey et al., 2012). Moreover, these vaccines have not yet provided effective cross-protection against multiple-serotype Salmonella infection (MacLennan et al., 2014).
Outer membrane vesicles (OMVs) are naturally released by Gram-negative bacteria such as Escherichia coli, S. enterica and Shigella spp (Mitra et al., 2012; Muralinath et al., 2011; Roy et al., 2011). OMVs are spherical structures that are predominantly composed of integral outer membrane components and periplasmic contents that are entrapped within the vesicle (Baker et al., 2014; Kulp and Kuehn, 2010). OMVs from Shigella spp, Vibrio cholerae, E. coli, Burkholderia pseudomallei and Acinetobacter baumannii, induce strong immunity and confer protection against bacterial challenge in animal models (McConnell et al., 2011; Mitra et al., 2012; Nieves et al., 2011; Roy et al., 2011; Roy et al., 2010), and OMVs derived from Haemophilus influenzae elicit cross-protective immunity against other serotypes (Roier et al., 2012). A vaccine based on OMVs from Neisseria meningitides has been globally licensed for use in preventing meningococcal B disease in children and adult humans (Holst et al., 2009). Therefore, an OMV-based vaccine represents a feasible approach for inducing protective immune responses against homologous and heterologous serotypes of Salmonella.
The natural OMVs produced by Salmonella are heterogeneous complexes that contain pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), lipoproteins, and outer membrane proteins (OMPs), which are the major components of OMVs (Beveridge, 1999; Kuehn and Kesty, 2005). OMPs isolated from rough-LPS mutants induce limited protective immune responses against Salmonella challenge (Isibasi et al., 1988; Liu et al., 2016; Ochoa-Repáraz et al., 2005; Udhayakumar and Muthukkaruppan, 1987). LPS, a major component of OMVs, is essential for the biosynthesis and assembly of the bacterial outer membrane, and is composed of three main parts, including conserved lipid A, core oligosaccharide and repeated O-antigen polysaccharide (Machtiger and Fox, 1973; Raetz and Whitfield, 2002). The genes encoding the enzymes required for synthesizing core oligosaccharide and O-antigen polysaccharide are clustered into two operons, waa and wba, respectively (Frirdich and Whitfield, 2005; Whitfield et al., 2003). Deletion of any of these genes results in LPS lacking full length O-antigen and/or an incomplete core (Kong et al., 2011c; Liu et al., 2016). We and others have demonstrated that LPS truncation results in remodeling of the outer membrane structure and composition (Ernst et al., 2001; Helander et al., 1998; Kong et al., 2011c). Thus, we hypothesized that membrane constituents derived from LPS mutants may be more effective at generating cross-protective immunity.
Previously, we investigated the immunogenicity of the OMPs from a set of Salmonella mutants with truncated LPS and demonstrated that OMPs from a waaC mutant could induce effective cross-protection against infection by multiple Salmonella serotypes (Liu et al., 2016). In this study, we extend that work by investigating the cross-protective potential of OMVs derived from a previously described set of rough S. Typhimurium with LPS truncations from heptose-less (ΔwaaC) to a single O-antigen unit (Δwzy) (Kong et al., 2011a,b,c). Our aim was to discover the ideal OMV-based vaccine candidate for controlling Salmonellosis caused by multiple serotypes of Salmonella.
2. Materials and methods
2.1. Bacterial strains, media, and growth conditions
The bacterial strains used in this study are listed in Table 1. All strains were grown in Luria-Bertani broth or agar (Difco, Detroit, MI, USA) at 37 °C. The mutant strains for isolating OMVs, including ΔwaaC41, ΔwaaF40, ΔwaaG42, ΔrfaH49, ΔwaaI43, ΔwaaJ44, ΔwaaL46, ΔrfbP45, and Δwzy-48, were derived from the Salmonella strain χ3761 (Kong et al., 2011c).
Table 1.
Bacterial strains used in this study.
| Strains | Description | Source or reference |
|---|---|---|
| χ3761 | S. Typhimurium UK-1 | (Hassan and Curtiss, 1990) |
| S100 | S. Typhimurium, clinical isolate from duck | (Liu et al., 2016) |
| S246 | S. Enteritidis, clinical isolate from chicken | (Liu et al., 2016) |
| S340 | S. Choleraesuis, clinical isolate from pig | (Liu et al., 2016) |
| χ12253 | ΔwaaC41 | χ3761 |
| χ12252 | ΔwaaF40 | χ3761 |
| χ11308 | ΔwaaG42 | (Kong et al., 2011c) |
| χ9945 | ΔrfaH49 | (Kong et al., 2011c) |
| χ11309 | ΔwaaI43 | (Kong et al., 2011c) |
| χ11310 | ΔwaaJ44 | (Kong et al., 2011c) |
| χ11312 | ΔwaaL46 | (Kong et al., 2011c) |
| χ11311 | ΔrfbP45 | (Kong et al., 2011c) |
| χ9944 | Δwzy-48 | (Kong et al., 2011c) |
2.2. Purification and quantification of OMVs
OMVs were isolated from Salmonella as described previously with some modifications (Muralinath et al., 2011). Briefly, culture supernatants were collected from 2 l bacteria cultures in the logarithmic phase (OD600 = 1) and filtered using a 0.45-μm Steritop bottle-top filter unit (Millipore, Bedford, MA, USA). The vesicles in the filtrate were then pelleted by centrifugation (2 h, 40,000 × g, 4 °C) and resuspended in Dulbecco’s phosphate-buffered saline (DPBS) (Mediatech, Manassas, VA, USA). The vesicles were further purified via density gradient centrifugation (overnight, 200,000 × g, 4 °C) on a discontinuous OptiPrep density gradient medium (Sigma-Aldrich, St. Louis, MO, USA). The density step gradient contained 2 ml each of 20%, 25%, 30%, 35%, 40% and 45% OptiPrep in 10 mM HEPES (pH 6.8) with 0.85% NaCl from top to bottom. The vesicle fractions were pooled, gently washed 3 times with DPBS and then dissolved in 1 ml DBPS and stored at −20 °C for future use.
The yield of OMV from the same cell mass of diverse truncated LPS mutants was determined by the protein content in the OMVs. The protein concentration was measured using a bicinchoninic acid (BCA) assay (Thermo Pierce, Rockford, IL, USA). All OMVs were isolated from the wild-type S. Typhimurium χ3761 and its derivatives (Table 1). All OMVs from the wild-type S. Typhimurium χ3761 and its derivatives were purified and quantified three times with similar results, as summarized in Fig. 1E. The quantification of LPS content in the same amount of each OMV sample (50 μg) was measured via Kdo (3-deoxy-D-manno-octulosonic acid) analysis, and commercial S. Typhimurium LPS purchased from Sigma-Aldrich (Saint Louis, MO, USA) was used as the standard (Osborn, 1963).
Fig. 1.
