Abstract
Leucine Rich Repeat Containing 8A (LRRC8A) is a required component of volume-regulated anion channels (VRACs). In vascular smooth muscle cells, tumor necrosis factor-α (TNFα) activates VRAC via type 1 TNFα receptors (TNFR1), and this requires superoxide (O2•−) production by NADPH oxidase 1 (Nox1). VRAC inhibitors suppress the inflammatory response to TNFα by an unknown mechanism. We hypothesized that LRRC8A directly supports Nox1 activity, providing a link between VRAC current and inflammatory signaling. VRAC inhibition by 4-(2-butyl-6,7-dichlor-2-cyclopentylindan-1-on-5-yl) oxobutyric acid (DCPIB) impaired NF-κB activation by TNFα. LRRC8A siRNA reduced the magnitude of VRAC and inhibited TNFα-induced NF-κB activation, iNOS and VCAM expression, and proliferation of VSMCs. Signaling steps disrupted by both siLRRC8A and DCPIB included; extracellular O2•− production by Nox1, c-Jun N-terminal kinase (JNK) phosphorylation and endocytosis of TNFR1. Extracellular superoxide dismutase, but not catalase, selectively inhibited TNFR1 endocytosis and JNK phosphorylation. Thus, O2•− is the critical extracellular oxidant for TNFR signal transduction. Reducing JNK expression (siJNK) increased extracellular O2•− suggesting that JNK provides important negative feedback regulation to Nox1 at the plasma membrane. LRRC8A co-localized by immunostaining, and co-immunoprecipitated with, both Nox1 and its p22phox subunit. LRRC8A is a component of the Nox1 signaling complex. It is required for extracellular O2•− production, which is in turn essential for TNFR1 endocytosis. These data are the first to provide a molecular mechanism for the potent anti-proliferative and anti-inflammatory effects of VRAC inhibition.
Keywords: Leucine Rich Repeat Containing 8, NADPH oxidase 1, Superoxide, Tumor necrosis factor-α, c-Jun N-terminal kinase
1. Introduction
Volume-regulated anion channels (VRACs) contribute to the control of cell size and produce swelling-activated anion currents (IClswell). In addition to an intrinsic ability to response to low ionic strength [1], this conductance can be activated by extracellular, NADPH oxidase-derived hydrogen peroxide (H2O2) produced in response to cell swelling or growth factors [2,3]. VRAC is required for proliferation of multiple cell types including vascular smooth muscle (VSMC) [4,5], where it has been well characterized [6,7]. It was proposed that changes in cell volume are associated with rapid nutrient uptake and cytokinesis which necessitates a concurrent regulatory volume decrease (RVD, reviewed in [8]). Consistent with this, IClswell magnitude and capacity to undergo an RVD vary with the cell cycle [9,10], becoming smaller as cells transition from a proliferative to a differentiated phenotype [11]. However, no specific biochemical link has been made between VRAC and proliferative or inflammatory signaling. TNFα, which causes NF-κB-dependent VSMC proliferation [12] activates VRAC under perforated patch recording conditions [13,14]. We sought to test the hypothesis that VRAC activation by cytokines represents a required step in oxidant-dependent signal transduction.
VRAC has been alternatively referred to as IClswell, the volume-activated anion current (IClvol), the Volume-Sensitive Outwardly Rectifying Anion Channel (VSOR), or the Voltage-Sensitive Osmolyte and Anion Channel (VSOAC). A lack of linkage between VRAC and a specific protein has limited understanding of its biologic role. Numerous candidate genes have failed to pass detailed testing, including; pICln, p-glycoprotien, phospholemmon, CLIC1, band 3, VDAC, TMEM16A-F and ClC-3 (reviewed in [15]). Recently, two labs independently concluded that the LRRC8 family of proteins comprises VRACs [16,17]. LRRC8A is required for the RVD elicited by hypotonicity and expressed LRRC8 proteins recapitulate the essential biophysical characteristics of VRACs including; time-dependent inactivation, ion selectivity (I > Br > Cl) and the ability to conduct large anions such as glutamate and taurine [17,18]. A mutation in human LRRC8A caused severely impaired B-cell function and introduction of this mutation into murine bone marrow disrupted both B- and T-cell development [19]. In cultured cells, this mutation in LRRC8A disrupted IClswell [17].
The LRRC8 family has 5 members (A–E). All contain four highly-conserved transmembrane domains which share homology with the pannexin family [17,20]. Like pannexins, LRRC8 proteins form multi-meric channels (6–8 subunits) whose properties depend upon the family members expressed [1]. Heterologously expressed LRRC8A is found both in intracellular vesicles and at the plasma membrane, but does not produce ion current. Other expressed LRRC8 isoforms (B–E) are primarily intracellular. However, co-expression of LRRC8A with LRRC8C, D or E induces membrane localization of both proteins and yields isoform-specific currents that differ in single channel conductance, open probability, rectification, ion selectivity and time-dependent inactivation [1]. Thus, LRRC8A is an obligatory component of VRACs [17]. It is not yet known if LRRC8 channels are active at intracellular sites. Although previous experiments have linked VRAC currents to proliferative signaling, the specific role of LRRC8 family proteins has not been assessed.
We combined targeted knockdown of LRRC8A protein with selective VRAC inhibition to assess the role of LRRC8A in the response of VSMCs to TNFα. Indices of the overall inflammatory response (NF-κB activation, iNOS and VCAM expression, proliferation) and specific proximal signaling steps (extracellular O2•− production by Nox1, JNK phosphorylation, TNFα receptor endocytosis) were measured. We found that LRRC8A and VRAC must both be present for TNFα to activate Nox1 in the plasma membrane. The O2•− that Nox1 produces extracellularly is required for signaling at both the plasma membrane (JNK phosphorylation) and in the cytoplasm (NF-κB activation). The cytoplasmic signaling impact of VRAC disruption is at least partially indirect, related to the fact that when extracellular O2•− is absent, TNFα receptor endocytosis does not occur. Therefore, VRAC potently affects both the plasmalemmal and endosomal phases of TNFα signaling.
2. Materials and methods
2.1. Reagents
DCPIB was purchased from Tocris Bioscience (United Kingdom). All other chemicals and enzymes were obtained from Sigma-Aldrich (St. Louis, MO).
2.2. Cell culture
NF-κB reporter (Luc)-HEK293 cells (BPS Bioscience, San Diego, CA) were maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. Primary aortic VSMC were obtained from C57/BL6 mice as described previously [21]. The cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM; Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 1× minimum essential medium non-essential amino acids, 1X vitamin, and 20 mM HEPES. The cells were incubated at 37 °C in a humidified 5% CO2 atmosphere.
2.3. siRNA transfection
siRNA (negative control, LRRC8A, Nox1, p22phox, JNK1) were purchased from Dharmacon (Lafayette, CO). siRNA (100 nM) was incubated with Lipofectamine 2000 (Life Technologies) in serum-free medium for 20 min. The resultant complex of siRNA-Lipofectamine 2000 was added to cells in DMEM containing 5% FBS and then maintained for 3 days before performing experiments.
