Abstract
IMPORTANCE
A common trigger for relapse in drug addiction is the experience of craving via exposure to cues previously associated with drug use. Preclinical studies have consistently demonstrated incubation of cue-induced drug-seeking during the initial phase of abstinence, followed by a decline over time. In humans, the incubation effect has been shown for alcohol, nicotine, and methamphetamine addictions, but not for heroin or cocaine addiction. Understanding the trajectory of cue-induced craving during abstinence in humans is of importance for addiction medicine.
OBJECTIVE
To assess cue-induced craving for cocaine in humans using both subjective and objective indices of cue-elicited responses.
DESIGN, SETTING, AND PARTICIPANTS
Seventy-six individuals addicted to cocaine with varying durations of abstinence (ie, 2 days, 1 week, 1 month, 6 months, and 1 year) participated in this laboratory-based cross-sectional study from June 19, 2007, to November 26, 2012. The late positive potential component of electroencephalography, a recognized marker of incentive salience, was used to track motivated attention to drug cues across these self-selected groups. Participants also completed subjective ratings of craving for cocaine before presentation of a cue, and ratings of cocaine “liking” (hedonic feelings toward cocaine) and “wanting” (craving for cocaine) after presentation of cocaine-related pictures. Data analysis was conducted from June 5, 2015, to March 30, 2016.
MAIN OUTCOMES AND MEASURES
The late positive potential amplitudes and ratings of liking and wanting cocaine in response to cocaine-related pictures were quantified and compared across groups.
RESULTS
Among the 76 individuals addicted to cocaine, 19 (25%) were abstinent for 2 days, 20 (26%) were abstinent for 1week, 15 (20%) were abstinent for 1 month, 12 (16%) were abstinent for 6 months, and 10 (13%) were abstinent for 1 year. In response to drug cues, the mean (SD) late positive potential amplitudes showed a parabolic trajectory that was higher at 1 (1.26 [1.36] µV) and 6 (1.17 [1.19] µV) months of abstinence and lower at 2 days (0.17 [1.09] µV), 1week (0.36 [1.26] µV), and 1 year (−0.27 [1.74] µV) of abstinence (P = .02, partial η2 = 0.16). In contrast, the subjective assessment of baseline craving (mean [SD] rating: 2 days, 26.05 [9.85]; 1week, 18.70 [11.01]; 1 month, 10.87 [10.70]; 6 months, 6.92 [8.47]; and 1 year, 3.00 [3.77]) and cue-induced liking (mean [SD] rating: 2 days, 3.06 [2.34]; 1week, 2.33 [2.87]; 1 month, 1.15 [2.03]; 6 months, 1.00 [2.24]; and 1 year, 1.00 [1.26]) and wanting (mean [SD] rating: 2 days, 3.44 [2.62]; 1week, 2.72 [2.87]; 1 month, 1.46 [2.33]; 6 months, 1.00 [2.16]; and 1 year, 1.00 [1.55]) of cocaine showed a linear decline from 2 days to 1 year of abstinence (P ≤ .001, partial η2 > 0.26).
CONCLUSIONS AND RELEVANCE
The late positive potential responses to drug cues, indicative of motivated attention, showed a trajectory similar to that reported in animal models. In contrast, we did not detect incubation of subjective cue-induced craving. Thus, the objective electroencephalographic measure may possibly be a better indicator of vulnerability to cue-induced relapse than subjective reports of craving, although this hypothesis must be empirically tested. These results suggest the importance of deploying intervention between 1 month and 6 months of abstinence, when addicted individuals may be most vulnerable to, and perhaps least cognizant of, risk of relapse.
A core challenge for treating drug addiction is the high propensity for relapse. The neuropsychological mechanisms of relapse remain poorly understood, but include the experience of intense craving for the drug elicited by cues or contexts previously associated with the drug.1 Gawin and Kleber2 first proposed that response to drug cues increases after some delay (which they termed as withdrawal) during abstinence. This seemingly counterintuitive notion was later empirically validated by preclinical studies showing a progressive increase in drug seeking during an individual’s early days of abstinence,3,4 followed by a decline after approximately 6 months of abstinence.5 Mechanistic studies have invoked abstinence-induced early adaptations in the ventral tegmental area6,7 and synaptic transmission changes in the nucleus accumbens,8 mediated via changes in composition of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subunit and silent synapse–based remodeling9,10 that strengthen the excitatory transmission to the medium spiny neurons, resulting in heightened reactivity to drug cues.11 In addition, selective gene expression in the striatal dopamine system has been implicated in incubation of drug seeking.12 In humans, incubation of craving (the subjectively assessed urge to use drugs) has been reported for methamphetamine,13 nicotine,14 and alcohol,15 but not for heroin,16 whereas the time-dependent trajectory of cue-induced craving in individuals addicted to cocaine is not known. More important, self-reported assessments of craving, as performed in these studies, are recognized for crucial pitfalls that affect their reliability and validity, including the influence of demand characteristics,17 social desirability bias,18 and compromised self-awareness.19 Moreover, subjective craving lacks direct translation to preclinical studies in which craving was defined by behavior and could not invoke introspection.
