Abstract
Objective
To use proton magnetic resonance spectroscopy (1H MRS) to investigate the effects of fish oil (FO) supplementation on cortical metabolite concentrations in adolescents with major depressive disorder (MDD).
Methods
Metabolite concentrations were determined by 1H MRS in the anterior cingulate cortex and bilateral dorsolateral prefrontal cortex (DLPFC) of adolescents with MDD before and following 10-week open-label supplementation with low (2.4 g/day, n = 7) or high (16.2 g/day, n = 7) dose FO. Depressive symptom severity scores and erythrocyte fatty acid levels were also determined.
Results
Baseline erythrocyte eicosapentaenoic acid (EPA) composition was positively correlated, and arachidonic acid (AA) and the AA/EPA ratio were inversely correlated, with choline (Cho) concentrations in the right DLPFC. Docosahexaenoic acid (DHA) composition was inversely correlated with myo-inositol (mI) concentrations in the left DLPFC. Erythrocyte EPA and DHA composition increased, and AA decreased, significantly following low-dose and high-dose FO supplementation. In the intent-to-treat sample, depressive symptom severity scores decreased significantly in the high-dose group (−40%, P < 0.0001) and there was a trend in the low-dose group (−20%, P = 0.06). There were no significant baseline–endpoint changes in metabolite levels in each voxel. In the low-dose group there were changes with large effect sizes, including a decrease in mI in the left DLPFC (−12%, P = 0.18, d = 0.8) and increases in glutamate + glutamine (Glx) (+12%, P = 0.19, d = 0.8) and Cho (+15%, P = 0.08, d = 1.2) in the right DLPFC. In the high-dose group, there was a trend for increases in Cho in the right DLPFC (+10%, P = 0.09, d = 1.2).
Discussion
These preliminary data suggest that increasing the LCn-3 fatty acid status of adolescent MDD patients is associated with subtle changes in Glx, mI, and Cho concentrations in the DLPFC that warrant further evaluation in a larger controlled trial.
Keywords: Docosahexaenoic acid, Adolescent, Dorsolateral prefrontal cortex, Anterior cingulated cortex, Proton magnetic resonance spectroscopy, Major depressive disorder
Introduction
Converging evidence suggests that the pathophysiology of major depressive disorder (MDD) is associated with a deficiency in long-chain omega-3 (LCn-3) fatty acids, including eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3).1–3 Case–control studies have consistently observed lower EPA and/or DHA levels in erythrocytes (red blood cells) or plasma phospholipids of adolescent and adult MDD patients.4–10 Some fatty acid composition studies,5,11,12 but not all,9,13 have observed an inverse correlation between plasma and/or erythrocyte LCn-3 fatty acid composition, or LCn-3 fatty acid composition relative to arachidonic acid (AA) composition (i.e. AA/EPA), and depression symptom severity scores. Independent meta-analyses of controlled trials have observed a significant advantage of fish oil (FO), a rich source of EPA and DHA, over placebo for reducing depressive symptoms in adult MDD patients.14–18 Moreover, preliminary FO trials have also observed reductions in depressive symptoms in children and adolescents with mood disorders.13,19–21 While these findings suggest that low LCn-3 fatty acid status may represent a modifiable risk factor for MDD, the central mechanisms mediating this relationship remains poorly understood.
The primary LCn-3 fatty acid found in mammalian brain gray matter is DHA, representing ~12% of adult human prefrontal cortex fatty acid composition.22,23 Although the role of DHA in cortical structure and function in humans remains poorly understood, preclinical studies suggest that DHA and its bioactive metabolites are neurotrophic,24,25 anti-inflammatory,26–28 neuroprotective,29,30 and promote neurovascular function.31–33 In the human frontal cortex, DHA levels increase rapidly during childhood and adolescence,22 a period associated with rapid changes in prefrontal cortex structural and functional maturation.34,35 Cross-sectional postmortem studies have observed lower DHA levels in prefrontal cortex and anterior cingulate cortex (ACC) of adults with MDD,36–38 but not in temporal lobe structures including the amygdala.39–42 Emerging neuroimaging evidence suggests that LCn-3 fatty acid status is positively correlated with frontolimbic structural and functional integrity in healthy subjects across the lifespan.43–49 Together these data suggest that there may be a relationship between low LCn-3 fatty acid status and abnormalities in cortical structure and function commonly observed in patient with MDD.50,51
Proton magnetic resonance spectroscopy (1H MRS) is an in vivo imaging technique that measures concentrations of metabolites associated with cortical structural and metabolic integrity.52 For example, N-acetyl aspartate (NAA) is primarily localized to neurons and translational studies have found that cortical NAA concentrations decrease in association with neuronal atrophy or dysfunction following acute ischemia.53–57 Myo-inositol (mI) is predominantly concentrated in astrocytes and is a principal product of phospholipase C-mediated phosphatidylinositol (PI) hydrolysis, and is also metabolized from glucose via 1L-myo-inositol 1-phosphate synthase.58 Choline (Cho)-containing compounds are enriched in membrane phospholipids and cytosolic compounds including glycerophosphocholine and phosphocholine.52 Some prior case–control 1H MRS studies,59–67 but not all,68,69 have observed significantly lower concentrations of NAA, mI, Cho, as well as glutamate+-glutamine (Glx) and/or glutamate (Glu) in different cortical and subcortical regions of patients with MDD. Other studies have observed changes in cortical metabolite levels in response to antidepressant treatments.68,70–73 However, there is currently nothing known about the relationship between reduced metabolite levels and LCn-3 fatty acid status and treatment response in patients with MDD.
We previously reported that deficits in cortical DHA accrual during perinatal development led to selective reductions in basal mI concentrations in the adult rat medial prefrontal cortex measured by 1H MRS at 7 T.74 More recently, we reported that healthy developing children with low erythrocyte LCn-3 fatty acid levels had significantly lower Cho, mI, NAA, and creatine (Cr) concentrations in the ACC, but not bilateral dorsolateral prefrontal cortex (DLPFC), compared with children with high erythrocyte LCn-3 fatty acid levels.75 A prior 1H MRS study found that 12-week ethyl-EPA supplementation selectively increased NAA concentrations in the ACC of medicated bipolar patients.76 These preliminary data support the hypothesis that there is a positive association between LCn-3 fatty acid status and cortical metabolite concentrations. To test this, the present study determined the effects of a 10-week open-label supplementation with one of two doses of FO (2.4 or 16.2 g/day) on metabolite concentrations in the bilateral DLPFC and ACC of moderately depressed adolescent MDD patients.
Materials and methods
Subjects
This trial was approved by the University of Cincinnati Institutional Review Board, and was registered at clinicaltrials.gov as NCT00511810. Written informed consent and assent were provided by a legal guardian and the patient, respectively. Patients were male or female adolescents (ages 8–24 years of age) diagnosed with MDD (DSM-IV-TR criteria), which was confirmed with the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS).77 All patients were assessed by a board-certified child and adolescent psychiatrist (J.R.S., M.P.D.). Patients were required to have residual depressive symptoms (baseline score of >28 and <40) on the Children’s Depression Rating Scale-Revised (CDRS-R) despite being administered a standard therapeutic dose of a selective serotonin reuptake inhibitor (SSRI) for a minimum of 6 weeks. Patients were maintained on their current SSRI and dose over the course of the trial. Subjects were screened to ensure that they could receive an 1H MRS examination safely (e.g. had no ferromagnetic metal in their body and were not claustrophobic), were right-hand dominant,78 and did not have a history of seizures, major medical illness, or traumatic brain injury. Subjects were excluded by a positive urine pregnancy test, having a urine drug screen which was positive for illicit substance use, greater than 1 year outside appropriate age/grade level, currently taking omega-3 fatty acid supplements, or having a seafood allergy.