2.4. Adenoviral-mediated gene transfer
Control virus (eGFP) and dominant-negative dynamin K44A were obtained from the Gene Transfer Vector Core at the University of Iowa (Iowa City, IA). Adenoviruses were added to VSMC (80% confluence, 10–30 MOI) in DMEM containing 5% FBS. Experiments were performed after 48 h.
2.5. NF-κB activity
NF-κB reporter (Luc)-HEK293 cells were used to quantify NF-κB activation in HEK293 cells. VSMC were infected with replication-deficient adenovirus expressing a luciferase reporter driven by NF-κB transcriptional activation for 40 h in DMEM containing 5% FBS followed by exposure to TNFα (10 ng/mL) in serum-free DMEM for 6 h. Inhibitors were incubated for 30 min prior to TNFα exposure. For siRNA transfected cells, adenovirus was infected two days after transfection of siRNA. Luciferase activity (relative light units) was quantified according to the manufacturer's protocol (Promega, Madison, WI) and normalized to protein concentration (BCA protein assay).
2.6. Patch-clamp recording
Three days following siRNA exposure cells were dissociated (trypsin) into fresh culture media, and stored for up to 4 h at 4 °C. Isotonic (300 mOsM) solution contained (mM): 130 NaCl, 1.8 MgCl2, 1.8 CaCl2, 10 HEPES, 1–2 NaOH, pH 7.4, and osmolality was adjusted to 300mosm with mannitol using a Precision Systems μOsmette osmometer (Natick, MA). Hypotonic external buffer contained no mannitol, and measured ~255mOsM. Pipette solution contained (mM): 120 CsCl, 4 TEACl, 2 MgCl2, 5 Na2ATP, 10 HEPES, 1–2 CsOH, 1.186 CaCl2, 5 EGTA, osmolality 290mosm (mannitol), estimated free Ca2+ of 61 μm using WEBMAXC; http://www.stanford.edu/~cpatton/webmaxc/webmaxcS.htm), pH 7.2. Pipette resistances were 2.5–4 MΩ⊡ Currents were filtered at 5 kHz and sampled at 100 kHz using an Axopatch 200B amplifier and pClamp 10 (Molecular Devices, Sunnyvale, CA). Whole-cell capacitance and series resistance (5–8 MΩ) was nulled and 60–65% series resistance compensation applied. Currents were recorded in response to 50 ms pulses (−100 mV to +160 mV, 20 mV increments) from a holding potential of −40 mV. Current amplitudes were averaged from a 2 ms segment at the end of each pulse in isotonic conditions, or mid-pulse in hypotonic conditions.
To achieve plasma membrane expression of ClC-3, a chimeric construct was created using the Stratagene (La Jolla, CA) QuikChange Lightning kit. A rat ClC-3-GFP fusion protein in pEGFP-N1 (Genebank XP_006253138.1, gift from S.A. Weinman, University of Kansas) was subcloned into pcDNA3.1. The first 59 N-terminal amino acids, which includes a clathrin-binding dileucine cluster (LLDLLD) which drives internalization, were replaced by the first 46 N-terminal amino acids of human ClC-5 (Genebank NP_000075.1) which has no internalization signal. Plasmids were transfected using Lipofectamine 2000 according to the manufacturer's instructions. This chimera is referred to as ClC-5/3.
2.7. Western blot analysis
Cells were serum-deprived (0.5% serum) for 3 h and then stimulated with TNFα (10 ng/mL). Protein extracts (40 μg) were separated by electrophoresis on a polyacrylamide gel (10%) and transferred to nitrocellulose membranes. Nonspecific binding was blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 h at room-temperature. Membranes were incubated with primary antibodies overnight at 4 °C. Antibodies included: p-JNK (#9255, Cell Signaling Technology, Danvers, MA), JNK (#9252, Cell Signaling Technology), p-ERK (#9101, Cell Signaling Technology), ERK (#9102, Cell Signaling Technology), Tubulin (Vanderbilt Antibody Core), iNOS (#610328, BD Transduction, San Jose, CA), VCAM (#AF643, R & D Systems, Minneapolis, MN), LRRC8A (#A304-175A, Bethyl Laboratories, Montgomery, TX), p22phox (#sc-271968, Santa Cruz Biotechnology, Dallas, TX), Nox1 (#SAB2501686, Sigma-Aldrich). Signals were developed using fluorescent secondary antibodies with the Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE) and quantified densitometrically.
2.8. Sulforhodamine B (SRB) assay
VSMCs grown on 96-well plates were treated with TNFα (10 ng/mL) for 24 h, fixed with 5% cold trichloroacetic acid for a 1 h at 4 °C, washed with water and air-dried at room temperature, and stained with 0.057% SRB solution for 30 min and rinsed with 1% acetic acid. After again drying, 10 mM Tris base (pH 10.5) was added and the OD was measured at 510 nm in a microplate reader (FLUOstar Omega, BMG Labtech).
2.9. Detection of TNFR endocytosis
Cells were grown on chamber slides, and incubated with siRNA for 3 days. Human TNFα conjugated with biotin (R & D Systems) was incubated with FITC-labeled avidin (Life Technologies) for 1 h at 4 °C, and then exposed to cells for 2 h at 4 °C. After washing with cold media, cells were warmed to 37 °C for 15 min. Cells were then fixed in 3.7% formaldehyde and nuclear counterstaining was performed with To-Pro-3 (Life Technologies) for 5 min. Cover-slides were mounted with ProLong Gold anti-fade reagent (Life Technologies). Cells were imaged by fluorescence confocal microscopy and FITC signal quantified using ImageJ software. For transferrin endocytosis, alexa fluor 546 conjugated transferrin (50 μg/mL, Life Technologies) was incubated instead of TNFα complex (biotin-avidin).
2.10. Detection of reactive oxygen species
Extracellular and endosomal superoxide was quantified using the membrane-impermeable electron spin resonance (ESR) probe 1-Hydroxy-2,2,6,6-tetramethylpiperidin-4-yl-trimethylammonium chloride (CAT1H; Enzo Life Sciences). Cells were incubated with Krebs/HEPES containing CAT1H (0.5 mmol/L) and TNFα (10 ng/mL) for 20 min then washed, scraped and snap frozen and placed in an ESR finger Dewar under liquid nitrogen. ESR spectra were recorded from the cell samples (endosomal) or from the Krebs buffer (extracellular) using the following settings: field sweep, 80 G; microwave frequency, 9.39 GHz; microwave power, 2 mW; modulation amplitude, 5 G; conversion time, 327.68 ms; time constant, 5242.88 ms; 512 points resolution; and receiver gain, 1×104. The ESR signal was normalized to protein concentration.
