Abstract
Sites of inflammation are defined by significant changes in metabolic activity. Recent studies have suggested that O2 metabolism and hypoxia play a prominent role in inflammation so-called “inflammatory hypoxia,” which results from a combination of recruited inflammatory cells (e.g., neutrophils and monocytes), the local proliferation of multiple cell types, and the activation of multiple O2-consuming enzymes during inflammation. These shifts in energy supply and demand result in localized regions of hypoxia and have revealed the important function off the transcription factor HIF (hypoxia-inducible factor) in the regulation of key target genes that promote inflammatory resolution. Analysis of these pathways has provided multiple opportunities for understanding basic mechanisms of inflammation and has defined new targets for intervention. Here, we review recent work addressing tissue hypoxia and metabolic control of inflammation and immunity.
Keywords: metabolism, inflammation, creatine, phosphocreatine, nucleotide, nucleoside, nucleotidase, mucosa, colitis, neutrophil, epithelium, murine model
INTRODUCTION
The inflammatory response is associated with profound shifts in tissue metabolism (1). These changes emanate from increased O2 consumption, the generation of large quantities of reactive O2 intermediates, and the local depletion of nutrients, resulting in profound tissue hypoxia (2). A number of recent studies have revealed that changes in tissue oxygenation are compounded by the recruitment of innate immune cells such as neutrophils, also known as polymorphonuclear leukocytes (PMN), and macrophages. Because the majority of inflammatory cells are recruited to inflammatory lesions, these cell types have the potential to substantially enhance ongoing metabolism and even shift metabolic demand beyond what can be provided by existing resources (3). Adaptive immunity, in contrast to innate immunity, is characterized by high rates of local T and B cell proliferation and has significantly different metabolic demands (4, 5). It is important to understand the interactions between metabolic changes in the local milieu [e.g., glucose, O2, and adenosine triphosphate (ATP)] and metabolic triggers and energy for immune cell recruitment/activation in these areas. Much recent evidence also suggests that metabolic shifts associated with ongoing inflammation and immunity may serve as important therapeutic targets.
Studies in the mucosa have provided important insight into metabolic demands associated with inflammatory responses. The gastrointestinal (GI) tract, for example, is characterized by a particularly unique oxygenation profile, experiencing profound fluctuations in blood perfusion at regular intervals (2). Even at baseline, epithelial cells lining the mucosa exist in a relatively low O2 tension environment, herein described as “physiological hypoxia.” Countercurrent O2 exchange mechanisms in the small intestine have revealed that O2 from the arterial blood supply diffuses to adjacent venules along the cryptvillus axis, resulting in graded hypoxia (6). A steep O2 gradient has also been documented in more distal, colonic regions of the GI tract, extending from the anaerobic lumen, across the epithelium, and to the richly vascularized subepithelial mucosa (7). Given the high energy requirements of the gut and the integral role of the epithelium in maintaining intestinal homeostasis, it is not surprising that these cells have evolved a number of mechanisms to cope with their austere metabolic environment. Here, we discuss how such metabolic shifts are regulated, particularly with regard to changes associated with the inflammatory response.
O2 METABOLISM IN HEALTHY AND INFLAMED TISSUES
Mucosal tissue oxygenation in the healthy state is a lesson in contrasts. Breathable air at sea level contains a partial O2 pressure (pO2) of ~145 mm Hg (approximately 21% O2). Measurements of the healthy lung alveolus have revealed a pO2 of 100–110 mm Hg (8). In stark contrast, the most luminal aspect of the healthy colon exists at a pO2 of less than 20 mm Hg (7, 9). Such differences reflect a combination of O2 sources, local metabolism, and the anatomy of blood flow (Figure 1).
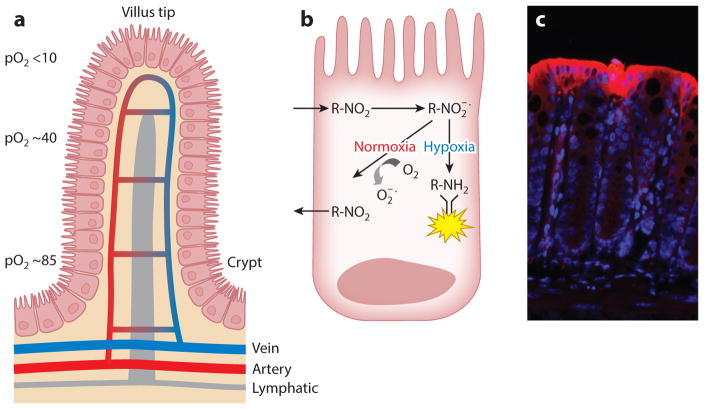
Figure 1.
Countercurrent blood flow and physiological hypoxia in the healthy intestinal mucosa. (a) A model of blood flow dynamics in the healthy intestinal mucosa. Countercurrent blood flow reduces local pO2 along the crypt-villus axis and results in low pO2 at the villus tip. (b) The mechanism of nitroimidazole dye retention in hypoxic regions. These imidazole derivatives (R-NO2) are taken into cells passively and reduced to highly reactive nitrogen intermediates ( ) within the cytoplasm. In the absence of adequate O2 to regenerate the native compound, these intermediates react with thiol groups in proteins, peptides, and amino acids to form adducts (R-NH2) where all atoms of the ring and side chain of the 2-nitroimidazole are retained at pO2 < 10 mm Hg. The adducts can be visualized through the use of labeled antibodies. (c) Physiological hypoxia. In the colonic mucosa of healthy mice, small amounts of nitroimidazole adduct (red ) are detected along the luminal aspect, suggesting a degree of physiological hypoxia in the normal colon. Abbreviation: pO2, partial oxygen pressure.
Tissue oxygenation, particularly low O2, has been tracked through the use of 2-nitroimidazole dyes, a class of compounds known to undergo intracellular metabolism dependent on levels of tissue oxygenation (10). These dyes were originally developed to image the low-O2 environment of growing tumors and to form adducts with thiol groups in proteins, peptides, and amino acids, where all atoms of the ring and side chain of the 2-nitroimidazole are retained at pO2 < 10 mm Hg (Figure 1). Mucosal tissue localization of these nitroimidazole dyes has revealed two profound observations. First, in the normal GI mucosa, particularly in the colon, physiological hypoxia pre-dominates (9) (Figure 1). Recent studies have shown that these low-O2 conditions are critical for the constitutive expression of certain innate immune factors found within the mucosa (e.g., human β defensin-1) (11). Second, the inflammatory lesions seen in mouse models [e.g., trinitrobenzene sulfonic (TNBS) colitis] are profoundly hypoxic or even anoxic, like some large tumors, and penetrate deep into the mucosal tissue. It is likely that multiple contributing factors (e.g., vasculitis, vasoconstriction, edema, and increased O2 consumption) predispose the inflamed intestinal epithelia to decreased O2 delivery and hypoxia (9). Although these 2-nitroimidazole compounds have not been used to image inflammatory lesions per se, they have shown significant clinical utility in tumor imaging and in the identification of stroke regions within the brains of patients (12). As opposed to other imaging techniques, these compounds have the advantages that they image only viable tissue and are not active within apoptotic or necrotic regions (13). Studies are under way to use these compounds as adjunct radiosensitizers for enhancing chemotherapy targeting (14).
Given the substantial shifts in metabolism and O2 availability during inflammation, a number of studies have shown that stabilization of hypoxia-inducible factor (HIF) under low-O2 conditions triggers the expression of genes that enable epithelial cells to function effectively as a barrier (15–18). HIF is a member of the Per-ARNT-Sim family of basic helix-loop-helix transcription factors and is considered one of the central regulators of overall tissue metabolism (19). HIF stabilization is dependent upon stabilization of an O2-dependent degradation domain expressed on the α-subunit and subsequent nuclear translocation to form a functional complex with HIF-1β and cofactors such as CREB-binding protein and its ortholog p300 (20). Under conditions of normal O2 supply, iron- and O2–dependent hydroxylation of two prolines (Pro564 and Pro402) within the O2-dependent degradation of HIF-α initiates the association with the von Hippel-Lindau tumor suppressor protein and rapid degradation via ubiquitin-E3 ligase proteasomal targeting (21, 22). A second hypoxic switch operates in the carboxy terminal transactivation domain of HIF-α, where hypoxia blocks the hydroxylation of Asp80, thereby facilitating the recruitment of CBP/p300 (23).
The spectrum of basal oxygenation within individual tissues is immense. Given the steep O2 gradient due to countercurrent blood flow, colonic and renal medullary epithelia exist at very low pO2 (24). These cells have proven to be remarkably resistant to hypoxia; even very low levels of oxygenation allow them to function normally (25, 26). The importance of HIF to epithelial function was originally shown by microarray analysis of intestinal epithelial cells cultured under low-O2 conditions (pO2 ~ 20 mm Hg) (15). These studies have subsequently been validated in animal models of intestinal inflammation (9, 27–31) and in observations of inflamed human tissues (32–34). Interestingly, many of the functional proteins encoded by HIF target genes localize to the most luminal aspect of the polarized epithelium—including mucins (35) and molecules that modify mucins [e.g., intestinal trefoil factor (36)], xenobiotic clearance by P-glycoprotein (16), and nucleotide metabolism/signaling (by ecto-5′-nucleotidase and CD73) (17, 18). Molecular studies of these hypoxia-regulated pathways have shown a dependence on HIF-mediated transcriptional responses. Extending these original studies to the in vivo setting, Karhausen et al. (9) generated mice expressing either mutant Hif1a (causing constitutive repression of Hif1a) or mutant von Hippel-Lindau (causing constitutive overexpression of HIF) targeted to the intestinal epithelial cells. Loss of epithelial HIF-1α resulted in a more severe colitic phenotype than is expressed by wild-type animals, with increased weight loss, decreased colon length, and, importantly, increased intestinal permeability. Conversely, constitutively active intestinal epithelial HIF was protective for each of the parameters studied. These findings were model dependent, given that epithelial HIF-based signaling has also been shown to promote inflammation in another study (31). However, the results confirmed that intestinal epithelial cells can adapt to hypoxia and indicated that HIF may contribute to such adaptation.
