Abstract
Advanced age is associated with a higher incidence of stroke and worse functional outcomes. Vagus nerve stimulation (VNS) paired with rehabilitative training has emerged as a potential method to improve recovery after brain injury but to date has only been evaluated in young rats. Here, we evaluated whether VNS paired with rehabilitative training would improve recovery of forelimb function after ischemic lesion of the motor cortex in rats 18 months of age. Rats were trained to perform the isometric pull task, an automated, quantitative measure of volitional forelimb strength. Once proficient, rats received an ischemic lesion of the motor cortex and underwent rehabilitative training paired with VNS for 6 weeks. VNS paired with rehabilitative training significantly enhances recovery of forelimb function after lesion. Rehabilitative training without VNS results in a 34% ± 19% recovery, whereas VNS paired with rehabilitative training yields a 98% ± 8% recovery of prelesion of forelimb function. VNS does not significantly reduce lesion size. These findings demonstrate that VNS paired with rehabilitative training enhances motor recovery in aged subjects in a model of stroke and may suggest that VNS therapy may effectively translate to elderly stroke patients.
Keywords: Ischemic stroke, Recovery, Vagus nerve stimulation, Motor function, Rehabilitation, Aging, Elderly
1. Introduction
Stroke is a leading cause of disability, with approximately 795,000 cases each year in the United States (Go et al., 2014). Age is the leading nonmodifiable risk factor; thus, the elderly population is disproportionately affected by stroke. In addition to increasing risk, advanced age negatively impacts functional recovery following stroke in animal models and patients (Alaverdashvili and Whishaw, 2010; Knoflach et al., 2012; Merrett et al., 2010). Despite physical rehabilitation, many elderly stroke patients are left with a significant degree of upper limb disability (Kelly-Hayes et al., 2003). The development of rehabilitative interventions to promote poststroke recovery, particularly in the elderly population, remains a significant clinical need.
Stimulation of the vagus nerve during rehabilitative training has emerged as a promising potential method to enhance recovery of forelimb function after brain injury. Vagus nerve stimulation (VNS) is believed to increase recovery by supporting neuroplasticity to enhance the benefits of rehabilitative training (Hays et al., 2013; Porter et al., 2011). VNS paired with rehabilitative training improves recovery of forelimb movement speed and volitional strength compared to rehabilitative training without VNS in models of cortical ischemic stroke (Hays et al., 2014b; Khodaparast et al., 2013, 2014) and even when initiated several weeks after lesion in a model of cortical and subcortical ischemic stroke (Khodaparast et al., 2015). Extending these findings, VNS therapy improves recovery in models of severe striatal intracerebral hemorrhage and traumatic brain injury (Hays et al., 2014a; Pruitt et al., 2015). Together, these findings indicate that VNS enhances recovery of forelimb function in a variety of mechanistically distinct models of acute and chronic brain injury. However, these proof-of-concept studies have all been performed in young rats, which limits the interpretation of these results in the context of the most likely target population.
As advanced age is associated with a reduction in neuroplasticity and poststroke recovery, it may occlude VNS-dependent enhancement of recovery. Following the Stroke Treatment Academic Industry Roundtable (STAIR) guidelines, we sought to evaluate whether VNS paired with rehabilitative training would enhance recovery after ischemic lesion of the motor cortex in rats aged at least 18 months at the time of stroke (Fisher et al., 2009). We find that VNS paired with rehabilitative training significantly enhances forelimb recovery compared with rehabilitative training alone. These findings provide initial evidence that VNS delivered during rehabilitation may represent a novel therapeutic intervention to increase motor recovery in elderly individuals after stroke.
2. Materials and methods
2.1. Subjects
Thirty-six female Fisher 344 rats, aged approximately 18 months at the time of motor cortex lesion, obtained from the NIA Charles River colony were used in this experiment. The rats were housed in a 12:12 hours reversed light cycle environment so that behavioral testing took place during the dark cycle to increase daytime activity levels. Rats were food deprived to no less than 85% of their normal body weight during training as motivation for the food pellet rewards. All handling, housing, and surgical procedures were approved by the University of Texas at Dallas Institutional Animal Care and Use Committee.
2.2. Isometric pull task training
The isometric pull task was performed similar to previous descriptions (Hays et al., 2012). The behavioral chamber consisted of an acrylic box (MotoTrak Rat System, Vulintus, Dallas, TX, USA) with a slot through which the rats could access an aluminum pull handle with only the right forelimb. The handle was centered in the slot at a height of 6.4 cm from the cage floor and 1.9 cm outside relative to the inner wall surface of the cage. The handle was affixed to a force transducer with a maximal load of 2 kg. Custom software was used to control the task and collect data. Forces readings were sampled at 100 Hz. A motor controller board relayed information to a custom MATLAB software which analyzed, displayed, and stored the data. Force values and corresponding time stamps were collected as continuous traces for each trial to allow for the analysis of force profiles over the course of a session. If a trial was successful, the software triggered an automated pellet dispenser to deliver a sucrose pellet (45 mg dustless precision pellet, BioServ, Frenchtown, NJ, USA) to a receptacle located in the front corner of the cage.
