Abstract
Hemorrhagic cystitis is a known complication of cyclophosphamide, an anti-neoplastic agent used to treat a variety of oncologic diseases in children. Hydration can prevent hemorrhagic cystitis; however, use varies in clinical practice. A team was assembled to develop evidence-based practice recommendations to address the following question: in a population of children with cancer, what is the appropriate pre and post hydration for the administration of different dose levels of intravenous cyclophosphamide to prevent bladder toxicity? The purpose was to identify the appropriate rate, duration and route of hydration to prevent bladder toxicity with low, intermediate and high dose cyclophosphamide. After a systematic search of the literature, 15 pieces of evidence were evaluated and used. There is a moderate level of quality evidence related to hydration for high dose cyclophosphamide and very low quality evidence related to intermediate or low dose cyclophosphamide. Three general recommendations were made for hydration associated with cyclophosphamide. There is a need for further research related to the prevention of bladder toxicity in children with cancer receiving cyclophosphamide.
Keywords: Cyclophosphamide, Hemorrhagic cystitis, Bladder toxicity, Pediatric, Cancer
Cyclophosphamide (CTX) is an antineoplastic alkylating agent used to treat a wide variety of malignant and non-malignant disorders (Frasier, Kanekal, & Kehrer, 1991). It is used in pediatric oncology as part of the standard treatment for leukemia, solid tumors and brain tumors. Cyclophosphamide is commonly administered intravenously but it is also available as an oral agent, although this is rarely used in children with cancer. The dosing for CTX ranges from low to intermediate to high dose, and is based on the diagnosis and specific treatment regimen. Cyclophosphamide is the most commonly used agent in stem cell transplant (SCT) preparative regimens, and is prescribed in high doses in this population (Hadjibabaie et al., 2008). One of the known side effects of CTX is hemorrhagic cystitis (West, 1997). Hydration is commonly administered in patients receiving CTX to prevent hemorrhagic cystitis; however, the hydration rate, timing, and duration vary significantly in clinical practice. The purpose of this review is to identify appropriate recommendations for hydration before and after low, intermediate, and high doses of CTX to prevent bladder toxicity in children and adolescents with cancer.
Background
Cyclophosphamide was first used for the treatment of cancer in 1958, and it was found to be associated with bladder injury, causing symptoms such as frequency, urgency, dysuria and hematuria (deVries & Freiha, 1990). Acrolein, one of the metabolites of CTX, has been identified as the causative agent responsible for damage to the bladder (Ramu, Fraiser, Mamiya, Ahmed & Kehrer, 1995). The acrolein metabolite can accumulate in the urine causing sloughing, thinning and inflammation of the bladder wall. This irritation causes bleeding of the endothelial lining in the bladder, known as hemorrhagic cystitis that may be initially microscopic, but can progress to gross hematuria and severe bleeding (West, 1997). The original grading scale for hemorrhagic cystitis developed by Droller, Saral and Santos (1982) defined 4 grades of severity ranging from microscopic hematuria (grade 1) to gross hematuria, clot formation and urinary tract obstruction (grade 4).
The definitions used for cyclophosphamide dosing levels for consistency in this evidence review and based on the literature (Droller et al., 1982; Hows et al., 1983; Shepard et al., 1991; Vose et al., 1993; Meisenberg et al., 1994; Ballen et al., 1999; Khojasteh, Zakerinia, Ramzi, & Haghshenas, 2000; Marshall et al., 2011; COG, 2010; Monach, Arnold, & Merkel, 2010) are:
High dose CTX >/= to 1500 mg/m2/dose
Intermediate dose CTX >/= to 750 mg to < 1500 mg/m2/dose
Low dose CTX < 750 mg/m2/dose
Significance
Hemorrhagic cystitis (HC) is a known complication of CTX that can cause distressing and painful symptoms for the child or adolescent. HC can cause significant morbidities such as severe bladder disease and damage to the renal system (McCarville, Hoffer, Gingrich & Jenkins, 2000). There are reported fatalities related to severe HC and bleeding complications (Droller, Saral, & Santos, 1982). Medical intervention can be invasive, aggressive and costly. HC can prolong hospitalization, significantly impact quality of life, and adversely affect patient outcomes including overall survival (Decker, Karam, & Wilcox, 2009; West, 1997).
The incidence of CTX-induced HC without preventative measures is estimated to be 25-60% (Stillwell, Benson, & Burgert, 1988; West, 1997). Lawrence, Simone and Aur (1975) reported HC in children with leukemia after repeated and prolonged exposure to both intravenous and oral CTX. Risk factors for pediatric HC in children with cancer include CTX or busulfan chemotherapy, age > 5 years, male sex, bone marrow or peripheral stem cell transplant recipients, and bladder irradiation (Riachy et al., 2013; Stillwell et al., 1988). Although some risk factors have been identified, any patient who receives CTX at any dose level has the potential to develop bladder toxicity at any time point (early or late in their treatment course).