1H MRS acquisition
Magnetic resonance imaging (MRI) and 1H MRS data were acquired on Varian 4 T whole-body scanner (Varian-Agilent Inc., Palo Alto, CA, USA). A 1H TEM (Transverse ElectroMagnetic) head coil was used as a transmitter/receiver. A multi-slice scout image was initially acquired for MRS voxel positioning. The scout image was followed by the acquisition of 3D whole head MRI using modified driven equilibrium Fourier transform (MDEFT) pulse sequence for tissue segmentation.79 After MRS voxel positioning, the magnetic field homogeneity was optimized using automatic shim method FASTMAP (Fast Automatic Shimming Technique by Mapping Along Projections).80 A typical water line width in the MRS voxel was 10–12 Hz. Three single-voxel PRESS (Point RESolved Spectroscopy) spectra were collected in the ACC (BA32/33) and left and right DLPFC (BA9) (Fig. 1). Spectra were acquired with repetition time (TR) 2000 ms, echo time (TE) 23 ms, voxel size 8 cc, and 64 averages with water suppression by VAPOR (Variable Pulse powers and Optimizing Relaxation delays) method.81 For computations of metabolite levels and eddy current correction, one reference spectrum without water suppression was collected at the same voxel position with the same parameters except four averages were acquired, and receiver gain reduced.
Figure 1.
Spectroscopic voxel placement in the left (A) and right (B) DLPFC (BA9), and ACC (BA32/33) (C).
To determine the tissue content within MRS voxels, MDEFT images were processed using a contrast-driven algorithm in statistical parametrical mapping (http://www.fil.ion.ucl.ac.uk/spm/). The tissue segmentation data are presented in percentage of gray matter, white matter, and cerebrospinal fluid. For the determination of metabolite levels, localized spectra were analyzed using LCModel (Linear Combination of Model spectra) with the water reference in unsuppressed-water spectra.82 All metabolite data except Glx and Glu were corrected with T1 and T2 relaxation losses using available values.83 Metabolite levels were also corrected with tissue segmentation data. The differences of water concentrations, T1 and T2 relaxation times in gray matter, white matter, and CSF were also taken into consideration for computation. Metabolite levels are presented as absolute concentrations (mM)
Treatment
Patients were randomized to open-label FO supplements (supplied by the Inflammation Research Foundation, Marblehead, MA, USA) at a fixed EPA+DHA dose of either 2.4 g/day (low dose: EPA 1.6 g +DHA 0.8 g; 4 capsules/day) or 16.2 g/day (high dose: EPA, 10.8 g +DHA 5.4 g; 2 tablespoons/day) for 10 weeks. The low dose (2.4 g/day) was selected based on previous findings that similar doses were safe and efficacious in pediatric and adolescent patients with mood disorders,19–21 and the high dose (16.2 g/day) based on efficacy and safety data in pediatric and adolescent attention deficit hyperactivity disorder (ADHD) patients.84 Independent analysis of the fatty acid composition of the FO confirmed that it was composed of ~45% EPA (20:5n-3) and 26% DHA (22:6n-3) wt% total fatty acids. Compliance was evaluated by determining capsule counts (low dose) or bottle volumes (high dose) at weekly visits.
Depressive symptom ratings
Depression symptom severity was determined at weekly visits with the CDRS-R, a 17-item observer-rated questionnaire.85,86 All patients were rated by a board-certified child and adolescent psychiatrist with established inter-rater reliabilities (κ > 0.9)
Fatty acid analysis
Whole venous blood (10 ml) was collected into EDTA-coated BD Vacutainer tubes, and centrifuged for 20 minutes (1500 g, 4°C). Plasma and buffy coat were removed and erythrocytes were washed three times with 0.9% NaCl and stored at −80°C. Lipid extraction was performed using the saponification and methylation procedure described previously.87 Briefly, erythrocyte samples were placed in a 20 ml glass vial into which 4 ml of 0.5 N methanolic sodium hydroxide was added, and the sample heated at 80°C for 5 minutes. Following a 10 minutes cooling period, 3 ml of boron trifluoride in methanol was added to methylate the sample. After an additional 5 minutes of heating in the water bath (80°C), the sample vial was allowed to cool, and 2 ml of a saturated solution (6.2 M) of sodium chloride and 5 ml of hexane was added. The samples were then mixed by vortex for 1 minute. The hexane fraction was then transferred into a 20 ml vial containing 10 mg of sodium sulfate to dry the sample. The hexane solution was then removed for gas chromatographic (GC) analysis. An injection volume of 1 µl of the hexane solution was analyzed. Total erythrocyte membrane fatty acid composition was determined with a Shimadzu GC-2010 equipped with an auto-injector (Shimadzu Scientific Instruments Inc., Columbia, MD, USA). The column was a DB-23 (123–2332): 30 m (length), ID (mm) 0.32 wide bore, film thickness of 0.25 µM (J&W Scientific, Folsom, CA, USA). The carrier gas was helium with a column flow rate of 2.5 ml/minute. Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap, PA, USA). Analysis of fatty acid methyl esters was based on areas calculated with Shimadzu Class VP 4.3 software. Data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). All samples were processed by a technician blinded to group assignment and treatment. Primary measures of interest were the major LCn-3 fatty acids, EPA, docosapentaenoic acid (DPA, 20:5n-3), DHA, and AA (20:4n-6), and the ratios of AA to EPA and/or DHA.
Statistical analyses
Differences between dose groups in demographic measures were evaluated using unpaired t-tests (two-tailed, α = 0.05) for continuous variables and Chi-square tests (two-tailed, α = 0.05) for dichotomous variables. For depressive symptom scores (CDRS) obtained at weekly visits, a mixed-effects regression model (PROC MIXED) that included terms for dose group, time, and group-by-time interaction was used to examine group difference in average rate of change over the 10-week study. Akaike’s Information Criterion was used to select the variance–covariance structure for this model (inclusion or exclusion of subject-level random intercepts and slopes and autoregressive structure for the residual covariances). Analyses of CDRS scores were performed on the intent-to-treat population, which included all patients who received at least one dose of study medication and also had at least one postbaseline efficacy assessment (n = 17). Relationships between baseline–endpoint changes in CDRS scores and metabolite levels were evaluated by multiple regression and adjusted for age and gender. For the analyses of metabolite levels within each voxel, we employed Bonferroni correction for multiple comparisons (α = 0.05/6 metabolites = 0.008). Partial Pearson correlations (i.e. between changes in the outcome and MRS variables, adjusted for baseline outcome levels) were used to summarize the magnitude of observed relationships. For primary outcome measures, effect sizes were calculated using Cohen’s d, with small, medium, and large effect sizes being equivalent to d-values of 0.30, 0.50, and 0.80, respectively. Statistical analyses were performed using SAS (version 9.2, Cary, NC, USA).
Results
Patient characteristics
A total of 24 patients were screened for study participation. Twenty patients met study criteria and completed baseline study assessments. A total of 14 patients completed the 10-week open-label intervention (low dose, n = 7; high dose, n = 7). A total of three patients were lost to follow-up post-randomization, and there were three patients randomized to low dose that terminated study participation early (two patients (weeks 3 and 6) due to a worsening of depressive symptoms, and one patient (week 9) declined to continue in the study due to claustrophobia). A flow diagram illustrating the sequence of subject recruitment and attrition has been published previously.13 Baseline demographic characteristics are presented in Table 1.
Table 1.