2.11. Immunoprecipitation (IP)
Cells were stimulated with TNFα (10 ng/mL) then lysed (0.01 M Tris base, 1 mM EDTA, 30 mM NaCl, 1% Nonidet P40, protease inhibitor cocktail, and phenylmethylsulfonyl fluoride (PMSF) at pH 7.4) for 1 h with nutation at 4 °C, and centrifuged for 30 min at 20,000g. Supernatants were pre-cleared with protein-G sepharose beads for 1 h at 4 °C and cleared-supernatants were incubated with antibody (2 μg) for 1.5 h, then incubated with protein-G sepharose for 1 h. Beads were washed with lysis buffer, resuspended in SDS sample buffer, boiled and the associated proteins were then analyzed by western blot. Antibodies for IP were used as follows: LRRC8A (#A304-175A, #A304-173A, Bethyl Laboratories), p22phox (#sc-20781, #sc-11712, Santa Cruz Biotechnology).
2.12. Immunofluorescence
VSMC were grown on glass coverslips and fixed in 3.7% formaldehyde in PBS for 10 min at room-temperature then washed with PBS. Cells were permeabilized with 1% BSA and 0.5% Triton-X 100 for 10 min, blocked with 1% BSA in PBS for 30 min, and then incubated with the primary antibodies in PBS+1% BSA for 2 h at 37 °C. Antibodies were used as follows: LRRC8A (1:200, #A304-173A, Bethyl Laboratories), Nox1 (1:100, Mox1 #sc-5821, Santa Cruz Biotechnology), p22phox (1:50, #sc-11712, Santa Cruz Biotechnology). After washing with PBS, secondary antibodies were incubated for 1 h at 37 °C and mounted in Prolong Gold Antifade reagent (ThermoFisher). Cover-slides were imaged by Leica SPE confocal laser microscope. The images were analyzed by ImageJ software.
2.13. Statistical analysis
Values are mean ± standard error of the mean (SEM), and `n' represents the number of independently performed experiments in cultured cells. Graphs were generated using Graph Pad Prism 5.0 (GraphPad Software, San Diego, CA). Statistical differences were assessed by Student's t-test or one-way ANOVA. Post hoc comparisons were performed using Newman-Keuls analysis to compare all groups. A P value less than 0.05 was considered to be statistically significant.
3. Results
To begin to explore the role of LRRC8A in inflammation, we initially used HEK293 cells that were engineered to endogenously express a transgene for the NF-κB luciferase reporter, (NF-κB reporter (Luc)-HEK293 cells). These cells were used as an initial screening tool to quantify the impact of two VRAC inhibitors, tamoxifen and DCPIB, on TNFα signaling. At a concentration that potently inhibits IClswell (10 μM, 80–90% inhibition [22]), tamoxifen significantly reduced NF-κB activation by TNFα (10 ng/mL) in HEK293 cells (Fig. 1A). Since tamoxifen also interacts with the estrogen receptor, we used 4-(2-Butyl-6,7-dichlor-2-cyclopentylindan-1-on-5-yl) oxybutyric acid (DCPIB), a potent (IC50 of ~ 3 μM [4]) and more selective inhibitor of IClswell. DCPIB caused concentration-dependent inhibition of NF-κB activation (Fig. 1B, IC50 =5.9 ± 1.9 μM, n =4–10). This effective concentration was consistent with DCPIB's documented ability to inhibit VSMC proliferation with an IC50 of ~7 μM [4]. DCPIB is quite selective for VRAC over other anion channels [23]. It can also inhibit connexin hemi-channels [24], but expression of these proteins is very low in VSMC [25]. Therefore, to enhance the specificity of VRAC inhibition we targeted LRRC8A protein using siLRRC8A. This also significantly inhibited NF-κB activation by TNFα in HEK293 cells (Fig. 1C).
Fig. 1.
Disruption of LRRC8A impairs NF-κB activation by TNFα. A–C: NF-κB activation in HEK293 cells. Tamoxifen (10 μM, A) and DCPIB (B) attenuate NF-κB activation. *p < 0.05 compared to Control in DMSO, †p < 0.05 compared to TNFα only (n=4 to 10). C: siLRRC8A reduces NF-κB activation in HEK293 cells. *p < 0.05 compared to siControl only, †p < 0.05 (n=5). D: In VSMC, DCPIB (30 μM) decreases NF-κB activation. *p < 0.05 compared to DMSO, †p < 0.05 (n=6). E: NF-κB activation was measured in the presence of siRNA targeting LRRC8A in VSMC. siLRRC8A decreases NF-κB activation. *p < 0.05 compared to siControl, †p < 0.05 (n=6). Results are mean ± SEM in each experimental group after normalization to control.
Since our primary interest is in TNFα-mediated vascular inflammatory signaling, we next extended these observations to VSMCs, a cell type in which we possess a more detailed understanding of the topology of TNFα signaling [21,26,27]. All subsequent data are from VSMCs. NF-κB activation in VSMC is almost completely attributable to the type 1 receptor subtype (TNFR1), and is largely dependent upon endosomal signaling (reduced ~60–70% by inhibition of endocytosis [21]). To determine if LRRC8A is required for IClswell in VSMC, we assessed the impact of siLRRC8A. It caused a very significant, but incomplete reduction in the response to hypotonic conditions (Fig. 2). The magnitude of the siRNA effect was variable between cells, as might be anticipated given the potential for cell-to-cell variation in siRNA uptake. Recent work has demonstrated a cell type-associated, variable dependence of IClswell on LRRC8A and suggested that other anion channels may also contribute to this response [28]. In contrast, IClswell was completely inhibited by 30 μM DCPIB. Both DCPIB and siLRRC8A, which reduced overall LRRC8A protein abundance by ~70% (Fig. 3A), significantly inhibited NF-κB activation by TNFα in VSMCs (Fig. 1D and E). To confirm the anti-inflammatory effects observed in the luciferase assays we also quantified the impact of siLRRC8A and DCPIB on NF-κB p65 protein phosphorylation. Very similar trends were observed (Suppl. Fig. S1).
Fig. 2.
Inhibition of VRAC by siLRRC8A or DCPIB in VSMC. A and B: Whole-cell currents recorded from cells treated with scrambled siRNA (siControl) (A), or siLRRC8A (B). Top: Baseline current level (50 ms pulses, −100 mV to +160 mV test potentials) in isotonic solution. Middle: Currents after 6–8 min exposure to hypotonic conditions. Bottom: Block of hypotonicity-induced currents by 30 μM DCPIB. Dotted lines show zero-current level. C: Current-voltage relationships. Mean current density of IClswell is significantly reduced across the entire voltage range in cells treated with siLRRC8A. *p < 0.05; Mann-Whitney test; siControl (n=7), siLRRC8A (n=10). D: Scatter plots of individual current densities recorded at −100 mV (left) and +120 mV (right), with mean and SEM values shown by lines. *p < 0.05.
Fig. 3.
siLRRC8A inhibits TNFα-induced inflammation in VSMC. A: Representative western blot images show expression of iNOS, VCAM, and LRRC8A after 48hrs of exposure to TNFα. B and C: Bar graphs show the relative abundance of proteins after normalization to tubulin expression. *p < 0.05 compared to Control in siControl. †p < 0.05 (n=4). D: siLRRC8A decreases TNFα-mediated cell survival in VSMC. Results are presented as mean ± SEM in each experimental group after normalization to control (siControl) levels. *p < 0.05 compared to Control in siControl, †p < 0.05 (n=10).