Standard practice to prevent HC (unless medically contraindicated) has included adequate hydration with or without diuretics to keep the urine dilute and urine flow adequate (Haselberger & Schwinghammer, 1995). Hydration has the potential to reduce or prevent bladder toxicity associated with CTX (Walker, 1999). There are wide variations in hydration recommendations and practices for children and adolescents with cancer receiving intravenous CTX. One publication reported significant variations of hydration recommendations across multiple pediatric oncology treatment protocols (Sievers, Lagan, Bartel, Rasco, & Blanding, 2001). Consistency in hydration practice recommendations for children and adolescents with cancer receiving CTX based on evidence could standardize practice and improve patient outcomes.
Evidenced Guideline Development Methods
EBP Review Team
Evidence-based practice (EBP) topics were developed in alignment with the Children's Oncology Group (COG) Nursing Discipline's blueprint and organizing framework (Landier, Leonard, & Ruccione, 2013; Kelly, Hooke, Ruccione, Landier, & Haase, 2013). These topics were vetted with COG leadership and other key stakeholders (i.e. COG committees such as Leukemia Committee), then a call for the EBP projects was disseminated through the COG Nursing Discipline membership. Multidisciplinary pediatric oncology healthcare providers from St. Louis Children's Hospital assembled as EBP team, and submitted an application. The EBP team consisted of two advanced practice pediatric oncology nurses, one pediatric oncology nurse coordinator, one pediatric oncology staff nurse, and one specialized oncology doctor of pharmacy (PharmD), all from a major academic pediatric children's hospital. After a competitive selection application process, the proposal was selected for development. A mentor was assigned to the group who was a doctoral prepared advanced practice pediatric oncology nurse with experience in evidence-based reviews. All team members volunteered for the project and participated in review of the evidence, determination of findings, and completion of the manuscript.
Question Development
Clinical questions can be phrased in a PICOT format to provide essential elements for the EBP review. PICOT stands for Patient, Intervention or Issue of Interest, Comparison, Outcome, and Time (Melnyk & Fineout-Overholt, 2011). The specific PICOT question that this guideline addressed was: In a population of children with cancer, what is the appropriate pre and post hydration for the administration of different dose levels of intravenous cyclophosphamide to prevent bladder toxicity? The question was developed to determine differences in hydration for low, intermediate and high dose cyclophosphamide. The goal of the project was to establish a standard practice recommendation for both rate and duration of hydration.
Literature Search Strategies
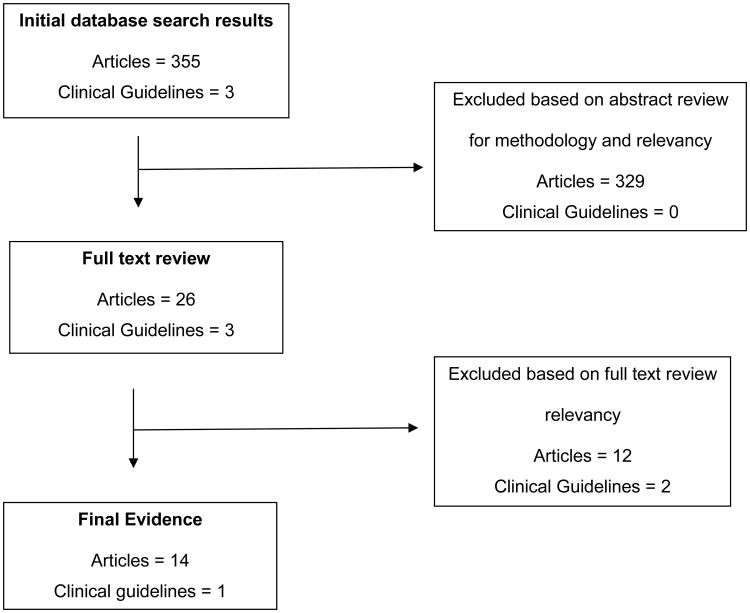
The literature search included an extensive search of major databases including PubMed, CINAHL, Embase, OVID, Cochrane, BestBETS, PedsCCM, and Up to Date. Key terms and MeSH terms used included: Cyclophosphamide, Intravenous hydration, Hemorrhagic Cystitis, Bladder, and MESNA (sodium-2-mercaptoethanesulfonate). The limits were set for English and human subjects. Due to the limited published evidence in the last ten years, the search was not restricted by time frame or patient age. Hand searching was also utilized. A medical librarian assisted with all literature searches. The initial search yielded 355 articles. Articles focused on cyclophosphamide-based regimens for efficacy or disease response were eliminated. Search results are illustrated in Figure 1.
Figure 1. Search Results.
