Abstract
Planar cell polarity (PCP)-signaling and associated tissue polarization are evolutionarily conserved. A well documented feature of PCP-signaling in vertebrates is its link to centriole/cilia positioning, although the relationship of PCP and ciliogenesis is still debated. A recent report in Drosophila established that Frizzled (Fz)-PCP core signaling has an instructive input to polarized centriole positioning in non-ciliated Drosophila wing epithelia as a PCP read-out. Here, we review the impact of this observation in the context of recent descriptions of the relationship(s) of core Fz-PCP signaling and cilia/centriole positioning in epithelial and non-epithelial cells. All existing data are consistent with a model where Fz-PCP signaling functions upstream of centriole/cilia positioning, independent of ciliogenesis. The combined data sets indicate that the Fz-Dsh PCP complex is instructive for centriole/ciliary positioning via an actin-based mechanism. Thereby, centriole/cilia/centrosome positioning can be considered an evolutionarily conserved readout and common downstream effect of PCP-signaling from flies to mammals.
Keywords: actin cytoskeleton, centriole/MTOC localization, cilia, epithelial polarity, Frizzled-Vangl signaling, microtubules, planar cell polarity
Introduction to centriole biology
A centriole, mother or daughter, is a highly conserved protein-based cellular organelle [1–4]. In its minimalistic form, centrioles are composed of short α, β, and γ-tubulin microtubules, and centrin [5], which are organized in a barrel-shape with a central cavity. Over the years many proteins have been closely associated with this cellular organelle. These proteins contribute to several aspects of centriole assembly and function, ranging from their maturation to connections between mother and daughter centrioles, and also to their positioning within a cell. Centrioles can appear in the cell cytosol or be associated with the plasma membrane in several specialized structures depending on the particular function in which they will participate, ranging from cell division, cell motility, cellular sensing, to cell polarity, among others [4, 6].
When this relatively small cylindrical structure, about 500 nm long and 200 nm in diameter, is associated with other proteins, which are generally thought to be part of the so-called peri-centriolar matrix (PCM), the centrioles become the functional microtubule organizing center (MTOC) or centrosome. It is important to mention that centrioles and their PCM are not the only MTOCs in eukaryotic cells, where non-centrosomal microtubules appear, and plasma membrane associated proteins, like calmodulin-regulated spectrin-associated protein 3 (CAMSAP3), can nucleate microtubules [7]. Also, it is worth mentioning that in Drosophila the interphase microtubule network does not strictly depend on centrioles [8, 9], and, strikingly, flies can develop to adulthood and are viable without centrioles [10]. Nonetheless, in addition to (i) serving widely/prominently as the MTOC connecting the polarized network of microtubules, the other principal functions of centrioles in cell division or during interphase are linked to; (ii) astral microtubules and the formation and orientation of the mitotic spindle; and (iii) forming basal bodies as part of the generation of cilia and the ciliary signaling hub [4, 6].
Do centrioles move in epithelial cells?
As presented in many cell biology text books, centrioles are usually located close to the nucleus near the center of a cell. Although this is true under many cell culture conditions and in non-epithelial cell types in vivo, it is also true that centriole movement can be (highly) dynamic in non-polarized, polarized, and dividing cells. Indeed, centriole positioning at the center of the cell is considered an actively maintained process, even though centrioles are localized near the cell centroid or geometric center of the cell. This central positioning depends on both, actin filament and microtubule (MT) dynamics and also actin and MT motors such as myosins, kinesins, and dyneins [11, 12].
During cell division, the two centrioles (mother and daughter) are at opposite cell poles (associated with the plasma membrane) ready to project their microtubules to establish the mitotic division spindle. However, during cell migration in vivo (e.g. convergent extension movements or cell intercalation) or in vitro in wound healing/scratch assays, a movement of centrioles becomes apparent [13, 14]. Here, centrioles reorient toward the leading edge of the migrating cells. In fully polarized epithelial cells in cell culture, a realistic description would be that centrioles are located close to the apical membrane while the nucleus is positioned in more basal planes [15, 16]. As described in 1990, when epithelial cells establish their functionally critical apical-basolateral polarity, centrioles migrate toward the apical membrane to complete the polarization of the epithelial cells [17, 18]. Consequently, in epithelial cells, centrioles can in fact be considered as a polarized organelle. This is even more apparent when centrioles engage as basal bodies in the formation and building of cilia (ciliogenesis). The centriole/basal body migration toward the apical membrane is a critical and well established process in ciliogenesis, but is still poorly understood [19–21].
During animal development, centrioles/basal bodies in epithelial cell layers thus appear in close proximity to the apical membrane and are also well separated from the nuclei (Fig. 1). For example, basal bodies in the floor plate of developing zebrafish or the node in mice are fully localized to the apical membrane from where the primary cilium extends [22, 23]. In Drosophila, where primary cilia are absent in epithelial cells, tissues such as the gut or wing are striking examples of centriolar location close to the apical membrane (Fig. 1A, i.e.). Both tissues reflect the apical-basal epithelial polarity and indicate that centrioles are inherently localized to the apical epithelial surface, independent of their role in ciliogenesis as basal bodies [24–26]. Indeed, one can easily guess where the apical membrane is by just looking at where centrioles are localized. How this centriolar localization and migration is achieved, when ciliogenesis is not the target function, remains unknown. The simplest prediction would be that the same mechanisms as described for basal body migration would be in place in non-ciliated Drosophila cells, in analogy to the ones described in Xenopus or zebrafish [19, 21, 27, 28].
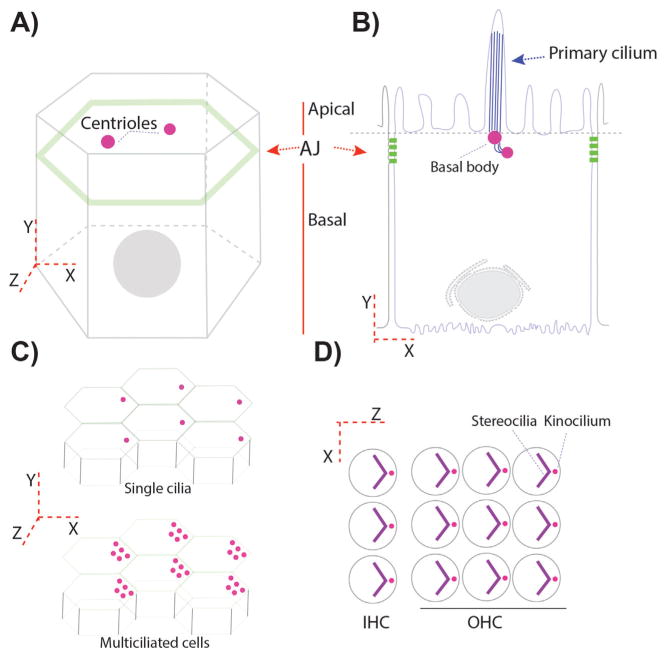
Figure 1.
Centriole/basal body positioning in polarized epithelial cells. A and B: Epithelial cells are like prisms with a belt of proteins connecting adjacent cells. These proteins form adherens junctions (AJ) in Drosophila (A) and AJ and tight junctions (TJ) in vertebrates (B). AJ or AJ/TJ are responsible for delimiting the apical and basal membrane domains in polarized epithelial cells. Centrioles, in polarized epithelial cells, are localized in the most apical planes (A and B). B: In a ciliated epithelium, centrioles migrate towards the apical membrane, where they contact the apical membrane becoming the “basal bodies”/BB, from which the cilia form and protrude into the apical space. C: Certain epithelia display a tissue-coordinated off-center positioning of BBs/centrioles, but still within the most apical planes. This is also observed in many multiciliated epithelia (bottom graph). D: In more specialized epithelial cells, like the outer and inner sensory hair cells (OHC and IHC) in the organ of Corti, the primary cilium, called kinocilium here, is also polarized in an off-center position in the apical plane, subsequently organizing an actin based V-shaped structure, the stereocilia bundle, which follows the polarization of the kinocilium.