ENERGY METABOLISM DURING INFLAMMATION
O2 is central to most metabolic processes but figures most prominently in the generation of energy though oxidative phosphorylation. One of the fundamental differences between the inflammatory response and the immune response is the means by which various leukocyte populations obtain energy. Cells of myeloid lineages derive their energy almost exclusively from glycolysis, whereas lymphocytes use predominantly oxidative phosphorylation (4, 37). In part, these differences shape the appropriateness of the immune response. For example, evidence in recent years indicates that the abilities of lymphocytes to proliferate and quiesce are strictly controlled by essential metabolites that support anabolic growth (4).
As opposed to lymphocytes, which proliferate within tissues, myeloid cells (e.g., PMN, macrophages, and dendritic cells) are recruited to sites of inflammation during immune responses. In transit, these cells expend copious amounts of energy. Cell migration, for example, requires large amounts of actin turnover, which by its nature is particularly ATP demanding (38). Once the cells reach sites of inflammation, nutrient, energy, and O2 demands increase to accomplish processes of phagocytosis and microbial killing. It has long been known that PMN are primarily glycolytic cells, have few mitochondria, and produce little energy from respiration (39). Predominantly glycolytic metabolism is believed to ensure that PMN can function at low O2 concentrations (even anoxia) associated with deep inflammatory lesions. In this regard, recent studies have revealed that PMN have unique mitochondrial properties, namely that the mitochondria maintain a transmembrane potential via the glycerol-3-phosphate shuttle, which functions to regulate aerobic glycolysis rather than produce energy (37). This unique mitochondrial phenotype appears to develop along the differentiation pathway from myeloid precursor cells.
T and B cells, by contrast, utilize amino acids, glucose, and lipids as energy sources during oxidative phosphorylation. As one might imagine, mitogenic stimulation of thymocytes or naïve T cells is a highly energy-demanding process. As lymphocytes proliferate, they become more and more dependent on glucose uptake. Stimulated proliferation of thymocytes can result in a nearly twenty-fold increase in glucose uptake, which is accomplished by high expression of glucose transporter-1 (40), which in turn is tightly controlled by HIF (see below). Nutrient uptake in naïve T cells is strictly instructed through interleukin (IL-)7- and IL-4-dependent pathways (41). During periods of high proliferation, even in the presence of adequate O2 concentration, lymphocytes become progressively more dependent on aerobic glycolysis for ATP synthesis. Lactate production from glycolysis can increase as much as 40 fold in mitogen-stimulated T cells. When glucose becomes limiting, as it often does at sites of high immune activity, T cells can utilize alternative energy sources, such as glutamine, within the tricarboxylic acid cycle (4).
TRANSCRIPTIONAL IMPRINTING BY INFILTRATING LEUKOCYTES DURING INFLAMMATION
Successful inflammatory responses are initiated by recruitment of leukocytes to sites of infection/injury, requiring leukocyte extravasation (diapedesis) out of the vasculature, through interstitial tissue, and into/across epithelial layers. This process of transmigration occurs through a sequence of highly coordinated cell-cell interaction steps, mediated by cell adhesion molecules and integrins. These steps have been extensively summarized elsewhere (42, 43). Given the adhesion-based interactions with bidirectional signaling capacity, it stands to reason that the process of transmigration could significantly impact gene expression of both the migrating cell and the substrate surface cell. Surprisingly few studies have explored the influence of transmigration on gene expression. However, some studies have examined the influence of transmigration on the gene expression profile of the migrating cell. One study examined the influence of transmigration of melanoma cells across endothelia and demonstrated an increase in β-catenin nuclear activity within the melanoma cells postmigration (44). A parallel study examined monocyte transendothelial migration and found that although an increase in nuclear β-catenin was evident within the monocyte, no observable change in Wnt target genes was detectable (45). Such divergent phenotypes may be due to the nature of the migrating cell or to the conditions used to elicit migration. Yet another study examined the influence of transendothelial migration on monocyte transcriptional imprinting and identified a dampening of apoptotic programing and, rather surprisingly, a decrease in antimicrobial peptides (46).
Studies involving the gene expression changes within the tissue or cells across which migration occurs are more scarce, likely due to the technical challenges involved in population purification. One group examined the role of PMN migration on gene expression within IL-1-stimulated human umbilical vein endothelial cells. Here the authors detected relatively few gene transcriptional changes, with hemoglobin beta being the most highly upregulated gene. Ultimately the authors concluded that this increase was due to contamination of their PMN preparation with reticulocytes (47). More recently, two studies examined the influence of PMN transepithelial migration on epithelial gene programming. The first was performed using lung epithelial cells and identified β-catenin signaling through T cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors as a prominent pathway (48). The second study investigated PMN transepithelial migration across colonic epithelial cells (49). The authors of this study attributed gene expression changes within the epithelium to the massive consumption of local O2 by PMN NADPH oxidase. These studies revealed that O2 consumption by activated PMN resulted in the stabilization of HIF within the epithelium, which was shown previously to regulate PMN transmigration (50). Utilizing murine models of colitis, the authors demonstrated that both the presence of PMN and PMN-elicited hypoxia were necessary for mucosal protection during inflammation. Depletion of PMN led to exacerbated tissue destruction during colitis (Figure 2).
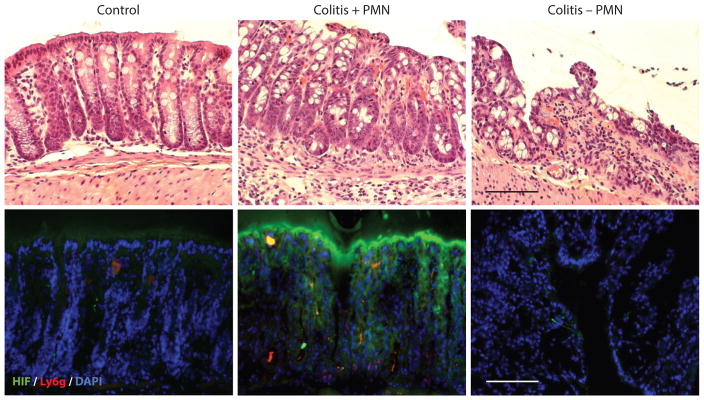
Figure 2.
Role of PMN in HIF stabilization during mucosal inflammation. Tissue from the distal colons of healthy mice and mice with active TNBS colitis, with and without depletion of systemic PMN through the use of anti-GR-1 antibody, reveals an increase in HIF among colitic mice with PMN, which is attenuated in the absence of PMN. Staining for HIF ( green) and Ly6g (red ) indicates accumulation of PMN in TNBS colitis. DAPI stains (blue) signify areas of high DNA concentration. Scale bars = 100 μm. Abbreviations: HIF, hypoxia-inducible factor; PMN, polymorphonuclear leukocytes; TNBS, trinitrobenzene sulfonic.
These studies have also been validated in human patients. Human inflammatory bowel disease (IBD) specimens containing crypt abscesses were examined for the localized expression of the quintessential HIF target gene Glut-1. As shown in Figure 3, areas adjacent to the human crypt abscess revealed marked upregulation of Glut-1 relative to control tissue. Of interest in this regard, patients who lack a functional NADPH oxidase [those with, for example, chronic granulomatous disease (CGD)] often present with an IBD-like syndrome (51). CGD patients exhibit congenital defects in genes coding the subunits that compose the neutrophil NADPH oxidase complex (e.g., mutations in CYBA, CYBB, multiple NCFs, RAC1, and RAC2). This NADPH oxidase complex is responsible for the generation of reactive O2 species and is used by innate immune cells (especially PMN) to kill invading pathogens. Approximately 40% of CGD patients develop IBD-like symptoms (52). Such clinical observations suggest that CGD-associated IBD represents a failure to resolve acute intestinal insults. Given the opposing roles of β-catenin and HIF in cell proliferation, coupled with reports that physical interaction between these factors occurs during hypoxia, sequestering β-catenin from its T cell factor–binding partners and enhancing HIF target gene expression (53), it is intriguing to speculate about whether PMN transmigration elicits simultaneous or temporally distinct signaling cascades through these mediators.
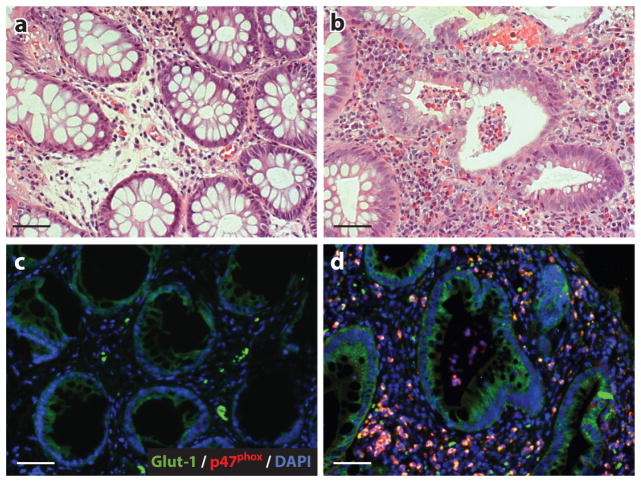
Figure 3.