To track human cue-induced craving in a manner akin to the preclinical literature, it may therefore be imperative to measure reactivity to drug-related cues more objectively. The late positive potential (LPP) component of the clinically translatable electroencephalogram (EEG) is an objective and temporally precise marker of motivated attention to salient stimuli20 that, in individuals with cocaine use disorders, correlates with subjectively assessed cue-induced craving,21predicts simulated drug-seeking behavior,22and is modulated by abstinence.23 Thus, we reasoned that the LPP-indexed motivated attention to drug cues, a known precursor to choice behavior,24 could be used to objectively track incubation of cue-induced craving. We hypothesized that, similar to the preclinical work, drug-related LPPs will show an inverted U-shaped trajectory as a function of duration of abstinence in individuals with cocaine use disorders. We further hypothesized that, consistent with the human literature to date, self-reported cue-induced craving will similarly show incubation across increasing durations of abstinence, whereas unprovoked baseline craving will show a steady decline.
Methods
Participants
Seventy-six individuals with cocaine use disorders recruited from the local community provided written consent to participate in accordance with Stony Brook University’s institutional review board, which approved this study. Exclusion criteria were head trauma with loss of consciousness for more than 30 minutes; current neurologic, medical, or psychiatric disorder that required hospitalization; use of any medications within the past 6 months; and positive urine test results for illicit drugs other than cocaine.
All individuals with cocaine use disorders underwent a comprehensive diagnostic interview consisting of the following components: Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Axis I Disorders25; Addiction Severity Index,26 to assess the severity and recency of alcohol- and drug-related problems; 18-item Cocaine Selective Severity Assessment,27 to evaluate withdrawal symptoms within the past 24 hours; 3-item Severity of Dependence Scale28; and 5-item Cocaine Craving Questionnaire.29 This interview established that the individuals with cocaine use disorders met criteria for current cocaine dependence (n = 59), or cocaine dependence in partial (n = 11) or sustained (n = 6) remission. Current comorbidities included marijuana abuse (n = 3), opiate dependence (n = 2), and alcohol abuse (n = 3) or dependence (n = 1) (nevertheless, a positive urine test result for any of these substances on the study day was exclusionary). Past comorbidities in the individuals with cocaine use disorders included fully sustained remission of alcohol use disorder (n = 28), marijuana abuse (n = 24), opiate dependence (n = 2), major depressive disorder (n = 6), or posttraumatic stress disorder (n = 3). In addition, 49 individuals with cocaine use disorders reported current cigarette smoking and 4 individuals with cocaine use disorders met criteria for antisocial personality disorder.
Before data analyses, individuals with cocaine use disorders were stratified into subgroups based on their self-reported length of abstinence on the study day. This stratification generally paralleled preclinical time windows,3 resulting in 5 subgroups with approximate mean abstinence durations of 2 days, 1 week, 1 month, 6 months, and 1 year. These subgroups did not differ on age, sex, lifetime duration of cocaine use, and severity of dependence (Table28).
Table.
Demographics, Measures of Severity and Recency of Cocaine Use, and Measures of Craving
| Characteristic | Duration of Abstinence, Valuea | ||||
|---|---|---|---|---|---|
| 2 d | 1 wk | 1 mo | 6 mo | 1 y | |
| Sample size, No. | 19 | 20 | 15 | 12 | 10 |
| Age, y | 45.16 (4.02) |
45.80 (6.46) |
40.60 (9.43) |
44.83 (2.92) |
43.60 (9.40) |
| Sex, No. (male/female) | 18/1 | 19/1 | 10/5 | 9/3 | 9/1 |
| Duration of cocaine use, y | 14.37 (6.88) |
17.60 (6.63) |
13.23 (6.86) |
15.83 (7.04) |
11.80 (9.37) |
| Abstinence, d | |||||
| Durationb | 1.58 (0.51) |
5.80 (4.1) |
36.60 (13.71) |
168.58 (29.02) |
368.30 (148.09) |
| Range | 1–2 | 3–15 | 18–65 | 121–206 | 215–570 |
| Severity of dependencec | 8.56 (3.59) |
7.50 (3.15) |
10.14 (2.88) |
9.00 (3.41) |
6.44 (4.50) |
| LPP amplitude, µVd | 0.17 (1.09) |
0.36 (1.26) |
1.26 (1.36) |
1.17 (1.19) |
−0.27 (1.74) |
| Self-reported baseline cravingb | 26.05 (9.85) |
18.70 (11.01) |
10.87 (10.70) |
6.92 (8.47) |
3.00 (3.77) |
| Self-reported cue-induced ratingsd | |||||
| Liking cocaine | 3.06 (2.34) |
2.33 (2.87) |
1.15 (2.03) |
1.00 (2.24) |
1.00 (1.26) |
| Wanting cocaine | 3.44 (2.62) |
2.72 (2.87) |
1.46 (2.33) |
1.00 (2.16) |
1.00 (1.55) |
Abbreviation: LPP, late positive potential.
Data are presented as mean (SD) unless otherwise indicated.
P < .01.