Training sessions lasted 30 minutes and were conducted twice daily, 5 days a week, with sessions on the same day separated by at least 2 hours. A trial was initiated when the rat generated a force of at least 10 grams on the handle. If pull force exceeded the 100-g threshold within 2 seconds of trial initiation, the trial was recorded as a success and a reward pellet was delivered. If the force did not exceed threshold within 2 seconds, the trial was recorded as a failure and no reward was given. Rats were held at the prelesion stage until they had 10 successive sessions averaging over 80% success rate. The prelesion data reported in this study is compiled from these 10 sessions. After this point, rats were given an ischemic lesion followed by 7 days of recovery, after which they returned for postlesion testing. All rats were tested until they had at least 4 sessions with greater than 10 trials each during the postlesion assessment, and a reliable baseline could be established. Based on postlesion hit rate, rats were assigned to balanced groups to receive rehabilitative training with or without paired VNS for 6 weeks.
2.3. Unilateral motor cortex ischemic lesion
Unilateral ischemic lesions of primary motor cortex were performed similar to a previously described method (Fang et al., 2010; Hays et al., 2012, 2014b; Khodaparast et al., 2013, 2014). Rats were anesthetized with ketamine hydrochloride (80 mg/kg, intraperitoneal) and xylazine (10 mg/kg, intraperitoneal) and given supplemental doses as needed. After placing the rat in a stereotaxic frame with a digital readout (David Kopf Instruments, Tujunga, CA, USA), a craniotomy was performed to expose motor cortex contralateral to the trained forelimb. Endothelin-1 (ET-1, Bachem, Torrance, CA, USA, 1 mg/mL in saline) was injected through a 26-gauge Hamilton syringe at 8 sites targeting forelimb area: anteroposterior 2.5 mm, 1.5 mm, 0.5 mm, and −0.5 mm and mediolateral 2.5 mm and 3.5 mm from bregma. The syringe was lowered to a depth of 1.6 mm from the cortical surface and 1.0 μL of ET-1 solution was applied at each injection location. The ET-1 solution was injected over a 2-minute period, and the syringe remained in the brain for an additional 3 minutes to allow perfusion. After the final injection, KwikCast silicone polymer (World Precision Instruments, Sarasota, FL, USA) was placed in the craniotomy and sealed with a thin layer of acrylic.
2.4. Vagus nerve cuff implantation and stimulation parameters
Following ischemic lesion, rats were implanted with a skull-mounted connector and a bipolar stimulating nerve cuff constructed with platinum-iridium leads (5–6 kΩ impedance). Implantations were performed as previously described (Hays et al., 2014b; Khodaparast et al., 2013, 2014). Four bone screws were manually drilled into the skull at points near the lambdoid suture and over the cerebellum. The 2-channel connector was attached to the cranial screws with acrylic. An incision and blunt dissection of the muscles in the neck exposed the left cervical vagus nerve. After isolation from the carotid artery, the vagus nerve was placed inside the cuff and the cuff was closed with sutures. Leads were tunneled subcutaneously and attached to the 2-channel connector atop the skull. All incisions were sutured, and the exposed 2-channel connector was encapsulated in acrylic. VNS was delivered identical to previous studies (Engineer et al., 2011; Hays et al., 2014a, 2014b; Khodaparast et al., 2013, 2014; Porter et al., 2011; Pruitt et al., 2015). Stimulation consisted of a 500 ms train of pulses at 30 Hz. Each biphasic pulse was 0.8 mA in amplitude and 100 μs in phase duration.
2.5. Treatment group assignment and exclusion criteria
Rats were assigned to balanced treatment groups based on postlesion hit rate. The Rehab group underwent rehabilitative training for 6 weeks, which consisted of freely performing the task during the training sessions (Fig. 1). The VNS+Rehab group underwent identical rehabilitative training but received stimulation of the vagus nerve on trials which exceeded the 100-g force threshold. No VNS was delivered on the sixth week, to allow assessment of persistent effects of VNS pairing. The 5-week course of VNS pairing is similar to previous studies (Hays et al., 2014a, 2014b; Khodaparast et al., 2013, 2014, 2015; Pruitt et al., 2015).
Fig. 1.
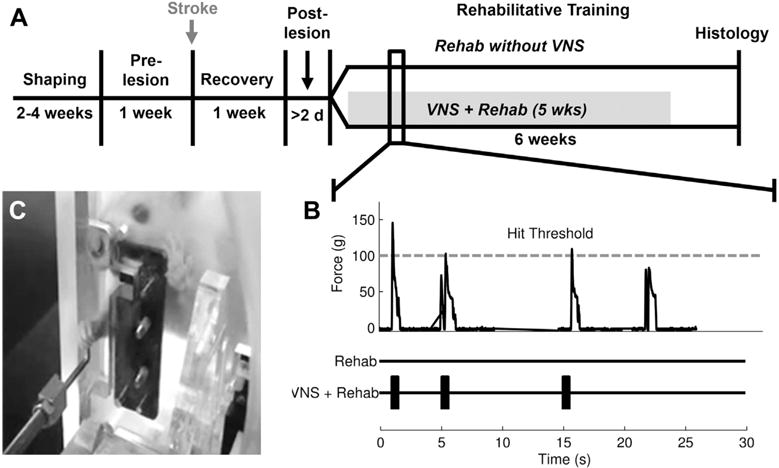
Experimental design. (A) Illustration of the experimental timeline. (B) Example isometric force task data from a behavioral session. The VNS+Rehab group received a brief burst of VNS paired with trials that exceeded the 100-g hit threshold. (C) A rat performing the isometric force task. Abbreviation: VNS, vagus nerve stimulation.