Patient demographic characteristics
| Variable* | All patients (n = 20) | Low dose** (n = 7) | High dose** (n = 7) | P-value*** |
|---|---|---|---|---|
| Age (years) | 15.2±3.4 | 15.6±3.4 | 13.6±3.4 | 0.30 |
| Gender (% female) | 70 | 71 | 71 | 1.0 |
| Race (n) | ||||
| Caucacian | 19 | 7 | 6 | 0.99 |
| African American | 1 | 0 | 1 | |
| Height (cm) | 162.8±13.7 | 164.8±11.1 | 162.7±18.6 | 0.79 |
| Weight (kg) | 63.5±19.6 | 54.6±3.7 | 67.1±25.7 | 0.23 |
| BMI (kg/m2) | 23.8±7.2 | 20.2±2.2 | 24.8±8.1 | 0.18 |
| BMI z score | 0.6±1.1 | −0.05±0.9 | 0.9±1.2 | 0.13 |
| BMI percentile | 65.1±32.4 | 47.7±31.9 | 72.9±34.9 | 0.20 |
| Age at onset (years) | 12.7±3.1 | 12.3±2.4 | 12.1±2.9 | 0.92 |
| CDRS-R | 32.5±5.3 | 34.1±3.8 | 33.9±3.1 | 0.94 |
| SSRI (n) | ||||
| Fluoxetine | 9 | 3 | 2 | – |
| Citalopram | 3 | 1 | 1 | – |
| Escitalopram | 2 | 0 | 1 | – |
| Sertraline | 6 | 3 | 3 | – |
| Erythrocyte fatty acids (wt% TTL) | ||||
| AA (20:4n-6) | 17.7±1.4 | 18.0±0.8 | 18.8±0.8 | 0.12 |
| EPA (20:5n-3) | 0.30±0.1 | 0.28±0.1 | 0.29±0.0 | 0.89 |
| DPA (20:5n-3) | 2.2±0.3 | 2.2±0.3 | 2.1±0.2 | 0.73 |
| DHA (22:6n-3) | 2.8±0.6 | 2.8±0.7 | 2.7±0.5 | 0.74 |
| Sum LCn-3 (EPA+DPA+DHA) | 5.4±0.6 | 5.3±0.5 | 5.1±0.4 | 0.54 |
| EPA+DHA | 3.2±0.6 | 3.1±0.6 | 3.0±0.5 | 0.75 |
| AA:EPA | 76.1±47.7 | 95.3±71.4 | 66.7±15.1 | 0.32 |
| AA:DHA | 6.5±1.3 | 6.7±1.4 | 7.1±0.9 | 0.62 |
| AA:EPA+DHA | 5.9±1.1 | 6.0±1.0 | 6.4±0.8 | 0.54 |
Values are group mean±SD or number of subjects (n).
Patients with both baseline and endpoint 1H MRS values.
Low dose vs. high dose (two-tailed t-tests).
Depressive symptom ratings
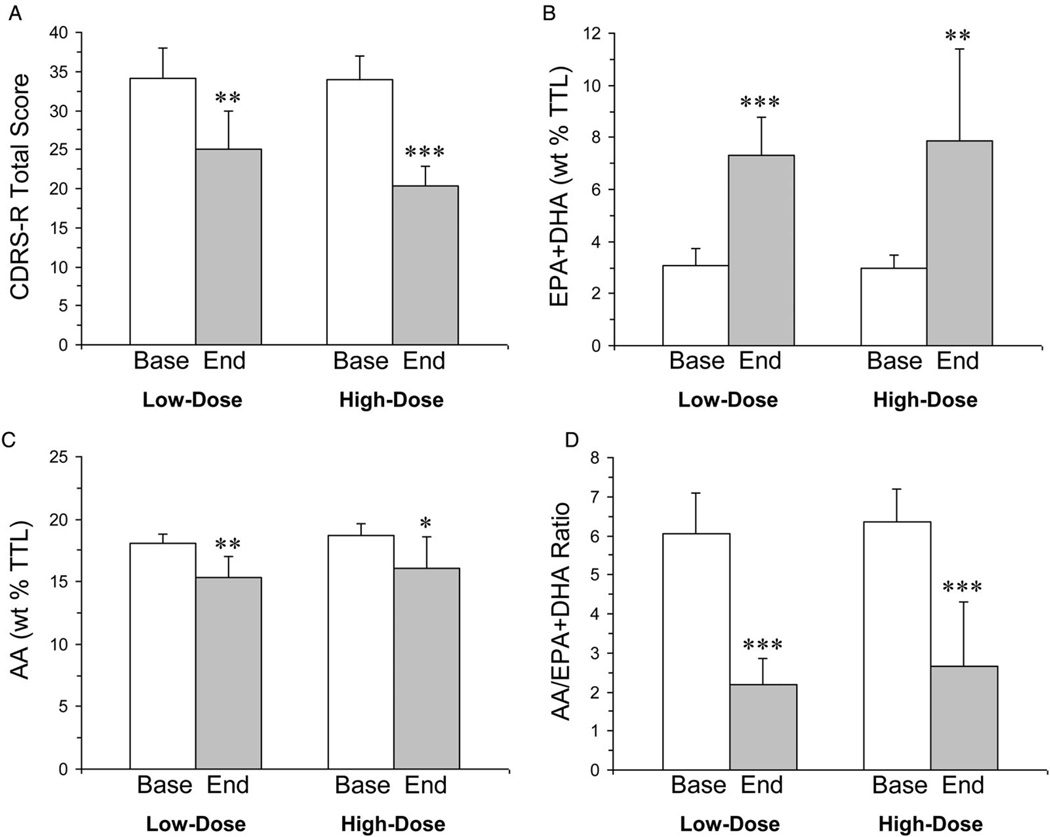
Baseline CDRS-R total scores in the low-dose and high-dose groups did not differ significantly (P = 0.94) (Table 1). For the CDRS mixed-effects model, the interaction term was not significant (P = 0.670). The baseline-last available CDRS-R score declined significantly in the high-dose group (−40%, P = 0.0001, d = 5.2) and there was a non-significant decrease in the low-dose group (−20%, P = 0.063, d = 0.93). After removal of the n = 3 patients in the low-dose group that did not complete the 10-week treatment trial, the baseline–endpoint decrease in CDRS-R total scores in the low-dose group became significant (−27%, P = 0.002, d = 2.2) (Fig. 2A).
Figure 2.
CDRS-R total scores (A), erythrocyte EPA+DHA composition (weight percent total fatty acid composition, wt% TTL) (B), erythrocyte AA composition (C), and the AA/EPA+DHA ratio in MDD patients treated with low-dose or high-dose FO and had baseline (Base) and endpoint (End, week 10) 1H MRS values (n = 7/dose group). Values are group means±SD. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.0001 vs. baseline.