Measurements via Severity of Dependence Scale.28
P < .05 (see the Results section and Figures for complete statistics and comparisons among groups).
Study Procedures
Electroencephalographic recordings were performed from June 19, 2007, to November 26, 2012, as participants viewed 4 types of pictures (30 pictures per category, each viewed for 2 seconds). Three picture types (pleasant, unpleasant, and neutral) were selected from the International Affective Picture System30 and the fourth picture type depicted cocaine and individuals preparing, using, or simulating use of cocaine.22,23
Electroencephalographic recordings were obtained using a cap with 64 electrodes, using a frontocentral electrode as ground and linked mastoids as reference. Electrodes were placed above and below the left eye to record vertical eye movements and were placed on the outer canthi of both eyes to record horizontal eye movements. The EEG was digitized at 500 Hz, amplified with a gain of 250, and bandpass filtered at 0 to 70 Hz. Electrode impedances did not exceed 20 kΩ for any electrodes used in the analysis.
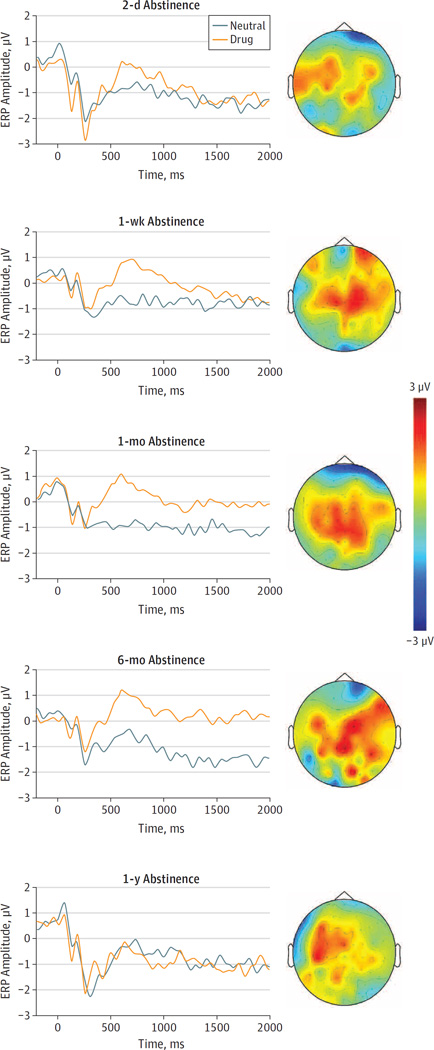
Offline analyses were conducted using Statistical Parametric Mapping, version 12 (Wellcome Department of Cognitive Neurology). Data were first high-pass filtered at 0.01 Hz, and were then rereferenced to the averaged electrical activity from all 64 electrodes. Artifact rejection procedures included the eye-blink correction using the partial signal space projection method31and a semiautomatic artifact rejection procedure using a voltage step of more than 75 µV between sample points, a peak-to-peak voltage difference of 150 µV within an epoch, and via visual inspection. Using robust averaging,32 the event-related potentials were constructed by separately averaging trials based on type of picture. The LPP for each type of picture was defined as the mean activity at the centroparietal (Cz, CP1, CPz, CP2, and Pz) electrodes. The LPP was scored as an averaged EEG activity across 400 to 2000 milliseconds after the onset of the stimulus. The mean EEG activity in the 200-millisecond window before picture onset served as the baseline (Figure 1).
Figure 1. Grand Averages of Event-Related Potentials (ERPs) in Response to Drug-Related and Neutral Cues at the Centroparietal Electrode Site.
Next to each waveform is the scalp topography (ie, a schematic map of the ERP amplitude at and around different electrode locations on the scalp, with the nose pointing north) of the drug cue reactivity (relative to neutral) in that group. The color intensity on the scalp topographies ranges from +3 µV (dark red) to −3 µV (dark blue), such that the red color intensity reflects increased amplitude in response to drug cues vs neutral cues and the blue color intensity reflects decreased amplitude in response to drug cues vs neutral cues.
Immediately following the EEG recordings, participants rated each picture on “liking” (hedonic feelings about cocaine after viewing cocaine images) by responding to the question, “Rate how much you like (or do not like) cocaine in response to this picture” and on “wanting” (craving for cocaine after viewing cocaine images) by responding to the question, “Rate how much you want (or do not want) cocaine in response to this picture,” on a scale of 1 to 9 (where 9 corresponded with most liking and most wanting, and 1 with least liking and least wanting). Separate ratings for liking and wanting cocaine were acquired to dissociate the cue-induced urge for using cocaine (wanting) from the hedonic experiences that drug cues elicit (liking); prior work has shown that self-reported liking and wanting can be dissociated in individuals with cocaine use disorders.33
Statistical Analyses
Data analysis was conducted from June 5, 2015, to March 30, 2016. The dependent variables were LPP amplitude in response to pleasant and unpleasant (all relative to neutral) cocaine-related cues and self-reported baseline craving (total Cocaine Craving Questionnaire score), cocaine liking, and cocaine wanting in response to cocaine-related (relative to neutral) cues (Table28). Between-group differences were analyzed using univariate analyses of variance (ANOVAs) with polynomial contrasts. In addition to the primary polynomial contrasts, Bonferroni corrected post hoc tests were used to further assess pairwise between-group differences. All data were assessed via the interquartile method34 for an objective assessment of outliers; no outliers were identified.