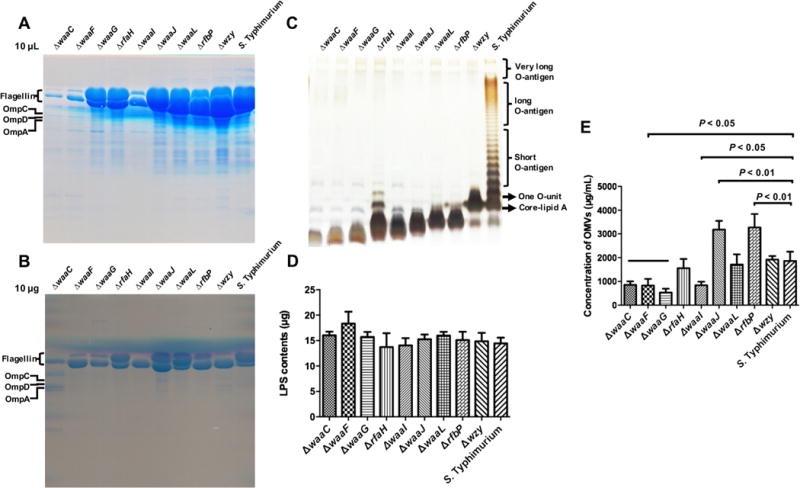
Quantity and characterization of OMVs derived from truncated-LPS mutant strains of Salmonella. (A) Proteins in each OMV samples as the same volume (10 μl) after purification were separated via SDS-PAGE on 12% gels and subjected to staining with GelCode™ Blue. The mutant strains are (from left to right): χ12253 (ΔwaaC41), χ12252 (ΔwaaF40), χ11308 (ΔwaaG42), χ9945 (ΔrfaH49), χ11309 (ΔwaaI43), χ11310 (ΔwaaJ44), χ11312 (ΔwaaL46), χ11311 (ΔrfbP45), χ9944 (Δwzy-48) and χ3761 (the wild-type strain). (B) Proteins in each OMV samples as the same amount (10 μg) based on the total protein contents after purification were separated via SDS-PAGE gel. (C) LPS profiles of OMVs derived from LPS mutants and their parental strain. LPS was visualized via silver staining of polyacrylamide gels. The expected locations of the O-antigen components and core are shown on the right. (D) Quantification of LPS levels in each OMV samples. The same amount of OMVs (50 μg) was measured using a Kdo (3-deoxy-d-manno-octulosonic acid) analysis. S. Typhimurium LPS was used as the standard. (E) Quantity of OMVs derived from LPS mutants and the parental strain. The concentration of OMVs isolated from 2-L bacterial culture in the logarithmic phase (OD600 = 1) was measured based on protein content using the BCA method.
2.3. LPS and protein profiles of OMVs derived from Salmonella mutant strains
The LPS profiles of OMVs were examined in the same manner as for the whole Salmonella cells (Hitchcock and Brown, 1983), and 10 μg of OMV sample, based on protein contents, were loaded to analyze the LPS profile The OMV samples were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained by silver staining. For protein analysis, 10 l of each OMV sample after density gradient centrifugation purified, or 10 μg of each OMV sample based on protein contents, were loaded into SDS-PAGE gel, and then stained with GelCode™ Blue Stain Reagent (Thermo Pierce, Rockford, IL, USA).
2.4. Animals
Six-week-old female BALB/c mice (Dashuo Biotechnology Co., Ltd., Chengdu, China) were used in all experiments and in accordance with the Animal Welfare Act (Ya’an, China; Approval No. 2011-028). The principles stated in the Guide for the Care and Use of Laboratory Animals were followed. The mice were housed with food and water and were monitored under the care of full-time staff. All efforts were made to minimize animal suffering during the experiments.
2.5. Immunization protocol and challenge
Six-week-old female mice were immunized intranasally (i.n.) or intraperitoneally (i.p.) on day 0 and were then boosted at day 21 (3 weeks) with 20 μg OMVs in 10 μl DPBS buffer per mouse (i.n.) or 5 μg OMVs in 100 μl DPBS buffer per mouse (i.p.). No adjuvant was used. An equivalent volume of DPBS buffer served as a control following the corresponding immunization route. To monitor the levels of systemic and mucosal immunity, blood samples were collected via mandibular vein puncture, and vaginal secretions were collected via repeated flushing using a total of 0.1 ml PBS buffer on days 14 (2 weeks) and 49 (7 weeks) after the first immunization. The serum and supernatant of the vaginal wash were stored at −80 °C for later use.
To determine protection rates, the mice were orally challenged with approximately 109 colony-forming units (CFU) of S100 (S. Typhimurium) in 20 μl buffered saline with 0.01% gelatin (BSG) 5 weeks after the booster immunization. The 50% lethal dose (LD50) of S100 is approximately 5 × 105 CFU in this mouse model.
In the second study, mice were immunized as described above and then orally challenged with either 107 CFU of S. Choleraesuis (~100-fold LD50) or 107 CFU of S. Enteritidis (~100-fold LD50) in 20 μl BSG buffer 5 weeks after the booster immunization. The challenged mice were monitored daily for 30 days.
2.6. Quantitative enzyme-linked immunosorbent assay (ELISA)
OMPs were isolated from Salmonella as previously described (Carlone et al., 1986). Recombinant soluble FliC protein with N-terminal His tag was purified by Ni-NTA chromatography (Flores-Langarica et al., 2015). Quantitative ELISA was used to analyze the antibody response according to the procedure described below. Briefly, 2 μg of OMPs from S. Typhimurium or other serotypes of Salmonella or 5 μg of FliC per well in 100 μl of sodium carbonate/bicarbonate coating buffer (pH 9.6) was used to coat NUNC MaxiSorp™ 96-well plates (Thermo Scientific, Waltham, MA, USA); the plates were then incubated overnight at 4 °C. To construct standard curves of each antiserum isotype and to quantify the concentrations of the antibody, the plates were coated in triplicate with two-fold dilutions of the appropriate purified mouse IgA and IgG isotype standard (BD Biosciences, San Jose, CA, USA), starting at 0.5 μg/l. The plate was washed 3 times with PBS containing 0.1% Tween 20 (PBST) and then blocked with 2% bovine serum albumin for 2 h at room temperature. A 100-μl volume of a suitably diluted sample was added to the individual wells in triplicate and incubated for 1 h at room temperature; after washing with PBST, biotinylated goat anti-mouse IgA and IgG (Southern Biotechnology Inc., Birmingham, AL, USA) were added to each well. The wells were then developed with a streptavidin-alkaline phosphatase conjugate (Southern Biotechnology Inc., Birmingham, AL, USA) and detected using a p-nitrophenylphosphate substrate (Sigma-Aldrich, St. Louis, MO, USA) in diethanolamine buffer (pH 9.8). Color development (absorbance) was measured at 405 nm using an automated ELISA plate reader (model EL311SX; BioTek, Winooski, VT, USA) for a suitable duration. The final Ig isotype concentration of the sample antibody was calculated using appropriate standard curves, and a log–log regression curve was calculated from at least 4 dilutions of the isotype standards.