In addition, the EBP team searched for chemotherapy guidelines and chemotherapy administration recommendations. The search included professional organizations linked to the topic: Oncology Nursing Society (ONS), Association of Pediatric Hematology/Oncology Nurses (APHON), Children's Oncology Group (COG), and the American Society of Clinical Oncology (ASCO). Additional exploration on the topic included searches within: Google Scholar and two National Guideline Databases.
Inclusion/Exclusion criteria
The inclusion criteria consisted of research articles that described the administration of hydration and supportive care with CTX. Systematic reviews, meta-analysis, research studies, and clinical guidelines were utilized. Due to the paucity of literature in pediatric oncology on this topic, all ages without restriction to disease and all oncologic diagnoses were included in the review.
Exclusion criteria included general background articles, basic review articles, disease-based review articles regarding the efficacy of CTX, and medical or surgical intervention for HC.
Review Approach
Each article was thoroughly reviewed by an EBP review team member and key information was extracted into a matrix evidence table. The articles and tables were then reviewed by the EB practice team leader and team mentor. Each study was analyzed and the following information was summarized: purpose, design, variables, population, interventions, instruments, measurements, results, implications, and confounding factors.
Analysis Techniques
The evidence was analyzed utilizing the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (Guyatt et al., 2011). Each article was given a grade of high, moderate, low or very low depending on the quality of evidence. If there was any question about the GRADE rating, the evidence was discussed among team members and a team consensus was reached. The team leader and team mentor synthesized all of the evidence and provided one overall GRADE rating for the quality of the evidence. Recommendation statements were developed from this synthesized evidence and labeled as strong or weak, as described in the introduction article of this journal issue. The strength of each recommendation was determined by the desirable and undesirable effects of the evidence and made independently of the quality level of the evidence (Andrews et al., 2013).
A total of three guidelines, all from ASCO were found. Two guidelines were updated versions of the same original guideline published in 1999 by Hensley et al. The EBP team used the 2002 guideline for this evidence review (Schuchter, Hensley, Meropol, & Winer, 2002) since the revised guideline in 2009 (Hensley et al., 2009) had no new evidence related to hydration with cyclophosphamide. The clinical guideline was reviewed and scored independently by the team leader and mentor using the Appraisal of Guidelines Research and Evaluation II (AGREE II) tool. Scores were averaged between the two raters and an overall mean score was obtained for each of the six domains (Cluzeau et al., 2003). These scores were discussed with the team leader and mentor for consensus and a decision was made about the inclusion of the guideline in the body of evidence.
Evidence Review
Fifteen pieces of evidence met the criteria for final inclusion in this review including: three systematic reviews, three randomized controlled trials, one non-randomized clinical trial, two descriptive studies, two retrospective studies, three case study series, and one clinical guideline. The evidence included patient populations of adults, adolescents, children, and combined age groups. Although the PICOT question focused on hydration with CTX, other interventions were reported in the hydration literature including the use of diuretics, MESNA, and continuous bladder irrigation (CBI).
Hydration Evidence
Four studies evaluated hydration and HC (Table 1). Droller et al. (1982) evaluated hyper hydration as a preventative method for HC among 198 adult patients receiving high dose CTX, and found a significant reduction in HC. The hydration was given 24 hours prior to CTX and every day of the regimen with hourly voiding. This study appeared to set the standard for hydration with high dose CTX. From a literature review performed in 1995, Haselberger and Schwinghammer concluded that hydration should be the key component of all prophylactic regimens with high dose intravenous cyclophosphamide. A study by Ballen and colleagues (1999) showed that hyper hydration alone was effective in preventing HC in 100 patients who received high dose CTX for SCT regimens.
Table 1. Hydration Evidence.
| First Author, Year Study Design | Subjects | Findings | GRADE quality level |
|---|---|---|---|
| Droller, 1982 Prospective, descriptive | 198 adult patients with oncology or aplastic anemia diagnosis | Significant HC prevalence difference between no hydration and IV fluids at 4-5 liters per day during CTX infusion (p< 0.01) | High |
| Haselberger, 1995 Literature review | Published evidence: high dose CTX in SCT | Hydration/adequate urine flow should be mandatory with high dose CTX | Moderate |
| Ballen, 1999 Prospective, descriptive | 100 SCT adult patients | Hydration with IV fluids at 250 ml/hr for 4 hours before through 24 hours after CTX effective in reducing HC | Moderate |
| Takamoto, 2004 Case study | 19 pediatric patients with solid tumor or hematological disease | Acrolein concentrations increased as urine volume decreased; peak times were 1-12 hours after CTX | Low |
Abbreviations: SCT = stem cell transplant; IV = intravenous; CTX = Cytoxan; HC = hemorrhagic cystitis
Takamoto and colleagues (2004) established the risk period for HC by measuring the acrolein concentrations of the urine in 36 occurrences of patients receiving intermediate or high dose CTX (1000-2100 mg/m2/dose). This paper demonstrated that high urine volume was important for reducing urinary acroleins. The urinary acrolein concentration is an index of hydration and is the key to the prevention of bladder toxicity. No clinical symptoms of HC occurred in this study, although 3 patients had hematuria. The highest risk for high acrolein concentrations occurred within the first several hours after CTX. The authors concluded that this is the most significant time to maintain urine flow to prevent HC.