Results
Late Positive Potential
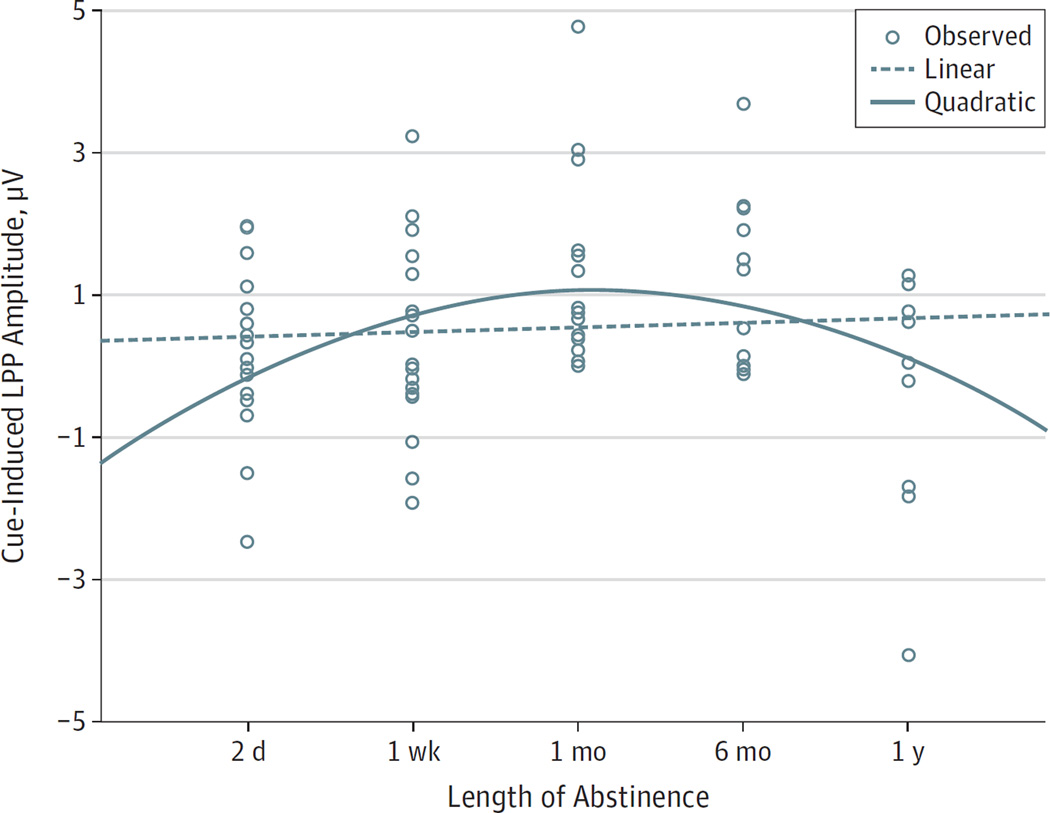
The univariate ANOVA for mean (SD) LPP showed a significant difference in amplitudes among the groups (2 days, 0.17 [1.09] µV; 1 week, 0.36 [1.26] µV; 1 month, 1.26 [1.36] µV; 6 months, 1.17 [1.19] µV; 1 year, −0.27 [1.74] µV; P = .02, partial η2 = 0.16). Consistent with our hypothesis, the polynomial contrast estimates showed that the group effect was quadratic (contrast estimate, −1.131; P = .002) and not linear (contrast estimate, −0.022; P = .95). Pairwise post hoc tests confirmed this parabolic association, showing that mean (SD) LPP-indexed cue reactivity was highest in the individuals with cocaine use disorders with 1 month and 6 months of abstinence compared with the shorter (2 days vs 1 month, 0.17 [1.09] vs 1.26 [1.36] µV; P = .02; 2 days vs 6 months, 0.17 [1.09] vs 1.17 [1.19] µV; P = .04; and 1 week vs 1 month, 0.36 [1.26] vs 1.26 [1.36] µV; P = .04) or longer durations of abstinence (1 month vs 1 year, 1.26 [1.36] vs −0.27 [1.74] µV; P = .005; and 6 months vs 1 year, 1.17 [1.19] vs −0.27 [1.74] µV; P = .01) (Figure 2).
Figure 2. Drug Cue Reactivity Measured With Late Positive Potential (LPP) Amplitude.
A quadratic (solid), rather than linear (dotted), trajectory is seen from 2 days to 1 year of abstinence. Circles indicate observed LPP amplitude.