2.7. Statistical analysis
Statistical analyses were performed using the GraphPad Prism 5 software package (Graph Software, San Diego, CA, USA). The data were expressed as the mean ± standard deviation. The means were evaluated with a one-way analysis of variance (ANOVA) between various vaccinated and control groups and were also compared using the least significant difference test. The differences of survival rates among all groups were analyzed by the log-rank sum test. P < 0.05 was considered a significant difference.
3. Results
3.1. Quantification and characterization of OMVs
Purified OMVs from the wild type and the mutant strains were evaluated via protein and LPS profile assays. Regardless whether loading was done based on equal volumes (10 μl) (Fig. 1A) or equal mass (10 μg) (Fig. 1B), flagellar proteins FliC and FljB were observed in the most OMV protein profiles. From the result of the same volume, the yield of OMVs from diverse truncated LPS mutants had obvious differences, and many protein bands were observed in the most OMV protein profiles (Fig. 1A). Furthermore, a number of other proteins were present in lower amounts, including bands corresponding to OmpC/F, OmpD and OmpA. (Fig. 1B). The LPS patterns of the truncated LPS mutants were consistent with those in our previous study (Kong et al., 2011c; Liu et al., 2016), showing distinct LPS lengths that ranged from one O-antigen unit in the wzy mutant to the presence of only Kdo in the waaC mutant (Fig. 1C). Similar levels of LPS were present in each preparation, roughly 15 μg LPS/50 μg of protein (Fig. 1D). The overall OMV yields were determined for each 2-l preparation grown to a final OD600 of 1.0. All mutant strains were grown in the same medium to the same OD and contained ~2 × 1013 CFU bacterial cells, and the cell mass achieved for each should be similar. Therefore, the results of OMV yield showed that the waaJ and rfbP mutations significantly enhanced OMV production (P < 0.01), whereas mutations in waaC, waaF and waaG resulted in reduced OMVs secretion compared with the wild-type strain (P < 0.05) (Fig. 1C). These results are consistent with previous results indicating that OMV formation is influenced by the outer membrane structure (McBroom et al., 2006).
3.2. OMVs derived from Salmonella mutants with truncated LPS elicit systemic and mucosal immune responses
Mice were inoculated i.n. or i.p. with the purified OMVs from the wild-type S. Typhimurium or its LPS mutants derivatives. A parallel, non-vaccinated control group was used as a negative control. During the period of immunization, the mice immunized with the wild-type OMVs by intraperitoneal route showed transient abdominal swelling after immunization; however, this mild symptom disappeared in three days. All mice that were immunized by intranasal administration remained in good health and exhibited no abnormal behavior. Most of OMVs elicited similar serum IgG responses in intranasally immunized mice at 2 weeks post-immunization, while those from the waaJ and rfbP mutants were significantly greater in induction of IgG production compared to the wild-type OMVs (P < 0.05; Fig. 2A). After boosting, only OMVs from the rfbP mutant elicited significantly higher anti-OMP serum IgG levels than the wild-type OMVs (P < 0.01), however OMVs from the waaF, waaG, waaI and waaL mutants induced significant lower levels of anti-OMP serum IgG than the wild-type OMVs (P < 0.05) (Fig. 2A). In mice immunized via the i.p. route, the IgG responses at 2 weeks were similar for all OMV preparations except the OMVs prepared from the waaI mutant, which was significantly less immunogenic (P < 0.05). After the booster immunization, the IgG responses in mice immunized with OMVs from the waaG, waaI and waaL mutants were significantly less than mice immunized with OMVs from any of the other strains (P < 0.01) (Fig. 2B). Regardless of immunization route, the levels of anti-OMP serum IgG in PBS control groups remained below the level of detection (data not shown).
Fig. 2.
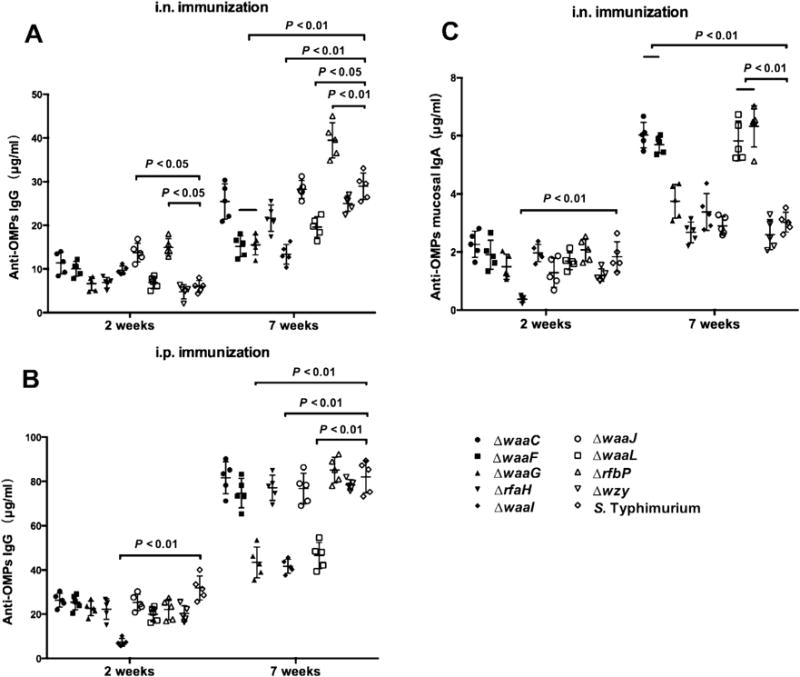
OMVs derived from Salmonella LPS mutant strains elicited strong systemic and mucosal immune responses via intranasal or intraperitoneal route. The total serum IgG specific for OMPs from S. Typhimurium after intranasal vaccination (A), IgG specific for OMPs from S. Typhimurium after intraperitoneal vaccination (B) and total IgA specific for OMPs from S. Typhimurium after intranasal vaccination (C) were measured using quantitative ELISA. Each group included 5 mice. The data represent the exact concentrations of IgG or mucosal IgA antibodies at the indicated number of weeks after immunization. The concentrations were quantified using the appropriate standard curve in individual sera or in vaginal washes from mice immunized i.n. or i.p. with OMVs derived from wild-type or mutant S. Typhimurium strains with truncated LPS core or O-antigen. The error bars represent variations among all mice in each group. The mice were boosted at week 5.
We also measured anti-OMP IgA in the vaginal secretions of immunized mice as an indicator of the mucosal response. At 2 weeks post-immunization, all mice had similarly low levels of mucosal IgA, except in the case of the rfaH mutant, which was significantly lower than the other groups (P < 0.05; Fig. 2C). After the booster immunization, the levels of mucosal IgA showed a robust increase, and the levels of mucosal IgA in mice immunized with OMVs derived from the waaC, waaF, waaL and rfbP mutants were significantly higher than those of wild-type OMV-immunized mice (P < 0.01) (Fig. 2C). No anti-OMP mucosal IgA antibodies were detected in any of the i.p. immunized groups (data not shown).