In fully differentiated cells, once centrioles are close or attached to the plasma membrane, they can also display polarized localization within a second tissue polarity axis. This information results in what is called translational polarity of the centrioles within the apical planes, which is an off-centered movement of the centrioles coordinated in the entire epithelial layer. In inner ear epithelial cells for example, the axoneme of the kinocilium (a specialized cilium) extends from the basal body, which is derived from the mother centriole of the centrosome attached to the apical membrane [6, 29]. Before the onset of actin-bundle morphogenesis, the kinocilium migrates from the center of the sensory hair cell apex to the lateral edge of the hair cell apex (Fig. 1D). The surrounding actin-based microvilli (also called stereocilia) then undergo selective elongation to form a V-shaped hair bundle, with the kinocilium at the vertex of the stereociliary chevron, next to the tallest stereocilium (Fig. 1D) [30, 31]. Likewise, in multi-ciliated cells, such as epithelial tracheal cells, ependymal cells, or epithelial cells lining the oviduct, centrioles functioning as basal bodies display polarization in two axes, first in the apical-basal axis with centrioles attached to the apical membrane and second off-centered in a coordinated manner (Fig. 1C) [32]. This coordinated display along the tissue contributes to the physiological function of these cells in their respective contexts, like for example removing the dust and mucus from the apical membrane in the trachea, or helping the cerebro-spinal fluid (CSF) flow inside the brain ventricles, or proper hearing in the inner ear cells [31–33].
How are the cilia positioned asymmetrically within the apical cellular apex? Which are the signaling pathways involved in ciliary positioning at the single cell level? How is the positioning of centrioles/basal bodies coordinated along an entire epithelial tissue? And what are the signals triggering this polarized centriole localization? These are exciting open questions at the core of many developmental, cellular, and disease related processes. Specifically, many cilia related diseases, generally referred to as ciliopathies, have been described through their effect on cilia assembly, positioning, or functioning [34–36].
Although mechanical forces, including directional fluid flow, are important in some situations like in ependymal cells in the brain, this is not the case for most examples during development. Over the last decade growing evidence has linked the planar cell polarity (PCP) pathway as a signaling system to regulate polarized ciliary localizations. PCP has been shown to be critical for proper positioning and orientation of cilia in multiciliated cells in the Xenopus larval skin [37–39], ependymal cells in mice [40], and epithelial cells in the oviduct [41, 42]. Similarly, primary cilia positioning is regulated by PCP in the mouse node [43], the outer hair cells in the mouse cochlea [30, 31], the floor plate from zebrafish [22], cell lens in mice [44], and planaria [45]. More recently, our work has demonstrated the PCP-signaling dependent polarized localization of centrioles in non-ciliated epithelial cells in the Drosophila wing epithelium [25]. Taken together with the involvement of PCP signaling in the orientation of the mitotic spindle in several contexts, this recent observation suggests that the planar cell polarity/PCP-pathway serves an evolutionarily conserved and universal function for polarized localization of centrioles [46–52].
In this review, we focus on these exciting interactions between PCP signaling and interphase centrioles, although some functional connections will also be made with the machinery involved in oriented cell division, where spindle interaction with the plasma membrane components of at least two cell polarity pathways take part.
The PCP pathway has different outcomes
The planar cell polarity pathway plays developmental/physiological functions
The planar cell polarity (PCP) signaling pathway is about cellular coordination and communication of cells across a whole tissue. In epithelial cells, PCP governs a second axis of polarity, which is orthogonal to the apical-basal axis. Like apical-basal polarity, PCP is a well conserved developmental process throughout the animal kingdom [32, 53–56]. Besides its “experimental origins” and detailed understanding from Drosophila [54, 57], many classical examples of planar polarization in mammals presented PCP, where it is linked it to several diseases [58]. Mammalian examples include (but are not restricted to) orientation and angle of skin hair follicles, oriented cell division in developing kidney tubules, or examples already mentioned above such as inner ear hair cell orientation, directional ciliary beating, cilia positioning in the node, and cilia sweeping in oviducts, trachea, and brain ventricles. Moreover, the PCP pathway has also been implicated in migrating cells (often also non-epithelial migrating cells), and hence this pathway coordinates the collective and directional movement of mesenchymal cells in convergent extension required for cell body elongation or neural tube closure in vertebrates and neuronal migration in developmental contexts [32, 53–56, 59].
The number of PCP associated processes in vertebrates is constantly growing, and now also includes PCP signaling functions in the cellular rearrangements during valve morphogenesis [60] or pancreatic beta cell differentiation and glucose homeostasis [61, 62], among others. Moreover, defects in the PCP pathway have been associated with several defined genetics syndromes [58]. Many of them associated with defects in cilia associated signaling and function (e.g. Meckel–Gruber syndrome) or neural tube closure defects like spina bifida or hydrocephalus, and ciliopathies in general [33,58,59,63–66]. On the other side of the evolutionary spectrum, in planaria, PCP signaling controls neuronal connectivity and animal body shape [45].
PCP studies in Drosophila pioneered the understanding of the molecular interactions of the core PCP factors [57, 67–69]. As such Drosophila remains the premier model system to study PCP signaling associated principles and its cell biology. The meticulous arrangement of photoreceptor cells in the fly compound eye or the highly regular orientation of bristles and cellular actin hairs/trichomes covering the Drosophila exoskeleton in wings, abdomens, or the thorax remain the most valuable tissues to study PCP [57, 69–71].
What are the PCP pathway mechanisms at the subcellular and molecular level?
PCP establishment requires two molecularly independent pathways that have nonetheless recently been shown to be also intercommunicating. The Fat (Ft) and Dachsous (Ds) pathway, known as the Ft-PCP pathway, and the Frizzled PCP pathway (Fz-PCP) or core PCP pathway. Although these have been defined as and are two independent pathways [69, 70, 72, 73], it is evident that there has to be crosstalk between the pathways and also that their cellular response(s) need to be coordinated [74–76]. The relationship and “inter-pathway” communication has been reviewed several times (please see for example [69, 70, 73]) and remains a very active field of research and debate. Two recent articles (from Drosophila) have placed the prickle (pk) gene (and its isoforms Sple and Pk) as a central player for the Ft-PCP and Fz core-PCP pathway crosstalk [75, 76].
Ft-PCP signaling includes the atypical cadherins Fat (Ft) and Dachsous (Ds) and the associated secreted/Golgi kinase Four-jointed (Fj). This cell-cell communication system is based on heterophilic interactions across cell contacts between the large extracellular domains of Ft and Ds, which is modulated by direct phosphorylation via Fj of both cadherins. Global signaling in this case is based on expression gradients of its different components [77–85].
The Fz core-PCP pathway (also known as non-canonical Wnt/Fz signaling) shares two membrane-associated components with canonical Wnt/β-catenin signaling, Frizzled (Fz) and Disheveled (Dsh) [56, 69, 86], and is globally regulated by Wnt-gradients as well [53,87,88]. Transmembrane interactions in the Fz-PCP system require and are transmitted by specific Fz family members, the 4-TM protein Van Gogh (Vang, a.k.a. Stbm/Strabismus, Vangl1/2 in vertebrates) and the atypical cadherin Flamingo (Fmi, a.k.a. Stan/Starry Night, Celsr1-3 in vertebrates) (Fig. 2). The cytosolic core components of this pathway are important to integrate, and stabilize, through feedback loops, the information at the single cell level. These include Dsh, Pk, and Diego (Dgo). Pk isoforms are key components interacting with Vang/Vangl and Dsh and Dgo with Fz [53, 69]. Positive and negative feedback loops by competitive interactions, contribute to the subcellular asymmetries of the Fz-PCP core components. The global cues are Wnt family members, which act as modulators for the extracellular interactions between Fz and Vang in flies [89] and likely also in vertebrates [53, 88]. Indeed, strong genetic support from both mouse and zebrafish model systems suggest, Wnt5a and Wnt11 are dedicated to the PCP context [53, 88, 90, 91]. Historicaly, Fz, Vang/Stbm, and Fmi (Celsr), and the cytoplasmic Dsh (Dvl), Pk, and Dgo (Diversin and Inversin in mammals) have been considered the core Fz-PCP components. However, recent work suggests a comparable core-like requirement of Scribble (Scrib) in Drosophila and mice [92–94] and roles for the Selectin family member Furrowed and a v-ATPase so far in Drosophila only [95–98]. Furthermore, there are many other regulatory factors involved in post-translational regulation of the core components (see above in general PCP signaling reviews for additional details).