The univariate ANOVAs for mean (SD) LPPs elicited by pleasant and unpleasant cues (relative to neutral) did not show significant group differences (pleasant: 2 days, 1.20 [2.06] µV; 1 week, 0.74 [0.85] µV; 1 month, 0.20 [1.42] µV; 6 months, 0.88 [1.09] µV; 1 year, 0.41 [1.17] µV; P = .32, partial η2 = 0.06; unpleasant: 2 days, 1.03 [1.25] µV; 1 week, 0.60 [1.17] µV; 1 month, 1.25 [1.10] µV; 6 months, 1.04 [1.58] µV; 1 year, 0.62 [1.65] µV; P = .59, partial η2 = 0.04).
Subjective Assessments
Baseline Craving
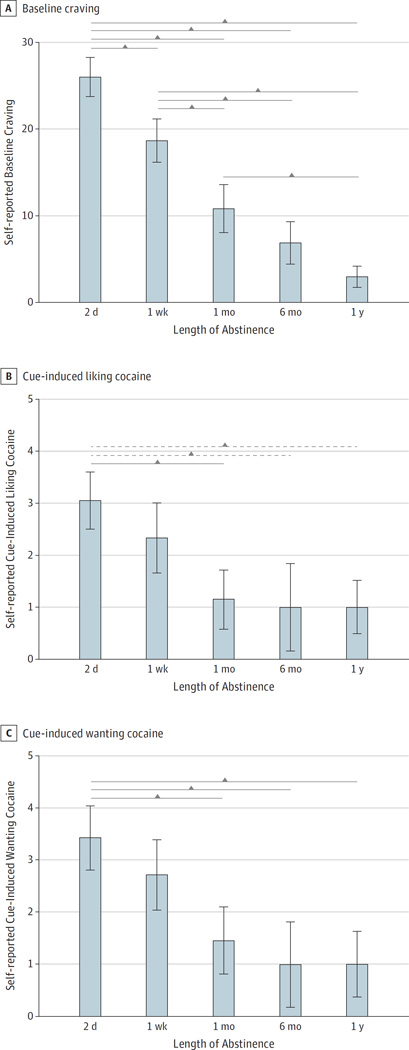
The univariate ANOVA for self-reported mean (SD) ratings of baseline craving showed a significant omnibus group effect (2 days, 26.05 [9.85]; 1 week, 18.70 [11.01]; 1 month, 10.87 [10.70]; 6 months, 6.92 [8.47]; 1 year, 3.00 [3.77]; P < .001, partial η2 = 0.44), such that the baseline craving declined linearly with increasing duration of abstinence (contrast estimate, −18.31; P < .001) and, expectedly, the parabolic quadratic contrast was not significant (contrast estimate, 2.87; P = .27).
Pairwise post hoc tests showed that mean (SD) ratings of baseline craving were significantly higher at 2 days (26.05 [9.85]) compared with 1 week (18.70 [11.01]; P = .02), 1 month (10.87 [10.70]; P < .001), 6 months (6.92 [8.47]; P < .001), and 1 year (3.00 [3.77]; P < .001). Mean (SD) ratings of baseline craving at 1 week (18.70 [11.01]) were higher than craving at 1 month (10.87 [10.70]; P = .02), 6 months (6.92 [8.47]; P = .001), and 1 year (3.00 [3.77]; P < .001) and baseline craving at 1 month (10.87 [10.70]) was higher than that at 1 year (3.00 [3.77]; P = .05) (Figure 3A).
Figure 3. Self-reported Craving and Cue-Induced Liking and Wanting Cocaine.
Ratings continue to decline during an extended duration of abstinence. Solid brackets indicate P < .05; dotted brackets, P < .10; and vertical lines within the bars, ±1 SEM.
Self-reported Liking
The univariate ANOVA for mean (SD) ratings of self-reported cue-induced liking of cocaine also showed a significant group effect (2 days, 3.06 [2.34]; 1 week, 2.33 [2.87]; 1 month, 1.15 [2.03]; 6 months, 1.00 [2.24]; 1 year, 1.00 [1.26]; P = .001, partial η2 = 0.26), such that cue-induced liking of cocaine also declined linearly with increasing duration of abstinence (contrast estimate, −2.71, P = .001). However, the parabolic quadratic contrast was not significant (contrast estimate, 1.11; P = .14).
Pairwise post hoc tests in mean (SD) ratings of cue-induced cocaine liking showed that individuals with cocaine use disorders with 2 days of abstinence reported liking cocaine (after seeing a cocaine picture, relative to a neutral picture) significantly more than did those with 1 month of abstinence (3.06 [2.34] vs 1.15 [2.03]; P = .03), and marginally more than did those with 6 months (3.06 [2.34] vs 1.00 [2.24]; P = .06) and 1 year (3.06 [2.34] vs 1.00 [1.26]; P = .07) of abstinence (Figure 3B).
Self-reported Wanting
The univariate ANOVA for mean (SD) ratings of cue-induced cocaine wanting (or craving) also showed a significant omnibus group effect (2 days, 3.44 [2.62]; 1 week, 2.72 [2.87]; 1 month, 1.46 [2.33]; 6 months, 1.00 [2.16]; 1 year, 1.00 [1.55]; P < .001, partial η2 = 0.30), such that this subjective measure of cue-induced craving declined linearly with increasing duration of abstinence (contrast estimate, −2.97; P = .001). Unexpectedly, and similar to subjective cocaine liking, the parabolic quadratic contrast for wanting was also not significant (contrast estimate, 1.19; P = .12).