As a large amount of the flagellin was present in the OMVs, the concentration of anti-FliC IgG in the serum from the immunized mice at 7 weeks after first immunization were also determined. We detected comparable anti-FliC IgG production in all groups of immunized mice, regardless of the immunization route. Intraperitoneal immunization induced relatively higher anti-FliC IgG levels than intranasal immunization (Fig. 3).
Fig. 3.
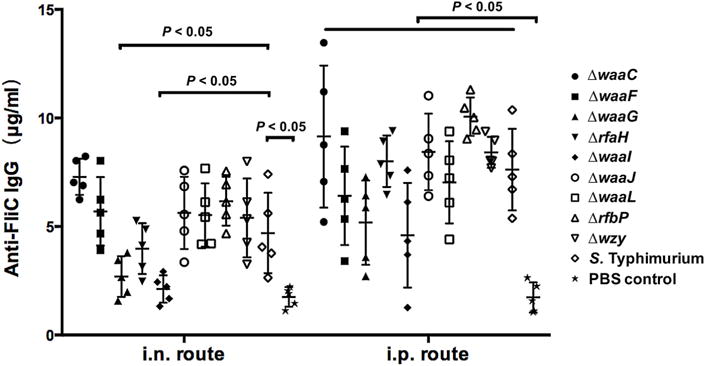
The total serum IgG specific for recombinant soluble FliC protein after intranasal or intraperitoneal vaccination with OMVs derived from diverse LPS mutant strains were measured using quantitative ELISA. Each group included 5 mice. The data represent the exact concentrations of IgG antibodies at the 7 weeks after first immunization. The concentrations were quantified using the appropriate standard curve in individual sera from mice immunized i.n. or i.p. with OMVs derived from wild-type or mutant S. Typhimurium strains with truncated LPS core or O-antigen. The mice were boosted at week 5. The error bars represent variations among all mice in each group.
3.3. Protective capacity of the truncated-LPS OMVs against infection with the wild-type S. Typhimurium
To evaluate protective efficiency, immunized mice were challenged with 1 × 109 CFU (approximately 2000 × LD50) of the wild-type S. Typhimurium S100 5 weeks after the booster. Immunization with OMVs from the wild type or the waaC, rfaH and rfbP mutants elicited effective protection against challenge, regardless of the route of immunization, although protection via the intranasal route (Table 2, Supplementary Fig. 1A) was slightly less than when mice were immunized i.p. (Table 2, Supplementary Fig. 1B) (80% vs 100%, respectively). Intranasal immunization with OMVs from the waaG and waaI mutants failed to protect the mice (0% survival), whereas intraperitoneal immunization with OMVs from these two strains conferred weak protection against Salmonella challenge (40% survival). All the mice in the control group (PBS group) succumbed after wild-type S. Typhimurium strain S100 infection.
Table 2.
Immunization with truncated LPS OMVs protected mice against oral challenge with S. Typhimurium strain S100.
| Groups Immunization administration |
No. of surviving mice/total No. of micea
|
|
|---|---|---|
| Intranasal route | Intraperitoneal route | |
| χ3761 (wild-type) | 4/5 (80%) | 5/5 (100%) |
| χ12253 (ΔwaaC12) | 4/5 (80%) | 5/5 (100%) |
| χ12252 (ΔwaaF15) | 2/5 (40%) | 3/5 (60%) |
| χ11308 (ΔwaaG42) | 0/5 (0%) | 2/5 (40%) |
| χ9945 (ΔrfaH49) | 3/5 (60%) | 5/5 (100%) |
| χ11309 (ΔwaaI43) | 0/5 (0%) | 2/5 (40%) |
| χ11310 (ΔwaaJ44) | 3/5 (60%) | 3/5 (60%) |
| χ11312 (ΔwaaL46) | 1/5 (20%) | 3/5 (60%) |
| χ11311 (ΔrfbP45) | 4/5 (80%) | 4/5 (80%) |
| χ9944 (Δwzy-48) | 1/5 (20%) | 3/5 (60%) |
| PBS group | 0/5 (0%) | 0/8 (0%) |
All vaccine groups except groups of intranasal immunization of χ11309 (ΔwaaI43) and χ11308 (ΔwaaG42) were significantly different from the PBS-vaccinated group (P < 0.01). There was no significant difference in protection among the other groups.
3.4. OMVs from the truncated-LPS mutants enhance cross-reactivity against OMPs from heterologous serotypes of Salmonella
We examined the capacity of the serum IgG elicited with our set of OMVs to cross-react with OMPs from the heterologous S. Choleraesuis (serogroup C1) and S. Enteritidis (serogroup D1). Regardless of the route of immunization, OMVs from the waaC mutant induced significantly higher serum IgG titers than the wild-type OMVs against OMPs from S. Choleraesuis (P < 0.05) (Fig. 4A). We also observed significant increases in cross-reactive responses to S. Choleraesuis OMPs in the sera from the mice immunized i.n. with OMVs from the waaJ, rfbP and wzy mutants (P < 0.01). In the i.p. group, OMVs from the waaF, waaG, waaI and waaL mutants elicited significantly lower anti-S. Enteritidis serum IgG responses than mice immunized with OMVs from the wild type strain (Fig. 4B). The serum IgG responses in all other groups was statistically indistinguishable from the wild type group.
Fig. 4.
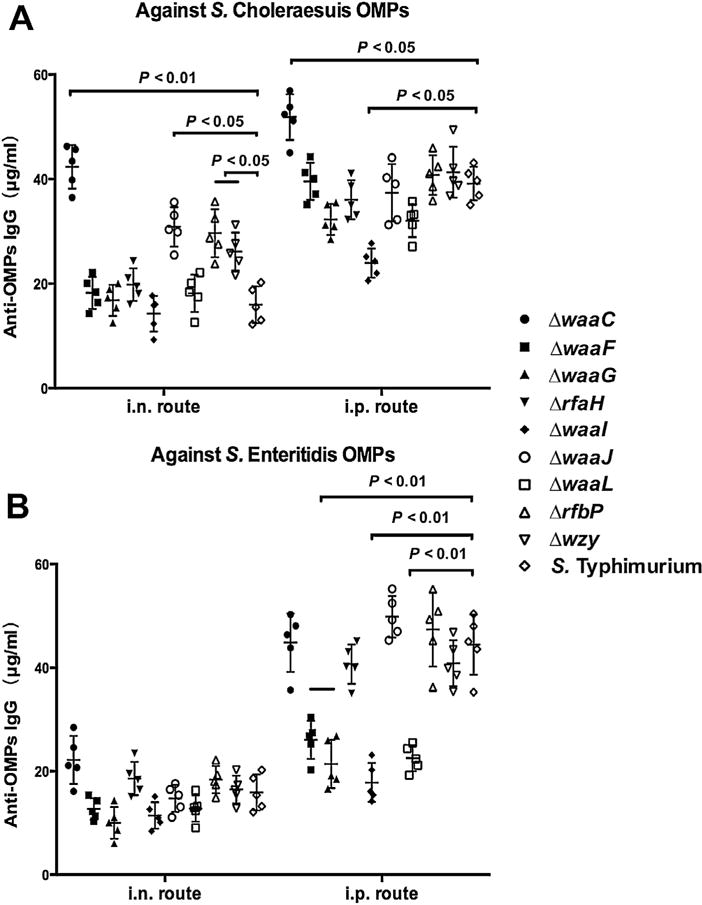
IgG cross-reactivity against OMPs from other Salmonella serogroups. Sera were obtained from mice (n = 10) that were immunized with OMVs from wild-type S. Typhimurium or from mutants with truncated LPS core or O-antigen at 7 weeks after the primary immunization. The sera were collected to evaluate IgG cross-reactivity against OMPs isolated from different Salmonella serogroups, including S. Choleraesuis (A) and S. Enteritidis (B). The error bars represent variations among all mice in each group.