Nineteen rats were excluded from the study based on the following criteria: (1) Did not reach a stable prelesion baseline (n=2); (2) Did not survive surgery (n = 6); (3) Did not display a reduction in hit rate of at least 20% compared to prelesion during postlesion assessment (n = 6); and (4) Were too impaired to perform the task following lesion (n = 5). All exclusions took place before group assignment and therefore could not bias interpretation of the effects of rehabilitative training or VNS.
2.6. Histological processing
Rats were transcardially perfused with 250 mL of 0.02% heparin/0.1 M phosphate buffer (PB) solution, followed by 450 mL of 4% paraformaldehyde/0.1 M PB solution. Brains were removed and postfixed in 4% paraformaldehyde/0.1 M PB solution, and then cryoprotected in a 30% sucrose/0.1 M PB solution. Coronal sections 100-μm thick were cryosectioned through the extent of the lesion. Sections were mounted serially and dehydrated in increasing concentrations of ethanol and xylene. Lesions and surrounding brain regions were visualized in dark field using a Nikon Eclipse Ni microscope. Contours of the lesion and remaining cortex and corpus callosum in every other section, spaced 200 μm apart, were traced using Neurolucida software program (MicroBrightfield Bioscience Williston, VT, USA). Lesion volume for each subject was estimated using Cavalieri analysis in MBF Stereo Investigator program. Ratios of the hemispheric cortex and corpus callosum were calculated by dividing volume of the ipsilesional by volume of contralesional tissue. Histology could not be performed on 5 subjects.
2.7. Statistics
All data are reported as the mean ± standard error of mean. Significant differences were determined using 1-way analysis of variance (ANOVA), 2-way ANOVA, Pearson correlation, and t tests where appropriate. Statistical tests for each comparison are noted in the text. Paired t tests were used to compare repeated measures over time within groups. Unpaired t tests were used for post hoc comparison across groups. Alpha level was set at 0.05 for all comparisons. Error bars indicate standard error of mean in all figures. In figures, asterisks indicate p < 0.05 between groups. Filled markers at each time point represent within-group significant differences compared to postlesion performance.
3. Results
3.1. Aged rats become highly proficient on the isometric pull task
Before lesion, subjects were highly proficient at the task (Fig. 2A, PRE; hit rate, Rehab: 92.2 ± 1.2%; VNS+Rehab: 87.6 ± 1.4%, n = 9, 8). A slight, but significant, difference in hit rate was observed between groups (Rehab vs. VNS+Rehab; unpaired t test, p = 0.020). Peak pull force significantly exceeded the 100-g threshold and was similar between groups (Fig. 2B, PRE; peak force, Rehab: 131.2 ± 3.4 g; VNS+Rehab: 127.8 ± 3.6 g; unpaired t test, p = 0.485). The distribution of peak pull forces on trials before lesion demonstrates the proportion of trials exceeding the hit threshold and was similar between groups (Fig. 3, PRE).
Fig. 2.
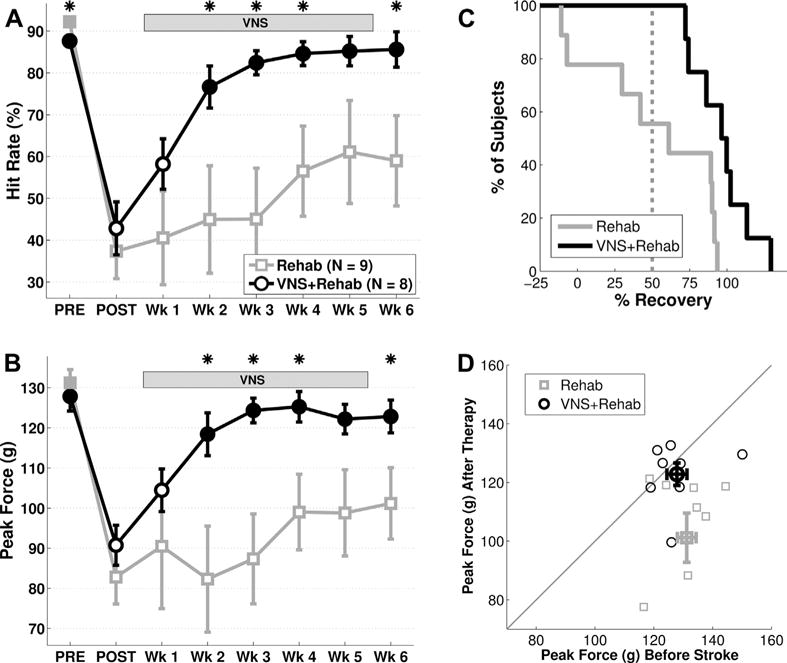
VNS paired with rehabilitative training improves forelimb function after stroke in aged rats. (A) VNS+Rehab improves recovery of hit rate performance on the isometric pull task compared to Rehab without VNS. (B) VNS+Rehab similarly enhances recovery of forelimb strength compared to the Rehab group. (C) All subjects that receive VNS+Rehab demonstrate a >50% recovery of hit rate at the end of therapy, while only a subset of subjects in the control groups demonstrate >50% recovery. (D) Peak force of individual subjects before lesion and on week 6 of therapy. Thin symbols represent individual subjects, and thick symbols represent the group mean. Note that subjects in the VNS+Rehab group tend to cluster near the unity line, consistent with a restoration of forelimb strength after therapy. *Denotes p < 0.05 between Rehab and paired VNS at each time point. Filled markers in (A) and (B) indicate p < 0.05 compared to postlesion performance (POST) for each group. Error bars indicate mean ± SEM. Abbreviations: SEM, standard error of mean; VNS, vagus nerve stimulation.