We next explored the relationship between LRRC8A abundance and downstream indices of inflammation. Both inducible nitric oxide synthase (iNOS) and vascular cell adhesion molecule-1 (VCAM-1) expression were increased following a 48hr exposure to TNFα (Fig. 3A–C) and these effects were inhibited by siLRRC8A. TNFα also caused a small, but significant increase in the expression of LRRC8A itself (Fig. 3A; ~1.3 fold increase). TNFα causes proliferation of VSMC and we observed an increase in the abundance of live VSMCs remaining in culture following a 24hr exposure to TNFα (Sulforhodamine B assay). This increase was blocked by siLRRC8A (Fig. 3D).
TNFα signaling occurs at both the plasma membrane and within endosomes [21]. Since targeting of LRRC8A clearly disrupted endosome-dependent signaling (NF-κB) it was important to determine if this compartment was properly assembled. We therefore assessed TNFα-dependent formation of “signaling endosomes”. TNFα-induced endocytic events were selectively quantified using biotin-labeled human TNFα and FITC-avidin [29]. Human TNFα selectively binds to TNFR1 in murine tissues [30]. Both siLRRC8A and DCPIB reduced the ability of VSMCs to form TNFα receptor-containing endosomes (Fig. 4). This was not a generalized effect on receptor endocytosis. DCPIB did not alter the uptake of labeled transferrin (Suppl. Fig. S2). Disruption of TNFα receptor endocytosis can account for inhibition of NF-κB activation and suggested that other strictly endosome-dependent effects of TNFα might also be inhibited. ERK phosphorylation is also very endocytosis-dependent [21], and was markedly reduced by siLRRC8A (Suppl. Fig. S3A).
Fig. 4.
Endocytosis of TNFR requires LRRC8A and Nox1-dependent O2−• production. Endocytosis was assessed using biotin-conjugated TNFα and FITC-avidin as described in Methods. A: Representative images show that TNFR endocytosis (white dots) is reduced by DCPIB (30 μM) in VSMC. B: After indicated siRNA transfection for 3days, TNFR endocytosis was assayed in VSMC. siLRRC8A and siNox1 attenuate TNFR endocytosis, but siJNK1 does not alter it. *p < 0.05 vs. siControl 0 min †p < 0.05. C: DCPIB (30 μM), SOD (500 U/mL), or catalase (1000 U/mL) was added right before 37 °C incubation (15 min). DCPIB and SOD reduce TNFR endocytosis, but catalase has no effect. Bar graphs reflect quantification of fluorescent dots per cell. *p < 0.05 vs. Control 0 min. †p < 0.05 compared to Control 15 min. Results are representative of at least three independent experiments.
To determine if receptor endocytosis requires Nox1 activation we employed siNox1, which also reduced TNFα endocytosis (Fig. 4B). Knockdown of Nox1 by siRNA was verified by western blotting (Suppl. Fig. S4A). To assess the role of O2−• in endocytosis, exogenous superoxide dismutase (SOD, 500 U/mL) was used. SOD strongly inhibited TNFα receptor endocytosis (Fig. 4C) and ERK phosphorylation (Suppl. Fig. S3B). In contrast, catalase (1000 U/mL) was completely without effect, suggesting that O2•−, not H2O2, is the critical signaling intermediate for the triggering of TNFR1 endocytosis. We next measured O2•− production directly using CAT1H. Extracellular O2•− was produced in response to TNFα, demonstrating that Nox1 activation precedes TNFR endocytosis. Both siLRRC8A and DCPIB impaired extracellular O2•− production (Fig. 5A and B), suggesting that VRAC current is required for Nox1 activity. Consistent with impaired endocytosis and Nox1 activity, the endosomal O2•− signal (cellular CAT1H) was also greatly reduced (Suppl. Fig. S5).
Fig. 5.
TNFα-induced extracellular O2−• production is associated with LRRC8A and JNK in VSMC. A: siLRRC8A impairs the increase in the CAT1H signal following TNFα exposure (n=4). B: DCPIB (30 μM) similarly reduces TNFα-induced extracellular O2−• production (n=4). C: siJNK1 potentiates TNFα-induced extracellular O2−• (n=5–7). D: Following adenoviral infection with eGFP (control) or DynK44A TNFα-induced extracellular superoxide is not altered (n=4). Results are presented as mean ± SEM in each experimental group after normalization to protein concentration. *p < 0.05 compared to Control (or siControl only), †p < 0.05.
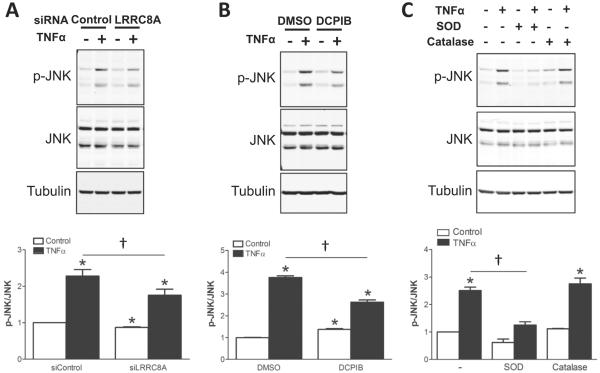
Failure of endosome formation in the absence of VRAC activity prevented further assessment of the contribution of LRRC8A to endosomal signaling, but this does not preclude an important role at that site. JNK activation is also Nox1-dependent, but occurs primarily at the plasma membrane. It may provide negative feedback regulation of Nox1 [21]. We therefore assessed the impact of siLRRC8A and DCPIB on JNK phosphorylation and determined that both were inhibitory (Fig. 6A and B). JNK phosphorylation also depended upon extracellular O2•− as it was inhibited by SOD but not catalase (Fig. 6C). We explored JNK regulation of Nox1 at the plasma membrane and observed that siJNK significantly enhanced extracellular O2•− production (Fig. 5C) but did not affect receptor endocytosis (Fig. 4B). Thus, endocytosis seems to be an unlikely mechanism by which extracellular O2•− production is terminated by JNK. Consistent with this, complete disruption of endocytosis using dominant negative dynamin (Dyn K44A) had no effect on extracellular O2•− (Fig. 5D).
Fig. 6.
JNK activation (phosphorylation) is associated with LRRC8A and extracellular O2−• in VSMC. A: siLRRC8A reduces JNK phosphorylation (p-JNK) by TNFα (10 ng/mL) for 10 min (n=7). B: DCPIB (30 μM) also attenuates JNK phosphorylation (n=3). C: SOD (500 U/mL, 10 min) reduces JNK phosphorylation, but catalase (1000 U/mL, 10 min) does not affect it (n=4–8). Bar graphs show the relative abundance of p-JNK after normalization to JNK expression. Results are presented as mean ± SEM in each experimental group after normalization to control. *p < 0.05 compared to Control. †p < 0.05.