3.5. Evaluation of cross-protection against heterologous-serotype Salmonella challenge
To evaluate cross-protective efficacy, we immunized groups of mice with OMVs from the waaC, rfaH or rfbP mutants. These mutants were chosen based on their ability to elicit both high levels of cross-reactivity against heterologous OMPs and the high survival rates after the wild-type S. Typhimurium challenge. OMVs from all four strains provided similar, strong protection against S. Typhimurium challenge, regardless of administration route, compared to the PBS control (P <0.01) (Fig. 5A and B). Mice immunized intranasally with OMVs from the waaC, rfaH or rfbP mutant strains showed higher survival rates against S. Choleraesuis challenge than mice immunized with the wild-type OMVs (Fig. 5C). Interestingly, protection provided by OMVs from the waaC mutant was significantly greater than by the wild-type OMVs (P < 0.05). All the OMVs administered i.p., conferred similar, strong protection against S. Choleraesuis challenge (Fig. 5D).
Fig. 5.
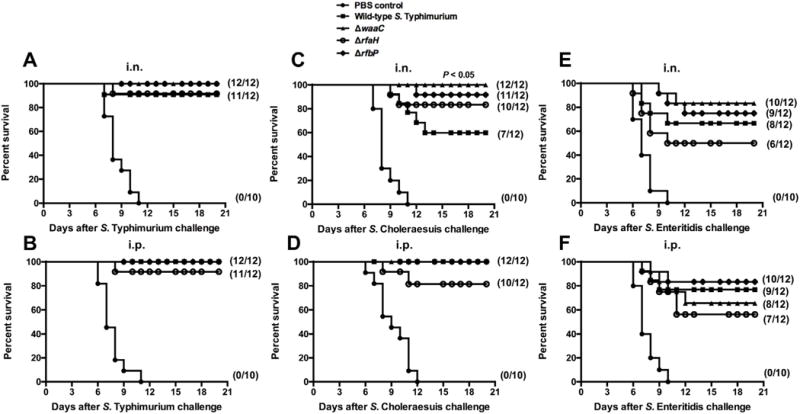
Cross-protective efficacy induced by truncated-LPS OMVs. 10 (control) or 12 (vaccine) BALB/c mice per group were i.n. (A, C, E) or i.p. (B, D, F) immunized twice, at 3-week intervals, with OMVs isolated from the indicated Salmonella mutants. The immunized mice were orally challenged with S. Typhimurium (A, B), S. Choleraesuis (C, D) or S. Enteritidis (E, F) 8 weeks after the first immunization. Mortality was monitored for 3 weeks after challenge. The numbers in parentheses indicate the number of surviving mice/total number of mice per group. All vaccine groups were significantly different from the PBS-vaccinated group (P < 0.01), and P < 0.05 compared with OMVs from wild-type S. Typhimurium.
Protection against S. Enteritidis challenge was more variable, although all the immunized groups achieved ≥ 50% survival rate. OMVs from the rfaH mutant induced the lowest level of protection against S. Enteritidis challenge (50% and 58.3% survival for intranasal and intraperitoneal routes, respectively), but no significant differences were observed among the immunized groups (Fig. 5E and F).
4. Discussion
Licensed, commercially produced Salmonella vaccines against S. Typhi infection are currently available for human use (Ochiai et al., 2014). Although there are many licensed anti-NTS Salmonella vaccines available for livestock (e.g. poultry and pigs), no licensed vaccine is available for the multiple serotypes of NTS that cause disease in humans (Gal-Mor et al., 2014). OMV-based vaccines that possess high immunogenicity but do not replicate are recognized as a good option for the development of vaccines against many bacterial infections (McConnell et al., 2011; Mitra et al., 2012; Roy et al., 2011; Roy et al., 2010). OMVs are composed of OMPs, LPS and other outer membrane and periplasmic components (Beveridge, 1999; Kuehn and Kesty, 2005). The LPS length affects the permeability of the bacterial membrane and the OMP composition (Helander et al., 1998). Thus, isolating OMVs from LPS mutants will alter OMV composition and this, in turn, will affect immunogenicity. In this study, we investigated the immunogenicity and efficacy of OMVs derived from a set of LPS mutants with progressively shorter LPS lengths, resulting from mutations in waaC, waaF, waaG, rfaH, waaI, waaJ, waaL, rfbP and wzy, against homologous and heterologous Salmonella challenge.
Targeted deletion of genes associated with outer membrane assembly can modulate bacterial vesiculation and OMV production (Baker et al., 2014), and vesiculation levels are also correlated with LPS biosynthesis in E. coli (McBroom et al., 2006). In our study, we observed that truncation of LPS does affect vesicle production, but there was not a strict correlation between vesicle production and LPS length (Fig. 1C). For instance, our rfbP mutant, which produces a complete lipid A core moiety but no O-antigen, increased vesicle production. In contrast, the waaC mutant, missing most of the LPS core, produced fewer OMVs than wild-type Salmonella (P < 0.01).
We also observed a large amount of flagellin present in the OMVs purified from the wild-type and mutant strains. Flagellin production in the OMVs varied among the LPS mutants (Fig. 1), indicating that LPS structure affects the flagellin production in Salmonella. This observation is consistent with the fact that most of these strains exhibit a range of defects in motility, with the waaI and waaG mutants having the greatest defect (Kong et al., 2011c; Muralinath et al., 2011). One striking result was the low levels of anti-flagellin serum IgG responses, particularly in the i.n. immunized group (Fig. 3). This can be explained if most of the flagellin that copurified with the OMVs was present as intact filaments, preventing its interaction with TLR5 to activate proinflammatory immune responses (Smith et al., 2003). Our result is also consistent with a previous report in which live Salmonella cells were administered by various routes (Sbrogio-Almeida and Ferreira, 2001). In that study, flagellin was poorly immunogenic as an intranasal antigen.