Pairwise post hoc tests for mean (SD) ratings of cue-induced cocaine wanting showed that individuals with cocaine use disorders with 2 days of abstinence wanted cocaine (after seeing a cocaine picture, relative to a neutral picture) significantly more than did those with 1month (3.44 [2.62] vs 1.46 [2.33]; P = .04), 6 months (3.44 [2.62] vs 1.00 [2.16]; P = .03), and 1 year (3.44 [2.62] vs 1.00 [1.55]; P = .04) of abstinence (Figure 3C).
Discussion
Using the LPP in response to drug cues as an objective marker of cue-induced craving, we showed that craving initially increases from 2 days to 1 week of abstinence and peaks at 1 month to 6 months before declining by 1 year of abstinence. These findings provide objective confirmation of a parabolic trajectory of cue reactivity as a function of duration of abstinence in individuals with cocaine use disorders, paralleling the time course of cue-induced craving suggested by preclinical reports.3 In contrast, when assessed subjectively, self-reported cue-induced craving (ie, ratings for cue-induced liking and wanting) showed a gradual decrease as a function of increasing duration of abstinence, as did the baseline non-provoked drug craving (assessed via the Cocaine Craving Questionnaire).
The novelty of this study is in its use of LPP amplitude as a proxy measure of motivated attention to drug cues, revealing an inverted U-shaped trajectory of motivated attention to drug cues as a function of duration of abstinence. The choice of using LPP amplitude was guided by previous studies that implicated the LPP in tracking biased cognitive processing of drug-related cues35 and cue-induced craving21 in individuals addicted to controlled substances. In addition, we posited that objective behavioral measures of drug seeking in animal models can be better approximated in humans addicted to substances of abuse by using objective markers of cue reactivity, such as the LPP, rather than subjective measures (eg, craving) that rely on higher-order cognitive functions (eg, introspection). Thus, expanding this type of study beyond self-reported craving allows us to make a more seamless translation between species, commonly a challenging goal.36 This choice was validated, as the LPPs elicited by pleasant and unpleasant (relative to neutral) cues did not show a comparable trajectory, reflecting the specificity of our findings to drug-related cues. Overall, the current findings in individuals with cocaine use disorders are consistent with those of previous reports of heightened behavioral responding for drug-related cues with increasing duration of abstinence in animal models of addictions to cocaine,4,37 heroin,38 methamphetamine,39 alcohol,40 nicotine,41 sucrose,42and saccharine,43with an eventual decline after a protracted period of abstinence.5
However, the results of cue-induced ratings of liking and wanting in our study were not consistent with the cue-induced reports of craving in previous studies13,14,16; instead, the cue-induced ratings in our study followed the same time course as the nonprovoked baseline craving. These inconsistent findings between studies perhaps stem from the variability in the methods used for subjectively quantifying craving: whereas our study inquired about liking and wanting cocaine while the participants viewed cocaine cues, other studies used the difference between cravings before and after cue exposure13,14,16 to quantify cue-induced craving. Additional sources of divergence are the differences in assessing duration of abstinence and group assignment. For example, whereas Bedi et al14 used biochemical assessments to quantify abstinence, others used self-reports and conducted their studies in controlled environments (hospitals and treatment centers) without access to substances of abuse.13,15,16Moreover, while some studies assigned participants randomly to each group,14,15 others, similar to our study, stratified the groups based on duration of abstinence at the time of the study.13,16
Some of the human studies used physiological signals of arousal (eg, heart rate, blood pressure, and skin conductance response) to objectively quantify reactivity to drug cues across durations of abstinence. Although they showed the expected significant response to drug cues compared with neutral cues, these physiological indices did not show differential responding across durations of abstinence.13,15,16 Previous reports have shown that heart rate and skin conductance habituate across repeated presentations of stimuli during a cue reactivity session,44–46 whereas the LPP has consistently shown resistance to habituation.45–47 Specifically, heart rate and skin conductance might reflect initial orienting responses (ie, sensory intake and preparation for action, respectively) to motivationally salient stimuli, which decrease with repeated stimulus presentation as it becomes clear that no adaptation to the initial orientation is necessary.48 In contrast, the LPP reflects stimulus detection and categorization that occurs every time a sensory stimulus is presented46; therefore, the LPP remains robustly detectable even when stimuli are repeated. Such sensitivity and reliability make the LPP amplitude an ideal candidate for detecting the time course of cue-induced reactivity. Nevertheless, the LPP amplitude in our study did not correlate with subjectively assessed cue-induced wanting of cocaine, further indicating the importance of objective measures of cue reactivity that more closely parallel preclinical work on drug-seeking. Indeed, the specificity of the LPP amplitude elicited by drug cues to eventually predicting relapse remains to be further investigated.