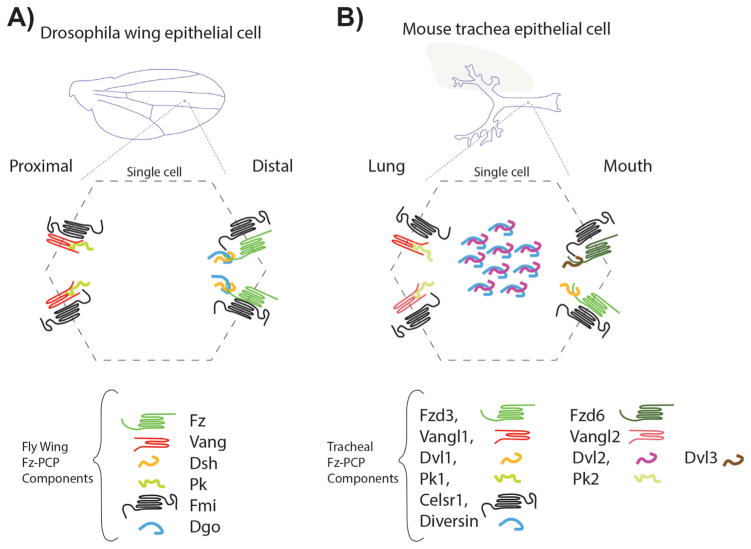
Figure 2.
Frizzled (Fz) planar cell polarity core components in Drosophila wings and mouse trachea. A: Drosophila wing blades are formed by two layers of polarized epithelial cells with the apical domain facing the wing surface. Following the proximal-distal axis, Fz-PCP core components are asymmetrically localized to a distal complex including Fz-Dsh-Fmi-Dgo and a proximal complex formed by Vang-Pk-Fmi. This pattern is reproduced in every single cell throughout the wing blade. B: In mouse tracheal epithelial cells, the apical domains face the lumen. In an analogous manner to Drosophila wing epithelial cells, the Fz-PCP core components are segregated in proximal (lung side) and distal (mouth side) complexes, containing the equivalent orthologues as in Drosophila. In addition to the several isoforms for most of the Fz-PCP core components, in mouse tracheal epithelial cells Diversin (the vertebrate Dgo homolog) and Dvl2 are detected near the basal bodies in these multiciliated cells.
At the core of both pathways is the asymmetrical subcellular localization of their protein components. Again, their interactions and localizations are best described in the Drosophila wing and eye epithelia for both pathways, Ft-PCP and Fz-PCP [69, 71]. The Drosophila wing epithelial model is arguably the most studied, and the wing blade is formed by two mono layers of epithelial cells. Each cell has at the junctional belt a distal domain enriched in Fz, Dsh, and Dgo complexes and a proximal domain where Vang/Stbm and Pk are interacting (Fig. 2). The cadherin Fmi completes the asymmetrical distribution as a component of both, the distal and proximal complexes, forming Fmi-Fmi interactions across the membrane, and interacting with Vang/Stbm and Fz in their respective domains. In the other system, Ft and Ds also display an asymmetric localization in Drosophila wing epithelial cells with Ds localization at the proximal side and Ft in the distal side of the cell. In vertebrates, comparable asymmetric distributions are observed in mouse trachea cells [99] with Fz/Dv1-Dvl3/Celsr on the oral side of the cells and Vangl2-Vangl1/Pk1-Pk2/Celsr on the pulmonary side (Fig. 2B). Partially described asymmetric localizations are found in most other vertebrate PCP model systems, such as the mouse inner ear, skin epidermal cells, or neural tube cells to mention just a few (e.g. see [32], for specific localization in other systems).
The planar cell polarity pathway connects with the cellular cytoskeleton
To understand the implications of PCP proteins in the context of centriole/centrosome/basal body positioning, it is necessary to briefly look at the functional and molecular connections between PCP proteins and cytoskeleton dynamics, including both the actin and microtubule networks.
PCP pathway impacts the actin cytoskeleton
The PCP pathway was discovered because of defects in cytoskeletal rearrangements as visible in the insect cuticle [100]. Initially discovered in Drosophila, the PCP mutants exhibit reorientation of the exocuticle structures in adult flies and actin-based hair (trichomes) and bristle polarity in wings and legs [100, 101]. As these cellular structures are a result of polymerized actin filaments and stable microtubules [25, 102, 103], an obvious connection could be established to the cytoskeleton. Genetic and molecular analyses further demonstrated that cytoskeletal actin rearrangements were indeed an important output of PCP signaling in several model systems [104, 105].
In Drosophila wing epithelial cells, once PCP components are fully asymmetrically localized (around 30–32 hours APF, after puparium formation), the actin-based hairs are projecting at their apical membrane, pointing towards the distal side of the wing (Fig. 3). Fz-PCP loss and gain-of-function alleles show a randomization of the positioning of the single actin-based hair within the apical membrane apex (see Fig. 3). In addition, multiple actin hairs can form as a consequence of the mislocalized (or even dispersed) actin nucleation machinery, often referred to as “multiple cellular hairs” or mch for short (see below for specifics about “mch-type” mutations and genes). Also, hairs growing at the center in loss-of-function (LOF) and late Fz over-expression (gain-of-function/GOF) experiments contribute to the idea of a dual function for Fz signaling. First Fz contributes defining the axis of polarity and later focusing the actin polymerization machinery to a specific cellular region [106], suggesting that the Fz-Dsh complex not only is critical for polarity axis establishment but also for the recruitment of critical downstream effector molecules [57].
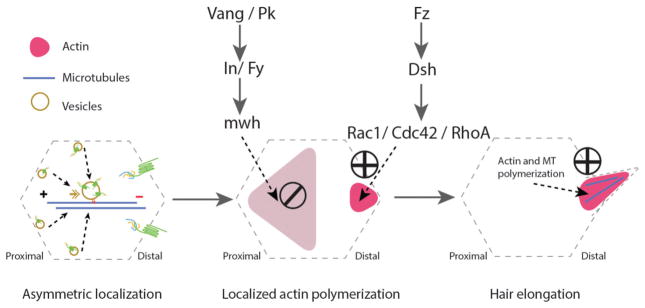
Figure 3.
Cytoskeletal arrangements during the planar cell polarization process in Drosophila pupal wing epithelial cells. uring pupal development, epithelial cells use essential cellular machinery, including the protein trafficking machinery which is involved in the asymmetric localization of the Fz-PCP core components in a polarized manner. This directed transport requires an alignment of well oriented microtubules (MT, blue line in left panel) with enriched minus-ends towards the distal side of each cell (left panel). This stage is followed by activation of actin polymerization in the distal side (via Fz-Dsh signaling), while blocking actin polymerization in the proximal side (via Vang-Pk and their effectors) of the same cell (middle panel). The last stage involves actin and stable microtubule polymerization at the base and inside trichomes (cellular hairs), which is essential for hair elongation (right panel).
A group of Fz-PCP effectors related to actin cytoskeleton regulation has been described, most of them causing a mch-type phenotype, these include the formin-family protein Multiple wing hair (Mwh), Inturned (In), Fuzzy, and Fritz. All of these are enriched at the proximal domain (the Vang/Stbm-Pk side) of the apical domain in any given epithelial wing cells, where they contribute to restrict/limit the actin polarization region to the distal apical domain (the Fz-Dsh-Dgo side) of the respective cell. Small Rho family GTPases regulate this localized actin polymerization process. Cdc42 (and RhoA), assumed to be recruited and/or activated by a Dsh-dependent process, nucleate actin polymerization contributing to hair extension, while Rac1 restricts the site at which actin hairs grow. Experimentally, expression of Cdc42 or RhoA dominant negative (DN) mutants in wing cells blocks hair growth, producing either stunted hairs or no hair at all [102, 107–109]. In contrast, over-expression of Rac1-DN produces multiple wing hair phenotypes, similar to those observed in mwh, in, fuzzy, or fritz mutants [57, 102, 107, 110–112]. Molecular connections between the Fz-PCP pathway and Rho family GTPases have been described besides the work in Drosophila, in several other model systems, such as Xenopus or chick embryos, and are generally consistent with the above described observations.