Ulcerative colitis patients with crypt abscesses reveal hypoxia-dependent target induction. Biopsies from uninflamed margins (panels a and c) and inflamed regions with active crypt abscesses (panels b and d ) in patients with ulcerative colitis were processed for H&E (top) and stained (bottom) for hypoxia-responsive Glut-1 ( green) and neutrophil p47phox (red ). DAPI stains (blue) indicate nuclei. Scale bars = 50 μm. Abbreviation: H&E, hematoxylin and eosin. Figure adapted from Reference 49 with permission.
Localized O2 depletion is not restricted to NADPH oxidase enzymes. Other oxygenase enzymes, such as the HIF target gene heme oxygenase-1, utilize molecular O2, heme, and NADPH to release carbon monoxide, biliverdin, and iron. Carbon monoxide in particular has been shown to have anti-inflammatory properties in macrophages (54). Likewise, nitric oxide (NO) synthase enzymes, including both endothelial and inducible types, utilize molecular O2 to catalyze the conversion of L-arginine to NO and L-citrulline (55). Locally generated NO acts on nearby cells to elicit paracrine signaling. In terms of metabolic regulation, NO can cause an inhibition of prolyl hydroxylase enzymes in normoxia, leading to HIF stabilization (56). Paradoxically, during hypoxia, mitochondrial cytochrome c can bind NO, resulting in O2 being shuttled away from mitochondria, which in turn provides sufficient substrate for PHD activity and HIF degradation (57).
Although PMN have a short circulating half-life, local tissue factors (e.g., GM-CSF) can significantly prolong PMN life span. Given the plethora of cytotoxic granule contents, rapid clearance of PMN from inflamed tissues is crucial to prevent mucosal damage from apoptotic or necrotic cell death. PMN transmigration is one possible fate to accelerate the clearance of PMN from the mucosa. Another, recently appreciated PMN fate is the generation of neutrophil extracellular traps (NETs). NETs are intricate matrices of decondensed chromatin studded with antimicrobial peptides (58) that trap and kill pathogens (59) (Figure 4). This novel PMN fate, distinct from apoptosis or necrosis, has been termed NETosis and may be employed as a last-ditch effort to control the spread of an infection (60). The conditions that dictate the release of NETs are still under investigation, but it is known that the process requires reactive O2 species (61), PMN elastase, and myeloperoxidase (62). Recently, HIF-1α signaling in PMN has been implicated in regulating the release of NETs (63). Although PMN have been implicated in establishing hypoxic microenvironments via NADPH oxidase activity within mucosal tissues (49), the PMN respiratory chain cannot function efficiently to kill bacteria in hypoxic environments (64). It is intriguing to postulate that in the event of extreme or prolonged conditions of hypoxia that preclude bacterial clearance by respiratory burst, resultant HIF stabilization in PMN may induce NETosis to compensate (Figure 4).
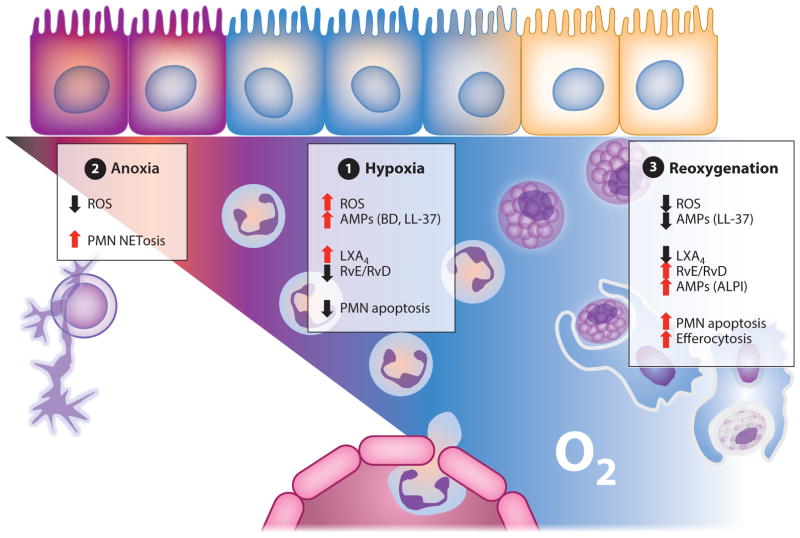
Figure 4.
O2-dependent regulation of AMPs; PMN fate in chronic inflammation and during resolution. (❶) Hypoxia: PMN induce localized hypoxia via NADPH oxidase activity, resulting in bacterial death by ROS generation. Hypoxia prolongs PMN life span, thereby preventing apoptosis. HIF-dependent AMPs are released and aid in antimicrobial activity. HIF-dependent glycolytic and proinflammatory genes (e.g., COX-2) are transcribed. RvE/RvD require the oxygenase activity of COX-2 and are synthesized. Activated PMN convert arachidonic acid to LXA4. (❷) Anoxia: In the absence of infection clearance, PMN accumulate and localized O2 depletion prevents NADPH oxidase activity. Under such conditions, PMN undergo NETosis or necrosis. (❶) Reoxygenation:With pathogenic bacterial clearance, PMN undergo apoptosis. RvE/RvD enhance clearance of apoptotic PMN through efferocytosis and induce intestinal ALPI expression on epithelia to restore mucosal homeostasis. Abbreviations: ALPI, alkaline phosphatase; AMP, antimicrobial peptide; HIF, hypoxia-inducible factor; PMN, polymorphonuclear leukocytes; ROS, reactive oxygen species; RvE/RvD, resolvins.
INFLAMMATORY HYPOXIA AND TRYPTOPHAN METABOLISM
Tryptophan (Trp) is an essential amino acid that is central to a productive inflammatory response. Trp metabolism leads to the production of serotonin, melatonin, and NAD+, as well as a growing number of biologically active catabolic intermediates (65). The first and rate-limiting step in Trp metabolism is catalyzed by the enzyme indoleamine 2,3 dioxygenase-1 (IDO1) resulting in the generation of kynurenine (Kyn) in an O2-dependent manner. Although IDO1 is not the only enzyme capable of generating Kyn through Trp catabolism (others being IDO2 and TDO2), IDO1 is the most widely expressed and the best studied of these enzymes. First identified in the rabbit intestine in the 1960s (66), IDO1 expression has long been known to be induced by proinflammatory signals, most prominently by interferon-gamma, and to have immune-modulating effects. The breakthrough observation that IDO activity plays a central role in promoting immune tolerance and preventing fetal rejection (67) served as a springboard for elucidating the role of this pathway in other disease states. The mechanisms through which IDO1 promotes immune tolerance are now known to be multifactorial and include the depletion of microenvironmental Trp and production of Kyns—which behave as activating ligands of the transcription factor aryl hydrocarbon receptor (AhR), discussed below, and serve to inhibit activated T cells, block TH17 differentiation (68, 69), and promote regulatory T cell differentiation and function (70, 71). Additionally, it is now appreciated that IDO1 possesses a nonenzymatic function, acting as a signal transducer in dendritic cells to contribute to TGF-β-driven tolerance in noninflammatory settings (72).
AhR is a ubiquitously expressed, ligand-activated member of the Per-Arnt-Sim family of basic helix-loop-helix transcription factors. Normally, AhR exists in the cytosol in complex with a number of proteins, including heat shock protein 90 (Hsp-90), AhR-interacting protein, and p23 (73–75). Ligand binding by AhR induces translocation of the AhR complex into the nucleus, where it complexes with AhR nuclear translocator (Arnt), also called Hif-1β . The AhR-Arnt heterodimers bind xenobiotic-responsive elements (XREs) in gene promoters, with members of the cytochrome P450 family being canonical targets (76–78). AhR is best known as the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). In response to TCDD binding, AhR activation leads to a variety of deleterious effects, including liver damage, epithelial changes, cancer, birth defects, and immunosuppression (79). Although AhR signaling has been well established in field toxicology, knockout mouse models suggest the presence of a physiologically important endogenous AhR ligand(s) (80, 81). A functional link between IDO1 and AhR was first suggested by the finding that AhR signaling induces the expression of IDO1 (82). More recently, it was shown that metabolites in the Trp catabolic pathway act as AhR ligands (83, 84). These initial observations led to a number of studies demonstrating the importance of Trp metabolites in promoting immune tolerance in an AhR-dependent manner. Mezrich and colleagues (85) demonstrated that Kyn specifically activated AhR and promoted the differentiation of Treg cells. Additionally, Kyn produced in a TDO-dependent manner was found to be immunosuppressive, and this activity was dependent on AhR (86). These critical observations led to a number of studies examining the role of Kyn-dependent AhR activation in inflammatory disease. Given its ability to modulate immune responses, IDO has been shown to play an important part in a number of disease models (Table 1).
Table 1.