MESNA with Hydration Evidence
The use and efficacy of MESNA with high dose CTX was a common theme in the literature review of hydration requirements with CTX and included eight pieces of evidence (Table 2). This made it difficult to separate the definitive intervention that was successful. One of the first articles to compare the use of MESNA versus forced diuresis was a randomized trial among 61 adult patients receiving high dose CTX (Hows et al., 1984). This trial showed that MESNA was more effective in the prevention of HC; however, both groups received hydration including 3 liters/day in the MESNA group and 6 liters/day in the hydration group. Conversely, a study by Shepard, Pringle, Barnett, Klingemann, Reece, and Phillips (1991) found MESNA to be equally effective as hyper hydration for the prevention of CTX induced HC among 100 patients with cancer.
Table 2. MESNA with Hydration Evidence.
| First Author, Year Study Design | Subjects | Findings | GRADE quality level |
|---|---|---|---|
| Hows, 1984 RCT | 61 SCT adult patients | Mesna plus 3 liters of IV hydration daily during CTX is more effective for HC than 6 liters of IV hydration plus diuresis (p<0.05) | Moderate |
| Shepard, 1991 RCT | 100 adult patients with cancer | No difference between mesna plus 1.5 liters/m2/day of IV fluids compared to 3 liters/m2/day of IV fluids plus lasix for severe HC (p=0.71) | High |
| Khojasteh, 2000 Case study | 18 pediatric and young adults with leukemia or hematological disease | Adding mesna with IV fluids at 3 liters/m2/day is effective in preventing HC | Low |
| Murphy, 1993 Retrospective | 217 adult patients with cancer | No HC prevalence difference between IV hydration at 3.6 liters/m2/day compared to same IV hydration plus mesna | Moderate |
| Marshall, 2011 Case study | 1 adult patient with breast cancer | CTX without hydration caused HC but mesna plus IV hydration given with subsequent CTX with no HC | Very Low |
| Monarch, 2010 Systematic review | 38 articles | No HC prevalence difference between IV hydration at 3 liters/m2/day compared to IV hydration of 1.5-3 liters/day plus mesna | Moderate |
| Schuchter, 2002 Clinical guideline | Update of the 1999 chemotherapy and radiotherapy protectants clinical guideline by ASCO; mesna plus saline diuresis or forced saline diuresis is recommended for high dose CTX | Not applicable | |
| Damron, 2009 Systematic Review | 54 articles | Mesna plus saline diuresis or forced diuresis with high dose CTX | High |
Abbreviations: RCT = randomized control trial; SCT = stem cell transplant; IV = intravenous; CTX = Cytoxan; HC = hemorrhagic cystitis; ASCO = American Society of Clinical Oncology
In 2000, Khojasteh and colleagues reported a prospective study with 18 subjects who received high dose CTX, and showed MESNA was effective in preventing HC, however all of these subjects also received 3 liters/m2/day of hydration. A retrospective analysis of 217 patients who received high dose CTX showed no significant difference in the incidence of HC among patients receiving MESNA plus hyper hydration versus hyper hydration alone (Murphy et al.,1993).
There is limited evidence regarding the use of MESNA with intermediate or low dose CTX. A single case study described the occurrence of HC in a patient with the first administration of low dose CTX (600 mg/m2/dose) (Marshall et al., 2011). Subsequent cycles were given with hydration and MESNA with no re-occurrence of the HC. A systematic review of 38 studies evaluated the evidence on bladder toxicity secondary to intermediate or high dose CTX in rheumatology patients (Monach, Arnold, & Merkel, 2010). The reviewers found that bladder toxicity was more likely to occur with daily long term oral CTX as compared to periodic intravenous use. Although there are no contraindications for use of MESNA among the rheumatology population, the routine use of MESNA could not be fully supported. Hydration with IV fluids prior to and for several hours after IV administration of cyclophosphamide is typically recommended in this population, as well as 72 hours of oral hydration after the dose.
In 1999, ASCO published evidence-based guidelines regarding the use of chemotherapy protectant agents (Hensley et al., 1999), which were updated in 2002 (Schuchter et al., 2002) and again in 2009 (Hensley et al., 2009). The guidelines recommended MESNA plus saline diuresis or forced saline diuresis for patients receiving high dose cyclophosphamide in the setting of SCT. Recommendations for surveillance from the ASCO guideline are based on the opinion of the expert panel and include baseline urinalysis, monitoring for hematuria and monitoring of urine output. There were no recommendations for hydration with low or intermediate dose CTX. In 2009, ONS published an evidence-based literature review regarding the prevention of bleeding in patients with cancer (Damron et al., 2009). The ONS review is similar to the ASCO guidelines with forced saline diuresis or MESNA with saline diuresis being recommended for high dose CTX in the SCT setting.