Erythrocyte fatty acid composition
At baseline erythrocyte fatty acid levels did not differ between the low-dose and high-dose groups (Table 1). Total erythrocyte LCn-3 fatty acid (EPA+DPA+DHA) composition increased significantly following low-dose (+51%, P<0.0001) and high-dose (+54%, P = 0.003) FO supplementation. More specifically, there was a 7-fold baseline–endpoint increase in erythrocyte EPA in the low-dose group (P = 0.003) and an 8-fold increase in the high-dose group (P = 0.001). DHA increased significantly in the low-dose (+40%, P < 0.0001) and high-dose (+52%, P = 0.001) groups. Erythrocyte EPA+DHA composition increased significantly following low-dose (+58%, P < 0.0001, d = 3.9) and high-dose (+62%, P = 0.003, d = 2.1) FO supplementation (Fig. 2B). Erythrocyte AA (20:4n-6) composition decreased significantly in the low-dose (−15%, P = 0.002) and high-dose (−14%, P = 0.019) groups (Fig. 2C), and the AA/EPA+DHA ratio decreased significantly in the low-dose (−64%, P < 0.0001) and high-dose (−58%, P = 0.0002) groups (Fig. 2D). A detailed description of baseline–endpoint changes in all fatty acid levels has been published previously.13
1H MRS
Baseline correlations
Among all patients with baseline metabolite and fatty acid levels, erythrocyte DHA composition was inversely correlated with mI concentrations in the left DLPFC (r = −0.55, P = 0.02) and there was a similar non-significant effect for mI in the right DLPFC (r = −0.40, P = 0.12). Erythrocyte DHA composition was not significantly correlated with other metabolites in the right or left DLPFC and the ACC. Erythrocyte EPA composition was positively correlated with Cho concentrations in the right DLPFC (r = +0.56, P = 0.022), and there were similar non-significant effects for mI (r = −0.44, P = 0.09) and NAA (r = +0.39, P = 0.13) in the right DLPFC. Erythrocyte EPA composition was not significantly correlated with other metabolite concentrations in the right or left DLPFC and the ACC. Erythrocyte AA was inversely correlated with Cho in the left DLPFC (r = −0.50, P = 0.03) and there was a similar trend in right DLPFC Cho (r = −0.49, P = 0.053). The AA/EPA ratio was inversely correlated with Cho concentrations in the right DLPFC (r = −0.57, P = 0.02), and there were trends for positive correlation between the AA/DHA ratio and mI in the left (r = +0.43, P = 0.09) and right (r = +0.38, P = 0.15) DLPFC. There were no correlations between these baseline fatty acid measures and any metabolite in the ACC.
Baseline–endpoint changes
Baseline and endpoint metabolite concentrations in the left and right DLPFC and ACC are presented in Table 2. For all voxels, the main effects of time and dose and the time-by-dose interaction were not significant for each metabolite. Including age and sex as covariates did not alter the results. In the low-dose group, there were non-significant changes with large effect sizes including a decrease in mI in the left DLPFC (−12%, P = 0.17, d = 0.8) and an increase in Glx in the right DLPFC (+12%, P = 0.19, d = 0.8). In the high dose group, there was a non-significant increase in NAA in the right DLPFC which had a large effect size (+7%, P = 0.16, d = 0.95). There were trends with large effect sizes for a baseline–endpoint increase in Cho in the right DLPFC in both the low-dose (+15%, P = 0.08, d = 1.1) and high-dose (+10%, P = 0.09, d = 1.2) groups. When the low-dose and high-dose groups were combined, there was a significant increase in Cho concentrations in the right DLPFC (+13%, P = 0.013, d = 1.1). There was an inverse correlation between baseline–endpoint change in the AA/EPA+DHA ratio and baseline– endpoint change in Glx concentrations in the right DLPFC (r = −0.61, P = 0.04). Endpoint erythrocyte EPA+DHA was positively correlated with ACC Glu (r = +0.68, P = 0.015), Glx (r = +0.61, P = 0.034), and Cr (r = +0.66, P = 0.021), and there was a positive trend for NAA (r = +0.52, P = 0.08).
Table 2.
Regional metabolite concentrations
| Metabolite* | Baseline | Week 10 | d** | P-value*** |
|---|---|---|---|---|
| Left DLPFC | ||||
| Low dose | ||||
| Cho | 1.6±0.1 | 1.5±0.1 | 0.45 | 0.47 |
| Cr | 6.6±0.8 | 6.5±0.7 | 0.14 | 0.82 |
| Glx | 5.9±1.5 | 5.8±0.9 | 0.11 | 0.86 |
| Glu | 5.3±1.2 | 5.0±0.6 | 0.33 | 0.60 |
| mI | 5.2±1.0 | 4.6±0.5 | 0.85 | 0.18 |
| NAA | 8.7±1.2 | 8.3±1.2 | 0.30 | 0.63 |
| High dose | ||||
| Cho | 1.6±0.2 | 1.5±0.1 | 0.23 | 0.71 |
| Cr | 6.6±0.9 | 6.8±0.4 | 0.28 | 0.66 |
| Glx | 6.9±2.4 | 6.8±0.6 | 0.08 | 0.90 |
| Glu | 6.0±1.4 | 5.8±0.3 | 0.19 | 0.90 |
| mI | 4.7±0.4 | 4.7±0.4 | 0.10 | 0.88 |
| NAA | 9.4±1.1 | 9.3±0.5 | 0.11 | 0.86 |
| Right DLPFC | ||||
| Low dose | ||||
| Cho | 1.4±0.2 | 1.7±0.3 | 1.20 | 0.08 |
| Cr | 6.4±0.8 | 6.7±0.9 | 0.33 | 0.60 |
| Glx | 5.5±0.9 | 6.3±1.1 | 0.84 | 0.19 |
| Glu | 5.3±0.9 | 5.5±0.8 | 0.34 | 0.59 |
| mI | 4.5±0.5 | 4.6±0.9 | 0.20 | 0.74 |
| NAA | 9.3±0.9 | 8.9±0.7 | 0.48 | 0.45 |
| High dose | ||||
| Cho | 1.6±0.1 | 1.8±0.2 | 1.18 | 0.09 |
| Cr | 7.1±0.8 | 7.2±0.7 | 0.09 | 0.88 |
| Glx | 6.3±0.6 | 6.6±1.1 | 0.33 | 0.61 |
| Glu | 5.6±0.7 | 5.7±0.6 | 0.12 | 0.87 |
| mI | 5.2±0.5 | 4.7±0.8 | 0.79 | 0.24 |
| NAA | 9.2±1.1 | 9.9±0.2 | 0.95 | 0.16 |
| ACC | ||||
| Low dose | ||||
| Cho | 1.8±0.6 | 1.8±0.3 | 0.04 | 0.95 |
| Cr | 7.3±2.3 | 6.1±1.2 | 0.77 | 0.28 |
| Glx | 6.5±2.5 | 6.4±0.8 | 0.63 | 0.95 |
| Glu | 5.0±1.8 | 4.8±1.0 | 0.20 | 0.77 |
| mI | 6.0±1.8 | 5.9±1.4 | 0.07 | 0.91 |
| NAA | 6.1±2.2 | 5.3±1.0 | 0.48 | 0.49 |
| High dose | ||||
| Cho | 1.8±0.5 | 1.9±0.2 | 0.25 | 0.66 |
| Cr | 6.8±1.5 | 7.0±1.1 | 0.20 | 0.74 |
| Glx | 8.2±2.2 | 7.7±1.3 | 0.30 | 0.61 |
| Glu | 5.6±1.5 | 5.6±1.1 | 0.05 | 0.93 |
| mI | 6.5±2.4 | 5.8±0.6 | 0.46 | 0.45 |
| NAA | 6.7±1.8 | 7.1±1.3 | 0.26 | 0.66 |
Values are mean concentration (mM) ± SD (see the Materials and methods section for abbreviations)
Cohens d.
Two-tailed t-test.
Relationships with depressive symptoms
Although there were no significant correlations between baseline CDRS-R total scores and metabolite concentrations in each voxel, there was a trend for an inverse correlation between left DLPFC mI and CDRS-R total scores (r = −0.34, P = 0.17). Multiple regression did not find any significant associations between baseline–endpoint changes in CDRS-R total scores and baseline–endpoint changes in metabolite levels in each voxel, before and after adjusting for age and gender. However, we did observe trends for mI in the left DLPFC (P = 0.17, d = 1.0) and Cho in the right DLPFC (P = 0.17, d = 1.0). Endpoint CDRS scores were inversely correlated with endpoint ACC Glx concentrations (r = −0.54, P = 0.055).