Influence of IDO1 and AhR modulation on model disease endpoints
| Disease model | IDO modulation | Impact on disease outcomes | Reference(s) |
|---|---|---|---|
|
| |||
| Arthritis | Inhibition (1MT) | Exacerbates disease | 162, 163 |
|
| |||
| Asthma | Activation (TLR9 ligand) | Ameliorates disease | 164 |
|
| |||
| EAE | Inhibition (1MT) | Exacerbates disease | 165 |
|
| |||
| Colitis | Inhibition (1MT) | Exacerbates disease | 92 |
| IDO1 knockout | Exacerbates disease | 166 | |
|
| |||
| EAE | Activation (TCDD) | Ameliorates disease | 100 |
| Activation (FICZ) | Exacerbates disease | 100, 167 | |
| Activation (ITE) | Ameliorates disease | 168 | |
|
| |||
| Arthritis | T cell–specific deletion | Ameliorates disease | 98 |
|
| |||
| Psoriasis | Activation (FICZ) | Ameliorates disease | 101 |
| Whole-body deletion | Exacerbates disease | 101 | |
| Cell-specific deletion(s) | Exacerbates disease | 101 | |
|
| |||
| Allergy-induced airway inflammation | Activation (TCDD) | Ameliorates disease | 169 |
| Activation (curcumin) | Ameliorates disease | 170 | |
|
| |||
| COPD | Whole-body deletion | Exacerbates disease | 171 |
|
| |||
| Pancreatitis | Activation (biliverdin) | Ameliorates disease | 172 |
|
| |||
| Colitis | Activation (FICZ) | Ameliorates disease | 103, 173 |
| Epithelial-specific deletion | Exacerbates disease | 174 | |
| T cell–specific deletion | Ameliorates disease | 174 | |
IDO1 is expressed at high levels at mucosal surfaces, likely through interferon-gamma signaling (87). As depicted in Table 1, a number of disease models have demonstrated the importance of this pathway in the modulation of mucosal inflammatory responses (88). Importantly, homeostatic expression in the intestine represents an important source of IDO1. Mouse studies have revealed that basal IDO1 expression is the highest expressed in the lamina propria of the small intestine. Interestingly, basal colonic IDO expression appears to occur largely in the epithelium (89). IDO is highly upregulated in response to inflammation, with expression being markedly induced in both mouse colitis models and human IBD (90, 91). A number of groups have demonstrated the protective nature of the IDO1 pathway in intestinal inflammation. Gurtner and colleagues (92) examined the impact of IDO1 in colonic inflammation using the IDO1 inhibitor 1-methyltryptophan in the TNBS mouse model of IBD. The results demonstrated that IDO1 inhibition significantly exacerbated the severity of inflammation in this model. Others have used a model of intestinal inflammation induced by graft-versus-host disease (GVHD) to demonstrate that IDO1-knockout mice exhibited more severe GVHD-associated colitis (93). The role of IDO1 signaling in the modulating lymphocyte responses was further demonstrated in a murine model of Citrobacter rodentium–induced colitis (89). The authors demonstrated that colitis in this model was improved in IDO1-knockout mice. This phenotype was attributed to increased levels of IgA in the mutant animals, leading to the proposal that B cell responses to the intestinal microbiota are regulated in an IDO1-dependent manner (89). These findings are particularly intriguing in light of recent data elucidating the importance of B cell–mediated IgA responses in shaping gut commensal communities (94–96). The role of IDO1 in the intestinal epithelium in both the basal conditions and particularly during inflammation remains an intriguingly open question. Our lab first described the concept of inflammatory hypoxia a number of years ago (9, 97). In this paradigm, the increased metabolic burden placed on a tissue during ongoing inflammation leads to O2 deficit and the stabilization of HIF-1α. This enhanced HIF-1α activation leads to the induction of gene transcription in a number of targets that have been shown to play a critical role in barrier reformation and tissue healing (25). Given the significant upregulation in expression of IDO1 in the intestinal mucosa during inflammation and the fact that IDO1 utilizes O2, it is enticing to speculate about whether IDO1 activity significantly contributes to the establishment of the local hypoxic environment. In this model, IDO1 is hypothesized both to contribute to the suppression of immune response and, in part through the stabilization of HIF-1α, to activate pathways crucial for the process of mucosal healing.
Analogous to the study of IDO1, AhR research has been conducted extensively in a number of autoimmune disease models, including collagen-induced arthritis (98) and experimental autoimmune encephalomyelitis (99) (see Table 1). The studies have produced conflicting data on the proinflammatory versus anti-inflammatory role of AhR in these models, perhaps due to the differential affinity of AhR for different ligands (100). Due to the presence of a broad range of antigens at mucosal surfaces, the role of AhR signaling in inflammation in barrier tissues has also been investigated in a number of models. For instance, Di Meglio and colleagues (101), using both mouse models and human tissue samples, demonstrated that AhR plays a critical role in controlling inflammation in psoriasis. Additionally, the role of AhR in lung inflammation has been investigated, and AhR has been shown to reduce eosinophilia and suppress TH2 cytokine production in a model of allergic asthma (102). AhR signaling has also been shown to play a critical role in intestinal inflammation. Monteleone et al. (103) demonstrated that AhR activation, through the administration of the AhR ligand FICZ, ameliorated disease in both acute TNBS and relapsing DSS models of colitis. The authors attributed this protective benefit in part to the AhR-dependent upregulation of IL-22. Finally, it was recently demonstrated that AhR activation plays a protective role in intestinal inflammation induced by opportunistic fungal infections (104). Again, the authors showed that these effects were due to the AhR-dependent upregulation of IL-22. Importantly, these studies also demonstrate that commensal bacterial species, specifically Lactobacillus reuteri, produce Trp metabolites that can act as AhR ligands. Administration of one of these compounds, indole-3-aldehyde, ameliorated disease in a DSS model of colitis (104). These results indicate that the IDO-AhR signaling axis may represent a key modulator of metabolic crosstalk between the microbiome and the host and in the integration of these signals in mucosal immune responses.
CREATINE METABOLISM AND MUCOSAL INFLAMMATION
Given the strong association between HIF and mucosal inflammation (2), Glover et al. (105) recently examined the differential contributions of HIF-1α and HIF-2α to transcriptional changes in intestinal epithelia. For these purposes, they performed chromatin immunoprecipitation (ChIP) with HIF-1α and HIF-2α antibodies, followed by hybridization to a promoter microarray. The log2 ratio (input-Cy3/HIF-ChIP-Cy5) was analyzed to identify sequences specific to HIF-1α and HIF-2α binding in hypoxia. Highly enriched subsets in the HIF-1α ChIP hits included multiple enzymes of the glycolytic pathway, autophagic targets, and jumonji domain containing histone demethylases. In addition, this analysis revealed prominent changes associated with immunity, transcription, and metabolism. Notably, promoter sequences for the cytosolic creatine kinase (CK) genes CKB (brain) and CKM (muscle) emerged as high-fidelity HIF-2α-selective targets. Likewise, two mitochondrial isoforms of CK (MTA1 and MT2), as well as the major creatine transporter (SLC6A8), were significantly enriched in HIF-2α ChIP. Morphological analysis revealed that CK isoforms prominently localize to tight junctional and adherens junctional regions of confluent intestinal epithelia (Figure 5).
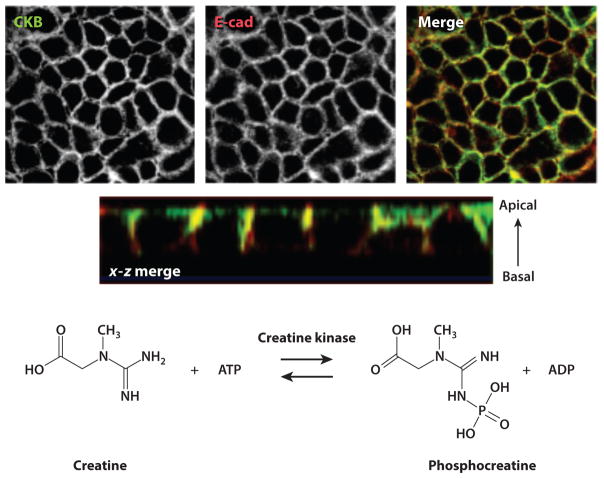
Figure 5.
Creatine kinase expression at epithelial junctions. The images in the upper panels, obtained through confocal microscopy, show CKB (top left), the adherens junction marker E-cad (top middle), and two views of a colocalized (merged) stain (top right and second row). At the bottom is the enzymatic reaction catalyzed by creatine kinase. Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; CKB, creatine kinase, brain isoform; E-cad, E-cadherin. Figure adapted from Reference 105 with permission.
Surprisingly little is known about CK function in the mucosa. The CK pathway is often neglected in energy metabolism, as it is assumed that high-energy phosphate transport between sites of ATP production (mitochondria) and ATP consumption (ATPases) relies solely on the diffusion of ATP and adenosine diphosphate (ADP). Although this may hold true in tissues devoid of CK and phosphocreatine (PCr), it is clearly inadequate for CK-containing tissues with high and fluctuating energy demands (e.g., skeletal and cardiac muscle, brain, retina, and spermatozoa) (106). In these latter tissues, four distinct types of CK subunits are expressed in a developmental stage–, species-, and tissue-specific manner (106). Our studies have revealed that each of these CK subunits is expressed in cultured intestinal epithelial cell lines as well as murine colonic epithelial cells in vivo and human colonic epithelia ex vivo (105). All CK isoenzymes catalyze the reversible transfer of the gamma-phosphate group of ATP to the guanidino group of Cr to yield ADP and PCr (Figure 3). In high CK–expressing tissues, a large pool of PCr is available for rapid regeneration of ATP hydrolyzed during high energy expenditure. During periods of high energy demand, the CK reaction remains in a near-equilibrium state, keeping the concentrations of ATP and ADP constant. In this regard, CK functions to buffer the cytosolic phosphorylation potential that appears to be critical for the proper functioning of a variety of cellular ATPases (106).