Similarities in the signaling impact of Nox1 [21] and LRRC8A deficiency on O2•− production, TNFR1 endocytosis and JNK activation suggested that LRRC8A is closely linked to Nox1-mediated O2•− production. We therefore sought to determine if LRRC8A is physically associated with the Nox1 multi-protein complex. Immunoprecipitation of LRRC8A and western blotting revealed an association between LRRC8A and Nox1-p22phox. These relationships were confirmed by immunoprecipitation with p22phox and blotting with anti-LRRC8A or anti-Nox1 (Fig. 7). Physical interaction between LRRC8A and Nox1-p22phox is independent of TNFα receptor activation. Finally, we performed immunofluorescent staining experiments to further explore the spatial relationship between LRRC8A with Nox1-p22phox. The proteins co-localize in a subset of individual cytoplasmic vesicles, and in multivesicular clusters (Fig. 8, antibody validation in Suppl. Fig. S6).
Fig. 7.
LRRC8A associates with the Nox1 complex. A: VSMC were stimulated with TNFα (10 ng/mL) for the indicated times, immunoprecipitated with anti-LRRC8A or anti-p22phox antibody, and immunoblotted with the same antibodies or with anti-Nox1. B: Bar graphs compare protein expression quantities of IP samples normalized by each protein of lysates (n=3–5).
Fig. 8.
LRRC8A co-localizes with Nox1 and p22phox. VSMC were immunostained using anti-LRRC8A in combination with anti-Nox1 (A), or anti-p22phox (B). A: Nox1 (green) co-localized with LRRC8A (red) and two representative areas (1 and 2) are magnified below. B: p22phox (green) and LRRC8A (red) co-localized and two representative areas (1 and 2) are shown below. Arrows (white) indicate areas of co-localization of two proteins. Results are representative of at least three independent experiments.
4. Discussion
Reducing the abundance of LRRC8A, or blocking VRAC with DCPIB mitigates TNFα-induced inflammation by reducing Nox1 activity. Extracellular O2•− supports JNK activation at the plasma membrane which provides important negative feedback regulation to Nox1. Surprisingly, extracellular O2•− is also required for TNFR endocytosis. Thus, by impairing endocytosis, LRRC8A disruption also interferes with subsequent TNFα signaling steps that occur in endosomes. Activated TNFR1 physically associates with Nox1 [31] as part of a large (1500–2000 kDa) signaling complex [32]. Co-immunoprecipitation of LRRC8A with Nox1 or its p22phox subunit revealed that this complex includes LRRC8A and this relationship was confirmed by immunofluorescence. Thus, VRAC supports Nox1 activity at the plasma membrane. The previously established ability of Nox1-derived H2O2 to activate VRAC [3] makes the two proteins functionally interdependent.
Effects of VRAC inhibitors on proliferation and inflammation led to the concept that control of cytoplasmic volume must be critical during proliferation and cytokinesis [8]. This explanation cannot account for the ability of anion channel blockers to selectively disrupt specific signaling events. VRAC is activated by TNFα in both vascular smooth muscle [22] and endothelial cells [14]. The associated inflammatory response is O2•−-dependent and sensitive to anion channel inhibitors, including compounds that are selective for VRAC [14,22,27] (reviewed in [33]). Our findings therefore demonstrate that VRAC inhibition disrupts redox signaling that is proximal to VRAC activation. VRAC has been characterized as being active at rest in VSMC [7] and may be further activated by both the oxidants produced by the TNFR-Nox1signaling complex and cytoplasmic acidification [34] associated with H+ production by Nox1 oxidation of NADPH [35] (See schematic, Fig. 9).
Fig. 9.

Schematic depiction of relationships between Nox1/p22phox, TNFR1 and VRAC. TNFα binds to TNFR1 which then associates with Nox1-p22phox and activates Nox1. O2•− is generated extracellularly as two electrons are passed across the plasma membrane from NADPH to O2. SOD dismutes O2•− to H2O2 which can activate VRAC, as can protons produced in the cytoplasm by NADPH oxidation. TNFR1-dependent activation of JNK provides negative feedback regulation of Nox1 activity. The basis for Nox1's dependence on VRAC current is not established. LRRC8A could also provide a conduit for O2•− to enter the cytoplasm, mediating targeted redox signaling at a molecular level.
The longstanding association between the Cl− Channel-3 (ClC-3) Cl−/H+ antiporter and VRAC, and the fact that ClC-3 null mice display impaired Nox1 activation and impaired TNFα signaling (27), make that protein relevant to this discussion. ClC-3 expression has been correlated with the magnitude of IClswell [36,37], and cytoplasmic anti-ClC-3 antibodies blocked IClswell activation [38]. However, there is overwhelming evidence that ClC-3 itself is not responsible for VRAC [1,39,40]. IClswell is preserved in ClC-3 null cells [41] including VSMC [22]. Expressed ClC-3 currents are biophysically distinct from VRAC [42], and are not inhibited by DCPIB (Suppl. Fig. S7). However, ClC-3 null cells completely lack TNFα-induced VRAC activation [14,22] and TNFα signaling is disrupted both in vitro [14,27] and in vivo [14,26]. So what is the basis for the ClC-3-dependance of VRAC activation and TNFα signaling? We previously proposed that ClC-3 provides charge compensation for Nox1 in endosomes [22,27]. We now observe that VRAC is required for O2•− production, and signaling endosomes never form when these channels are inhibited. The physical association between Nox1 and LRRC8A suggests that VRAC is a superior candidate for the role of charge compensator, but this relationship remains purely speculative. ClC-3 disruption could also impact endosomal trafficking of VRAC, as did dominant negative Rab5 and Rab11, both of which impaired TNFα-induced VRAC activation [22].
To our knowledge this work represents the first report of selective O2•−-dependence of receptor endocytosis (Fig. 4). Uptake of TNFR was inhibited by disruption of extracellular O2•− (siNox1, siLRRC8A, DCPIB, SOD). The absence of an effect of catalase points to extracellular O2•− as the trigger for endocytosis, but its signaling target is unknown. We cannot rule out the possibility that the O2•− produced by Nox1 enters the cell and is converted to H2O2 intracellularly. Endocytosis of TNFR1 is generally thought to be mediated by clathrin-coated vesicles [29], although caveolar uptake may also be involved [43]. We are unaware of an established paradigm for redox control of either endocytic mechanism.
Quantification of O2•− using CAT1H revealed that more O2•− was produced extracellularly (~400–700pmol/mg, Fig. 5) than within endosomes (~100 pmol/mg, Suppl. Fig. S5 and [21]) in response to TNFα. Thus, Nox1 is clearly active prior to endocytosis. The TNFR1 signaling complex is known to increase in mass following agonist stimulation, likely through recruitment of both TNFR1 (TRAF, TRADD) and Nox1/p22phox associated proteins (p47phox or NoxO1, NoxA1, Rac1). We now add VRAC to this protein cluster. Our data cannot address whether association with Nox1/p22phox is direct or through other members of the complex.