Discussion
This study investigated the effects of 10-week adjunctive supplementation with either low- or high-dose FO on regional cortical metabolite concentrations in depressed adolescents with MDD. We found that baseline erythrocyte DHA composition was inversely correlated with mI concentrations in left DLPFC and there was a similar non-significant trend in the right DLPFC. EPA was positively correlated, and AA and the AA/EPA ratio were inversely correlated, with Cho concentrations in the right DLPFC. Supplementation with either low- or high-dose FO significantly increased erythrocyte EPA and DHA levels, and depression symptom severity scores decreased significantly in the high-dose group and there was a non-significant trend in the low-dose group. Contrary to our hypothesis, however, increases in erythrocyte EPA and DHA composition and reductions in depressive symptoms were not associated with robust changes in metabolite levels. However, in the low-dose group we observed non-significant trends with large effect sizes for a baseline–endpoint decrease in mI in the leftDLPFC and a baseline–endpoint increase in Glx and Cho in the right DLPFC. In the high-dose group, there was a trend for a baseline–endpoint increase in Cho in the right DLPFC. Moreover, among all subjects endpoint erythrocyte EPA+DHA was positively correlated with ACC Glu, Glx, and Cr, and there was a positive trend for NAA. Together these preliminary data suggest that increasing the LCn-3 fatty acid status in adolescent MDD patients is associated with subtle changes in metabolite concentrations in the ACC and DLPFC that warrant further evaluation. To our knowledge, this is the first 1H MRS study to investigate the effects of FO supplementation on cortical metabolite concentrations in MDD patients.
Baseline erythrocyte DHA composition was inversely correlated with mI concentrations in left DLPFC, and there was a similar trend in the right DLPFC. We also observed a trend for a baseline–endpoint decrease in mI in the left DLPFC in the low-dose group. These findings suggest that in adolescent MDD patients higher DHA levels are associated with lower mI concentrations in the DLPFC. This finding contrasts with our previous study in healthy children in which DLPFC mI concentrations were not correlated with erythrocyte DHA composition.75 Although this previous study employed younger (8–10 years of age) male subjects, compared with predominantly female adolescents in the present study, it may be relevant that a previous study found that 8-week antidepressant treatment increased mI/Cr levels in the left DLPFC of medication-naïve MDD patients.68 The latter finding suggests that antidepressant exposure may increase DLPFC mI levels, and that this effect is attenuated by higher DHA levels. This is supported in part by our finding that stimulation of phospholipase C-mediated PI hydrolysis led to significant increases in mI levels in the mPFC of DHA-deficient rats but not in control rats.74 Interestingly, prior case–control studies have observed elevated mI or mI/Cr levels in the PFC or ACC of adolescents with bipolar depression or mania which were reduced by lithium treatment.88–90 Additional studies are warranted to determine whether elevations in PFC mI concentrations in response to antidepressant treatment are modulated by DHA, and whether this relationship is relevant to antidepressant efficacy and tolerability.
Because mI concentrations are several-fold greater in astrocytes than neurons, the astrocyte mI pool is likely the major contributor to the mI peak at 4 T.58 Astrocytes play a critical role in glucose uptake from blood vessels, and postmortem studies have observed significantly lower astrocyte markers and astrocyte endfeet contact with blood vessels in the PFC of MDD patients.91,92 Because mI is metabolized from glucose via 1L-myo-inositol 1-phosphate synthase, the observed inverse correlation between DHA status and DLPFC mI concentrations is consistent with the negative modulation of DLPFC glucose metabolism by DHA. This is supported by a previous positron emission tomography study that found that plasma DHA levels were inversely correlated with resting glucose metabolic rates in the PFC and ACC of medication-free MDD patients.93 Moreover, preclinical data suggest that DHA is required for the normal growth and maturation of cortical astrocyte-mediated glucose uptake and neurovascular coupling.32,33,94,95 While these findings suggest that DHA may moderate astrocyte-mediated glucose uptake and metabolism in the PFC of MDD patients, additional studies will be required to directly evaluate this mechanism.
We also observed a trend with a large effect size for a baseline–endpoint increase in Glx in the right DLPFC, and that endpoint erythrocyte EPA+DHA was positively correlated with Glx and Glu concentrations in the ACC. Prior 1H MRS studies have observed lower Glx or Glu concentrations in the ACC of patients with MDD.65 These data suggest that increasing LCn-3 fatty acid status may promote Glu uptake and storage in the ACC of MDD patients. However, a previous 12-week ethyl-EPA (2 g/day) supplementation trial did not observe changes in Glx concentrations in the ACC of medicated bipolar patients,76 and we previously found that ACC Glx concentrations were not correlated with erythrocyte EPA or DHA composition in healthy children.75 In view of preclinical evidence suggesting that lower cortical DHA levels are associated with deficits in Glu synaptic integrity and astrocyte-mediated Glu uptake,96–98 additional studies are warranted to elucidate the relationships between LCn-3 fatty acid status and cortical Glu activity in MDD.
Prior studies have found that NAA concentrations decrease in association with neuronal atrophy or dysfunction.53–57 We previously reported that erythrocyte DHA composition was positively correlated with NAA concentrations in the ACC of healthy children,75 and another study found that 12-week ethyl-EPA (2 g/day) supplementation selectively increased (+9%) NAA concentrations in the ACC of medicated bipolar patients.76 Although we did not observe significant baseline–endpoint changes in ACC NAA, we did observe a trend for a positive correlation between endpoint ACC NAA concentrations and erythrocyte EPA+DHA composition. Moreover, we observed a trend with a large effect size for a baseline–endpoint increase in right DLPFC NAA in the high-dose group. In view of evidence that DHA has neurotrophic24,25 and neuroprotective29,30 effects, the observed trend for increased NAA in the right DLPFC following high-dose FO supplementation warrants additional investigation.
Although Cho is enriched in the membrane phospholipids phosphatidylcholine and sphingomyelin, cytosolic Cho compounds including glycerophosphocholine and phosphocholine are thought to be the major contributors to the Cho signal.52 The Cho signal may therefore represent an index of membrane phospholipid metabolism, with increases reflecting elevated membrane turnover. A previous study found that the Cho signal in the hippocampus of MDD patients was significantly lower than healthy controls and normalized following successful electroconvulsive therapy.61 Other studies similarly found that the Cho increases in the basal ganglia or hippocampus following successful fluoxetine treatment.70,73 In the present study, baseline erythrocyte EPA composition was positively correlated, and AA and the AA/EPA ratio inversely correlated, with Cho concentrations in the right DLPFC. Moreover, we observed trends with large effect sizes for a baseline–endpoint increase in Cho in the right DLPFC in both dose groups which became statistically significant when both groups were combined. These data are in general agreement with our previous finding that Cho concentrations in the ACC were positively correlated with erythrocyte DHA,75 and suggest that the positive association between LCn-3 fatty acid status and right DLPFC Cho concentrations in adolescent MDD patients may be relevant to antidepressant response.
This preliminary neuroimaging study has several important limitations. First, patients were being treated with an SSRI prior to and during study participation. Therefore, it is possible that normalizing effects of SSRIs on metabolite levels precluded observation of more robust changes in response to FO supplementation.68,71,72 Second, the relatively small number of patients randomized to each treatment group may have been underpowered to detect baseline–endpoint changes in metabolite concentrations. Third, the present study did not include a healthy comparison group to determine whether baseline metabolite levels observed in MDD patients were abnormal. Fourth, the duration of FO supplementation was relatively short (10 weeks), and a longer intervention period may be required to produce more robust changes in metabolite concentrations. Therefore, larger and longer intervention studies employing medication-naive patients and healthy controls are warranted to confirm and extend the present findings.