To evaluate the immunogenic and protective properties of OMVs isolated from various rough Salmonella mutants, we compared immunization by intranasal and intraperitoneal routes. Previous studies have suggested that intranasal immunization is an effective route to induce a protective immunity at both systemic and mucosal sites (Holmgren and Czerkinsky, 2005; Kiyono and Fukuyama, 2004), and the intranasal route has received considerable attention for vaccine delivery (Partidos, 2000). Moreover, intraperitoneal immunization is a fairly standard route for immunizing mice with OMVs because this route induces robust humoral immune responses (Alaniz et al., 2007; Nieves et al., 2014; Schild et al., 2009). Therefore, we chose these two routes of administration to evaluate immunogenicity and protective efficacy. Previous studies indicate that high doses of antigen administered by intranasal route are likely to reach the intestinal tract or be drained directly by the posterior cervical lymph nodes (Partidos, 2000). Thus, we immunized intranasally with 20 μg of OMV, a higher dose than we used for intraperitoneal immunization.
Many pathogens, including Salmonella, typically initiate the infection process by interacting with a mucosal surface. Therefore, the development of a vaccine that induces mucosal immunity at such sites would represent an optimal strategy to control enteric bacterial infection (Capozzo et al., 2004; Pasetti et al., 2003). The mucosal IgA antibody responses observed in the vaginal washes are important indicators of mucosal immunity (Verweij et al., 1998), and the mucosal immune defense mediated by IgA antibodies is likely to be associated with protection against Salmonella infection (Holmgren and Czerkinsky, 2005). Our results showed that the vaginal IgA levels induced by OMVs derived from the mutants waaC, waaF, waaL and rfbP were significantly higher than those induced by the wild-type OMVs via the intranasal route (Fig. 2C). However, the protection rate was not consistent with the mucosal IgA titer (Fig. 4), indicating that other arms of immunity are also critically important for conferring protection against high dose challenge of Salmonella by oral infection.
Intraperitoneal immunization using ΔwaaC, ΔrfaH and wild-type OMVs provided 100% protection while intranasal immunization provided 80% survival (Table 2). This difference in protection may be due to the ability of intraperitoneal immunization to elicit stronger humoral immunity than intranasal immunization. However, intraperitoneal immunization with OMVs induced mild symptoms as a consequence of the LPS component of OMVs (Pridmore et al., 2001). This problem can be overcome using one of several genetic strategies to detoxify the LPS in living Salmonella cells without compromising its adjuvant effect (Kong et al., 2011a; Kong et al., 2012; Kong et al., 2011b), facilitating the purification of OMVs with reduced toxicity for future vaccine development.
The results of our cross-protection study showed that OMVs, particularly those derived from the waaC and rfbP mutants, were able to promote cross-reactive antibodies and cross-protection against S. Choleraesuis and S. Enteritidis infections (Fig. 5Band C ). We attribute this to two major OMV antigen types, the conserved OMPs and the conserved lipid A core moiety. OMPs isolated from the wild-type Salmonella or from mutants with truncated LPS were capable of inducing cross-protective immunity against S. Typhimurium and heterologous serovars (Isibasi et al., 1988; Ochoa-Repáraz et al., 2005; Udhayakumar and Muthukkaruppan, 1987). Our recent study also demonstrated that OMPs from waaC mutant could induce effective cross-protection against infection of multiple Salmonella serotypes (Liu et al., 2016), suggesting that OMPs in OMV-based vaccines have essential roles in inducing protective immunity. It is unknown from the present study which kind of protein components in OMVs play leading roles in inducing cross immunity and conferring cross-protection (Fig. 4). Conserved LPS oligosaccharides in OMVs also contribute to inducing protective immunity (Di Lorenzo et al., 2015). However, these sugar epitopes may provide limited broad-spectrum protection against smooth Gram-negative bacterial infections (Gigliotti and Shenep, 1985; Johns et al., 1983; Siber et al., 1985). Flagellin in the OMVs induced comparable immune responses (Fig. 3); however, these anti-flagellin antibodies may play non-essential roles in providing cross-protection against oral infection by heterologous Salmonella serovars because many of these serovars produce immunologically distinct flagellin proteins (Ramachandran et al., 2016). We have observed that OMVs derived from the mutants lacking flagellin may possess advantages over OMVs derived from the wild-type Salmonella in inducing protective immunity against heterologous Salmonella infection (unpublished data). In the future, we plan to investigate in detail the immunogenicity of the OMVs derived from truncated LPS mutants with deletion of flagellin genes.
In conclusion, in this work, we systemically investigated the effect of truncated LPS on the production of OMVs and found that deletion of the rfbP gene could enhance OMV production in Salmonella. We also found that truncated-LPS OMVs display strong immunogenicity and protective efficacy against wild-type S. Typhimurium. Further, OMVs derived from waaC and rfbP mutant strains elicited greater cross-protection against S. Choleraesuis than wild-type OMVs when administered as an intranasal vaccine.
Supplementary Material
Acknowledgments
We thank Dr. Roy Curtiss III to provide the laboratory equipments for purifying and characterizing the OMVs samples. This study was supported by the National Natural Science Foundation of China (31570928, 31472179, 31270981), and by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (Grant NIH R01 AI112680 to Q.K.).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijmm.2016.08.004.