Disruption of LRRC8A, or scavenging of O2•−, reduced TNFα-induced JNK phosphorylation (Fig. 6). So how does extracellular O2•− signal to the cytoplasm? O2•− cannot cross lipid bilayers by simple diffusion, it must either act extracellularly to trigger a secondary signaling process, or cross the membrane by a facilitated mechanism. A simple hypothesis is that VRAC could act as a O2•− conductance, allowing it to be delivered directly to a cytoplasmic redox-sensitive target (Fig. 9). The anion selectivity of VRACs is I > Cl > Br > F with I− having the largest anionic radius at 2.2 Å [44]. By comparison, the distance between the center of dissolved O2•− and the nearest deuteron is 2.4 Å [45], and VRAC can conduct other large anions including bicarbonate, lactate and amino acids [35]. A DIDS-sensitive anion channel facilitates the release of O2•− from signaling endosomes and this could be an endosomal VRAC [46]. Unfortunately, our experiments cannot address this question directly because VRAC inhibition blocks O2•− generation and endocytosis.
We previously showed that JNK provides feedback inhibition to Nox1 [21]. This idea is further supported by the increase in extracellular O2•− induced by siJNK (Fig. 5C). JNK does not terminate extracellular O2•− production by triggering endocytosis, since siJNK had no effect on this process (Fig. 4B), and directly impairing endocytosis via dynamin inhibition did not increase extracellular O2•− production (Fig. 5D). The regulatory impact of JNK could be based on phosphorylation of Nox1 or an associated protein that supports Nox1 activity, such as LRRC8A, but direct regulation by JNK has never been demonstrated.
VRAC inhibition is also anti-inflammatory in vivo. DCPIB reduced microglial inflammation and neuronal injury following cerebral ischemia [47], and infarct size after middle cerebral artery occlusion in rats [48] or hypoxia in mice [49]. Mitigation of cytokine signaling provides an appealing mechanism for these results since TNFα plays a critical role in ischemic brain injury [50]. There may be a clinical clue in human cardiovascular disease to the anti-inflammatory effects of VRAC inhibition. Tamoxifen inhibits VRAC with EC50 values as low as 1.3 μM [51]. Typical oral tamoxifen regimens in breast cancer patients yield serum levels of ~1 μM, high dose regimens ~5 μM [52], with tissue concentrations that can be 10–60× higher [53]. Concern that estrogen receptor blockade would increase cardiovascular risk for women led to detailed follow-up studies of cardiovascular health in women receiving this drug. Surprisingly, inflammatory indicators such as C-reactive protein were reduced [54], as was risk for adverse cardiovascular events [55,56]. Cardiovascular protection by tamoxifen has also been modeled in animals, where it prevented the development of atherosclerotic plaques in ApoE−/− mice [57] and reduced plaque formation in rabbits on a high cholesterol diet [58].
Our work sheds important new light on molecular mechanisms of TNFα signal transduction. The functional inter-dependence and physical association between Nox1, which transfers electrons across an insulating membrane to create a new anion (O2•−), and LRRC8A anion channels suggests an important relationship. Defining the biophysical basis for this will be the focus of future experimentation.
Supplementary Material
Acknowledgments
This project was supported by a post-doctoral fellowship grant from the American Heart Association (H. Choi; 13POST16950048).
Abbreviations
- Ad
adenovirus
- BCA
bicinchoninic acid
- BSA
bovine serum albumin
- CAT1H
1-Hydroxy-2,2,6,6-tetramethylpiperidin-4-yl-trimethylammonium chloride
- DMEM
Dulbecco's modified Eagle's medium
- DynK44A
dominant-negative dynamin
- eGFP
enhanced green fluorescent protein
- ERK
extracellular signal-regulated kinase
- ESR
electron spin resonance
- FBS
fetal bovine serum
- FITC
Fluorescein isothiocyanate
- iNOS
inducible nitric oxide synthase
- JNK
c-Jun N-terminal kinase
- LRRC8
Leucine Rich Repeat Containing 8
- MAPK
mitogen activated protein kinase
- MOI
multiplicity of infection
- NADPH
reduced nicotinamide-adenine dinucleotide phosphate
- NF-κB
nuclear factor-kappa B
- Nox
NADPH oxidase
- ROS
reactive oxygen species
- RVD
regulatory volume decrease
- SEM
standard error of the mean
- siRNA
small interfering ribonucleic acid
- SOD
superoxide dismutase
- TNFα
tumor necrosis factor-α
- TNFR
tumor necrosis factor-α receptor
- TRADD
TNFR1-associated death domain protein
- TRAF
TNFR-associated factor
- VCAM
vascular cell adhesion molecule
- VRAC
volume-regulated anion channel
- VSMC
vascular smooth muscle cell
Footnotes
Conflict of interest None declared.
Appendix A. Supplementary material Supplementary data associated with this article can be found in the online version at doi:10.1016/j.freeradbiomed.2016.11.003.
References
- [1].Syeda R, Qiu Z, Dubin AE, Murthy SE, Florendo MN, Mason DE, Mathur J, Cahalan SM, Peters EC, Montal M, Patapoutian A. LRRC8 proteins form volume-regulated anion channels that sense ionic strength. Cell. 2016;164:499–511. doi: 10.1016/j.cell.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ren Z, Raucci FJ, Jr., Browe DM, Baumgarten CM. Regulation of swelling-activated Cl(−) current by angiotensin II signalling and NADPH oxidase in rabbit ventricle. Cardiovasc. Res. 2008;77:73–80. doi: 10.1093/cvr/cvm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Varela D, Simon F, Riveros A, Jorgensen F, Stutzin A. NAD(P)H oxidase-derived H(2)O(2) signals chloride channel activation in cell volume regulation and cell proliferation. J. Biol. Chem. 2004;279:13301–13304. doi: 10.1074/jbc.C400020200. [DOI] [PubMed] [Google Scholar]
- [4].Liang W, Huang L, Zhao D, He JZ, Sharma P, Liu J, Gramolini AO, Ward ME, Cho HC, Backx PH. Swelling-activated Cl− currents and intracellular CLC-3 are involved in proliferation of human pulmonary artery smooth muscle cells. J. Hypertens. 2014;32:318–330. doi: 10.1097/HJH.0000000000000013. [DOI] [PubMed] [Google Scholar]
- [5].Qian JS, Pang RP, Zhu KS, Liu DY, Li ZR, Deng CY, Wang SM. Static pressure promotes rat aortic smooth muscle cell proliferation via upregulation of volume-regulated chloride channel. Cell Physiol. Biochem. 2009;24:461–470. doi: 10.1159/000257485. [DOI] [PubMed] [Google Scholar]