In conclusion, this preliminary 1H MRS study found that open-label 10-week adjunctive supplementation with low- or high-dose FO significantly increased LCn-3 fatty acid status in adolescent MDD patients, and reduced depression symptom severity scores in the high-dose group with a similar trend in the low-dose group. Although these changes were not associated with robust alterations in cortical metabolite concentrations, we did observe baseline and endpoint correlations between metabolite concentrations and LCn-3 fatty acid levels as well as trends for baseline–endpoint changes in mI, Glx, and Cho concentrations in the ACC and DLPFC. Despite the limitations of this preliminary study, the results identify candidate metabolites and cortical regions that may be more sensitive to LCn-3 fatty acid status that warrant further evaluation in a larger controlled FO trial employing medication-naive patients with MDD.
Acknowledgments
This study was supported in part by an investigator-initiated research grant from the Inflammation Research Foundation (IRF) to R.K.M., National Institute of Health grants MH083924 to R.K.M. and M.P.D. (Co-PIs), and DK59630 to P.T. The IRF and NIH did not have any role in the analysis or interpretation of the research. This trial was registered at clinicaltrials.gov as NCT00511810.
R.K.M. has received research support from Martek Biosciences Inc., Inflammation Research Foundation (IRF), Ortho-McNeil Janssen, AstraZeneca, Eli Lilly, and has served on the IRF scientific advisory board. M.P.D. has received research support from AstraZeneca, Eli Lilly, Martek, Johnson & Johnson, Shire, Ortho-McNeil Janssen, Pfizer, Otsuka, Shire, Amylin, Lundbeck, Novartis, Somerset, Sumitomo, Thrasher Foundation, GlaxoSmithKline, NARSAD, and NIMH, NIDA, NIAAA, and is a consultant for GlaxoSmithKline, Eli Lilly, Pfizer, and Merck. C.M.A. has received research support from AstraZeneca, Schering Plough/Merck, Somerset, Otsuka, Eli Lilly, Ortho-McNeil Janssen, GlaxoSmithKline, Pfizer, Shire, and Repligen, and is a consultant for Schering Plough/Merck. J.R.S. has received research support from Eli Lilly, Shire, and from the American Academy of Child and Adolescent Psychiatry.
Footnotes
Conflicts of interest R.J., T.R., P.T., W.-J.C., W.A.W., and J.A.W have no conflicts to declare.
Contributors
Dr McNamara designed the study and wrote the manuscript. Drs Jandacek and Tso and Therese Rider performed the gas chromatography. Dr Chu and Mr Weber participated in the collection and analyses of the 1H MRS data. Drs Adler and DelBello participated in the design of the study and in the interpretation of the data. All authors contributed to the writing and editing of this manuscript.
Disclaimer statements
Ethics approval This trial was approved by the University of Cincinnati Institutional Review Board.
References
- 1.McNamara RK. Long-chain omega-3 fatty acid deficiency in mood disorders: rationale for treatment and prevention. Curr Drug Discov Technol. 2013;10:233–244. doi: 10.2174/1570163811310030006. [DOI] [PubMed] [Google Scholar]
- 2.Hibbeln JR. Depression, suicide and deficiencies of omega-3 essential fatty acids in modern diets. World Rev Nutr Diet. 2009;99:17–30. doi: 10.1159/000192992. [DOI] [PubMed] [Google Scholar]
- 3.Su K-P, Balanza-Martinez V. Role of omega-3 fatty acids in mood disorders. In: McNamara RK, editor. The omega-3 fatty acid deficiency syndrome. USA: Nova Science Publishers, Inc.; pp. 315–336. [Google Scholar]
- 4.Assies J, Pouwer F, Lok A, Mocking RJ, Bockting CL, Visser I, et al. Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS ONE. 2010;5:e10635. doi: 10.1371/journal.pone.0010635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 6.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 7.Riemer S, Maes M, Christophe A, Rief W. Lowered omega-3 PUFAs are related to major depression, but not to somatization syndrome. J Affect Disord. 2010;123:173–180. doi: 10.1016/j.jad.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 9.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pottala JV, Talley JA, Churchill SW, Lynch DA, von Schacky C, Harris WS. Red blood cell fatty acids are associated with depression in a case-control study of adolescents. Prostaglandins Leukot Essent Fatty Acids. 2012;86:161–165. doi: 10.1016/j.plefa.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;(31 Suppl):S157–S161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 12.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. 2008;43:1031–1038. doi: 10.1007/s11745-008-3224-z. [DOI] [PubMed] [Google Scholar]
- 13.McNamara RK, Strimpfel J, Jandacek R, Rider T, Tso P, Welge JA, et al. Detection and treatment of long-chain omega-3 fatty acid deficiency in adolescents with SSRI-resistant major depressive disorder. Pharma Nutr. 2014;2:38–46. doi: 10.1016/j.phanu.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91:757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- 15.Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 16.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 17.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2009;28:525–542. doi: 10.1080/07315724.2009.10719785. [DOI] [PubMed] [Google Scholar]
- 18.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72:1577–1584. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63:1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- 20.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163:1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- 21.Wozniak J, Biederman J, Mick E, Waxmonsky J, Hantsoo L, Best C, et al. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17:440–447. doi: 10.1016/j.euroneuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 23.McNamara RK, Liu Y, Jandacek R, Rider T, Tso P. The aging human orbitofrontal cortex: decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins Leukot Essent Fatty Acids. 2008;78:293–304. doi: 10.1016/j.plefa.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci Lett. 2007;415:154–158. doi: 10.1016/j.neulet.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coti Bertrand P, O’Kusky JR, Innis SM. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr. 2006;136:1570–1575. doi: 10.1093/jn/136.6.1570. [DOI] [PubMed] [Google Scholar]
- 26.Lu DY, Tsao YY, Leung YM, Su KP. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for ω-3 fatty acids. Neuropsychopharmacology. 2010;35:2238–2248. doi: 10.1038/npp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orr SK, Palumbo S, Bosetti F, Mount HT, Kang JX, Greenwood CE, et al. Unesterified docosahexaenoic acid is protective in neuroinflammation. J Neurochem. 2013;127:378–393. doi: 10.1111/jnc.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 29.Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009;40:3121–3126. doi: 10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozyurt B, Sarsilmaz M, Akpolat N, Ozyurt H, Akyol O, Herken H, et al. The protective effects of omega-3 fatty acids against MK-801-induced neurotoxicity in prefrontal cortex of rat. Neurochem Int. 2007;50:196–202. doi: 10.1016/j.neuint.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Ellis EF, Police RJ, Dodson LY, McKinney JS, Holt SA. Effect of dietary n-3 fatty acids on cerebral microcirculation. Am J Physiol. 1992;262:1379–1386. doi: 10.1152/ajpheart.1992.262.5.H1379. [DOI] [PubMed] [Google Scholar]
- 32.Tsukada H, Kakiuchi T, Fukumoto D, Nishiyama S, Koga K. Docosahexaenoic acid (DHA) improves the age-related impairment of the coupling mechanism between neuronal activation and functional cerebral blood flow response: a PET study in conscious monkeys. Brain Res. 2000;862:180–186. doi: 10.1016/s0006-8993(00)02115-6. [DOI] [PubMed] [Google Scholar]