PCP pathway remodels the microtubule network
The interactions or cross-talk between microtubules and the PCP pathways are more complex. These can be divided into microtubule requirements for correct PCP axis establishment (upstream of PCP) and microtubule dynamics as an output of PCP signaling (downstream effectors of PCP).
Microtubules themselves are polarized structures, with plus and minus ends. In both vertebrates and flies, correct asymmetric localization of Fz-PCP components requires this microtubule polarity. Again, most of our knowledge comes from studies of Drosophila pupal wings, where an enrichment of MT plus-ends was detected toward the distal domain. Fmi-Fz-Dsh containing vesicles use these microtubule “roads” to move to the right target location [113]. Treatments with the MT dissociating drug Nocodazole, which blocks polymerization of microtubules, causes mislocalization of core PCP proteins in pupal wing cells [113]. Similar results were obtained in planar polarized tracheal cells, where polarized microtubules are also detected and Nocodazole treatment causes aberrant intracellular accumulation of Vangl1 and Pk2 vesicles [99].
Direct interactions of microtubule dynamics and Fz-PCP or Ft-PCP [114] proteins are evident in several model systems. Dvl proteins can influence microtubule stability [115, 116]. On the other hand, isoforms of the pk gene (Pk and Sple) can control the polarity of the microtubule network in PCP at least in flies [74].
Planar cell polarity instructs centriole/basal body positioning
A connection between asymmetrically localized centrioles and PCP signaling was first discovered in 2003 in the mouse inner ear [94]. Here, in the cochlear hair cells in the organ of Corti, a mutant allele of a core PCP mutant (the Looptail mutant of Vangl2, VanglLp/Lp) affected cellular positioning of the kinocilium and hence the orientation of the stereocilia hair bundles (Figs 1 and 5). Subsequently, many studies followed looking at the kinocilium and stereocilia hair bundle localization, morphology, shape, and relative position in PCP core and related factors. In general, core PCP protein mutants including, Vangl2 [94], Dvl, Fzd3, and Fzd6 double mutants [117], and Flamingo/Celsr1[118] were shown to display random positioning of the basal body/kinocilia within the apical membrane, while maintaining the relative relationship between the actin-based stereocilia and the kinocilium.
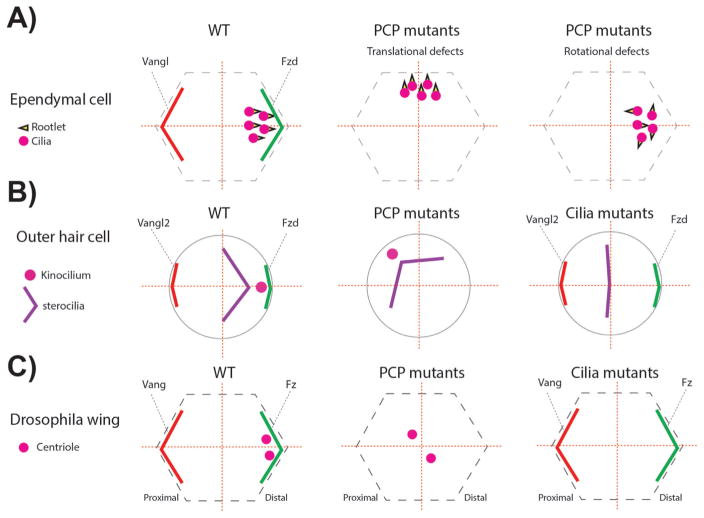
Figure 5.
PCP and cilia mutants associated phenotypes related to centriole/basal body (BB) A: In ependymal cells BBs are localized near the Fzd expression domain. In addition, the basal foot of the cilia, called rootlet and critical for ciliary beating, is aligned in the same direction (left panel). In one set of specific PCP mutants (check text for specific mutants) the localization of the BBs leading to so-called “translational defects” (middle panel). In other PCP mutants, although the translational polarity is correct, the alignment of the ciliary rootlets is uncoordinated (right panel). B: In the inner ear, outer hair cells (OHCs) position the BBs of the kinocilium (a specialized primary cilium) near the Fzd-Dvl localization domain, similar to ependymal cells (left panel). In PCP mutants the off-centered positioning of the BB is affected, but the V-shaped stereocilia bundle is not affected, following the position of the BB/kinocilium (middle panel). In cilia mutants, the V-shape of the stereocilia is affected and the structure remains central. The stereocilia bundle can appear as a line or circular shape depending on the severity of the ciliary mutant, in which the kinoclium is missing (right panel). C: Once fully polarized in Drosophila pupal wing cells, centrioles are localized near the Fz-Dsh domain, similar to mouse ependymal cells and OHCs (left panel). In PCP core and effector mutants, centrioles remain centered (middle panel). In Sas4 mutants where centrioles are absent, PCP remains mostly normal and actin polymerization in trichomes is largely unaffected (right panel).
In general, PCP model systems to study the positioning of centrioles/basal bodies can be divided into multi-ciliated cells (MCCs) and mono-ciliated (primary cilium only) cells. Among the MCCs, ependymal cells in the brain ventricles and Xenopus epidermal cells have thus far been the most productive model systems to describe and analyze ciliary positioning. In the apical membrane of ependymal cells, cilia have an additional polarity, called “rotational polarity” apart from the already mentioned translational polarity. Rotational polarity refers to the orientation of a single cilium and can be visualized by the orientation of its ciliary rootlet. PCP mutants of Celsr1, Fzd3, and Vangl2 from the core Fz-PCP system, which coordinates translational polarity in ependymal cells, in fact show defects in both cilia polarity processes, as rotational polarity is dependent on the proper establishment of translational polarity [119] (see Fig. 4 for details). Celsr2, Celsr3, Frizzled3 (Fzd3) and Vangl2 also organize multicilia in individual cells (single-cell polarity) [120] with cilia located on the opposite side of Vangl2 [119] (Fig. 4). Celsr1 knock-out mice also have ciliary orientation defects in cells lining the oviduct [41]. While more recently, Dvl triple knock-out mice showed consistent hydrocephalus due to abnormal cilia patch positioning of ependymal cells [121]. Additionally, knock-downs of individual and combined Dvl’s (Dvl1, Dvl2, and Dvl3) in MCCs of the Xenopus epidermis showed ciliary polarity defects [37], which is a phenotype also observed in PCP effector protein interference, for example Inturned, or Fuzzy [38].
Figure 4.
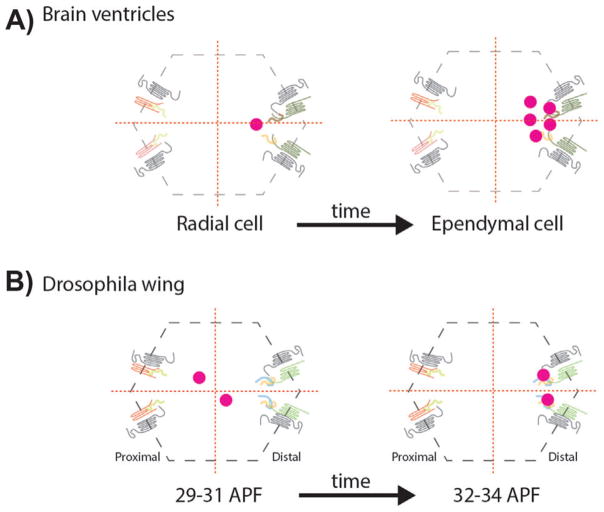
Planar polarized centriole positioning. A: Centrioles, as basal bodies (BBs) at the apical plane lining the brain ventricle cells, are planar cell polarized, first in radial cells and followed by their differentiation to ependymal cells, where BBs are multiplied generating a multiciliated epithelium. The off-centered distribution in both, radial and ependymal cells, is controlled by the Fz-PCP signaling pathway. B: During Drosophila pupal wing development, centrioles get polarized along the Fz-PCP polarization axis, migrating towards the Fz-Dsh-Fmi-Dgo distal domain. APF: After puparium formation. Protein cartoons are as in Fig. 2 with the Fz-Dsh/Dvl complexes on the right in each cell.