Energy expenditure within the mucosa (and the need for an ATP buffering system coordinated by CK) is particularly high during the restitution of epithelial cells following insult (e.g., active inflammation). The dynamic regulation of epithelial junctions is tightly linked to the circumferential F-actin belt (107). Such F-actin contractility is highly dependent on the adequate availability of high-energy phosphates and on a ready supply of ATP. The dynamic nature of this F-actin-ATP interaction has been elegantly demonstrated through epithelial models of ATP depletion (107), mutagenesis of myosin light chain kinase (108), and pharmacological targeting of actin polymerization (e.g., phalloidin) (109). G-actin monomers assemble into filaments with distinct ends (107). Each monomer contains one nucleotide-binding cleft where ATP is converted to ADP and inorganic phosphate (Pi) via two processes: ATP cleavage, which produces ADP Pi-actin, and Pi release, which leads to ADP-actin. ATP cleavage is slow for actin monomers but is strongly increased after the monomer has been incorporated into a filament and resultant actin protomer. Notably, this process requires a large amount of cellular energy. It is estimated, for example, that platelets expend as much as 50% of cellular ATP (110) in actin-myosin-related regulation of the cytoskeleton. From this perspective, it is not surprising that Cr-PCr may well be central to energy homeostasis and tissue barrier function during episodes of mucosal inflammation.
HOST-MICROBIAL METABOLISM AND TISSUE HYPOXIA
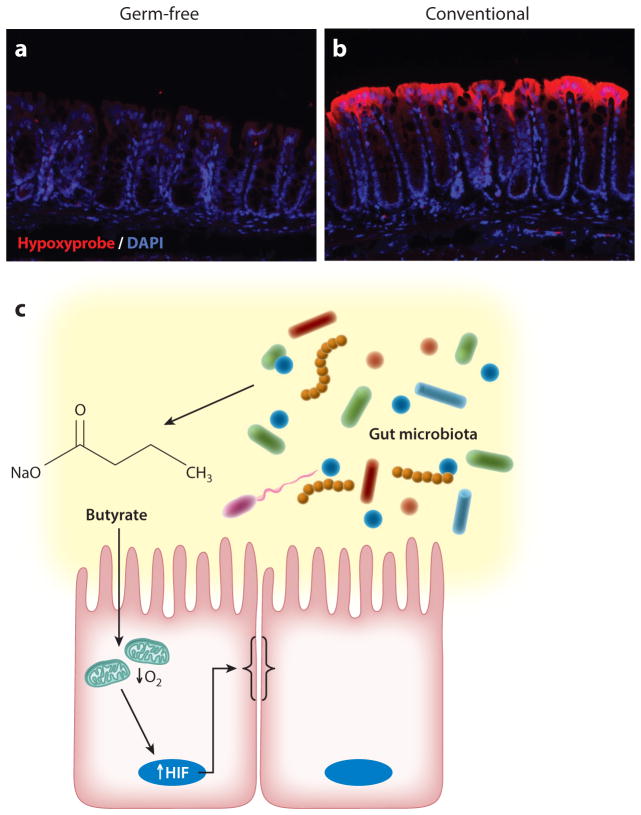
The mammalian gastrointestinal tract is home to trillions of bacteria. A finely regulated commensal relationship exists within the intestinal mucosa, where microbes, essential for host health, can also initiate and perpetuate mucosal disease (111). These microbes, in addition to aiding in digestion, produce a number of vitamins and benefit the host through the local synthesis of short-chain fatty acids (SCFAs), including butyrate, propionate, and acetate. Butyrate, for example, can reach luminal concentrations of 30 mM in the colon and serves as a preferred metabolic substrate for colonic epithelial cells (112). In contrast to other SCFAs, butyrate is efficiently absorbed and metabolized by the epithelium. Very little butyrate is released into portal circulation (112). One factor contributing to the preference of the colonic epithelium for butyrate is that butyrate stimulates expression of pyruvate dehydrogenase kinases, which inhibit the pyruvate dehydrogenase complex (113). This inhibition prevents conversion of glucose-derived pyruvate to acetyl-CoA. Yet, because formation of acetyl-CoA from butyrate is not dependent on pyruvate dehydrogenase, butyrate-derived acetyl-CoA is available for oxidative phosphorylation. Kelly et al. (114) recently demonstrated that SCFAs (especially butyrate) increase epithelial O2 consumption and deplete local O2 to the extent that HIF is stabilized. These same studies revealed that in vivo depletion of microbiota through antibiotics reduced colonic butyrate and HIF expression, both of which were restored by butyrate supplementation. Analysis of germ-free mice revealed nearly no retention of O2-sensitive dyes and significantly decreased HIF stabilization at baseline (Figure 6). Moreover, the level of barrier protection afforded by butyrate was lost in cells lacking HIF, a fact that links butyrate metabolism to stabilized HIF and overall barrier function. Given the multiple levels of protection afforded by HIF within the mucosa (115), these findings implicate the butyrate-HIF axis as a novel host-microbe crosstalk pathway wherein SCFAs signal protective distal gut functions (Figure 6).
Figure 6.
Microbial-derived signals deplete local O2. The top panels depict hypoxia localization (red ), illuminated by the nitroimidazole dye pimonidazole, in (a) germ-free and (b) conventional animals. DAPI stains (blue) indicate areas of high DNA concentration. (c) A model in which microbial-derived short-chain fatty acids (e.g., butyrate) stimulate epithelial metabolism and deplete intracellular O2 to the extent that HIF-1α is stabilized and promotes epithelial barrier function. Abbreviation: HIF, hypoxia-inducible factor.
Significant literature supports a homeostatic role for SCFAs in the distal gut during inflammation (112, 116). For example, the protection elicited by fiber and resistant starch in experimental colitis is thought to be dependent on SCFA production (117–119), and administration of exogenous butyrate promotes resistance to experimental colitis (120, 121). Recent studies investigating dysbiosis in inflammatory bowel disease identified lower concentrations of luminal butyrate and reduced abundance of butyrate-producing organisms (e.g., certain Roseburia and Faecalibacterium genera) with disease (122–124). The importance of butyrate as the preferred epithelial substrate has been highlighted by the demonstration that pharmacological inhibition of β-oxidation induces colitis (125) and mice with mitochondrial polymorphisms that maintain increased oxidative phosphorylation activity are resistant to colitis (126). Several trials have evaluated the efficacy of butyrate in the treatment of human disease, primarily ulcerative colitis, with mixed results (112).
The intestinal microbiota shift in fundamental ways during inflammation. It remains unclear exactly what these shifts in the microbiota might mean to tissue and immune function (127). Microbial signals, such as those delivered by a mix of Clostridial species, induce mucosal tolerance by promoting the formation of regulatory T cells (128). Moreover, studies have implicated SCFAs as critical products of tolerogenic Clostridial species (129). In addition to functioning as a direct energy source, SCFAs can signal through a series of G protein–coupled receptors (GPR) to mediate their biological functions (130, 131). In mice, deletion of Gpr41 and Gpr43 mediates protective immunity in inflammatory models (130, 131). Also notable is the observation that treatment of mice with propionate promotes colonic protection during inflammation (131) and that the major butyrate receptor (GPR109a) functions to suppress colonic carcinogenesis and inflammation (132).
THERAPEUTIC IMPLICATIONS OF INFLAMMATORY HYPOXIA
The molecular mechanisms of HIF stabilization have been significantly clarified by the discovery of critical posttranslational modifications to the α-subunit of HIF. Three HIF prolyl hydroxylases have been shown to be central to the hypoxic stabilization of HIF (133). Prolyl hydroxylases were discovered in a search of the C. elegans genome database for sequences that might encode members of the 2-oxoglutarate-dependent oxygenase family based on the structural existence of a β-barrel motif that represents the catalytic site of these oxygenase enzymes (134). This search led to the discovery of the egl-9 gene. egl-9 mutant worms constitutively expressed the C. elegans ortholog to mammalian HIF-1α, a finding suggesting that egl-9 plays an important role in the regulation of the hypoxia response. Use of the EGL-9 sequence resulted in the identification of three ubiquitously expressed EGL-9 orthologs in mammals, designated PHD (prolyl hydroxylase domain–containing protein) 1, 2, and 3, each of which hydroxylates human HIF-1α at Pro-564. The proline residues that are hydroxylated in both worm and mammalian HIF-1α proteins are expressed within the amino acid motif LXXLAP. Each of the PHD enzymes is encoded by different genes, and their gene product enzymes demonstrate tissue-specific expression patterns (133). All three PHDs are found in the mucosa (27, 30, 135). Significantly different phenotypes exist in mice genetically lacking individual isoforms of the hydroxylases. Studies in PHD1−/− mice, for example, have revealed decreased exercise performance, abnormal basal metabolism (136, 137), and increased intestinal barrier function due to decreased epithelial apoptosis (138). By contrast, homozygous PHD2-knockout is embryonically lethal due to abnormal developmental angiogenesis (139, 140), and PHD2-heterozygous knockout animals show enhanced tumor angiogenesis (139). PHD3-homozygous knockout mice show reduced neuronal apoptosis, abnormal sympathoadrenal development, and reduced blood pressure (141). These diverse phenotypes strongly suggest distinct, isoform-specific functions in vivo.
The identification of HIF-selective PHDs as controlling HIF expression has provided the basis for the exploration of PHD-based molecular tools and therapies (142, 143). Pharmacological inactivation of PHDs by 2-oxoglutarate analogues is sufficient to stabilize HIF-1α (142) but is nonspecific for individual PHD isoforms. In vitro studies suggest some significant differences in PHD isoform substrate specificity. For instance, PHD3 does not hydroxylate proline 564 on HIF-α, and comparison of enzyme activity in vitro has shown that the O2-dependent degradation sequence is hydroxylated most efficiently by PHD2 (144, 145). These observations have generated significant interest in identifying enzyme-modifying therapeutics. A number of PHD inhibitors have been described, including antagonists of alpha-keto-glutarate (142), analogs of naturally occurring cyclic hydroxamates (146), and direct inhibitors of prolyl hydroxylases (147). For these purposes, prolyl hydroxylase inhibitors that stabilize HIF-1α and induce the expression of downstream HIF target genes have been used in a variety of settings. In the mucosa, PHD inhibition has been shown to provide an overall beneficial influence on clinical symptoms, most likely through actions on barrier function (27, 30).