Footnotes
Author contributions
Qing Liu and Qingke Kong conceived and designed the experiments; Qiong Liu, Jie Yi, Tian Liu, Kang Liang and Yanlong Jiang performed the experiments. Qing Liu, Qiong Liu and Qingke Kong analyzed the data; Qiong Liu, Kenneth L. Roland and Qingke Kong wrote the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Alaniz RC, Deatherage BL, Lara JC, Cookson BT. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol. 2007;179:7692–7701. doi: 10.4049/jimmunol.179.11.7692. [DOI] [PubMed] [Google Scholar]
- Baker JL, Chen L, Rosenthal JA, Putnam D, DeLisa MP. Microbial biosynthesis of designer outer membrane vesicles. Curr Opin Biotechnol. 2014;29:76–84. doi: 10.1016/j.copbio.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725–4733. doi: 10.1128/jb.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzo AVE, Cuberos L, Levine MM, Pasetti MF. Mucosally delivered Salmonella live vector vaccines elicit potent immune responses against a foreign antigen in neonatal mice born to naive and immune mothers. Infect Immun. 2004;72:4637–4646. doi: 10.1128/IAI.72.8.4637-4646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlone G, Thomas M, Rumschlag HS, Sottnek FO. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J Clin Microbiol. 1986;24:330–332. doi: 10.1128/jcm.24.3.330-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo F, Silipo A, Lanzetta R, Parrilli M, Molinaro A. Bacterial lipopolysaccharides: an overview of their structure, biosynthesis and immunological activity, carbohydrate chemistry: state of the art and challenges for drug Development: an overview on structure, biological roles, synthetic methods and application as therapeutics. World Scientific; 2015. pp. 57–89. [Google Scholar]
- Eng SK, Pusparajah P, Ab Mutalib NS, Ser HL, Chan KG, Lee LH. Salmonella A review on pathogenesis, epidemiology and antibiotic resistance. Front Life Sci. 2015;8:284–293. [Google Scholar]
- Ernst RK, Guina T, Miller SI. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect. 2001;3:1327–1334. doi: 10.1016/s1286-4579(01)01494-0. [DOI] [PubMed] [Google Scholar]
- Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012;379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira RB, Valdez Y, Coombes BK, Sad S, Gouw JW, Brown EM, Li Y, Grassl GA, Antunes LC, Gill N, Truong M, Scholz R, Reynolds LA, Krishnan L, Zafer AA, Sal-Man N, Lowden MJ, Auweter SD, Foster LJ, Finlay BB. A highly effective component vaccine against nontyphoidal Salmonella enterica infections. mBio. 2015;6:e01421–01415. doi: 10.1128/mBio.01421-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Langarica A, Bobat S, Marshall JL, Yam-Puc JC, Cook CN, Serre K, Kingsley RA, Flores-Romo L, Uematsu S, Akira S. Soluble flagellin coimmunization attenuates Th1 priming to Salmonella and clearance by modulating dendritic cell activation and cytokine production. Eur J Immunol. 2015;45:2299–2311. doi: 10.1002/eji.201545564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frirdich E, Whitfield C. Review: lipopolysaccharide inner core oligosaccharide structure and outer membrane stability in human pathogens belonging to the Enterobacteriaceae. J Endotoxin Res. 2005;11:133–144. doi: 10.1179/096805105X46592. [DOI] [PubMed] [Google Scholar]
- Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti F, Shenep JL. Failure of monoclonal antibodies to core glycolipid to bind intact smooth strains of Escherichia coli. J Infect Dis. 1985;151:1005–1011. doi: 10.1093/infdis/151.6.1005. [DOI] [PubMed] [Google Scholar]
- Girard MP, Steele D, Chaignat CL, Kieny MP. A review of vaccine research and development: human enteric infections. Vaccine. 2006;24:2732–2750. doi: 10.1016/j.vaccine.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Hassan J, Curtiss R. Control of colonization by virulent Salmonella typhimurium by oral immunization of chickens with avirulent Δcya Δcrp S.typhimurium. Res Microbiol. 1990;141:839–850. doi: 10.1016/0923-2508(90)90119-b. [DOI] [PubMed] [Google Scholar]
- Helander IM, Latva-Kala K, Lounatmaa K. Permeabilizing action of polyethyleneimine on Salmonella typhimurium involves disruption of the outer membrane and interactions with lipopolysaccharide. Microbiology. 1998;144:385–390. doi: 10.1099/00221287-144-2-385. [DOI] [PubMed] [Google Scholar]
- Hitchcock PJ, Brown TM. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- Holst J, Martin D, Arnold R, Huergo CC, Oster P, O’Hallahan J, Rosenqvist E. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009;27:B3–B12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- Isibasi A, Ortiz V, Vargas M, Paniagua J, Gonzalez C, Moreno J, Kumate J. Protection against Salmonella typhi infection in mice after immunization with outer membrane proteins isolated from Salmonella typhi 9, 12, d, Vi. Infect Immun. 1988;56:2953–2959. doi: 10.1128/iai.56.11.2953-2959.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M, Skehill A, McCabe WR. Immunization with rough mutants of Salmonella minnesota. IV. Protection by antisera to O and rough antigens against endotoxin. J Infect Dis. 1983;147:57–67. doi: 10.1093/infdis/147.1.57. [DOI] [PubMed] [Google Scholar]
- Kiyono H, Fukuyama S. NALT-versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Six DA, Liu Q, Gu L, Roland KL, Raetz CR, Curtiss R. Palmitoylation state impacts induction of innate and acquired immunity by the Salmonella enterica serovar Typhimurium msbB mutant. Infect Immun. 2011a;79:5027–5038. doi: 10.1128/IAI.05524-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Six DA, Roland KL, Liu Q, Gu L, Reynolds CM, Wang X, Raetz CR, Curtiss R. Salmonella synthesizing 1-monophosphorylated lipopolysaccharide exhibits low endotoxic activity while retaining its immunogenicity. J Immunol. 2011b;187:412–423. doi: 10.4049/jimmunol.1100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Yang J, Liu Q, Alamuri P, Roland KL, Curtiss R. Effect of deletion of genes involved in lipopolysaccharide core and O-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar Typhimurium. Infect Immun. 2011c;79:4227–4239. doi: 10.1128/IAI.05398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Six DA, Liu Q, Gu L, Wang S, Alamuri P, Raetz CR, Curtiss R. Phosphate groups of lipid A are essential for Salmonella enterica serovar Typhimurium virulence and affect innate and adaptive immunity. Infect Immun. 2012;80:3215–3224. doi: 10.1128/IAI.00123-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak GK, MacDonald D, Landry L, Farber JM. Foodborne outbreaks in Canada linked to produce: 2001 through 2009. J Food Protect. 2013;76:173–183. doi: 10.4315/0362-028X.JFP-12-126. [DOI] [PubMed] [Google Scholar]
- Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Gene Dev. 2005;19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Liu Q, Zhao X, Liu T, Yi J, Liang K, Kong Q. Immunogenicity and cross-protective efficacy induced by outer membrane proteins from Salmonella Typhimurium mutants with truncated LPS in mice. Int J Mol Sci. 2016;17:416. doi: 10.3390/ijms17030416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan CA, Martin LB, Micoli F. Vaccines against invasive Salmonella disease: current status and future directions. Hum Vacc Immunother. 2014;10:1478–1493. doi: 10.4161/hv.29054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtiger N, Fox CF. Biochemistry of bacterial membranes. Annu Rev Biochem. 1973;42:575–600. doi: 10.1146/annurev.bi.42.070173.003043. [DOI] [PubMed] [Google Scholar]