Continuous Bladder Irrigation with Hydration Evidence
Three studies evaluated the effect of hydration along with continuous bladder irrigation (CBI) for the prevention of HC in patients receiving CTX (Table 3). Through a non-randomized clinical trial, Hajibabaie et al. (2008) demonstrated that hydration with MESNA and CBI was effective in reducing the rate and duration of HC in 80 patients who received high dose CTX for a SCT preparative regimen. Vose et al. (1993) compared MESNA with CBI in a randomized clinical trial of 200 SCT patients receiving high dose CTX. Both arms of the study received hyper hydration and the results showed no significance difference between CBI or MESNA in preventing HC. Meisenberg and colleagues (1994) evaluated CBI and hyper hydration together (both interventions in all subjects) and found it to be labor-intensive but highly effective in the prevention of HC associated high dose CTX.
Table 3. Continuous Bladder Irrigation with Hydration Evidence.
| First Author, Year Study Design | Subjects | Findings | GRADE quality level |
|---|---|---|---|
| Hajibabaie, 2008 Non-randomized trial with historical controls | 80 adult SCT patients | No HC prevalence difference between IV hydration versus IV hydration with CBI (p=0.11) | Moderate |
| Vose, 1993 RCT | 200 adult SCT patients | No HC prevalence difference between IV hydration plus mesna versus IV hydration plus CBI but more bladder spasm, discomfort, and urinary tract infections in CBI group | High |
| Meisenberg, 1994 Retrospective | 303 adult SCT patients | CBI plus IV hydration at 200ml/m2/hour is effective in preventing HC | Moderate |
Abbreviations: RCT = randomized control trial; SCT = stem cell transplant; IV = intravenous; CTX = Cytoxan; HC = hemorrhagic cystitis; CBI = continuous bladder irrigation
Best Practices
The EBP team, who are active members of COG, reviewed 44 COG protocols which contained CTX infusions to assess hydration evidence. The recommended hydration ranged from 2-24 hours after low/intermediate dose CTX, and 24 hours after high dose CTX in these protocols. Almost all COG protocols for low, intermediate and high dose CTX suggest achieving a dilute specific gravity less than or equal to 1.010 prior to any CTX administration (Children's Oncology Group, 2013). An effort is currently underway within COG to standardize chemotherapy administration practices, including associated hydration, across COG protocols (R. Womer, personal communication, January 3, 2014).
Rapid pre-hydration protocols have been implemented in some institutions to reduce the duration of hydration required prior to the administration for some chemotherapy agents, including CTX. A rapid pre-hydration protocol for CTX, ifosfamide, cisplatin, and methotrexate was evaluated in one children's hospital and demonstrated a significant reduction in pre-hydration time (6.75 hours to 1.57 hours) without any adverse side effects (Womer et al., 2002; Tracy, DiTaranto & Wormer, 2004). Fratino, Daniel, Cohen, and Chen (2008) also implemented an aggressive pre-hydration protocol which resulted in a reduction of the time to achieve adequate hydration status (4.9 hours to 1.4 hours) prior to the initiation of chemotherapy.
Confounding Factors
There are numerous confounding factors for HC in the literature that include: low platelet count which increases the risk of bleeding; viral causes of HC such as BK or adenovirus; previous radiation to the bladder; repeated doses of CTX; and other chemotherapy agents that cause HC such as busulfan. Some of the literature accounts for these confounding factors but many articles did not address these issues. The overall evidence can be difficult to interpret due to numerous variables and combined interventions to prevent HC. These factors make definitive conclusions about a single intervention more challenging.
Overall Summary of Recommendations
Based on the effects of the synthesized evidence, the following recommendations are made.
Recommendation #1
The EBP team makes a strong recommendation with an overall moderate quality of evidence that administering at least 3 liters/m2/day of intravenous fluids is the standard pre and post hydration rate for patients receiving high dose CTX. The evidence recommends achieving a dilute specific gravity prior to high dose CTX and 24 hours of hydration post high dose CTX.
Recommendation #2
The EBP team makes a strong recommendation with an overall low quality of evidence that pre and post hydration be administered to patients who are receiving low or intermediate doses of intravenous CTX. There is insufficient evidence to recommend intravenous versus oral hydration, intravenous fluid infusion rate, or duration of hydration in patients receiving low or intermediate CTX.
Recommendation # 3
The EBP team makes a strong recommendation with an overall moderate quality of evidence that forced saline diuresis or MESNA with forced saline diuresis in patients receiving high dose intravenous CTX is effective in reducing the incidence of hemorrhagic cystitis.