- 33.Ximenes da Silva A, Lavialle F, Gendrot G, Guesnet P, Alessandri JM, Lavialle M. Glucose transport and utilization are altered in the brain of rats deficient in n-3 polyunsaturated fatty acids. J Neurochem. 2002;81:1328–1337. doi: 10.1046/j.1471-4159.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 34.Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, et al. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- 36.Conklin SM, Runyan CA, Leonard S, Reddy RD, Muldoon MF, Yao JK. Age-related changes of n-3 and n-6 polyunsaturated fatty acids in the anterior cingulate cortex of individuals with major depressive disorder. Prostaglandins Leukot Essent Fatty Acids. 2010;82:111–119. doi: 10.1016/j.plefa.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNamara RK, Hahn C-G, Jandacek R, Rider T, Tso P, Stanford K, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 38.McNamara RK, Jandacek R, Tso P, Dwivedi Y, Ren X, Pandey GN. Lower docosahexaenoic acid concentrations in the postmortem prefrontal cortex of adult depressed suicide victims compared with controls without cardiovascular disease. J Psychiatr Res. 2013;47:1187–1191. doi: 10.1016/j.jpsychires.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamazaki K, Choi KH, Kim HY. Phospholipid profile in the postmortem hippocampus of patients with schizophrenia and bipolar disorder: no changes in docosahexaenoic acid species. J Psychiatr Res. 2010;44:688–693. doi: 10.1016/j.jpsychires.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamazaki K, Hamazaki T, Inadera H. Fatty acid composition in the postmortem amygdala of patients with schizophrenia, bipolar disorder, and major depressive disorder. J Psychiatr Res. 2012;46:1024–1028. doi: 10.1016/j.jpsychires.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Hamazaki K, Hamazaki T, Inadera H. Abnormalities in the fatty acid composition of the postmortem entorhinal cortex of patients with schizophrenia, bipolar disorder, and major depressive disorder. Psychiatry Res. 2013;210:346–350. doi: 10.1016/j.psychres.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 42.McNamara RK, Rider T, Jandacek R, Tso P. Abnormal fatty acid pattern in the superior temporal gyrus distinguishes bipolar disorder from major depression and schizophrenia and resembles multiple sclerosis. Psychiatry Res. 2014;215:560–567. doi: 10.1016/j.psychres.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conklin SM, Gianaros PJ, Brown SM, Yao JK, Hariri AR, Manuck SB, et al. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett. 2007;421:209–212. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 44.McNamara RK, Able J, Jandacek R, Rider T, Tso P, Eliassen JC, et al. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr. 2010;91:1060–1067. doi: 10.3945/ajcn.2009.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samieri C, Maillard P, Crivello F, Proust-Lima C, Peuchant E, Helmer C, et al. Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology. 2012;79:642–650. doi: 10.1212/WNL.0b013e318264e394. [DOI] [PubMed] [Google Scholar]
- 46.Titova OE, Sjögren P, Brooks SJ, Kullberg J, Ax E, Kilander L, et al. Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. Age (Dordr) 2013;35:1495–1505. doi: 10.1007/s11357-012-9453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walhovd KB, Storsve AB, Westlye LT, Drevon CA, Fjell AM. Blood markers of fatty acids and vitamin D, cardiovascular measures, body mass index, and physical activity relate to longitudinal cortical thinning in normal aging. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.11.011. S0197–4580(13)00579–4. [DOI] [PubMed] [Google Scholar]
- 48.Witte AV, Kerti L, Hermannstädter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, et al. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex. 2014 doi: 10.1093/cercor/bht163. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Virtanen JK, Siscovick DS, Lemaitre RN, Longstreth WT, Spiegelman D, Rimm EB, et al. Circulating omega-3 polyunsaturated fatty acids and subclinical brain abnormalities on MRI in older adults: the Cardiovascular Health Study. J Am Heart Assoc. 2013;2:e000305. doi: 10.1161/JAHA.113.000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 51.Sacher J, Neumann J, Fünfstück T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord. 2012;140:142–148. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Curr Top Behav Neurosci. 2014 doi: 10.1007/7854_2011_197. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Demougeot C, Marie C, Giroud M, Beley A. N-acetylaspartate: a literature review of animal research on brain ischaemia. J Neurochem. 2004;90:776–783. doi: 10.1111/j.1471-4159.2004.02583.x. [DOI] [PubMed] [Google Scholar]
- 54.Duijn JH, Matson GB, Maudsley AA, Hugg JW, Weiner MW. Human brain infarction: proton MR spectroscopy. Radiology. 1992;183:711–718. doi: 10.1148/radiology.183.3.1584925. [DOI] [PubMed] [Google Scholar]
- 55.Federico F, Simone IL, Lucivero V, Giannini P, Laddomada G, Mezzapesa DM, et al. Prognostic value of proton magnetic resonance spectroscopy in ischemic stroke. Arch Neurol. 1998;55:489–494. doi: 10.1001/archneur.55.4.489. [DOI] [PubMed] [Google Scholar]
- 56.Lanfermann H, Kugel H, Heindel W, Herholz K, Heiss WD, Lackner K. Metabolic changes in acute and subacute cerebral infarctions: findings at proton MR spectroscopic imaging. Radiology. 1995;196:203–210. doi: 10.1148/radiology.196.1.7784568. [DOI] [PubMed] [Google Scholar]
- 57.Malisza KL, Kozlowski P, Peeling J. A review of in vivo 1H magnetic resonance spectroscopy of cerebral ischemia in rats. Biochem Cell Biol. 1998;76:487–496. doi: 10.1139/bcb-76-2-3-487. [DOI] [PubMed] [Google Scholar]
- 58.Griffin JL, Bollard M, Nicholson JK, Bhakoo K. Spectral profiles of cultured neuronal and glial cells derived from HRMAS(1) H NMR spectroscopy. NMR Biomed. 2002;15:375–384. doi: 10.1002/nbm.792. [DOI] [PubMed] [Google Scholar]
- 59.Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- 60.Coupland NJ, Ogilvie CJ, Hegadoren KM, Seres P, Hanstock CC, Allen PS. Decreased prefrontal myo-inositol in major depressive disorder. Biol Psychiatry. 2005;57:1526–1534. doi: 10.1016/j.biopsych.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 61.Ende G, Braus DF, Walter S, Weber-Fahr W, Henn FA. The hippocampus in patients treated with electroconvulsive therapy: a proton magnetic resonance spectroscopic imaging study. Arch Gen Psychiatry. 2000;57:937–943. doi: 10.1001/archpsyc.57.10.937. [DOI] [PubMed] [Google Scholar]
- 62.Gruber S, Frey R, Mlynárik V, Stadlbauer A, Heiden A, Kasper S, et al. Quantification of metabolic differences in the frontal brain of depressive patients and controls obtained by 1H-MRS at 3 Tesla. Invest Radiol. 2003;38:403–408. doi: 10.1097/01.rli.0000073446.43445.20. [DOI] [PubMed] [Google Scholar]
- 63.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 64.Järnum H, Eskildsen SF, Steffensen EG, Lundbye-Christensen S, Simonsen CW, Thomsen IS, et al. Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatr Scand. 2011;124:435–446. doi: 10.1111/j.1600-0447.2011.01766.x. [DOI] [PubMed] [Google Scholar]