HIF-stabilizing agents have been shown to promote erythropoietin (Epo) production accompanied by significant increases in red blood cell production. Several studies indicate that Epo production is preferentially regulated by HIF-2α and not HIF-1α. In mice, for example, extrarenal Epo production in the liver has been shown to require HIF-2α but not HIF-1α (148). Among humans, a family expressing a mutation in the HIF2A gene has been reported to exhibit stabilization of the HIF2A protein and erythrocytosis (149). The most mature work toward development of an HIF stabilizer/PHD inhibitor for therapeutic purposes is in the area of renal failure and erythropoiesis. The HIF-stabilizing agent FG-4592 is currently in phase 2 and 3 clinical trials for the treatment of renal anemia in patients with end-stage kidney disease. There are likely a number of indications where uncontrolled stimulation of erythropoiesis (e.g., with HIF-2α stabilizer) is unwarranted. Some recent work has identified PHD inhibitors with relative selectivity for HIF-1α versus HIF-2α. AKB-4924, a relatively HIF-1α-selective PHD inhibitor, has been explored in studies of mucosal infection and inflammation (150, 151). The basis for HIF-1α over HIF-2α selectivity is not known at the present time. AKB-4924 has been shown to enhance the antibacterial function of phagocytes and skin keratinocytes against a variety of mucosal pathogens and holds promise for enhancing overall innate immune response to microbial threats (150, 151). Use of AKB-4924 in models of murine colitis has augmented epithelial barrier function and led to an approximately 50-fold reduction in serum endotoxin during colitis. AKB-4924 also decreased cytokines involved in pyrogenesis and hypothermia, significantly reducing serum levels of proinflammatory cytokines while increasing anti-inflammatory IL-10. AKB-4924 offered no protection against colitis in epithelial-specific-HIF-1α-deficient mice, strongly implicating epithelial-specific HIF-1α as the tissue target for AKB-4924-mediated protection in colitis. Such findings may provide the basis for a therapeutic use of PHD inhibitors in inflammatory mucosal disease.
Recent studies have also shown that pathways other than PHD inhibition may be viable approaches to target HIF stabilization. An example is inhibition of the HIF E3 ubiquitin ligase. The E3 SCF ubiquitin ligase specific to HIF-α-family members is composed of Elongin B/C, RBX, CUL2, and the F-box domain of VHL and is responsible for the polyubiquitination and degradation of HIF (152). Tight regulation of the E3 SCF is attained by the covalent modification of the ubiquitin-like protein NEDD8. A functional E3 SCF complex requires the COP9 signalosome to bind Nedd8 to Cul2 and can be deneddylated by the Nedd8-specific dual protease Den1 (also called SenP8). A small-molecule inhibitor targeting Nedd8 conjugation to the cullins has become commercially available. This compound, MLN-4924, functions to inhibit Nedd8-activating enzyme and causes the deneddylation of Cul1 and Cul2 (153, 154). MLN-4924 is an adenosine monophosphate analog (153, 154), and, given previous observations that adenosine deneddylates cullin proteins (155), MLN-4924 could prove to be a useful investigative tool. Recent studies have shown, for example, that MLN-4924 is a potent HIF stabilizer in vitro and in vivo (156, 157).
HIF inhibitors have also been identified. Based largely on chemical compound screens, original studies by Kong et al. (158) identified echinomycin (NSC-13502) as a potent HIF-1α inhibitor. Accordingly, echinomycin has been profiled among a number of direct and indirect HIF-1α target drugs that demonstrate promise for cancer therapeutics (159). Cardiac glycosides, for example, have been shown to potently inhibit HIF (160). A screen of the Hopkins Drug Library of FDA-approved drugs revealed twenty drugs that inhibit HIF-1α. Eleven of the most potent inhibitors were cardiac glycosides (e.g., digoxin, proscillaridin A, and ouabain) and were revealed to act as inhibitors of HIF-1α protein synthesis. Other HIF inhibitors include Hsp90 inhibitors, HDAC inhibitors, topoisomerase inhibitors, and PI3K/mTOR inhibitors and have recently been detailed elsewhere (161).
CONCLUSIONS
Differences in baseline O2 tension in mucosal tissues and the profound increase in energy demand within inflammatory lesions provide a unique opportunity to understand tissue metabolism in health and disease. Studies in model disease systems have provided new insights toward a better understanding of inflammatory responses and the mechanisms that promote resolution. Of particular relevance is the shift in tissue oxygenation toward hypoxia, specifically as it relates to HIF target pathways that are strongly associated with tissue barrier function and metabolic pathways that contribute fundamentally to inflammatory resolution. Profound interest in the development of HIF-stabilizing agents (especially PHD inhibitors) has provided keen insight and shows promise for clinical development. Ongoing studies to better define localized metabolomic signatures hold promise in elucidating new targets for therapeutic development.
Acknowledgments
This work was supported by NIH grants DK50189, DK104713, DK103639, DK099452, UL1RR025780, and DK095491; VA Merit Award 1I01BX002182; and the Crohn’s and Colitis Foundation of America.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol. 2010;184:4062–68. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–87. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- 4.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–52. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 5.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1α and adenosine receptors. Nat Rev Immunol. 2005;5:712–21. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd AP. Metabolic control of intestinal oxygenation and blood flow. Fed Proc. 1982;41:2084–89. [PubMed] [Google Scholar]
- 7.Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;18:1055–63. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaible B, Schaffer K, Taylor CT. Hypoxia, innate immunity and infection in the lung. Respir Physiol Neurobiol. 2010;174:235–43. doi: 10.1016/j.resp.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Investig. 2004;114:1098–106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans SM, Hahn S, Pook DR, Jenkins WT, Chalian AA, et al. Detection of hypoxia in human squamous cell carcinoma by EF5 binding. Cancer Res. 2000;60:2018–24. [PubMed] [Google Scholar]
- 11.Kelly CJ, Glover LE, Campbell EL, Kominsky DJ, Ehrentraut SF, et al. Fundamental role for HIF-1α in constitutive expression of humanβ defensin-1. Mucosal Immunol. 2013;6:1110–18. doi: 10.1038/mi.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takasawa M, Moustafa RR, Baron JC. Applications of nitroimidazole in vivo hypoxia imaging in ischemic stroke. Stroke. 2008;39:1629–37. doi: 10.1161/STROKEAHA.107.485938. [DOI] [PubMed] [Google Scholar]
- 13.Kizaka-Kondoh S, Konse-Nagasawa H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Sci. 2009;100:1366–73. doi: 10.1111/j.1349-7006.2009.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guise CP, Mowday AM, Ashoorzadeh A, Yuan R, Lin WH, et al. Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia. Chin J Cancer. 2014;33:80–86. doi: 10.5732/cjc.012.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–34. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–94. [PubMed] [Google Scholar]
- 17.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Investig. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Ex Med. 2003;198:783–96. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol 2011. 2011:22. doi: 10.1101/sqb.2011.76.010678. [DOI] [PubMed] [Google Scholar]
- 20.Semenza GL. HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 21.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–75. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 22.Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lando D, Peet DJ, Whelan DA, Gorman JJ, Murray LW. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science. 2002;295:858–61. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 24.Karhausen J, Stafford-Smith M. The role of nonocclusive sources of acute gut injury in cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28:379–91. doi: 10.1053/j.jvca.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Colgan SP, Curtis VF, Lanis JM, Glover LE. Metabolic regulation of intestinal epithelial barrier during inflammation. Tissue Barriers. 2015;3(1–2):e970936. doi: 10.4161/21688362.2014.970936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pannabecker TL, Layton AT. Targeted delivery of solutes and oxygen in the renal medulla: role of microvessel architecture. Am J Physiol Ren Physiol. 2014;307:F649–55. doi: 10.1152/ajprenal.00276.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–65. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Han IO, Kim HS, Kim HC, Joe EH, Kim WK. Synergistic expression of inducible nitric oxide synthase by phorbol ester and interferon-γ is mediated through NF-κB and ERK in microglial cells. J Neurosci Res. 2003;73:659–69. doi: 10.1002/jnr.10706. [DOI] [PubMed] [Google Scholar]
- 29.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136:607–18. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 30.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–55. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah YM, Ito S, Morimura K, Chen C, Yim SH, et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036–48. e3. doi: 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, et al. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56:209–13. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariani F, Sena P, Marzona L, Riccio M, Fano R, et al. Cyclooxygenase-2 and Hypoxia-Inducible Factor-1alpha protein expression is related to inflammation, and up-regulated since the early steps of colorectal carcinogenesis. Cancer Lett. 2009;279:221–29. doi: 10.1016/j.canlet.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Matthijsen RA, Derikx JP, Kuipers D, van Dam RM, Dejong CH, Buurman WA. Enterocyte shedding and epithelial lining repair following ischemia of the human small intestine attenuate inflammation. PLOS ONE. 2009;4:e7045. doi: 10.1371/journal.pone.0007045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem. 2006;99:1616–27. doi: 10.1002/jcb.20947. [DOI] [PubMed] [Google Scholar]
- 36.Furuta GT. Clinicopathologic features of esophagitis in children. Gastrointest Endosc Clin N Am. 2001;11:683–715. vii. [PubMed] [Google Scholar]
- 37.van Raam BJ, Sluiter W, de Wit E, Roos D, Verhoeven AJ, Kuijpers TW. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PLOS ONE. 2008;3:e2013. doi: 10.1371/journal.pone.0002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 39.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Investig. 1982;70:550–57. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greiner EF, Guppy M, Brand K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J Biol Chem. 1994;269:31484–90. [PubMed] [Google Scholar]