- Mahan MJ, Heithoff DM, House JK. Salmonella cross-protective vaccines: fast-forward to the next generation of food safety. Future Microbiol. 2012;7:805–808. doi: 10.2217/fmb.12.60. [DOI] [PubMed] [Google Scholar]
- Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, Jones TF, Fazil A, Hoekstra RM, Burd I.C.E.D. The global burden of Nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J Bacteriol. 2006;188:5385–5392. doi: 10.1128/JB.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell MJ, Rumbo C, Bou G, Pachón J. Outer membrane vesicles as an acellular vaccine against Acinetobacter baumannii. Vaccine. 2011;29:5705–5710. doi: 10.1016/j.vaccine.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Mitra S, Barman S, Nag D, Sinha R, Saha DR, Koley H. Outer membrane vesicles of Shigella boydii type 4 induce passive immunity in neonatal mice. FEMS Immunol Med Microbiol. 2012;66:240–250. doi: 10.1111/j.1574-695X.2012.01004.x. [DOI] [PubMed] [Google Scholar]
- Muralinath M, Kuehn MJ, Roland KL, Curtiss R. Immunization with Salmonella enterica serovar Typhimurium-derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae. Infect Immun. 2011;79:887–894. doi: 10.1128/IAI.00950-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves W, Asakrah S, Qazi O, Brown KA, Kurtz J, Aucoin DP, McLachlan JB, Roy CJ, Morici LA. A naturally derived outer-membrane vesicle vaccine protects against lethal pulmonary Burkholderia pseudomallei infection. Vaccine. 2011;29:8381–8389. doi: 10.1016/j.vaccine.2011.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves W, Petersen H, Judy BM, Blumentritt CA, Russell-Lodrigue K, Roy CJ, Torres AG, Morici LA. A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin Vaccine Immunol. 2014;21:747–754. doi: 10.1128/CVI.00119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai RL, Khan MI, Soofi SB, Sur D, Kanungo S, You YA, Habib MA, Sahito SM, Manna B, Dutta S, Acosta CJ, Ali M, Bhattacharya SK, Bhutta ZA, Clemens JD. Immune responses to Vi capsular polysaccharide typhoid vaccine in children 2–16 years old in Karachi, Pakistan, and Kolkata, India. Clin Vaccine Immunol. 2014;21:661–666. doi: 10.1128/CVI.00791-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Repáraz J, García B, Solano C, Lasa I, Irache JM, Gamazo C. Protective ability of subcellular extracts from Salmonella Enteritidis and from a rough isogenic mutant against Salmonellosis in mice. Vaccine. 2005;23:1491–1501. doi: 10.1016/j.vaccine.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Osborn MJ. Studies on the gram-Negative cell wall. I. Evidence for the role of 2-Keto- 3-Deoxyoctonate in the lipopolysaccharide of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1963;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partidos CD. Intranasal vaccines: forthcoming challenges. Pharma Sci Technol Today. 2000;3:273–281. doi: 10.1016/s1461-5347(00)00281-9. [DOI] [PubMed] [Google Scholar]
- Pasetti MF, Barry EM, Losonsky G, Singh M, Medina-Moreno SM, Polo JM, Ulmer J, Robinson H, Sztein MB, Levine MM. Attenuated Salmonella enterica serovar Typhi and Shigella flexneri 2a strains mucosally deliver DNA vaccines encoding measles virus hemagglutinin, inducing specific immune responses and protection in cotton rats. J Virol. 2003;77:5209–5217. doi: 10.1128/JVI.77.9.5209-5217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridmore AC, Wyllie DH, Abdillahi F, Steeghs L, van der Ley P, Dower SK, Read RC. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via toll-like receptor (TLR) 2 but not via TLR4/MD2. J Infect Dis. 2001;183:89–96. doi: 10.1086/317647. [DOI] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran G, Tennant SM, Boyd MA, Wang JY, Tulapurkar ME, Pasetti MF, Levine MM, Simon R. Functional activity of antibodies directed towards flagellin proteins of Non-Typhoidal Salmonella. PLoS One. 2016;11:e0151875. doi: 10.1371/journal.pone.0151875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roier S, Leitner DR, Iwashkiw J, Schild-Prufert K, Feldman MF, Krohne G, Reidl J, Schild S. Intranasal immunization with nontypeable Haemophilus influenzae outer membrane vesicles induces cross-protective immunity in mice. PLoS One. 2012;7:e42664. doi: 10.1371/journal.pone.0042664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N, Barman S, Ghosh A, Pal A, Chakraborty K, Das SS, Saha DR, Yamasaki S, Koley H. Immunogenicity and protective efficacy of Vibrio cholerae outer membrane vesicles in rabbit model. FEMS Immunol Med Microbiol. 2010;60:18–27. doi: 10.1111/j.1574-695X.2010.00692.x. [DOI] [PubMed] [Google Scholar]
- Roy K, Hamilton DJ, Munson GP, Fleckenstein JM. Outer membrane vesicles induce immune responses to virulence proteins and protect against colonization by enterotoxigenic Escherichia coli. Clin Vaccine Immunol. 2011;18:1803–1808. doi: 10.1128/CVI.05217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrogio-Almeida M, Ferreira L. Flagellin expressed by live Salmonella vaccine strains induces distinct antibody responses following delivery via systemic or mucosal immunization routes. FEMS Immunol Med Microbiol. 2001;30:203–208. doi: 10.1111/j.1574-695X.2001.tb01571.x. [DOI] [PubMed] [Google Scholar]
- Schild S, Nelson EJ, Bishop AL, Camilli A. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect Immun. 2009;77:472–484. doi: 10.1128/IAI.01139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siber GR, Kania SA, Warren HS. Cross-reactivity of rabbit antibodies to lipopolysaccharides of Escherichia coli J5 and other gram-negative bacteria. J Infect Dis. 1985;152:954–964. doi: 10.1093/infdis/152.5.954. [DOI] [PubMed] [Google Scholar]
- Simon R, Wang JY, Boyd MA, Tulapurkar ME, Ramachandran G, Tennant SM, Pasetti M, Galen JE, Levine MM. Sustained protection in mice immunized with fractional doses of Salmonella Enteritidis core and O polysaccharide-flagellin glycoconjugates. PLoS One. 2013;8:e64680. doi: 10.1371/journal.pone.0064680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B. Salmonella vaccines for animals and birds and their future perspective. Open Vac J. 2009;2:100–112. [Google Scholar]
- Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247–1253. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- Tennant SM, Wang JY, Galen JE, Simon R, Pasetti MF, Gat O, Levine MM. Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect Immun. 2011;79:4175–4185. doi: 10.1128/IAI.05278-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udhayakumar V, Muthukkaruppan VR. Protective immunity induced by outer-membrane proteins of Salmonella typhimurium in mice. Infect Immun. 1987;55:816–821. doi: 10.1128/iai.55.3.816-821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij WR, de Haan L, Holtrop M, Agsteribbe E, Brands R, van Scharrenburg GJ, Wilschut J. Mucosal immunoadjuvant activity of recombinant Escherichia coli heat-labile enterotoxin and its B subunit: induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with influenza virus surface antigen. Vaccine. 1998;16:2069–2076. doi: 10.1016/s0264-410x(98)00076-0. [DOI] [PubMed] [Google Scholar]
- Whitfield C, Kaniuk N, Frirdich E. Molecular insights into the assembly and diversity of the outer core oligosaccharide in lipopolysaccharides from Escherichia coli and Salmonella. J Endotoxin Res. 2003;9:244–249. doi: 10.1179/096805103225001440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