- 65.Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, et al. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36:198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 66.Mirza Y, Tang J, Russell A, Banerjee SP, Bhandari R, Ivey J, et al. Reduced anterior cingulate cortex glutamatergic concentrations in childhood major depression. J Am Acad Child Adolesc Psychiatry. 2004;43:341–348. doi: 10.1097/00004583-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 67.Rosenberg DR, Macmaster FP, Mirza Y, Smith JM, Easter PC, Banerjee SP, et al. Reduced anterior cingulate glutamate in pediatric major depression: a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:700–704. doi: 10.1016/j.biopsych.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Kaymak SU, Demir B, Oğuz KK, Sentürk S, Uluğ B. Antidepressant effect detected on proton magnetic resonance spectroscopy in drug-naïve female patients with first-episode major depression. Psychiatry Clin Neurosci. 2009;63:350–356. doi: 10.1111/j.1440-1819.2009.01951.x. [DOI] [PubMed] [Google Scholar]
- 69.Nery FG, Stanley JA, Chen HH, Hatch JP, Nicoletti MA, Monkul ES, et al. Normal metabolite levels in the left dorsolateral prefrontal cortex of unmedicated major depressive disorder patients: a single voxel (1)H spectroscopy study. Psychiatry Res. 2009;174:177–183. doi: 10.1016/j.pscychresns.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 70.Block W, Träber F, von Widdern O, Metten M, Schild H, Maier W, et al. Proton MR spectroscopy of the hippocampus at 3T in patients with unipolar major depressive disorder: correlates and predictors of treatment response. Int J Neuropsychopharmacol. 2009;12:415–422. doi: 10.1017/S1461145708009516. [DOI] [PubMed] [Google Scholar]
- 71.Caverzasi E, Pichiecchio A, Poloni GU, Calligaro A, Pasin M, Palesi F, et al. Magnetic resonance spectroscopy in the evaluation of treatment efficacy in unipolar major depressive disorder: a review of the literature. Funct Neurol. 2012;27:13–22. [PMC free article] [PubMed] [Google Scholar]
- 72.Gonul AS, Kitis O, Ozan E, Akdeniz F, Eker C, Eker OD, et al. The effect of antidepressant treatment on N-acetyl aspartate levels of medial frontal cortex in drug-free depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:120–125. doi: 10.1016/j.pnpbp.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 73.Sonawalla SB, Renshaw PF, Moore CM, Alpert JE, Nierenberg AA, Rosenbaum JF, et al. Compounds containing cytosolic choline in the basal ganglia: a potential biological marker of true drug response to fluoxetine. Am J Psychiatry. 1999;156:1638–1640. doi: 10.1176/ajp.156.10.1638. [DOI] [PubMed] [Google Scholar]
- 74.McNamara RK, Able J, Jandacek R, Rider T, Tso P, Lindquist DM. Perinatal n-3 fatty acid deficiency selectively reduces myoinositol levels in the adult rat PFC: an in vivo 1H-MRS study. J Lipid Res. 2009;50:405–411. doi: 10.1194/jlr.M800382-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McNamara RK, Jandacek R, Rider T, Tso P, Weber W, Chu W-J, et al. Low docosahexaenoic acid status is associated with reduced indices of cortical integrity in the anterior cingulate of healthy male children: a 1H MRS study. Nutr Neurosci. 2013;16:183–190. doi: 10.1179/1476830512Y.0000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frangou S, Lewis M, Wollard J, Simmons A. Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J Psychopharmacol. 2007;21:435–439. doi: 10.1177/0269881106067787. [DOI] [PubMed] [Google Scholar]
- 77.Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 78.Crovitz HF, Zener K. A group-test for assessing hand- and eye-dominance. Am J Psychol. 1962;75:271–276. [PubMed] [Google Scholar]
- 79.Lee J-H, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, et al. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- 80.Gruetter R, Boesch C. Fast, noniterative shimming of spatially localized signals. In vivo analysis of the magnetic field along axes. J Magn Reson. 1992;96:323–334. [Google Scholar]
- 81.Tkac I, Staruck Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 82.Provencher SW. Estimation of metabolite concentration from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 83.Hetherington HP, Mason GF, Pan JW, Ponder SL, Vaughan JT, Twieg DB, et al. Evaluation of cerebral gray and white matter metabolite differences by spectroscopic imaging at 4.1T. Magn Reson Med. 1994;32:565–571. doi: 10.1002/mrm.1910320504. [DOI] [PubMed] [Google Scholar]
- 84.Sorgi PJ, Hallowell EM, Hutchins HL, Sears B. Effects of an open-label pilot study with high-dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutr J. 2007;6:16. doi: 10.1186/1475-2891-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie GJ. Psychometric properties of the Children’s Depression Rating Scale-Revised in adolescents. J Child Adolesc Psychopharmacol. 2010;20:513–516. doi: 10.1089/cap.2010.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poznanski EO, Cook SC, Carroll BJ, Corzo H. Use of the Children’s Depression Rating Scale in an inpatient psychiatric population. J Clin Psychiatry. 1983;44:200–203. [PubMed] [Google Scholar]
- 87.Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:514–515. [Google Scholar]
- 88.Davanzo P, Thomas MA, Yue K, Oshiro T, Belin T, Strober M, et al. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology. 2001;24:359–369. doi: 10.1016/S0893-133X(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 89.Davanzo P, Yue K, Thomas MA, Belin T, Mintz J, Venkatraman TN, et al. Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. Am J Psychiatry. 2003;160:1442–1452. doi: 10.1176/appi.ajp.160.8.1442. [DOI] [PubMed] [Google Scholar]
- 90.Patel NC, Cecil KM, Strakowski SM, Adler CM, DelBello MP. Neurochemical alterations in adolescent bipolar depression: a proton magnetic resonance spectroscopy pilot study of the prefrontal cortex. J Child Adolesc Psychopharmacol. 2008;18:623–627. doi: 10.1089/cap.2007.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rajkowska G, Hughes J, Stockmeier CA, Javier Miguel-Hidalgo J, Maciag D. Coverage of blood vessels by astrocytic endfeet is reduced in major depressive disorder. Biol Psychiatry. 2013;73:613–621. doi: 10.1016/j.biopsych.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. 2013;14:1225–1236. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sublette ME, Milak MS, Hibbeln JR, Freed PJ, Oquendo MA, Malone KM, et al. Plasma polyunsaturated fatty acids and regional cerebral glucose metabolism in major depression. Prostaglandins Leukot Essent Fatty Acids. 2009;80:57–64. doi: 10.1016/j.plefa.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Joardar A, Sen AK, Das S. Docosahexaenoic acid facilitates cell maturation and beta-adrenergic transmission in astrocytes. J Lipid Res. 2006;47:571–581. doi: 10.1194/jlr.M500415-JLR200. [DOI] [PubMed] [Google Scholar]
- 95.Pifferi F, Roux F, Langelier B, Alessandri JM, Vancassel S, Jouin M, et al. (n-3) polyunsaturated fatty acid deficiency reduces the expression of both isoforms of the brain glucose transporter GLUT1 in rats. J Nutr. 2005;135:2241–2246. doi: 10.1093/jn/135.9.2241. [DOI] [PubMed] [Google Scholar]
- 96.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, et al. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Latour A, Grintal B, Champeil-Potokar G, Hennebelle M, Lavialle M, Dutar P, et al. Omega-3 fatty acids deficiency aggravates glutamatergic synapse and astroglial aging in the rat, hippocampal CA1. Aging Cell. 2013;12:76–84. doi: 10.1111/acel.12026. [DOI] [PubMed] [Google Scholar]
- 98.Moreira JD, Knorr L, Ganzella M, Thomazi AP, de Souza CG, de Souza DG, et al. Omega-3 fatty acids deprivation affects ontogeny of glutamatergic synapses in rats: relevance for behavior alterations. Neurochem Int. 2010;56:753–759. doi: 10.1016/j.neuint.2010.02.010. [DOI] [PubMed] [Google Scholar]