Fig. 3.
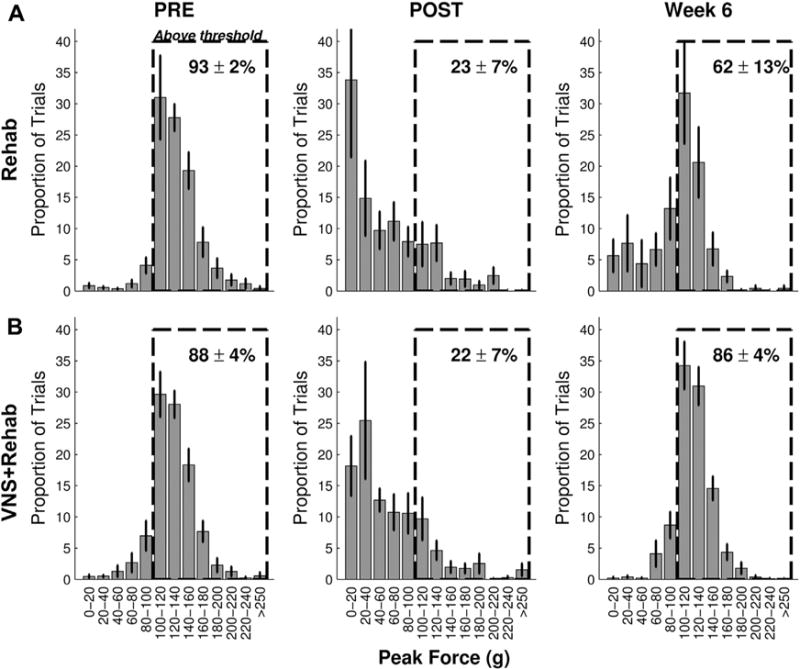
Distribution of pull forces. Probability distribution histograms of pull forces before lesion (PRE, left column), after lesion (POST, middle column), and on the sixth week of therapy (week 6, right column) for the Rehab (A) and VNS+Rehab groups (B). The numbers in the dashed box indicates the percent of trials that exceeded the 100-g hit threshold. Note the similarity of the distributions of pull forces at PRE and week 6 in the VNS+Rehab group consistent with a restoration of forelimb strength. Abbreviation: VNS, vagus nerve stimulation.
3.2. Ischemic lesion of motor cortex significantly impairs forelimb performance
Once proficient on the task, rats received an ischemic lesion of motor cortex to impair use of the trained forelimb. As expected, ischemic lesions substantially impaired forelimb performance. One week after lesion, hit rate was significantly reduced in both groups compared to prelesion performance (Fig. 2A, POST, Rehab: 37.4% ± 6.6%; paired t test vs. PRE, p = 2.67 × 10−5; VNS+Rehab: 42.8% ± 6.3%; p = 6.12 × 10−5). No difference in hit rate was observed between groups (Rehab vs. VNS+Rehab, unpaired t test, p = 0.535). Peak pull force was also reduced after lesion (Fig. 2B, POST, Rehab: 82.8 ± 6.7 g; paired t test vs. PRE, p = 1.36 × 10−4; VNS+Rehab: 90.8±5.0 g; p = 1.79 × 10−5). No difference was observed between groups (Rehab vs. VNS+Rehab, unpaired t test, p = 0.33), indicating that both groups displayed comparable forelimb weakness. The distribution of peak pull forces illustrated a notable leftward shift compared to prelesion (Fig. 3, POST). This shift was similar between groups and is consistent with a reduction in forelimb strength.
Rehabilitative training is a common poststroke intervention and can promote benefits in the elderly (Denti et al., 2008). We evaluated whether 6 weeks of rehabilitative training would improve forelimb function in aged rats. Rehabilitative training without VNS did not result in a significant improvement of function. ANOVA on hit rate during the therapy period failed to reveal a significant effect of time (Fig. 2A; 1-way ANOVA, F[5,53] = 0.62, p = 0.687). Examining individual performance at the completion of therapy, only 5 of 9 subjects demonstrated a >50% recovery (Fig. 1C). Similar results were observed for volitional forelimb strength. ANOVA on peak force also failed to reveal a significant effect of time (Fig. 2B; 1-way ANOVA, F[5,53] = 0.47, p = 0.794) but post hoc comparison with postlesion performance failed to reach significance (POST vs. wks 1–6; Rehab, paired t test, all p > 0.05). A notable reduction in volitional strength compared to prelesion levels was observed in most subjects even after extensive rehabilitative training (Fig. 2D). Distribution of peak forces on the sixth week of rehabilitative training demonstrated a rightward shift compared to postlesion, suggesting a partial recovery (Fig. 3; week 6). These findings suggest that rehabilitative training yields little improvement in forelimb strength after stroke in aged rats.