Among the mono-ciliated cells, lens cells in the eye [44], epithelial/neuropeithelial cells in the Zebrafish embryo [22], and mouse node cells [23], all showed PCP signaling dependent polarization of basal bodies. Ironically, the last model system to show the connection between PCP signaling and centrioles is the Drosophila wing (Fig. 4). A possible explanation is because its cells do not have primary cilia and hence nobody bothered to look [25]. However it was shown before in Drosophila gut cells that centriole polarization was not affected in PCP mutants [24]. Our data in Drosophila wings emphasize the intimate relationship between PCP signaling and centriole polarization and, importantly, incorporate to a certain degree, centriole positioning as a universal read-out for PCP signaling [25].
This could be extended to MTOCs/centrosomes as a component of the spindle orientation during asymmetric cell division in Drosophila. In this context Fz, Fmi, and Vang contribute to regulate the localization of cell-fate determinants, like Numb, and regionalization of proteins at the cell cortex [50, 51, 122]. Indeed, Vang and Pk co-localize and promote the localization of Partner of Inscuteable (Pins) [48]. Pins together with Dlg and Gαi then settle the axis orientation for the spindle, which thus has been aligned with the PCP axis.
What is the mechanism by which PCP signaling could position centrioles or orient cilia?
As of now and as outlined above, all published data support the idea of PCP, actin, and microtubules cooperating in the positioning of centrioles/basal bodies. It is established that correct actin filament polymerization is required for proper positioning of ciliary basal bodies in apical-basal polarized epithelial cells. Cytochalasin D, an inhibitor of actin polymerization, interferes with basal body migration and ciliary development in epithelial cells [123]. Also in planar cell polarized epithelial cells, like the node in mouse embryos, the posterior localization of cilia is impaired by Rac1 inactivation, via the Rac1 inhibitor NSC23766, with cilia remaining in a central apical position [23]. Furthermore, NSC23766 treatment leads to basal body positioning defects in auditory hair cells. IPA-3, while a small-molecule inhibitor of PAK, which is a cytoskeletal regulator downstream of the small GTPases Rac and Cdc42 [124], impaired ciliary positioning in inner ear hair cells [125]. Moreover, in the Xenopus epidermis, expression of a dominant-negative RhoA (RhoA-N19) caused a failure of basal bodies to align, but ciliogenesis was completed [37], indicating that actin-polymerization interference affects positioning but not the morphogenesis of cilia. Similarly in Xenopus epidermal cells, Dvl, inturned, and fuzzy loss-of-function scenarios cause a decrease in apical actin assembly, which is important for the orientation of basal bodies and cilia [13, 37, 38]. Dvl and Inturned are localized near the basal apparatus of cilia and regulate Rho activation and localization with Dvl activating Rho, while Inturned is required for Rho localization [37, 38]. Also, directed apical actin polymerization cooperates in the process of apical docking of basal bodies, as reflected by the interference in RhoA-N19 expressing cells [37].
In Drosophila pupal wing cells, Nocodazole treatment during wing hair development at 26–34 hours APF induces formation of multiple cellular hairs (mch) in each cell [103], as also observed in Rac1DN expression or the overexpression of the Fz-Dsh core PCP complex factors and effectors, in, fy, and mwh. In this model, mch-phenotypes as seen in mwh loss-of-function backgrounds in wing epithelial cells are functionally linked to the randomization of basal body positioning in epidermal cells. In ependymal cells, non-muscle myosin-II regulates the translational, but not the rotational, polarizarion of basal bodies, blocked by using the myosin II inhibitor Blebbistatin. In planarians, actin polymerization decrease also correlates with centriole polarization defects [45]. These observations are all consistent with a model where localized/organized actin polymerization influences the positioning of centrioles or basal bodies. Further studies will be needed to assess if centrioles can still migrate towards the cellular periphery in these contexts.
PCP and cilia proteins are connected
There are partially contradictory and many not fully explained results regarding the ciliary protein network, cilia function, and their links to core-PCP proteins, most likely because it is difficult to separate the localization of cilia/basal bodies from ciliary function.
To date, the clearest data set on the role of core PCP proteins in cilia positioning and/or function comes from genetic work on the Vangl proteins, which appear exclusively dedicated to PCP signaling (unlike Fz and Dsh proteins for example that either also act in other signaling pathways or are localized to basal bodies) [22, 94]. Analyses of Vangl mutants in mice and zebrafish revealed that cilia form indistinguishably from wild-type, but are mis-positioned within the apical cell apex [22], giving rise for example to left-right asymmetry defects, neural tube closure defects, misaligned inner ear sensory cells, etc. [94]. These data strongly argue for a role of core PCP-signaling upstream of ciliary positioning, but not in ciliogenesis per se. Still in Vangl2 there some discrepancies, since cilia defect were reported in kupffeŕs vesicle in zebrafish [126]. Despite the clarity of these data sets, the field is confused as there are other convincing data sets linking the core PCP factors also to ciliogenesis. Among these, several PCP loss-of-function (LOF) alleles display ciliogenesis defects by themselves, these include for example Celsr2, Celsr3 mutants (mice) and Dvl1, Dvl2, and Dvl3 interference in Xenopus [37]. Moreover, PCP and ciliary mutants present common phenotypes at the organ/tissue level, including hearing defects, neural tube closure, left-right asymmetry, open eye lids, dyskinesia, or convergent extension (cochlea and mediolateral C&E) defects. There are also genetic interactions between Mks and Bbs (Bardet-Biedl syndrome/BBS) mutants, and Vangl2 Looptail mutant [127]. However, once examined closer at the subcellular level the individual mutants revealed that they do not share the same defects, for example, the inner ear sensory defects examined in detail at the level of stereocilia and the kinocilium are clearly distinct. Importantly, in PCP LOF conditions (e.g. Vangl alleles) the stereocilia bundle is still arranged in a V-shape (like in wild-type), but the kinocilium is randomly positioned, while in mutants affecting ciliogenesis the kinocilium remains at the center and importantly the stereocilia bundles appear in many (random) forms (circular or flat). Such stereocilia defects are also apparent in Gαi3-KO and mPins–KO, where stereociliary hair bundles display flattened shapes and the kinocilium is more centrally positioned in the outer hair cells [128]. Importantly, in these mutants the Vangl2 asymmetric localization persists and is indistinguishable from wild-type, indicating that the core PCP factor localization is upstream and independent of ciliogenesis. This type of relationship between PCP and Gα/Pins signaling is also observed in asymmetric cell division in Drosophila [48, 49]. Accordingly, in Ift88 mutant mice, with IFT88 being a classical ciliogenesis factor [129], localization of all core PCP proteins remained normal. Thus, with both, a closer examination at the cellular level and a step-back “aerial” view at the signaling level, the conclusion is that core PCP signaling factors act “upstream” in positioning of cilia/basal bodies within the apical apex of any given cell and are independent of ciliogenesis.
Our recent data [25] extends these studies to non-ciliated epithelial cells, and firmly establishes that core PCP signaling acts upstream of centriole positioning in general. Specifically, centrioles localize to the side of the cell that is enriched in Fz-Dsh complexes (and devoid of Vang-Pk complexes) (Fig. 4). This observation is consistent with the regulation of ciliary positioning in vertebrates, where for example in the zebrafish neural tube, cilia are localized in a PCP-signaling dependent manner to the posterior cellular side, the cell side enriched with Fz-Dvl proteins, and away from the Vangl-Pk cell side (anterior) [22]. Consistent with the PCP upstream of centriole/cilia model is the observation that in Drosophila, ablation of centrioles (in sas4 mutants) does not generate PCP phenotypes and wing hairs are positioned properly and point in the right (distal) direction, together with Fmi displaying a mostly perfect asymmetric localization in these acentriolar cells [25]. Thus taken together, all these data are consistent with one simple model: (i) the core PCP factors localize centrioles/basal bodies within the apical cellular apex; and (ii) centrioles/basal bodies get positioned to the side of the cell that is enriched in Fz-Dsh/Dvl complexes (Fig. 5). This model is also consistent with the PCP regulated mitotic spindle orientation, where centrioles/centrosomes are aligned with the PCP axis via interactions with the Fz-Dsh and/or Vang complexes [46, 50].