Considerations
Patients enrolled on clinical trials should follow protocol guidelines for the use of pre and post hydration with CTX, and protocol recommendations for MESNA dosing and administration. Consistency in the care of patients enrolled on clinical trials is crucial to accurately report grades of toxicity and study outcomes.
Individual patient factors need to be considered in all patients receiving CTX. There may be specific co-morbidities present that impact a patient's ability to tolerate recommended hydration. These factors include but are not limited to: age, disease status, mobility, renal function, and cardiac status. Nausea and fatigue induced by chemotherapy may further decrease the likelihood of sufficient oral hydration.
Conclusion and Discussion
Hydration is a crucial component of supportive care for the patient receiving intravenous CTX. Overall, the evidence in pediatric oncology patients related to HC from high dose CTX is very limited. In addition, there is limited or no research based evidence regarding hydration with low or intermediate dose CTX. There was limited information about duration of pre and post hydration for different dose levels of CTX. There were no recommendations related to oral versus intravenous hydration for CTX. In addition, the administration of hydration in the outpatient clinic for CTX was not addressed in the literature. Although the quality of evidence is low, the EBP team chooses to make a strong recommendation (#2) with a low quality of evidence because hydration with CTX has become part of the clinical standard of care and the benefits of hydration greatly outweigh any associated risks.
There is a need for research-based recommendations in the pediatric population that includes:
pre and post hydration with low or intermediate dose cyclophosphamide;
outpatient administration of hydration with cyclophosphamide;
comparisons of intravenous versus oral hydration; and
supportive care measures that may prevent or reduce HC (such as timing of administration, frequent voiding, use of diuretics).
Best current practices for CTX hydration are currently used at most pediatric oncology centers that include following protocol recommendations or adhering to individual institutional policies. A consistent approach for pre and post hydration with CTX would simplify prescribing and administration of this agent; provide important outcome measures; improve patient safety; reduce costs; and could decrease the incidence of bladder toxicity.
Acknowledgments
Funding: Supported by the National Cancer Institute – Children's Oncology Group Chair's Grant (U10 CA098543) and Alex's Lemonade Stand Foundation.
Biographies
Deborah Robinson is an advanced practice nurse at Saint Louis Children's Hospital.
Ginny Schulz is an advanced practice nurse at Saint Louis Children's Hospital.
Rachel Langley is a clinical pharmacist at Saint Louis Children's Hospital.
Kevin Donze is a nurse clinician at Saint Louis Children's Hospital.
Kari Winchester is a staff nurse at Washington University School of Medicine.
Cheryl Rodgers is an assistant professor at Duke University School of Nursing.
Footnotes
Authors' Disclosure of potential conflicts of interests: None of the authors have personal or financial conflicts of interests with this project or publication.
Contributor Information
Deborah Robinson, Hematology/Oncology, Saint Louis Children's Hospital, One Children's Place, St. Louis, MO 63110.
Ginny Schulz, Hematology/Oncology, Saint Louis Children's Hospital, One Children's Place, St. Louis, MO 63110.
Rachel Langley, Hematology/Oncology, Saint Louis Children's Hospital, One Children's Place, St. Louis MO 63110.
Kevin Donze, Hematology/Oncology, Saint Louis Children's Hospital, One Children's Place, St. Louis, MO 63110.
Kari Winchester, Hematology/Oncology, Washington University School of Medicine, One Children's Place, St. Louis, MO 63110.
Cheryl Rodgers, Duke University School of Nursing, 307 Trent Drive, Durham, NC 27572.