- 41.Plas DR, Rathmell JC, Thompson CB. Homeostatic control of lymphocyte survival: potential origins and implications. Nat Immunol. 2002;3:515–21. doi: 10.1038/ni0602-515. [DOI] [PubMed] [Google Scholar]
- 42.Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu Rev Pathol Mech Dis. 2007;2:111–43. doi: 10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- 43.Voisin MB, Nourshargh S. Neutrophil transmigration: emergence of an adhesive cascade within venular walls. J Innate Immun. 2013;5:336–47. doi: 10.1159/000346659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi J, Chen N, Wang J, Siu CH. Transendothelial migration of melanoma cells involves N-cadherin-mediated adhesion and activation of the beta-catenin signaling pathway. Mol Biol Cell. 2005;16:4386–97. doi: 10.1091/mbc.E05-03-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tickenbrock L, Schwable J, Strey A, Sargin B, Hehn S, et al. Wnt signaling regulates transendothelial migration of monocytes. J Leukoc Biol. 2006;79:1306–13. doi: 10.1189/jlb.0905539. [DOI] [PubMed] [Google Scholar]
- 46.Williams MR, Sakurai Y, Zughaier SM, Eskin SG, McIntire LV. Transmigration across activated endothelium induces transcriptional changes, inhibits apoptosis, and decreases antimicrobial protein expression in human monocytes. J Leukoc Biol. 2009;86:1331–43. doi: 10.1189/jlb.0209062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams MR, Kataoka N, Sakurai Y, Powers CM, Eskin SG, McIntire LV. Gene expression of endothelial cells due to interleukin-1 beta stimulation and neutrophil transmigration. Endothelium. 2008;15:73–165. doi: 10.1080/10623320802092443. [DOI] [PubMed] [Google Scholar]
- 48.Zemans RL, Briones N, Campbell M, McClendon J, Young SK, et al. Neutrophil transmigration triggers repair of the lung epithelium via β-catenin signaling. PNAS. 2011;108:15990–95. doi: 10.1073/pnas.1110144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colgan SP, Dzus AL, Parkos CA. Epithelial exposure to hypoxia modulates neutrophil transepithelial migration. J Exp Med. 1996;184:1003–15. doi: 10.1084/jem.184.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang JS, Noack D, Rae J, Ellis BA, Newbury R, et al. Chronic granulomatous disease caused by a deficiency in p47phox mimicking Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:690–95. doi: 10.1016/s1542-3565(04)00292-7. [DOI] [PubMed] [Google Scholar]
- 52.Werlin SL, Chusid MJ, Caya J, Oechler HW. Colitis in chronic granulomatous disease. Gastroenterology. 1982;82:328–31. [PubMed] [Google Scholar]
- 53.Kaidi A, Williams AC, Paraskeva C. Interaction between β-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–17. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 54.Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1–dependent pathway. J Exp Med. 2005;202:1703–13. doi: 10.1084/jem.20051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–20. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 56.Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14:3470–81. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α. Science. 2003;302:1975–78. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 58.Jann NJ, Schmaler M, Kristian SA, Radek KA, Gallo RL, et al. Neutrophil antimicrobial defense against Staphylococcus aureus is mediated by phagolysosomal but not extracellular trap-associated cathelicidin. J Leukoc Biol. 2009;86:1159–69. doi: 10.1189/jlb.0209053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–35. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 60.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–88. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–91. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McInturff AM, Cody MJ, Elliott EA, Glenn JW, Rowley JW, et al. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1α. Blood. 2012;120:3118–25. doi: 10.1182/blood-2012-01-405993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGovern NN, Cowburn AS, Porter L, Walmsley SR, Summers C, et al. Hypoxia selectively inhibits respiratory burst activity and killing of Staphylococcus aureus in human neutrophils. J Immunol. 2011;186:453–63. doi: 10.4049/jimmunol.1002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–20. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto S, Hayaishi O. Tryptophan pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme or enzymes. J Biol Chem. 1967;242:5260–66. [PubMed] [Google Scholar]
- 67.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–93. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 68.Keller TL, Zocco D, Sundrud MS, Hendrick M, Edenius M, et al. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat Chem Biol. 2012;8:311–17. doi: 10.1038/nchembio.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–38. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–61. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 71.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Investig. 2007;117:2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–78. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 73.Kazlauskas A, Poellinger L, Pongratz I. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (aryl hydrocarbon) receptor. J Biol Chem. 1999;274:13519–24. doi: 10.1074/jbc.274.19.13519. [DOI] [PubMed] [Google Scholar]
- 74.Ma Q, Whitlock JP., Jr A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1997;272:8878–84. [PubMed] [Google Scholar]
- 75.Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988;263:13802–5. [PubMed] [Google Scholar]
- 76.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. PNAS. 1992;89:8185–89. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schrenk D. Impact of dioxin-type induction of drug-metabolizing enzymes on the metabolism of endo- and xenobiotics. Biochem Pharmacol. 1998;55:1155–62. doi: 10.1016/s0006-2952(97)00591-1. [DOI] [PubMed] [Google Scholar]
- 78.Tomita S, Jiang HB, Ueno T, Takagi S, Tohi K, et al. T cell-specific disruption of arylhydrocarbon receptor nuclear translocator (Arnt) gene causes resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced thymic involution. J Immunol. 2003;171:4113–20. doi: 10.4049/jimmunol.171.8.4113. [DOI] [PubMed] [Google Scholar]
- 79.Mandal PK. Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J Comp Physiol B. 2005;175:221–30. doi: 10.1007/s00360-005-0483-3. [DOI] [PubMed] [Google Scholar]
- 80.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–6. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. PNAS. 1996;93:6731–36. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375:331–35. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rieber N, Belohradsky BH. AHR activation by tryptophan—pathogenic hallmark of Th17-mediated inflammation in eosinophilic fasciitis, eosinophilia-myalgia-syndrome and toxic oil syndrome? Immunol Lett. 2010;128:154–55. doi: 10.1016/j.imlet.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 85.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–98. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 87.Colgan SP, Parkos CA, Matthews JB, D’Andrea L, Awtrey CS, et al. Interferon-γ induces a surface phenotype switch in intestinal epithelia: downregulation of ion transport and upregulation of immune accessory ligands. Am J Physiol Cell Physiol. 1994;267:C402–C10. doi: 10.1152/ajpcell.1994.267.2.C402. [DOI] [PubMed] [Google Scholar]
- 88.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–43. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harrington L, Srikanth CV, Antony R, Rhee SJ, Mellor AL, et al. Deficiency of indoleamine 2,3-dioxygenase enhances commensal-induced antibody responses and protects against Citrobacter rodentium-induced colitis. Infect Immun. 2008;76:3045–53. doi: 10.1128/IAI.00193-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hansen JJ, Holt L, Sartor RB. Gene expression patterns in experimental colitis in IL-10-deficient mice. Inflamm Bowel Dis. 2009;15:890–99. doi: 10.1002/ibd.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolf AM, Wolf D, Rumpold H, Moschen AR, Kaser A, et al. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol. 2004;113:47–55. doi: 10.1016/j.clim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 92.Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF. Inhibition of in-doleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–73. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 93.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Mellor AL, Munn DH, Blazar BR. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood. 2009;114:5062–70. doi: 10.1182/blood-2009-06-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, et al. Foxp3+ T cells regulate immunoglobulin A selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity. 2014;41:152–65. doi: 10.1016/j.immuni.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 95.Moon C, Baldridge MT, Wallace MA, Burnham CA, Virgin HW, Stappenbeck TS. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 2015;521:90–93. doi: 10.1038/nature14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–10. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karhausen J, Haase VH, Colgan SP. Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle. 2005;4:256–58. [PubMed] [Google Scholar]
- 98.Nakahama T, Kimura A, Nguyen NT, Chinen I, Hanieh H, et al. Aryl hydrocarbon receptor deficiency in T cells suppresses the development of collagen-induced arthritis. PNAS. 2011;108:14222–27. doi: 10.1073/pnas.1111786108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–32. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 100.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 101.Di Meglio P, Duarte JH, Ahlfors H, Owens ND, Li Y, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989–1001. doi: 10.1016/j.immuni.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jeong KT, Hwang SJ, Oh GS, Park JH. FICZ, a tryptophan photoproduct, suppresses pulmonary eosinophilia and Th2-type cytokine production in a mouse model of ovalbumin-induced allergic asthma. Int Immunopharmacol. 2012;13:377–85. doi: 10.1016/j.intimp.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 103.Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–48. e1. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 104.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–85. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 105.Glover LE, Bowers BE, Saeedi B, Ehrentraut SF, Campbell EL, et al. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. PNAS. 2013;110:19820–25. doi: 10.1073/pnas.1302840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 107.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–24. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 109.Song JC, Hrnjez BJ, Farokhzad OC, Matthews JB. PKC-epsilon regulates basolateral endocytosis in human T84 intestinal epithelia: role of F-actin and MARCKS. Am J Physiol Cell Physiol. 1999;277:C1239–49. doi: 10.1152/ajpcell.1999.277.6.C1239. [DOI] [PubMed] [Google Scholar]