Is it really that clear or are there complications? Although Vangl2, Dvl2, and Diversin (Dgo homolog) can localize to the base of cilia, near the basal bodies not at the basal body itself [37, 99, 127, 130], the asymmetric localization of core PCP proteins is not affected. So the localization to the base of cilia could be considered a second downstream function of core PCP factors, similar to effects of late Fz or Dsh overexpression in Drosophila wing cells, inducing multiple cellular hairs (see above), but not affecting the PCP polarity axes. Such observations might also be tissue-specific (as a downstream PCP read-out), as Vangl2 for example does not localize at the ciliary base in tracheal cells [99] or in inner ear sensory cells [30, 31, 131].
Taken altogether, the available results indicate that cilia function downstream (or maybe in parallel in some contexts) of the core Fz-PCP pathway and that the core PCP signaling factors are required for correct positioning of cilia (basal bodies), centrioles, or centrosomes. The functional interactions are relatively easy to visualize in terms of PCP signaling implication in the positioning of centrioles/centrosomes/cilia.
Conclusions
Data acquired over the past decade allows several conclusions about the intimate relationship between core PCP-signaling and centrioles/basal bodies (BBs) polarization/positioning. The connection between centriole polarization/localization and PCP-signaling has been studied in several systems, and in all systems where centrioles/BBs and PCP have been analyzed together, polarization of PCP complexes precedes and is independent of centrioles/BBs positioning. The models tested now also include Drosophila wing epithelia (a simpler model as no cilia form here), and this model is consistent with observations in vertebrate node, neural tube, tracheal epithelia, ependymal cells, and inner ear sensory hair cells and supportive cells [22, 31–33, 53, 132]. All data from these models are consistent with core Fz-PCP signaling acting upstream of centriole or ciliary polarization and positioning.
How does PCP-signaling impact the positioning of centrioles/BBs? The consensus argues for a critical involvement of actin in this process. This might be obvious since a connection between actin and centriole movements had been established both in non-polarized and epithelial apical-basal polarized cells [11, 12, 17]. Implications of small Rho-family GTPases (Rac, Rho, or Cdc42) are also consistent with these observations, although direct molecular connections to the core PCP components might differ from tissue to tissue, or model to model. Nonetheless, Dsh/Dvl proteins have been suggested to serve an “organizer function” and recruit these GTPases, their activators, and other actin-polymerization factors.
It will be more difficult to uncover precise connections between microtubules and cilia/BB positioning as a PCP output. The problem is the upstream and downstream implications of microtubular networks in PCP establishment and maintenance. Nonetheless, with the inclusion of Drosophila wing epithelia to the list of model systems to study the link(s) between core PCP signaling and centriole localization/polarization, new doors are open to analyze cilia “translational polarity” or planar polarity of centrioles and centrosomes in the absence of cilia.
Acknowledgments
We thank Carlo Iomini and all members of the Mlodzik lab for helpful discussions, and Ashley Humphries for helpful comments on the manuscript. J.M. C-G is a recipient of an Atracción y Retención de talento contract from the GOBEX (Extremadura government). This work was supported by NIGMS and NEI grants from the National Institutes of Health to M.M and BFU2014-54699-P grant from the Ministry of Economy to J.M. C-G.
The authors declare no competing financial interests.
Abbreviations
- MCC
multi-ciliated cell
- MT
microtubule
- MTOC
microtubule organizing center
- PCP
planar cell polarity
References
- 1.Bornens M. The centrosome in cells and organisms. Science. 2012;335:422–6. doi: 10.1126/science.1209037. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M. Evolution: tracing the origins of centrioles, cilia, and flagella. J Cell Biol. 2011;194:165–75. doi: 10.1083/jcb.201011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azimzadeh J, Marshall WF. Building the centriole. Curr Biol. 2010;20:R816–25. doi: 10.1016/j.cub.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007;8:451–63. doi: 10.1038/nrm2180. [DOI] [PubMed] [Google Scholar]
- 5.Beisson J, Wright M. Basal body/centriole assembly and continuity. Curr Opin Cell Biol. 2003;15:96–104. doi: 10.1016/s0955-0674(02)00017-0. [DOI] [PubMed] [Google Scholar]
- 6.Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139:663–78. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka N, Meng W, Nagae S, Takeichi M. Nezha/CAMSAP3 and CAMSAP2 cooperate in epithelial-specific organization of non-centrosomal microtubules. Proc Natl Acad Sci USA. 2012;109:20029–34. doi: 10.1073/pnas.1218017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gogendeau D, Basto R. Centrioles in flies: the exception to the rule? Semin Cell Dev Biol. 2010;21:163–73. doi: 10.1016/j.semcdb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Rogers GC, Rusan NM, Peifer M, Rogers SL. A multicomponent assembly pathway contributes to the formation of acentrosomal microtubule arrays in interphase Drosophila cells. Mol Biol Cell. 2008;19:3163–78. doi: 10.1091/mbc.E07-10-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basto R, Lau J, Vinogradova T, Gardiol A, et al. Flies without centrioles. Cell. 2006;125:1375–86. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Euteneuer U, Schliwa M. Evidence for an involvement of actin in the positioning and motility of centrosomes. J Cell Biol. 1985;101:96–103. doi: 10.1083/jcb.101.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J, Burakov A, Rodionov V, Mogilner A. Finding the cell center by a balance of dynein and myosin pulling and microtubule pushing: a computational study. Mol Biol Cell. 2010;21:4418–27. doi: 10.1091/mbc.E10-07-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner ME, Mitchell JW, Putzbach W, Bacon E, et al. Radial intercalation is regulated by the Par complex and the microtubule-stabilizing protein CLAMP/Spef1. J Cell Biol. 2014;206:367–76. doi: 10.1083/jcb.201312045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blitzer AL, Panagis L, Gusella GL, Danias J, et al. Primary cilia dynamics instruct tissue patterning and repair of corneal endothelium. Proc Natl Acad Sci USA. 2011;108:2819–24. doi: 10.1073/pnas.1016702108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15:225–42. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Fraticelli AE, Auzan M, Alonso MA, Bornens M, et al. Cell confinement controls centrosome positioning and lumen initiation during epithelial morphogenesis. J Cell Biol. 2012;198:1011–23. doi: 10.1083/jcb.201203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buendia B, Bre MH, Griffiths G, Karsenti E. Cytoskeletal control of centrioles movement during the establishment of polarity in Madin-Darby canine kidney cells. J Cell Biol. 1990;110:1123–35. doi: 10.1083/jcb.110.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez Bay By AE, Schreiner R, Mazzoni F, Carvajal-Gonzalez JM, et al. The kinesin KIF16B mediates apical transcytosis of transferrin receptor in AP-1B-deficient epithelia. EMBO J. 2013;32:2125–39. doi: 10.1038/emboj.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012;13:608–18. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avasthi P, Marshall WF. Stages of ciliogenesis and regulation of ciliary length. Differentiation. 2012;83:S30–42. doi: 10.1016/j.diff.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–77. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol. 2010;12:407–12. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto M, Shinohara K, Wang J, Ikeuchi S, et al. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol. 2010;12:170–6. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- 24.Taniguchi K, Maeda R, Ando T, Okumura T, et al. Chirality in planar cell shape contributes to left-right asymmetric epithelial morphogenesis. Science. 2011;333:339–41. doi: 10.1126/science.1200940. [DOI] [PubMed] [Google Scholar]