- [6].Yamazaki J, Duan D, Janiak R, Kuenzli K, Horowitz B, Hume JR. Functional and molecular expression of volume-regulated chloride channels in canine vascular smooth muscle cells. J. Physiol. 1998;507(Pt 3):729–736. doi: 10.1111/j.1469-7793.1998.729bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Greenwood IA, Large WA. Properties of a Cl− current activated by cell swelling in rabbit portal vein vascular smooth muscle cells. Am. J. Physiol. 1998;275:H1524–H1532. doi: 10.1152/ajpheart.1998.275.5.H1524. [DOI] [PubMed] [Google Scholar]
- [8].Matchkov VV, Secher Dam V, Bodtkjer DM, Aalkjaer C. Transport and function of chloride in vascular smooth muscles. J. Vasc. Res. 2013;50:69–87. doi: 10.1159/000345242. [DOI] [PubMed] [Google Scholar]
- [9].Wang L, Chen L, Zhu L, Rawle M, Nie S, Zhang J, Ping Z, Kangrong C, Jacob TJ. Regulatory volume decrease is actively modulated during the cell cycle. J. Cell Physiol. 2002;193:110–119. doi: 10.1002/jcp.10156. [DOI] [PubMed] [Google Scholar]
- [10].Chen L, Wang L, Zhu L, Nie S, Zhang J, Zhong P, Cai B, Luo H, Jacob TJ. Cell cycle-dependent expression of volume-activated chloride currents in nasopharyngeal carcinoma cells. Am. J. Physiol. Cell Physiol. 2002;283:C1313–C1323. doi: 10.1152/ajpcell.00182.2002. [DOI] [PubMed] [Google Scholar]
- [11].Yin Z, Tong Y, Zhu H, Watsky MA. ClC-3 is required for LPA-activated Cl− current activity and fibroblast-to-myofibroblast differentiation. Am. J. Physiol. Cell Physiol. 2008;294:C535–C542. doi: 10.1152/ajpcell.00291.2007. [DOI] [PubMed] [Google Scholar]
- [12].Selzman CH, Shames BD, McIntyre RC, Jr, Banerjee A, Harken AH. The NFkappaB inhibitory peptide, IkappaBalpha, prevents human vascular smooth muscle proliferation. Ann. Thorac. Surg. 1999;67:1227–1231. doi: 10.1016/s0003-4975(99)00252-0. discussion 1231–1222. [DOI] [PubMed] [Google Scholar]
- [13].Matsuda JJ, Filali MS, Volk KA, Lamb FS. Activation of IClswell by TNFalpha requires ClC-3-dependent ROS production. FASEB J. 2008;22(937):918. [Google Scholar]
- [14].Yang H, Huang LY, Zeng DY, Huang EW, Liang SJ, Tang YB, Su YX, Tao J, Shang F, Wu QQ, Xiong LX, Lv XF, Liu J, Guan YY, Zhou JG. Decrease of intracellular chloride concentration promotes endothelial cell inflammation by activating nuclear factor-kappaB pathway. Hypertension. 2012;60:1287–1293. doi: 10.1161/HYPERTENSIONAHA.112.198648. [DOI] [PubMed] [Google Scholar]
- [15].Pedersen SF, Klausen TK, Nilius B. The identification of a volume-regulated anion channel: an amazing Odyssey. Acta Physiol. 2015;213:868–881. doi: 10.1111/apha.12450. [DOI] [PubMed] [Google Scholar]
- [16].Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157:447–458. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344:634–638. doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- [18].Hyzinski-Garcia MC, Rudkouskaya A, Mongin AA. LRRC8A protein is indispensable for swelling-activated and ATP-induced release of excitatory amino acids in rat astrocytes. J. Physiol. 2014;592:4855–4862. doi: 10.1113/jphysiol.2014.278887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sawada A, Takihara Y, Kim JY, Matsuda-Hashii Y, Tokimasa S, Fujisaki H, Kubota K, Endo H, Onodera T, Ohta H, Ozono K, Hara J. A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans. J. Clin. Investig. 2003;112:1707–1713. doi: 10.1172/JCI18937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Abascal F, Zardoya R. LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell-cell communication. Bioessays. 2012;34:551–560. doi: 10.1002/bies.201100173. [DOI] [PubMed] [Google Scholar]
- [21].Choi H, Dikalova A, Stark RJ, Lamb FS. c-Jun N-terminal kinase attenuates TNFalpha signaling by reducing Nox1-dependent endosomal ROS production in vascular smooth muscle cells. Free Radic. Biol. Med. 2015;86:219–227. doi: 10.1016/j.freeradbiomed.2015.05.015. [DOI] [PubMed] [Google Scholar]
- [22].Matsuda JJ, Filali MS, Moreland JG, Miller FJ, Lamb FS. Activation of swelling-activated chloride current by tumor necrosis factor-alpha requires ClC-3-dependent endosomal reactive oxygen production. J. Biol. Chem. 2010;285:22864–22873. doi: 10.1074/jbc.M109.099838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Decher N, Lang HJ, Nilius B, Bruggemann A, Busch AE, Steinmeyer K. DCPIB is a novel selective blocker of I(Cl,swell) and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br. J. Pharm. 2001;134:1467–1479. doi: 10.1038/sj.bjp.0704413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bowens NH, Dohare P, Kuo YH, Mongin AA. DCPIB, the proposed selective blocker of volume-regulated anion channels, inhibits several glutamate transport pathways in glial cells. Mol. Pharmacol. 2013;83:22–32. doi: 10.1124/mol.112.080457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hakim CH, Jackson WF, Segal SS. Connexin isoform expression in smooth muscle cells and endothelial cells of hamster cheek pouch arterioles and retractor feed arteries. Microcirculation. 2008;15:503–514. doi: 10.1080/10739680801982808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chu X, Filali M, Stanic B, Takapoo M, Sheehan A, Bhalla R, Lamb FS, Miller FJ., Jr. A critical role for chloride channel-3 (CIC-3) in smooth muscle cell activation and neointima formation. Arterioscler Thromb. Vasc. Biol. 2011;31:345–351. doi: 10.1161/ATVBAHA.110.217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Miller FJ, Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ. Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- [28].Sirianant L, Wanitchakool P, Ousingsawat J, Benedetto R, Zormpa A, Cabrita I, Schreiber R, Kunzelmann K. Non-essential contribution of LRRC8A to volume regulation. Pflug. Arch. 2016;468:805–816. doi: 10.1007/s00424-016-1789-6. [DOI] [PubMed] [Google Scholar]
- [29].Schneider-Brachert W, Tchikov V, Neumeyer J, Jakob M, Winoto-Morbach S, Held-Feindt J, Heinrich M, Merkel O, Ehrenschwender M, Adam D, Mentlein R, Kabelitz D, Schutze S. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–428. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- [30].Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Wong GH, Chen EY, Goeddel DV. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc. Natl. Acad. Sci. USA. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yazdanpanah B, Wiegmann K, Tchikov V, Krut O, Pongratz C, Schramm M, Kleinridders A, Wunderlich T, Kashkar H, Utermohlen O, Bruning JC, Schutze S, Kronke M. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature. 2009;460:1159–1163. doi: 10.1038/nature08206. [DOI] [PubMed] [Google Scholar]
- [32].Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J, Inoue J, Ichijo H. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J. Biol. Chem. 2005;280:37033–37040. doi: 10.1074/jbc.M506771200. [DOI] [PubMed] [Google Scholar]