- 110.Daniel JL, Molish IR, Robkin L, Holmsen H. Nucleotide exchange between cytosolic ATP and F-actin-bound ADP may be a major energy-utilizing process in unstimulated platelets. Eur J Biochem. 1986;156:677–84. doi: 10.1111/j.1432-1033.1986.tb09631.x. [DOI] [PubMed] [Google Scholar]
- 111.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–19. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 113.Blouin JM, Penot G, Collinet M, Nacfer M, Forest C, et al. Butyrate elicits a metabolic switch in human colon cancer cells by targeting the pyruvate dehydrogenase complex. Int J Cancer. 2011;128:2591–601. doi: 10.1002/ijc.25599. [DOI] [PubMed] [Google Scholar]
- 114.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–71. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–87. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ploger S, Stumpff F, Penner GB, Schulzke JD, Gabel G, et al. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- 117.Ito H, Tanabe H, Kawagishi H, Tadashi W, Yasuhiko T, et al. Short-chain inulin-like fructans reduce endotoxin and bacterial translocations and attenuate development of TNBS-induced colitis in rats. Dig Dis Sci. 2009;54:2100–8. doi: 10.1007/s10620-008-0599-x. [DOI] [PubMed] [Google Scholar]
- 118.Morita T, Tanabe H, Sugiyama K, Kasaoka S, Kiriyama S. Dietary resistant starch alters the characteristics of colonic mucosa and exerts a protective effect on trinitrobenzene sulfonic acid-induced colitis in rats. Biosci Biotechnol Biochem. 2004;68:2155–64. doi: 10.1271/bbb.68.2155. [DOI] [PubMed] [Google Scholar]
- 119.Videla S, Vilaseca J, Antolin M, Garcia-Lafuente A, Guarner F, et al. Dietary inulin improves distal colitis induced by dextran sodium sulfate in the rat. Am J Gastroenterol. 2001;96:1486–93. doi: 10.1111/j.1572-0241.2001.03802.x. [DOI] [PubMed] [Google Scholar]
- 120.Cresci G, Nagy LE, Ganapathy V. Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. JPEN J Parenter Enteral Nutr. 2013;37:763–74. doi: 10.1177/0148607113486809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leonel AJ, Teixeira LG, Oliveira RP, Santiago AF, Batista NV, et al. Antioxidative and immunomodulatory effects of tributyrin supplementation on experimental colitis. Br J Nutr. 2013;109:1396–407. doi: 10.1017/S000711451200342X. [DOI] [PubMed] [Google Scholar]
- 122.Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275–83. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 123.Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut. 2013;62:1745–52. doi: 10.1136/gutjnl-2012-303611. [DOI] [PubMed] [Google Scholar]
- 124.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–89. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 125.Roediger WE, Nance S. Metabolic induction of experimental ulcerative colitis by inhibition of fatty acid oxidation. Br J Exp Pathol. 1986;67:773–82. [PMC free article] [PubMed] [Google Scholar]
- 126.Bar F, Bochmann W, Widok A, von Medem K, Pagel R, et al. Mitochondrial gene polymorphisms that protect mice from colitis. Gastroenterology. 2013;145:1055–63. e3. doi: 10.1053/j.gastro.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 127.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–36. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 129.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 131.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–86. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–39. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 134.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 135.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, et al. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. PNAS. 2006;103:18154–59. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–80. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- 137.Schneider M, Van Geyte K, Fraisl P, Kiss J, Aragones J, et al. Loss or silencing of the PHD1 prolyl hydroxylase protects livers of mice against ischemia/reperfusion injury. Gastroenterology. 2009;38:1143–54. e1–2. doi: 10.1053/j.gastro.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 138.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 139.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–51. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ozolins TR, Fisher TS, Nadeau DM, Stock JL, Klein AS, et al. Defects in embryonic development of EGLN1/PHD2 knockdown transgenic mice are associated with induction of Igfbp in the placenta. Biochem Biophys Res Commun. 2009;390:372–76. doi: 10.1016/j.bbrc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 141.Bishop T, Gallagher D, Pascual A, Lygate CA, de Bono JP, et al. Abnormal sympathoadrenal development and systemic hypotension in PHD3−/− mice. Mol Cell Biol. 2008;28:3386–400. doi: 10.1128/MCB.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mole DR, Schlemminger I, McNeill LA, Hewitson KS, Pugh CW, et al. 2-oxoglutarate analogue inhibitors of HIF prolyl hydroxylase. Bioorg Med Chem Lett. 2003;13:2677–80. doi: 10.1016/s0960-894x(03)00539-0. [DOI] [PubMed] [Google Scholar]
- 143.Masson N, Ratcliffe PJ. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O(2) levels. J Cell Sci. 2003;116:3041–49. doi: 10.1242/jcs.00655. [DOI] [PubMed] [Google Scholar]
- 144.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–54. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 145.Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–23. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 146.Schlemminger I, Mole DR, McNeill LA, Dhanda A, Hewitson KS, et al. Analogues of dealanylalahopcin are inhibitors of human HIF prolyl hydroxylases. Bioorg Med Chem Lett. 2003;13:1451–54. doi: 10.1016/s0960-894x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 147.Nwogu JI, Geenen D, Bean M, Brenner MC, Huang X, Buttrick PM. Inhibition of collagen synthesis with prolyl 4-hydroxylase inhibitor improves left ventricular function and alters the pattern of left ventricular dilatation after myocardial infarction. Circulation. 2001;104:2216–21. doi: 10.1161/hc4301.097193. [DOI] [PubMed] [Google Scholar]
- 148.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Investig. 2007;117:1068–77. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358:162–68. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Keely S, Campbell EL, Baird AW, Hansbro PM, Shalwitz RA, et al. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 2014;7:114–23. doi: 10.1038/mi.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Okumura CY, Hollands A, Tran DN, Olson J, Dahesh S, et al. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J Mol Med. 2012;28:1079–89. doi: 10.1007/s00109-012-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mikus P, Zundel W. COPing with hypoxia. Semin Cell Dev Biol. 2005;16:462–73. doi: 10.1016/j.semcdb.2005.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Boh BK, Smith PG, Hagen T. Neddylation-induced conformational control regulates cullin RING ligase activity in vivo. J Mol Biol. 2011;409:136–45. doi: 10.1016/j.jmb.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 154.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–36. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 155.Khoury J, Ibla JC, Neish AS, Colgan SP. Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J Clin Investig. 2007;117:703–11. doi: 10.1172/JCI30049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Curtis VF, Ehrentraut SF, Campbell EL, Glover LE, Bayless AJ, et al. Stabilization of HIF through inhibition of Cullin-2 neddylation is protective in mucosal inflammatory responses. FASEB J. 2015;29:208–15. doi: 10.1096/fj.14-259663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ehrentraut SF, Kominsky DJ, Glover LE, Campbell EL, Kelly CJ, et al. Central role for endothelial human deneddylase-1/SENP8 in fine-tuning the vascular inflammatory response. J Immunol. 2013;190:392–400. doi: 10.4049/jimmunol.1202041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–55. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- 159.Wang R, Zhou S, Li S. Cancer therapeutic agents targeting hypoxia-inducible factor-1. Curr Med Chem. 2011;18:3168–89. doi: 10.2174/092986711796391606. [DOI] [PubMed] [Google Scholar]
- 160.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. PNAS. 2008;105:19579–86. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xia Y, Choi HK, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem. 2012;49:24–40. doi: 10.1016/j.ejmech.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 162.Jaen O, Rulle S, Bessis N, Zago A, Boissier MC, Falgarone G. Dendritic cells modulated by innate immunity improve collagen-induced arthritis and induce regulatory T cells in vivo. Immunology. 2009;126:35–44. doi: 10.1111/j.1365-2567.2008.02875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Szanto S, Koreny T, Mikecz K, Glant TT, Szekanecz Z, Varga J. Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice. Arthritis Res Ther. 2007;9:R50. doi: 10.1186/ar2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, et al. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J Clin Investig. 2004;114:270–79. doi: 10.1172/JCI21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 2005;19:1347–49. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 166.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Taylor PA, Mellor AL, et al. Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood. 2008;111:3257–65. doi: 10.1182/blood-2007-06-096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–9. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 168.Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. PNAS. 2010;107:20768–73. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Luebke RW, Copeland CB, Daniels M, Lambert AL, Gilmour MI. Suppression of allergic immune responses to house dust mite (HDM) in rats exposed to 2,3,7,8-TCDD. Toxicol Sci. 2001;62:71–79. doi: 10.1093/toxsci/62.1.71. [DOI] [PubMed] [Google Scholar]
- 170.Moon DO, Kim MO, Lee HJ, Choi YH, Park YM, et al. Curcumin attenuates ovalbumin-induced airway inflammation by regulating nitric oxide. Biochem Biophys Res Commun. 2008;375:275–79. doi: 10.1016/j.bbrc.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 171.Rico de Souza A, Zago M, Pollock SJ, Sime PJ, Phipps RP, Baglole CJ. Genetic ablation of the aryl hydrocarbon receptor causes cigarette smoke-induced mitochondrial dysfunction and apoptosis. J Biol Chem. 2011;286:43214–28. doi: 10.1074/jbc.M111.258764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xue J, Nguyen DT, Habtezion A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology. 2012;143:1670–80. doi: 10.1053/j.gastro.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ji T, Xu C, Sun L, Yu M, Peng K, et al. Aryl hydrocarbon receptor activation down-regulates IL-7 and reduces inflammation in a mouse model of DSS-induced colitis. Dig Dis Sci. 2015;60:1958–66. doi: 10.1007/s10620-015-3632-x. [DOI] [PubMed] [Google Scholar]
- 174.Chinen I, Nakahama T, Kimura A, Nguyen NT, Takemori H, et al. The aryl hydrocarbon receptor/microRNA-212/132 axis in T cells regulates IL-10 production to maintain intestinal homeostasis. Int Immunol. 2015;27:405–15. doi: 10.1093/intimm/dxv015. [DOI] [PubMed] [Google Scholar]