References
- Andrews J, Guyatt G, Oxman A, Alderson P, Dahm P, Falck-Ytter Y, et al. Schunemann HJ. Grade guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology. 2013;66:719–725. doi: 10.1016/j.jclinepi.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Ballen KK, Becker P, Levebvre K, Emmons R, Lee K, Levy W, et al. Lowry P. Safety and cost of hyper hydration for the prevention of hemorrhagic cystitis in bone marrow transplant recipients. Oncology. 1999;57(4):287–292. doi: 10.1159/000012062. [DOI] [PubMed] [Google Scholar]
- Children's Oncology Group. Members only website. 2013 Retrieved from https://members.childrensoncologygroup.org/
- Cluzeau FA, Burgers JS, Brouwers M, Grol R, Mäkelä M, Littlejohns P, et al. Hunt C. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Quality and Safety in Health Care. 2003;12(1):18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damron BH, Brant JM, Belansky HB, Friend PJ, Samsonow S, Schaal A. Putting evidence into practice: Prevention and management of bleeding in patients with cancer. Clinical Journal of Oncology. 2009;13(5):573–583. doi: 10.1188/09.CJON.573-583. [DOI] [PubMed] [Google Scholar]
- Decker DB, Karam JA, Wilcox DT. Pediatric hemorrhagic cystitis. Journal of Pediatric Urology. 2009;5:254. doi: 10.1016/j.jpurol.2009.02.199. [DOI] [PubMed] [Google Scholar]
- deVries CR, Freiha FS. Hemorrhagic cystitis: A review. The Journal of Urology. 1990;143(1):1–9. doi: 10.1016/s0022-5347(17)39848-8. [DOI] [PubMed] [Google Scholar]
- Droller MJ, Saral R, Santos G. Prevention of cyclophosphamide-induced hemorrhagic cystitis. Urology. 1982;20(3):256–8. doi: 10.1016/0090-4295(82)90633-1. [DOI] [PubMed] [Google Scholar]
- Fraiser LH, Kanekal S, Kehrer JP. Cyclophosphamide toxicity: Characterizing and avoiding the problem. Drugs. 1991;42(5):781–95. doi: 10.2165/00003495-199142050-00005. [DOI] [PubMed] [Google Scholar]
- Fratino LM, Daniel DA, Cohen KJ, Chen AR. Evaluation of quality improvement initiative in pediatric oncology: Implementation of aggressive hydration protocol. Journal of Nursing Care Quality. 2008;24(2):153–159. doi: 10.1097/01.NCQ.0000347453.13547.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. Schunemann HJ. GRADE guidelines: 1. Introduction – GRADE evidence profiles. Journal of Clinical Epidemiology. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Hadjibabaie M, Alimoghaddam K, Shamshiri AR, Iravani M, Bahar B, Mousavi A, et al. Ghavamzadeh A. Continuous bladder irrigation prevents hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation. Urologic Oncology. 2008;26(1):43–46. doi: 10.1016/j.urolonc.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Haselberger MB, Schwinghammer TL. Efficacy of MESNA for prevention of hemorrhagic cystitis after high-dose cyclophosphamide therapy. Annals of Pharmacotherapeutics. 1995;9:918–921. doi: 10.1177/106002809502900914. [DOI] [PubMed] [Google Scholar]
- Hensley M, Hagerty K, Kewalramani T, Green D, Meropol N, Wasserman T, et al. Schuchter L. American Society of Clinical Oncology 2008 Clinical Practice Guideline: Use of chemotherapy and radiation therapy protectants. Journal of Clinical Oncology. 2009;27(1):127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- Hensley M, Schuchter LM, Lindley C, Meropol NJ, Cohen GI, Broder G, et al. Winer E. American Society of Clinical Oncology: Clinical practice guidelines for the use of chemotherapy and radiotherapy protectants. Journal of Clinical Oncology. 1999;17(10):3333–3355. doi: 10.1200/JCO.1999.17.10.3333. [DOI] [PubMed] [Google Scholar]
- Hows JM, Mehta A, Ward L, Woods K, Perez R, Gordon MY, Gordon-Smith EC. Comparison of mesna with forced diuresis to prevent cyclophosphamide induced hemorrhagic cystitis in marrow transplantation: A prospective randomized study. British Journal of Cancer. 1984;50:753–756. doi: 10.1038/bjc.1984.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KP, Hooke MC, Ruccione K, Landier W, Haase J. Developing an organizing framework to guide nursing research in the Children's Oncology Group (COG) Seminars in Oncology Nursing. 2013 doi: 10.1016/j.soncn.2013.12.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khojasteh NH, Zakerinia M, Ramzi M, Haghshenas M. A new regimen of MESNA effectively prevents cyclophosphamide-induced hemorrhagic cystitis in bone marrow recipients. Transplantation Proceedings. 2000;32:596. doi: 10.1016/s0041-1345(00)00906-4. [DOI] [PubMed] [Google Scholar]