- 25.Carvajal-Gonzalez JM, Roman AC, Mlodzik M. Positioning of centrioles is a conserved readout of Frizzled planar cell polarity signalling. Nat Commun. 2016;7:11135. doi: 10.1038/ncomms11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matis M, Russler-Germain DA, Hu Q, Tomlin CJ, et al. Microtubules provide directional information for core PCP function. Elife. 2014;3:e02893. doi: 10.7554/eLife.02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallingford JB. Planar cell polarity signaling, cilia and polarized ciliary beating. Curr Opin Cell Biol. 2010;22:597–604. doi: 10.1016/j.ceb.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–13. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–44. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones C, Chen P. Primary cilia in planar cell polarity regulation of the inner ear. Curr Top Dev Biol. 2008;85:197–224. doi: 10.1016/S0070-2153(08)00808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ezan J, Montcouquiol M. Revisiting planar cell polarity in the inner ear. Semin Cell Dev Biol. 2013;24:499–506. doi: 10.1016/j.semcdb.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–53. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 33.Ohata S, Alvarez-Buylla A. Planar organization of multiciliated ependymal (E1) cells in the brain ventricular epithelium. Trends Neurosci. 2016;39:543–51. doi: 10.1016/j.tins.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valente EM, Rosti RO, Gibbs E, Gleeson JG. Primary cilia in neurodevelopmental disorders. Nat Rev Neurol. 2014;10:27–36. doi: 10.1038/nrneurol.2013.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bettencourt-Dias M, Hildebrandt F, Pellman D, Woods G, et al. Centrosomes and cilia in human disease. Trends Genet. 2011;27:307–15. doi: 10.1016/j.tig.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–43. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park TJ, Mitchell BJ, Abitua PB, Kintner C, et al. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–9. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–11. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell B, Stubbs JL, Huisman F, Taborek P, et al. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol. 2009;19:924–9. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guirao B, Meunier A, Mortaud S, Aguilar A, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–50. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- 41.Shi D, Komatsu K, Hirao M, Toyooka Y, et al. Celsr1 is required for the generation of polarity at multiple levels of the mouse oviduct. Development. 2014;141:4558–68. doi: 10.1242/dev.115659. [DOI] [PubMed] [Google Scholar]
- 42.Shi D, Usami F, Komatsu K, Oka S, et al. Dynamics of planar cell polarity protein Vangl2 in the mouse oviduct epithelium. Mech Dev. 2016;141:78–89. doi: 10.1016/j.mod.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Song H, Hu J, Chen W, Elliott G, et al. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–82. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugiyama Y, Stump RJ, Nguyen A, Wen L, et al. Secreted frizzled-related protein disrupts PCP in eye lens fiber cells that have polarised primary cilia. Dev Biol. 2010;338:193–201. doi: 10.1016/j.ydbio.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almuedo-Castillo M, Salo E, Adell T. Dishevelled is essential for neural connectivity and planar cell polarity in planarians. Proc Natl Acad Sci USA. 2011;108:2813–8. doi: 10.1073/pnas.1012090108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segalen M, Bellaiche Y. Cell division orientation and planar cell polarity pathways. Semin Cell Dev Biol. 2009;20:972–7. doi: 10.1016/j.semcdb.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 47.Besson C, Bernard F, Corson F, Rouault H, et al. Planar cell polarity Breaks the symmetry of PAR protein distribution prior to mitosis in Drosophila sensory organ precursor cells. Curr Biol. 2015;25:1104–10. doi: 10.1016/j.cub.2015.02.073. [DOI] [PubMed] [Google Scholar]
- 48.Bellaiche Y, Beaudoin-Massiani O, Stuttem I, Schweisguth F. The planar cell polarity protein strabismus promotes Pins anterior localization during asymmetric division of sensory organ precursor cells in Drosophila. Development. 2004;131:469–78. doi: 10.1242/dev.00928. [DOI] [PubMed] [Google Scholar]
- 49.Bellaiche Y, Radovic A, Woods DF, Hough CD, et al. The partner of inscuteable/discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell. 2001;106:355–66. doi: 10.1016/s0092-8674(01)00444-5. [DOI] [PubMed] [Google Scholar]
- 50.Bellaiche Y, Gho M, Kaltschmidt JA, Brand AH, et al. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat Cell Biol. 2001;3:50–7. doi: 10.1038/35050558. [DOI] [PubMed] [Google Scholar]
- 51.Gho M, Schweisguth F. Frizzled signalling controls orientation of asymmetric sense organ precursor cell divisions in Drosophila. Nature. 1998;393:178–81. doi: 10.1038/30265. [DOI] [PubMed] [Google Scholar]
- 52.Ciruna B, Jenny A, Lee D, Mlodzik M, et al. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–4. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, Mlodzik M. Wnt-frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt) Annu Rev Cell Dev Biol. 2015;31:623–46. doi: 10.1146/annurev-cellbio-100814-125315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–38. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 55.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–92. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–33. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adler PN. The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr Top Dev Biol. 2012;101:1–31. doi: 10.1016/B978-0-12-394592-1.00001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–40. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JE, Gleeson JG. Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol. 2011;24:98–105. doi: 10.1097/WCO.0b013e3283444d05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tatin F, Taddei A, Weston A, Fuchs E, et al. Planar cell polarity protein Celsr1 regulates endothelial adherens junctions and directed cell rearrangements during valve morphogenesis. Dev Cell. 2013;26:31–44. doi: 10.1016/j.devcel.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cortijo C, Gouzi M, Tissir F, Grapin-Botton A. Planar cell polarity controls pancreatic beta cell differentiation and glucose homeostasis. Cell Rep. 2012;2:1593–606. doi: 10.1016/j.celrep.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bader E, Migliorini A, Gegg M, Moruzzi N, et al. Identification of proliferative and mature beta-cells in the islets of Langerhans. Nature. 2016;535:430–4. doi: 10.1038/nature18624. [DOI] [PubMed] [Google Scholar]
- 63.Schnell U, Carroll TJ. Planar cell polarity of the kidney. Exp Cell Res. 2016;343:258–66. doi: 10.1016/j.yexcr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McNeill H. Planar cell polarity and the kidney. J Am Soc Nephrol. 2009;20:2104–11. doi: 10.1681/ASN.2008111173. [DOI] [PubMed] [Google Scholar]
- 65.Chai G, Goffinet AM, Tissir F. Celsr3 and Fzd3 in axon guidance. Int J Biochem Cell Biol. 2015;64:11–4. doi: 10.1016/j.biocel.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 66.Tissir F, Goffinet AM. Shaping the nervous system: role of the core planar cell polarity genes. Nat Rev Neurosci. 2013;14:525–35. doi: 10.1038/nrn3525. [DOI] [PubMed] [Google Scholar]
- 67.Singh J, Mlodzik M. Planar cell polarity signaling: coordination of cellular orientation across tissues. Wiley Interdiscip Rev Dev Biol. 2012;1:479–99. doi: 10.1002/wdev.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jenny A, Mlodzik M. Planar cell polarity signaling: a common mechanism for cellular polarization. Mt Sinai J Med. 2006;73:738–50. [PubMed] [Google Scholar]
- 69.Carvajal-Gonzalez JM, Mlodzik M. Mechanisms of planar cell polarity establishment in Drosophila. F1000Prime Rep. 2014;6:98. doi: 10.12703/P6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–72. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jenny A. Planar cell polarity signaling in the Drosophila eye. Curr Top Dev Biol. 2010;93:189–227. doi: 10.1016/B978-0-12-385044-7.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donoughe S, DiNardo S. Dachsous and frizzled contribute separately to planar polarity in the Drosophila ventral epidermis. Development. 2011;138:2751–9. doi: 10.1242/dev.063024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–63. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olofsson J, Sharp KA, Matis M, Cho B, et al. Prickle/spiny-legs isoforms control the polarity of the apical microtubule network in planar cell polarity. Development. 2014;141:2866–74. doi: 10.1242/dev.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merkel M, Sagner A, Gruber FS, Etournay R, et al. The balance of prickle/spiny-legs isoforms controls the amount of coupling between core and fat PCP systems. Curr Biol. 2014;24:2111–23. doi: 10.1016/j.cub.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Ayukawa T, Akiyama M, Mummery-Widmer JL, Stoeger T, et al. Dachsous-dependent asymmetric localization of spiny-legs determines planar cell polarity orientation in Drosophila. Cell Rep. 2014;8:610–21. doi: 10.1016/j.celrep.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 77.Brittle A, Thomas C, Strutt D. Planar polarity specification through asymmetric subcellular localization of fat and dachsous. Curr Biol. 2012;22:907–14. doi: 10.1016/j.cub.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brittle AL, Repiso A, Casal J, Lawrence PA, et al. Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr Biol. 2010;20:803–10. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mao Y, Mulvaney J, Zakaria S, Yu T, et al. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development. 2011;138:947–57. doi: 10.1242/dev.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–94. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- 81.Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–24. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- 82.Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–84. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- 83.Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of fat: dachsous binding by the cadherin domain kinase four-jointed. Curr Biol. 2010;20:811–7. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zeidler MP, Perrimon N, Strutt DI. The four-jointed gene is required in the Drosophila eye for ommatidial polarity specification. Curr Biol. 1999;9:1363–72. doi: 10.1016/s0960-9822(00)80081-0. [DOI] [PubMed] [Google Scholar]