3.3. VNS paired with rehabilitation significantly enhances recovery of forelimb function
VNS paired with rehabilitative training enhances recovery of forelimb function in multiple models of brain injury in young rats (Hays et al., 2014a, 2014b; Khodaparast et al., 2013, 2014, 2015; Pruitt et al., 2015); therefore, we sought to examine whether VNS-dependent benefits would extend to aged subjects. Similar to previous studies, the present design evaluated VNS delivery to enhance the benefits of rehabilitative training (Hays et al., 2014a, 2014b; Khodaparast et al., 2013, 2014, 2015; Pruitt et al., 2015). Thus, VNS was paired with rehabilitative training sessions and began at least 1 week after lesion (Fig. 1). VNS paired with rehabilitative training significantly improved recovery of forelimb function after stroke in aged rats. Subjects in the VNS group received an average of 5438 ± 516 stimulations over the 5 weeks of therapy. ANOVA on hit rate revealed a significant effect of time (Fig. 2A; 1-way ANOVA, F[5,47] = 7.14, p = 6.54 × 10−5). Significant improvements compared to postlesion performance were observed beginning on week 2 of therapy (POST vs. wks 2–6; Rehab, paired t test, all p > 0.05). All subjects that received VNS paired with rehabilitative training exhibited >50% recovery of function, a significantly greater proportion than subjects that receive rehabilitative training alone (Fig. 2C; Rehab vs. VNS+Rehab; χ2 = 5.63, p = 0.018). ANOVA on peak force also revealed a significant effect of time (Fig. 2B; 1-way ANOVA, F[5,47] = 3.74, p = 6.89 × 10−3). Peak force was significantly improved compared to postlesion levels beginning on week 2 of therapy (POST vs. wks 2–6; Rehab, paired t test, all p > 0.05). Both hit rate and peak force remained recovered on week 6 after the cessation of VNS (wk 5 vs. wk 6, paired t test; hit rate: p = 0.94; peak force: p = 0.90), potentially indicating a lasting effect of therapy. Most subjects that received VNS paired with rehabilitative training exhibit peak force comparable to prelesion levels at the end of therapy (Fig. 2D). The distribution of peak forces on week 6 demonstrated a marked rightward shift compared to postlesion (Fig. 3). Moreover, the distribution on the sixth week of therapy was highly similar to that before lesion, consistent with a restoration of volitional forelimb strength. These findings suggest that VNS paired with rehabilitative training significantly enhances recovery of forelimb function after ischemic lesion of motor cortex in aged rats.
To determine whether VNS paired with rehabilitative training yields greater recovery of forelimb function compared to rehabilitative training alone, we compared forelimb performance between groups during the therapy period. VNS paired with rehabilitative training resulted in significantly improved performance compared to rehabilitative training without VNS (Fig. 2A; 2-way ANOVA, F [1,101] = 31.15, p = 2.25 × 10−7). Improved performance was observed in the VNS+Rehab group compared to the Rehab group on most weeks during the therapy period (Rehab vs. VNS+Rehab at wks 1–6; unpaired t test; weeks 2–4, 6, p < 0.05; weeks 1, 5 p > 0.05). VNS paired with rehabilitative training also significantly increased peak force (Fig. 2B; 2-way ANOVA, F[1,101] = 28.02, p= 7.71 × 10−7). Peak pull force was significantly greater on most weeks during the therapy period in the VNS+Rehab group (Rehab vs. VNS+Rehab at wks 1–6; unpaired t test; weeks 2–4, 6, p < 0.05; weeks 1, 5 p > 0.05). These findings indicate that VNS paired with rehabilitative training yields significantly greater recovery of forelimb function compared to rehabilitative training without VNS after stroke in aged rats.
3.4. Lesion size is not significantly influenced by VNS
Previous studies indicate that higher intensity VNS delivered within an hour after ischemic lesion can promote neuroprotection and reduce lesion volume (Ay et al., 2009; Hiraki et al., 2012). We sought to examine whether the stimulation paradigm used in this study, consisting of short bursts of VNS paired with rehabilitative training beginning at least 9 days after motor cortex lesion, would reduce lesion size. No significant difference in average lesion volume was observed between groups (Fig. 4A–C; Rehab: 1.49 ± 0.12 mm3, VNS+Rehab: 1.01 ± 0.21 mm3, n = 5, 7; unpaired t test, p = 0.10). Lesion volume was not correlated with recovery (Pearson linear correlation; r = −0.37, p = 0.231). In addition, no differences were observed in hemispheric ratio of cortex or corpus callosum area (ratio of ipsilesional/contralesional cortical area, Rehab: 0.85 ± 0.04, VNS+Rehab: 0.90 ± 0.05, p = 0.4188; ratio of ipsilesional/contralesional corpus callosum area, Rehab: 1.010 ± 0.02, VNS+Rehab: 1.02 ± 0.04, p = 0.766). These findings corroborate previous reports (Hays et al., 2014a; Khodaparast et al., 2013, 2014, 2015; Pruitt et al., 2015) and indicate that VNS delivered using the paradigm in this study does not offer gross neuroprotection.