- Landier W, Leonard M, Ruccione KS. Children's Oncology Group's 2013 blueprint for research: Nursing discipline. Pediatric Blood & Cancer. 2013;60(6):1031–1036. doi: 10.1002/pbc.24415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence HJ, Simone J, Aur RA. Cyclophosphamide-induced hemorrhagic cystitis in children with leukemia. Cancer. 1975;36:1572–1576. doi: 10.1002/1097-0142(197511)36:5<1572::aid-cncr2820360506>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Marshall A, McGrath C, Torigian D, Papanicolaou N, Lal P, Tweed CK. Low-dose cyclophosphamide associated with hemorrhagic cystitis in a breast cancer patient. The Breast Journal. 2011;18:272–275. doi: 10.1111/j.1524-4741.2011.01161.x. [DOI] [PubMed] [Google Scholar]
- McCarville MB, Hoffer FA, Gingrich JR, Jenkins JJ. Imaging findings of hemorrhagic cystitis in pediatric oncology patients. Pediatric Radiology. 2000;30:131–138. doi: 10.1007/s002470050031. [DOI] [PubMed] [Google Scholar]
- Meisenberg B, Lassiter M, Hussein A, Ross M, Vredenburgh JJ, Peters WP. Prevention of hemorrhagic cystitis after high-dose alkylating agent chemotherapy and autologous bone marrow transplant. Bone Marrow Transplant. 1994;14(2):287–91. [PubMed] [Google Scholar]
- Melynk BM, Fineout-Overholt E. Evidence-Based Practice in Nursing & Healthcare. 2nd. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- Monach PA, Arnold LM, Merkel PA. Incidence and prevention of bladder toxicity from cyclophosphamide in the treatment of rheumatic diseases. Arthritis & Rheumatism. 2010;62(1):9–21. doi: 10.1002/art.25061. [DOI] [PubMed] [Google Scholar]
- Murphy C, Harden E, Stevens D, Lynch JP, Montes V, Herzig RH. The addition of mesna to hyperhydration does not decrease the incidence of hemorrhagic cystitis in patients receiving high dose cyclophosphamide. Oncology Reports. 1993;1:265–266. doi: 10.3892/or.1.1.265. [DOI] [PubMed] [Google Scholar]
- Ramu K, Fraiser LH, Mamiya B, Ahmed T, Kehrer JP. Acrolein mercapturates: synthesis, characterization, and assessment of their role in the bladder toxicity of cyclophosphamide. Chemical Research Toxicology. 1995;8:515–524. doi: 10.1021/tx00046a005. [DOI] [PubMed] [Google Scholar]
- Riachy E, Krauel L, Rich BS, McEvoy MP, Honeyman JN, Boulad F, LaQuaglia MP. Risk factors and predictors of severity scores in pediatric hemorrhagic cystitis. Journal of Urology. 2013 doi: 10.1016/j.juro.2013.08.007. in press. [DOI] [PubMed] [Google Scholar]
- Schuchter LM, Hensley M, Meropol NJ, Winer EP. Update of Recommendations for the use of chemotherapy and radiotherapy protectants: Clinical practice guidelines of the American Society of Clinical Oncology. Journal of Clinical Oncology. 2002;20(12):2895–2903. doi: 10.1200/JCO.2002.04.178. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Pringle LE, Barnett MJ, Klingemann HG, Reece DE, Phillips GL. MESNA versus hyper hydration for the prevention of cyclophosphamide-induced hemorrhagic cystitis in bone marrow transplantation. Journal of Clinical Oncology. 1991;9(11):2016–2020. doi: 10.1200/JCO.1991.9.11.2016. [DOI] [PubMed] [Google Scholar]
- Sievers TD, Lagan MA, Bartel SB, Rasco C, Blanding PJ. Variation in administration of cyclophosphamide and MESNA in the treatment of childhood malignancies. Journal of Pediatric Oncology Nursing. 2001;18(1):37–45. doi: 10.1177/104345420101800105. [DOI] [PubMed] [Google Scholar]
- Stillwell TJ, Benson RC, Burgert O. Cyclophosphamide-induced hemorrhagic cystitis in Ewing's sarcoma. Journal of Clinical Oncology. 1988;6:76–82. doi: 10.1200/JCO.1988.6.1.76. [DOI] [PubMed] [Google Scholar]
- Takamoto S, Sakura N, Namera A, Yashiki M. Monitoring of urinary acrolein concentration in patients receiving cyclophosphamide and ifosphamide. Journal of Chromatography B. 2004;806:59–63. doi: 10.1016/j.jchromb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Tracy E, DiTaranto S, Womer R. Evolution of a rapid hydration protocol. Journal of Pediatric Oncology. 2004;21(1):22–26. doi: 10.1177/1043454203259956. [DOI] [PubMed] [Google Scholar]
- Vose JM, Reed EC, Pippert GC, Anderson JR, Bierman PJ, Kessinger A, Armitage JO. Mesna compared with continuous bladder irrigation as uroprotection during high-dose chemotherapy and transplantation: A randomized trial. Journal of Clinical Oncology. 1993;11(7):1306–1310. doi: 10.1200/JCO.1993.11.7.1306. [DOI] [PubMed] [Google Scholar]
- Walker RD. Cyclophosphamide induced hemorrhagic cystitis. Journal of Urology. 1999;161:1747. [PubMed] [Google Scholar]
- West NJ. Prevention and treatment of hemorrhagic cystitis. Pharmacotherapy. 1997;17(4):696–706. [PubMed] [Google Scholar]
- Womer RB, Tracy E, Soo-Hoo W, Bickert B, DiTaranto S, Barnsteiner JH. Multidisciplinary systems approach to chemotherapy safety: Rebuilding processes and holding the gains. Journal of Clinical Oncology. 2002;20(24):4705–4712. doi: 10.1200/JCO.2002.04.108. [DOI] [PubMed] [Google Scholar]