- [33].Okada Y, Sato K, Numata T. Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J. Physiol. 2009;587:2141–2149. doi: 10.1113/jphysiol.2008.165076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nilius B, Prenen J, Droogmans G. Modulation of volume-regulated anion channels by extra- and intracellular pH. Pflug. Arch. 1998;436:742–748. doi: 10.1007/s004240050697. [DOI] [PubMed] [Google Scholar]
- [35].Grinstein S, Furuya W. Cytoplasmic pH regulation in phorbol ester-activated human neutrophils. Am. J. Physiol. 1986;251:C55–C65. doi: 10.1152/ajpcell.1986.251.1.C55. [DOI] [PubMed] [Google Scholar]
- [36].Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- [37].Zhou JG, Ren JL, Qiu QY, He H, Guan YY. Regulation of intracellular Cl− concentration through volume-regulated ClC-3 chloride channels in A10 vascular smooth muscle cells. J. Biol. Chem. 2005;280:7301–7308. doi: 10.1074/jbc.M412813200. [DOI] [PubMed] [Google Scholar]
- [38].Wang GX, Hatton WJ, Wang GL, Zhong J, Yamboliev I, Duan D, Hume JR. Functional effects of novel anti-ClC-3 antibodies on native volume-sensitive osmolyte and anion channels in cardiac and smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1453–H1463. doi: 10.1152/ajpheart.00244.2003. [DOI] [PubMed] [Google Scholar]
- [39].Wang J, Xu H, Morishima S, Tanabe S, Jishage K, Uchida S, Sasaki S, Okada Y, Shimizu T. Single-channel properties of volume-sensitive Cl− channel in ClC-3-deficient cardiomyocytes. Jpn. J. Physiol. 2005;55:379–383. doi: 10.2170/jjphysiol.S655. [DOI] [PubMed] [Google Scholar]
- [40].Gong W, Xu H, Shimizu T, Morishima S, Tanabe S, Tachibe T, Uchida S, Sasaki S, Okada Y. ClC-3-independent, PKC-dependent activity of volume-sensitive Cl channel in mouse ventricular cardiomyocytes. Cell Physiol. Biochem. 2004;14:213–224. doi: 10.1159/000080330. [DOI] [PubMed] [Google Scholar]
- [41].Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- [42].Guzman RE, Grieschat M, Fahlke C, Alekov AK. ClC-3 is an intracellular chloride/proton exchanger with large voltage-dependent nonlinear capacitance. ACS Chem. Neurosci. 2013;4:994–1003. doi: 10.1021/cn400032z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].D'Alessio A, Al-Lamki RS, Bradley JR, Pober JS. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am. J. Pathol. 2005;166:1273–1282. doi: 10.1016/S0002-9440(10)62346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ahrens LH. The use of ionization potentials. Part 1. Ionic radii of the elements. Geochim. Cosmochim. Acta. 1952;2:155–169. [Google Scholar]
- [45].Narayana PA, Suranarayana D, Kevan L. Electron-spin echo studies of the solvation structure of O-(2-) in water. Abstr. Pap. Am. Chem. Soc. 1982;183 56-Phys. [Google Scholar]
- [46].Mumbengegwi DR, Li Q, Li C, Bear CE, Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol. Cell Biol. 2008;28:3700–3712. doi: 10.1128/MCB.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Han Q, Liu S, Li Z, Hu F, Zhang Q, Zhou M, Chen J, Lei T, Zhang H. DCPIB, a potent volume-regulated anion channel antagonist, attenuates microglia-mediated inflammatory response and neuronal injury following focal cerebral ischemia. Brain Res. 2014;1542:176–185. doi: 10.1016/j.brainres.2013.10.026. [DOI] [PubMed] [Google Scholar]
- [48].Zhang Y, Zhang H, Feustel PJ, Kimelberg HK. DCPIB, a specific inhibitor of volume regulated anion channels (VRACs), reduces infarct size in MCAo and the release of glutamate in the ischemic cortical penumbra. Exp. Neurol. 2008;210:514–520. doi: 10.1016/j.expneurol.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Alibrahim A, Zhao LY, Bae CY, Barszczyk A, Sun CL, Wang GL, Sun HS. Neuroprotective effects of volume-regulated anion channel blocker DCPIB on neonatal hypoxic-ischemic injury. Acta Pharmacol. Sin. 2013;34:113–118. doi: 10.1038/aps.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tuttolomondo A, Pecoraro R, Pinto A. Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: a review of the evidence to date. Drug Des. Dev. Ther. 2014;8:2221–2238. doi: 10.2147/DDDT.S67655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wondergem R, Gong W, Monen SH, Dooley SN, Gonce JL, Conner TD, Houser M, Ecay TW, Ferslew KE. Blocking swelling-activated chloride current inhibits mouse liver cell proliferation. J. Physiol. 2001;532:661–672. doi: 10.1111/j.1469-7793.2001.0661e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].O'Day SJ, Boasberg PD, Kristedja TS, Martin M, Wang HJ, Fournier P, Cabot M, DeGregorio MW, Gammon G. High-dose tamoxifen added to concurrent biochemotherapy with decrescendo interleukin-2 in patients with metastatic melanoma. Cancer. 2001;92:609–619. doi: 10.1002/1097-0142(20010801)92:3<609::aid-cncr1361>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- [53].Lien EA, Solheim E, Ueland PM. Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res. 1991;51:4837–4844. [PubMed] [Google Scholar]
- [54].Romero WG, Da Silva FB, Borgo MV, Bissoli NS, Gouvea SA, Abreu GR. Tamoxifen alters the plasma concentration of molecules associated with cardiovascular risk in women with breast cancer undergoing chemotherapy. Oncologist. 2012;17:499–507. doi: 10.1634/theoncologist.2011-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bradbury BD, Lash TL, Kaye JA, Jick SS. Tamoxifen-treated breast carcinoma patients and the risk of acute myocardial infarction and newly-diagnosed angina. Cancer. 2005;103:1114–1121. doi: 10.1002/cncr.20900. [DOI] [PubMed] [Google Scholar]
- [56].Hackshaw A, Roughton M, Forsyth S, Monson K, Reczko K, Sainsbury R, Baum M. Long-term benefits of 5 years of tamoxifen: 10-year follow-up of a large randomized trial in women at least 50 years of age with early breast cancer. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2011;29:1657–1663. doi: 10.1200/JCO.2010.32.2933. [DOI] [PubMed] [Google Scholar]
- [57].Reckless J, Metcalfe JC, Grainger DJ. Tamoxifen decreases cholesterol sevenfold and abolishes lipid lesion development in apolipoprotein E knockout mice. Circulation. 1997;95:1542–1548. doi: 10.1161/01.cir.95.6.1542. [DOI] [PubMed] [Google Scholar]
- [58].Kallas Hueb C, Aldrighi JM, Kallas E, Franchini Ramires JA. Repercussions of raloxifen, tamoxifen and estrogen on aortic atherosclerotic lesions of female rabbits submitted to ovariectomy and hypercholesterol diet. Maturitas. 2005;50:30–38. doi: 10.1016/j.maturitas.2004.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.