- 85.Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev Biol. 2000;228:181–96. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- 86.Devenport D. The cell biology of planar cell polarity. J Cell Biol. 2014;207:171–9. doi: 10.1083/jcb.201408039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sagner A, Merkel M, Aigouy B, Gaebel J, et al. Establishment of global patterns of planar polarity during growth of the Drosophila wing epithelium. Curr Biol. 2012;22:1296–301. doi: 10.1016/j.cub.2012.04.066. [DOI] [PubMed] [Google Scholar]
- 88.Gao B, Song H, Bishop K, Elliot G, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–76. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu J, Roman AC, Carvajal-Gonzalez JM, Mlodzik M. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nat Cell Biol. 2013;15:1045–55. doi: 10.1038/ncb2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, et al. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–46. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- 91.Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol. 2009;20:986–97. doi: 10.1016/j.semcdb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Courbard JR, Djiane A, Wu J, Mlodzik M. The apical/basal-polarity determinant Scribble cooperates with the PCP core factor Stbm/Vang and functions as one of its effectors. Dev Biol. 2009;333:67–77. doi: 10.1016/j.ydbio.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Montcouquiol M, Sans N, Huss D, Kach J, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–75. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, et al. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–7. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 95.Chin ML, Mlodzik M. The Drosophila selectin furrowed mediates intercellular planar cell polarity interactions via frizzled stabilization. Dev Cell. 2013;26:455–68. doi: 10.1016/j.devcel.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hermle T, Saltukoglu D, Grunewald J, Walz G, et al. Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol. 2010;20:1269–76. doi: 10.1016/j.cub.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 97.Hermle T, Guida MC, Beck S, Helmstadter S, et al. Drosophila ATP6AP2/VhaPRR functions both as a novel planar cell polarity core protein and a regulator of endosomal trafficking. EMBO J. 2013;32:245–59. doi: 10.1038/emboj.2012.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buechling T, Bartscherer K, Ohkawara B, Chaudhary V, et al. Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr Biol. 2010;20:1263–8. doi: 10.1016/j.cub.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 99.Vladar EK, Bayly RD, Sangoram AM, Scott MP, et al. Microtubules enable the planar cell polarity of airway cilia. Curr Biol. 2012;22:2203–12. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- 101.Held LI, Jr, Duarte CM, Derakhshanian K. Extra joints and misoriented bristles on Drosophila legs. Prog Clin Biol Res. 1986;217A:293–6. [PubMed] [Google Scholar]
- 102.Eaton S, Wepf R, Simons K. Roles for Rac1 and Cdc42 in planar polarization and hair outgrowth in the wing of Drosophila. J Cell Biol. 1996;135:1277–89. doi: 10.1083/jcb.135.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Turner CM, Adler PN. Distinct roles for the actin and microtubule cytoskeletons in the morphogenesis of epidermal hairs during wing development in Drosophila. Mech Dev. 1998;70:181–92. doi: 10.1016/s0925-4773(97)00194-9. [DOI] [PubMed] [Google Scholar]
- 104.Devenport D. Tissue morphodynamics: translating planar polarity cues into polarized cell behaviors. Semin Cell Dev Biol. 2016;55:99–110. doi: 10.1016/j.semcdb.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vladar EK, Antic D, Axelrod JD. Planar cell polarity signaling: the developing cell’s compass. Cold Spring Harb Perspect Biol. 2009;1:a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krasnow RE, Adler PN. A single frizzled protein has a dual function in tissue polarity. Development. 1994;120:1883–93. doi: 10.1242/dev.120.7.1883. [DOI] [PubMed] [Google Scholar]
- 107.Eaton S, Auvinen P, Luo L, Jan YN, et al. CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing disc epithelium. J Cell Biol. 1995;131:151–64. doi: 10.1083/jcb.131.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yan J, Lu Q, Fang X, Adler PN. Rho1 has multiple functions in Drosophila wing planar polarity. Dev Biol. 2009;333:186–99. doi: 10.1016/j.ydbio.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–5. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- 110.Lu Q, Yan J, Adler PN. The Drosophila planar polarity proteins inturned and multiple wing hairs interact physically and function together. Genetics. 2010;185:549–58. doi: 10.1534/genetics.110.114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yun UJ, Kim SY, Liu J, Adler PN, et al. The inturned protein of Drosophila melanogaster is a cytoplasmic protein located at the cell periphery in wing cells. Dev Genet. 1999;25:297–305. doi: 10.1002/(SICI)1520-6408(1999)25:4<297::AID-DVG3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 112.Yan J, Huen D, Morely T, Johnson G, et al. The multiple-wing-hairs gene encodes a novel GBD-FH3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics. 2008;180:219–28. doi: 10.1534/genetics.108.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shimada Y, Yonemura S, Ohkura H, Strutt D, et al. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–22. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 114.Harumoto T, Ito M, Shimada Y, Kobayashi TJ, et al. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell. 2010;19:389–401. doi: 10.1016/j.devcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krylova O, Messenger MJ, Salinas PC. Dishevelled-1 regulates microtubule stability: a new function mediated by glycogen synthase kinase-3beta. J Cell Biol. 2000;151:83–94. doi: 10.1083/jcb.151.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ciani L, Krylova O, Smalley MJ, Dale TC, et al. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J Cell Biol. 2004;164:243–53. doi: 10.1083/jcb.200309096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Curtin JA, Quint E, Tsipouri V, Arkell RM, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–33. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 119.Boutin C, Labedan P, Dimidschstein J, Richard F, et al. A dual role for planar cell polarity genes in ciliated cells. Proc Natl Acad Sci USA. 2014;111:E3129–38. doi: 10.1073/pnas.1404988111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tissir F, Qu Y, Montcouquiol M, Zhou L, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13:700–7. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- 121.Ohata S, Nakatani J, Herranz-Perez V, Cheng J, et al. Loss of Dishevelleds disrupts planar polarity in ependymal motile cilia and results in hydrocephalus. Neuron. 2014;83:558–71. doi: 10.1016/j.neuron.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu B, Usui T, Uemura T, Jan L, et al. Flamingo controls the planar polarity of sensory bristles and asymmetric division of sensory organ precursors in Drosophila. Curr Biol. 1999;9:1247–50. doi: 10.1016/s0960-9822(99)80505-3. [DOI] [PubMed] [Google Scholar]
- 123.Boisvieux-Ulrich E, Laine MC, Sandoz D. Cytochalasin D inhibits basal body migration and ciliary elongation in quail oviduct epithelium. Cell Tissue Res. 1990;259:443–54. doi: 10.1007/BF01740770. [DOI] [PubMed] [Google Scholar]
- 124.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–81. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 125.Sipe CW, Lu X. Kif3a regulates planar polarization of auditory hair cells through both ciliary and non-ciliary mechanisms. Development. 2011;138:3441–9. doi: 10.1242/dev.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.May-Simera HL, Kai M, Hernandez V, Osborn DP, et al. Bbs8, together with the planar cell polarity protein Vangl2, is required to establish left-right asymmetry in zebrafish. Dev Biol. 2010;345:215–25. doi: 10.1016/j.ydbio.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 127.Ross AJ, May-Simera H, Eichers ER, Kai M, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–40. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 128.Ezan J, Lasvaux L, Gezer A, Novakovic A, et al. Primary cilium migration depends on G-protein signalling control of subapical cytoskeleton. Nat Cell Biol. 2013;15:1107–15. doi: 10.1038/ncb2819. [DOI] [PubMed] [Google Scholar]
- 129.Jones C, Roper VC, Foucher I, Qian D, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- 130.Yasunaga T, Itoh K, Sokol SY. Regulation of basal body and ciliary functions by Diversin. Mech Dev. 2011;128:376–86. doi: 10.1016/j.mod.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu DK, Kelley MW. Molecular mechanisms of inner ear development. Cold Spring Harb Perspect Biol. 2012;4:a008409. doi: 10.1101/cshperspect.a008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vladar EK, Brody SL. Analysis of ciliogenesis in primary culture mouse tracheal epithelial cells. Methods Enzymol. 2013;525:285–309. doi: 10.1016/B978-0-12-397944-5.00014-6. [DOI] [PubMed] [Google Scholar]