Fig. 4.
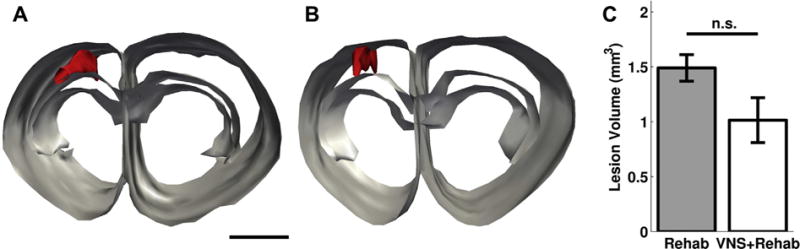
VNS does not affect lesion size. Lesion reconstructions from representative subjects from the Rehab group (A) and the VNS+Rehab group (B). Red represents lesion area, and gray lines outline corpus callosum and cortex. Scale bar is 1 mm. (C) No significant difference in lesion volume was observed between groups. Abbreviations: n.s., not significant; VNS, vagus nerve stimulation. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In this study, we evaluated whether VNS therapy would improve recovery of forelimb function after stroke in aged rats. We find that VNS paired with rehabilitative training significantly increases forelimb motor recovery compared to equivalent rehabilitative training without VNS. VNS-dependent enhancement of recovery persists for at least 1 week after the cessation of stimulation, potentially suggesting a lasting improvement. No significant differences were observed between groups in lesion size. The findings from this study further support VNS paired with rehabilitative training as a poststroke intervention and indicate that VNS therapy may be effective in elderly stroke patients.
Similar to proof-of-concept studies in young rats (Hays et al., 2014a, 2014b; Khodaparast et al., 2013, 2014, 2015; Pruitt et al., 2015), we find that VNS paired with rehabilitative training improves forelimb recovery compared to rehabilitative training alone in aged rats. The magnitude of recovery in aged rats that receive VNS paired with rehabilitative training (98% ± 8% recovery) is comparable to that observed in young rats that receive the same treatment in a previous study (see Supplementary Data; 96% ± 3% recovery; aged vs. young, unpaired t test, p = 0.892; Khodaparast et al., 2013). This likely suggests that, in this model of advanced age in rats, VNS paired with rehabilitative training remains efficacious. It should be noted that while these 18–20 month old animals were significantly older than rats used in most stroke studies, we cannot rule out that the benefits of VNS therapy would be reduced in very old animals. Intensive rehabilitative training without VNS in aged rats resulted in modest improvements in forelimb recovery (34% ± 19%) that is comparable to that observed for an equivalent intervention in young rats (see Supplementary Data; 40% ± 17%; aged vs. young, unpaired t test, p = 0.817). These findings are consistent with studies evaluating the benefits of rehabilitative interventions in the elderly which suggest that age alone is not a determinant in the benefits of rehabilitative therapies (Bagg et al., 2002). The present study does not include a group that receives VNS without rehabilitative training, thus precluding direct evaluation of the effects of VNS alone to enhance recovery. However, several previous studies have indicated that VNS must be paired with rehabilitative training to yield significant benefits (Hays et al., 2014b; Khodaparast et al., 2014, 2015), suggesting that VNS likely acts through a timing-dependent mechanism to improve recovery. Together, these findings suggest that VNS effectively enhances recovery of forelimb function in a model of stroke in aged rats.
VNS is believed to improve recovery after brain injury by enhancing neuroplasticity to support the benefits of rehabilitative training (Hays et al., 2013). Most fibers of the cervical vagus nerve are afferent projections that terminate in the central nervous system (Foley and DuBois, 1937). VNS drives neural activity in proplasticity neuromodulatory centers in the brain, including the noradrenergic locus coeruleus and cholinergic basal forebrain (Detari et al., 1983; Dorr and Debonnel, 2006; Groves et al., 2005). Stimulation of the vagus nerve increases levels of these neuromodulators, as well as brain derived neurotrophic factor, providing a clear link to plasticity and recovery after injury (Conner et al., 2005; Follesa et al., 2007; Furmaga et al., 2012; Roosevelt et al., 2006). A reduction in either noradrenergic or cholinergic function prevents the effects of VNS on the central nervous system, including VNS-dependent enhancement of cortical plasticity (Hulsey et al., 2016; Krahl et al., 1998; Nichols et al., 2011). Temporal dissociation of VNS delivery and rehabilitative training significantly decreases VNS-dependent recovery after stroke, providing further indication that enhanced-plasticity underlies recovery (Khodaparast et al., 2014, 2015). Together, these lines of evidence provide potential pathways by which VNS can promote recovery after stroke. However, additional studies are needed to directly define the molecular and neuronal mechanisms that underlie VNS-dependent enhancement of recovery and examine the efficacy of VNS therapy under conditions that limit or perturb plasticity.