More important, and taken together with the LPP results, our findings suggest that subjective measures of craving, which are used clinically to assess treatment outcome, may not be comprehensively assessing objectively quantified cue reactivity. Cue reactivity, which is associated well with animal models of drug-seeking behavior, is perhaps a more translationally valid approach to identify the time course of cue-induced responses and vulnerability to relapse in abstinent individuals addicted to controlled substances. That is, because elevated drug cue reactivity is associated with subsequently increased drug use or relapse in initially abstinent individuals addicted to controlled substances,49 by inference, the time course of cue reactivity identified in our study may also reflect the time course of vulnerability to relapse in these individuals. Therefore, our results are also clinically crucial as they highlight an abstinence period (ie, 1–6 months) during which individuals addicted to controlled substances may be most vulnerable to relapse and may benefit most from a targeted intervention, thereby providing clinicians with better treatment planning. This period of vulnerability may occur without conscious awareness, and may perhaps be associated with reduced vigilance of one’s heightened reactivity to drug cues, which may contribute to relapse.
The current study replicates preclinical reports of a parabolic trajectory of cue reactivity using a cross-sectional design in humans. Such a design, especially when group membership is based on self-reported duration of abstinence and without randomization, can potentially lead to a self-selection bias, even when the groups are matched on demographics and drug use history, as in our study. As reflected in an associated limitation of this study, the between-group variability in duration of abstinence was large. For example, the range of duration of abstinence in the groups with 2 days and 1week of abstinence was different than that in the groups with 6 months and 1 year of abstinence (1–2 and 3–15 days vs 121–206 and 215–570 days, respectively). Future longitudinal studies are therefore warranted to replicate the results of our study in a randomized and within-individuals design and to extend our results by leveraging individual variability in the trajectory of cue reactivity for personalized prediction of relapse and intervention planning. Our study also does not inform about the specific neural mechanisms underlying the identified quadratic shifts in cue reactivity. Futures studies may build on the current findings and use spatially precise neuroimaging techniques, such as functional magnetic resonance imaging, to further our understanding of the neural mechanisms underlying incubation of cue reactivity and its eventual decline. However, the goal of our study was to leverage the high temporal resolution and easier clinical translatability of EEG results, which may soon be implemented in clinical settings for timely quantification of temporally precise cue reactivity and to facilitate personalized treatment in individuals addicted to controlled substances.
Taken together, our results may have identified an objectively ascertained period of high vulnerability to relapse that coincides with the window of discharge from most treatment programs, perhaps increasing the propensity for relapse. Therefore, our results could help guide the implementation of alternative individually tailored and optimally timed intervention, prevention, and treatment strategies. Indeed, EEG is highly affordable and portable and therefore deployable in treatment centers for online assessment of cue-induced craving. More broadly, our results promise comparisons between species, from rodents to nonhuman primates to humans,50 to enhance understanding of the basic neurobiological mechanisms underlying the incubation of cue-induced craving in drug addiction.
Conclusions
Our study objectively measures cue-induced craving in individuals with cocaine use disorders using an EEG marker to support the parabolic trajectory of incubated craving observed in preclinical studies, an effect that was not observed with subjective assessments of craving, indicating a vulnerability that may operate below conscious awareness. These clinically significant results highlight 1month and 6months of abstinence as the period during which abstaining individuals addicted to controlled substances may be most vulnerable to, and perhaps least cognizant of, the risk of relapse.
Key Points.
Question
Does cue-induced craving follow a parabolic trajectory as a function of duration of abstinence in humans with cocaine use disorders, as previously shown in preclinical studies?
Findings
The results of this laboratory-based cross-sectional study confirm the prior preclinical reports of a parabolic trajectory of cue-induced craving, but only when assessed objectively using the electroencephalographic measures of cue reactivity and not via subjective reports of craving.
Meaning
These translational findings highlight an abstinence period during which individuals addicted to cocaine are perhaps most vulnerable to relapse and show that the use of objective assessments is important to uncover such a clinically crucial phenomenon in individuals addicted to cocaine who are seeking treatment.
Acknowledgments
Funding/Support: This study was supported by grants F32DA033088 (Dr Parvaz), 1K01DA037452 and 1R21DA40046 (Dr Moeller), and R01DA023579, 1R21DA034954-01, and R01DA041528-01 (Dr Goldstein) from the National Institute on Drug Abuse.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Parvaz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: All authors.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Parvaz.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: All authors.
Obtaining funding: Moeller, Goldstein.
Administrative, technical, or material support: Goldstein.
Study supervision: Goldstein.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229(3):453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers: clinical observations. Arch Gen Psychiatry. 1986;43(2):107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 3.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation: incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 2004;176(1):101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- 6.Lu L, Wang X, Wu P, et al. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66(2):137–145. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24(7):1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mameli M, Halbout B, Creton C, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12(8):1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 9.Conrad KL, Tseng KY, Uejima JL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BR, Ma YY, Huang YH, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16(11):1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016;17(6):351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Rubio FJ, Zeric T, et al. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and Trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci. 2015;35(21):8232–8244. doi: 10.1523/JNEUROSCI.1022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8(7):e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedi G, Preston KL, Epstein DH, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69(7):708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li P, Wu P, Xin X, et al. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol. 2015;20(3):513–522. doi: 10.1111/adb.12140. [DOI] [PubMed] [Google Scholar]
- 16.Wang GB, Zhang XL, Zhao LY, et al. Drug-related cues exacerbate decision making and increase craving in heroin addicts at different abstinence times. Psychopharmacology (Berl) 2012;221(4):701–708. doi: 10.1007/s00213-011-2617-5. [DOI] [PubMed] [Google Scholar]
- 17.Williamson A. Using self-report measures in neurobehavioural toxicology: can they be trusted? Neurotoxicology. 2007;28(2):227–234. doi: 10.1016/j.neuro.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. J Consult Psychol. 1960;24:349–354. doi: 10.1037/h0047358. [DOI] [PubMed] [Google Scholar]
- 19.Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci. 2014;18(12):635–641. doi: 10.1016/j.tics.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajcak G, MacNamara A, Foti D, Ferri J, Keil A. The dynamic allocation of attention to emotion: simultaneous and independent evidence from the late positive potential and steady state visual evoked potentials. Biol Psychol. 2013;92(3):447–455. doi: 10.1016/j.biopsycho.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Franken IH, Dietvorst RC, Hesselmans M, Franzek EJ, van de Wetering BJ, Van Strien JW. Cocaine craving is associated with electrophysiological brain responses to cocaine-related stimuli. Addict Biol. 2008;13(3–4):386–392. doi: 10.1111/j.1369-1600.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 22.Moeller SJ, Hajcak G, Parvaz MA, Dunning JP, Volkow ND, Goldstein RZ. Psychophysiological prediction of choice: relevance to insight and drug addiction. Brain. 2012;135(pt 11):3481–3494. doi: 10.1093/brain/aws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunning JP, Parvaz MA, Hajcak G, et al. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users—an ERP study. Eur J Neurosci. 2011;33(9):1716–1723. doi: 10.1111/j.1460-9568.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krajbich I, Armel C, Rangel A. Visual fixations and the computation and comparison of value in simple choice. Nat Neurosci. 2010;13(10):1292–1298. doi: 10.1038/nn.2635. [DOI] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 26.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 27.Kampman KM, Volpicelli JR, McGinnis DE, et al. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23(4):449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 28.Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br J Addict. 1992;87(11):1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 29.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34(1):19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- 30.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Gainsville: University of Florida; 2008. [Google Scholar]
- 31.Nolte G, Hämäläinen MS. Partial signal space projection for artefact removal in MEG measurements: a theoretical analysis. Phys Med Biol. 2001;46(11):2873–2887. doi: 10.1088/0031-9155/46/11/308. [DOI] [PubMed] [Google Scholar]
- 32.Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26(1):99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein RZ, Woicik PA, Moeller SJ, et al. Liking and wanting of drug and non-drug rewards in active cocaine users: the STRAP-R questionnaire. J Psychopharmacol. 2010;24(2):257–266. doi: 10.1177/0269881108096982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilcox RR. Fundamentals of Modern Statistical Methods. 2nd. New York, NY: Springer; 2010. [Google Scholar]
- 35.Littel M, Euser AS, Munafò MR, Franken IH. Electrophysiological indices of biased cognitive processing of substance-related cues: ameta-analysis. Neurosci Biobehav Rev. 2012;36(8):1803–1816. doi: 10.1016/j.neubiorev.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Moeller SJ, Stoops WW. Cocaine choice procedures in animals, humans, and treatment-seekers: can we bridge the divide? Pharmacol Biochem Behav. 2015;138:133–141. doi: 10.1016/j.pbb.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19(1):48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- 38.Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 2001;156(1):98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- 39.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55(11):1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 40.Bienkowski P, Rogowski A, Korkosz A, et al. Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur Neuropsychopharmacol. 2004;14(5):355–360. doi: 10.1016/j.euroneuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31(4):733–741. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84(1):73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aoyama K, Barnes J, Grimm JW. Incubation of saccharin craving and within-session changes in responding for a cue previously associated with saccharin. Appetite. 2014;72:114–122. doi: 10.1016/j.appet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breiter HC, Etcoff NL, Whalen PJ, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 45.Codispoti M, Ferrari V, Bradley MM. Repetition and event-related potentials: distinguishing early and late processes in affective picture perception. J Cogn Neurosci. 2007;19(4):577–586. doi: 10.1162/jocn.2007.19.4.577. [DOI] [PubMed] [Google Scholar]
- 46.Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: autonomic and cortical correlates. Brain Res. 2006;1068(1):213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Olofsson JK, Polich J. Affective visual event-related potentials: arousal, repetition, and time-on-task. Biol Psychol. 2007;75(1):101–108. doi: 10.1016/j.biopsycho.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty, and the startle reflex: habituation in humans. Behav Neurosci. 1993;107(6):970–980. doi: 10.1037//0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- 49.Courtney KE, Schacht JP, Hutchison K, Roche DJ, Ray LA. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol. 2016;21(1):3–22. doi: 10.1111/adb.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Caprioli D, Marchant NJ. Recent updates on incubation of drug craving: a mini-review. Addict Biol. 2015;20(5):872–876. doi: 10.1111/adb.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]