Most evidence suggest that neural plasticity is attenuated in aged animal models and humans (Burke and Barnes, 2006; Freitas et al., 2011; Müller-Dahlhaus et al., 2008; Sawaki et al., 2003; Tennant et al., 2012). This age-related reduction in plasticity could prevent the therapeutic benefits of VNS. However, the significant VNS-dependent enhancement of recovery observed in this study largely excludes this possibility. Based on these findings, we predict that VNS paired with rehabilitative training is able to drive plasticity through partially diminished mechanisms or through alternative mechanisms that do not display age-related reduction. Future studies should investigate the neuronal and molecular changes that support VNS-dependent recovery in the context of healthy advanced age and stroke.
A recent pilot clinical trial provides initial evidence that VNS paired with rehabilitative training holds potential as a poststroke intervention. In this open-label study, patients who received VNS paired with rehabilitation demonstrated 3-fold greater improvement in Upper Extremity Fugl-Meyer score compared to patients who received rehabilitation without VNS (Dawson et al., 2015), providing an initial demonstration that VNS may improve post-stroke recovery. The present preclinical study provides additional proof-of-concept support and suggests that VNS therapy may provide benefits for elderly patients as it translated to the broader clinical population of stroke patients. In addition, as suggested in previous studies evaluating rehabilitation in the elderly, advanced age itself should likely not be a selection criterion for VNS therapy (Bagg et al., 2002; Nakayama et al., 1994).
In this study, we report that VNS paired with rehabilitative training enhances recovery of forelimb function compared to rehabilitative training without VNS after stroke in aged rats. Recent reports in patients have demonstrated that targeted plasticity therapies using VNS paired with rehabilitative regimens hold promise to treat neurological disorders associated with age, including stroke and tinnitus (Dawson et al., 2015; De Ridder et al., 2013; De Ridder et al., 2015). The findings from this study provide additional support for VNS and indicate that the therapy may effectively translate to the elderly population. Although these results are promising, it is important to test VNS therapy in the context of unhealthy aging, and other age-related complicating factors that may interfere with recovery, such as hypertension, diabetes, and neurodegenerative diseases.
Supplementary Material
Acknowledgments
The authors would like to thank Andrew Sloan for engineering support and Reema Casavant for assistance with surgeries. They thank Michael Borland, John Buell, and Mark Lane for help with electronics construction. In addition, they thank Elizabeth Nutting, Xavier Carrier, Meera Iyengar, Priyanka Das, Brian Nguyen, Iqra Qureshi, Sabiha Sultana, and Virginia Land for assistance with behavioral testing. This project was supported in part by funding from the Defense Advanced Research Projects Agency HR0011-15-2-0017, NIH NINDS R01 NS085167, NIH NIDCD R01 DC010433, and the Texas Biomedical Device Center.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neurobiolaging.2016.03.030.
Footnotes
Disclosure statement
Navid Khodaparast is a consultant for, and Michael P. Kilgard is a consultant for and has a financial interest in MicroTransponder, Inc, a company which is developing VNS-based therapies. Robert L. Rennaker owns Vulintus, Inc. Other authors declare no conflicts of interest.
References
- Alaverdashvili M, Whishaw I. Compensation aids skilled reaching in aging and in recovery from forelimb motor cortex stroke in the rat. Neuroscience. 2010;167:21. doi: 10.1016/j.neuroscience.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Ay I, Lu J, Ay H, Gregory Sorensen A. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia. Neurosci Lett. 2009;459:147–151. doi: 10.1016/j.neulet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Bagg S, Pombo AP, Hopman W. Effect of age on functional outcomes after stroke rehabilitation. Stroke. 2002;33:179–185. doi: 10.1161/hs0102.101224. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, Hilmi O, McLean J, Forbes K, Kilgard MP, Rennaker RL, Cramer SC, Walters M, Engineer N. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke. 2015;47:143–150. doi: 10.1161/STROKEAHA.115.010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Kilgard M, Engineer N, Vanneste S. Placebo-controlled vagus nerve stimulation paired with tones in a patient with refractory tinnitus: a case report. Otol Neurotol. 2015;36:575–580. doi: 10.1097/MAO.0000000000000704. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S, Engineer ND, Kilgard MP. Safety and efficacy of vagus nerve stimulation paired with tones for the treatment of tinnitus: a case series. Neuromodulation. 2013;17:170–179. doi: 10.1111/ner.12127. [DOI] [PubMed] [Google Scholar]
- Denti L, Agosti M, Franceschini M. Outcome predictors of rehabilitation for first stroke in the elderly. Eur J Phys Rehabil Med. 2008;44:3–11. [PubMed] [Google Scholar]
- Detari L, Juhasz G, Kukorelli T. Effect of stimulation of vagal and radial nerves on neuronal activity in the basal forebrain area of anaesthetized cats. Acta Physiol Hung. 1983;61:147–154. [PubMed] [Google Scholar]
- Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 2006;318:890–898. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Barbay S, Plautz EJ, Hoover E, Strittmatter SM, Nudo RJ. Combination of NEP 1–40 treatment and motor training enhances behavioral recovery after a focal cortical infarct in rats. Stroke. 2010;41:544–549. doi: 10.1161/STROKEAHA.109.572073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JO, DuBois FS. Quantitative studies of the vagus nerve in the cat. I. The ratio of sensory to motor fibers. J Comp Neurol. 1937;67:49–67. [Google Scholar]
- Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, Puligheddu M, Marrosu F, Biggio G. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, Bashir S, Vernet M, Peña-Gómez C, Pascual-Leone A. Changes in cortical plasticity across the lifespan. Front Aging Neurosci. 2011;3:5. doi: 10.3389/fnagi.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmaga H, Carreno FR, Frazer A. Vagal nerve stimulation rapidly activates brain-derived neurotrophic factor receptor TrkB in rat brain. PLoS One. 2012;7:e34844. doi: 10.1371/journal.pone.0034844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statisticse2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves DA, Bowman EM, Brown VJ. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci Lett. 2005;379:174–179. doi: 10.1016/j.neulet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, II, Kilgard MP. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke. 2014a;45:3097–3100. doi: 10.1161/STROKEAHA.114.006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Khodaparast N, Ruiz A, Sloan AM, Hulsey DR, Rennaker RL, Kilgard MP. The timing and amount of vagus nerve stimulation during rehabilitative training affect post-stroke recovery of forelimb strength. Neuroreport. 2014b;25:676–682. doi: 10.1097/WNR.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Khodaparast N, Sloan AM, Hulsey DR, Pantoja M, Ruiz AD, Kilgard MP, Rennaker RL., II The isometric pull task: a novel automated method for quantifying forelimb force generation in rats. J Neurosci Methods. 2012;212:329–337. doi: 10.1016/j.jneumeth.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Hays SA, Rennaker RL, II, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res. 2013;207:275–299. doi: 10.1016/B978-0-444-63327-9.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraki T, Baker W, Greenberg JH. Effect of vagus nerve stimulation during transient focal cerebral ischemia on chronic outcome in rats. J Neurosci Res. 2012;90:887–894. doi: 10.1002/jnr.22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, Kilgard MP. Reorganization of motor cortex by vagus nerve stimulation requires cholinergic innervation. Brain Stimul. 2016;9:174–181. doi: 10.1016/j.brs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12:119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, Rennaker RL, II, Kilgard MP. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis. 2013;60:80–88. doi: 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Hays SA, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, II, Kilgard MP. Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil Neural Repair. 2014;28:698–706. doi: 10.1177/1545968314521006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, Rennaker RL, 2nd, Hays SA. Vagus nerve stimulation during rehabilitative training improves forelimb recovery after chronic ischemic stroke in rats [e-pub ahead of print] Neurorehabil. Neural Repair. 2015 doi: 10.1177/1545968315616494. http://dx.doi.org/10.1177/1545968315616494. [DOI] [PMC free article] [PubMed]
- Knoflach M, Matosevic B, Rücker M, Furtner M, Mair A, Wille G, Zangerle A, Werner P, Ferrari J, Schmidauer C. Functional recovery after ischemic stroke—a matter of age data from the Austrian stroke Unit Registry. Neurology. 2012;78:279–285. doi: 10.1212/WNL.0b013e31824367ab. [DOI] [PubMed] [Google Scholar]
- Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39:709–714. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- Merrett DL, Kirkland SW, Metz GA. Synergistic effects of age and stress in a rodent model of stroke. Behav Brain Res. 2010;214:55–59. doi: 10.1016/j.bbr.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Dahlhaus JFM, Orekhov Y, Liu Y, Ziemann U. Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Exp Brain Res. 2008;187:467–475. doi: 10.1007/s00221-008-1319-7. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcome. The Copenhagen Stroke Study. Stroke. 1994;25:808–813. doi: 10.1161/01.str.25.4.808. [DOI] [PubMed] [Google Scholar]
- Nichols J, Nichols A, Smirnakis S, Engineer N, Kilgard M, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience. 2011;189:207–214. doi: 10.1016/j.neuroscience.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, Rennaker RL, II, Kilgard MP. Repeatedly pairing vagus nerve stimulation with a movement Reorganizes primary motor cortex. Cereb Cortex. 2011;22:2365–2374. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- Pruitt D, Schmid A, Kim L, Abe C, Trieu J, Choua C, Hays S, Kilgard M, Rennaker RL., II Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury [e-pub ahead of print] J Neurotrauma. 2015 doi: 10.1089/neu.2015.3972. http://dx.doi.org/10.1089/neu.2015.3972. [DOI] [PMC free article] [PubMed]
- Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006;1119:124–132. doi: 10.1016/j.brainres.2006.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki L, Yaseen Z, Kopylev L, Cohen LG. Age-dependent changes in the ability to encode a novel elementary motor memory. Ann Neurol. 2003;53:521–524. doi: 10.1002/ana.10529. [DOI] [PubMed] [Google Scholar]
- Tennant KA, Adkins DL, Scalco MD, Donlan NA, Asay AL, Thomas N, Kleim JA, Jones TA. Skill learning induced plasticity of motor cortical representations is time and age-dependent. Neurobiol Learn Mem. 2012;98:291–302. doi: 10.1016/j.nlm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


