Abstract
Purpose
Erythropoietin (EPO) is a promising neuroprotective agent and is currently in Phase III clinical trials for the treatment of traumatic brain injury. The goal of this study was to determine if EPO is also protective in traumatic eye injury.
Methods
The left eyes of anesthetized DBA/2J or Balb/c mice were exposed to a single 26psi over-pressure air-wave while the rest of the body was shielded. DBA/2J mice were given intraperitoneal injections of EPO or buffer and analyses were performed at 3 or 7 days post-blast. Balb/c mice were given intramuscular injections of rAAV.EpoR76E or rAAV.eGFP either pre-or post-blast and analyses were performed at 1-month post-blast.
Results
EPO had a bi-modal effect on cell death, glial reactivity, and oxidative stress. All measures were increased at 3 days post-blast and decreased at 7-days post-blast. Increased retinal ferritin and NADPH oxygenases were detected in retinas from EPO-treated mice. The gene therapy approach protected against axon degeneration, cell death and oxidative stress when given after blast, but not before.
Conclusions
Systemic, exogenous, EPO and EPO-R76E protects the retina after trauma even when initiation of treatment is delayed by up to 3 weeks. Systemic treatment with EPO or EPO-R76E beginning before or soon after trauma may exacerbate protective effects of EPO within the retina as a result of increased iron levels from erythropoiesis and thus, increased oxidative stress within the retina. This is likely overcome with time as a result of an increase in levels of antioxidant enzymes. Either intraocular delivery of EPO or treatment with non-erythropoietic forms of EPO may be more efficacious.
Keywords: erythropoietin, eye trauma, blast, neuroprotection
Eye trauma occurs in 1.5-2 million people world-wide annually.1 From 2007-2010 the incidence of eye trauma related emergency room visits in the United States was 37.6 per 10,000 persons.2 This is in addition to vision loss that occurs in the military population as a result of exposure to improvised explosive devices. In the years spanning 2000 to 2010, 186,555 U.S. service members suffered eye injuries.3 Eye injuries are a common comorbidity of blast-induced mild traumatic brain injury, the signature injury of recent wars.4-7 Unfortunately, there are no medical therapies currently available for patients with traumatic injury to the retina or optic nerve due, in part, to a lack of understanding of the underlying cellular and molecular etiology of the vision loss.
We developed a model to test if retinal injury and vision loss could be induced directly by the over-pressure air-wave aspect of an IED, independently of damage to the rest of the body, including the brain.8-10 We have tested the effects of this injury on three mouse strains. The mildest effect was seen in the C57Bl/6 mouse, which had focal oxidative stress in the first week and focal cell death at 1 month after injury. Due to the lack of cell death prior to one month, this strain was not used for the current study. In contrast, focal oxidative stress, glial reactivity, cell death, and retinal detachments were present after an eye-directed air blast in both the Balb/c and DBA/2J mouse strains.8, 11 In the DBA/2J mouse, cell death is greatest at 3 and 28-days post-blast, with very little cell death detected at 7-days post-blast.8 Even at 3-days post-blast only about 10% of the retina contains TUNEL-positive cells. In the Balb/c mouse, cell death is greatest at 7-days post-blast with an average of 50% of the retina containing TUNEL-positive cells.11 Less of the retina is affected at 3 and 28-days post-blast. The effect of blast is most striking in the DBA/2J mouse, which has a high neuroinflammatory state.8 Interestingly, oxidative stress as detected by anti-nitrotyrosine, a marker for peroxynitrite activity, spreads to encompass the entire retina by one month post-injury, corresponding to the timing of vision loss in this model.8 The delay in vision loss is consistent with reports in service members and veterans who have mild TBI.4, 5 Our results suggest that oxidative stress and neuroinflammation may play key roles in the spread of an injury signal that leads to vision loss after eye trauma.
Erythropoietin (EPO) was originally characterized for its role in the production of red blood cells by its action in blocking apoptosis of progenitor cells (for review see 12). It is FDA approved and used in the clinic for the treatment of anemia. EPO and its receptor are also present in the central nervous system where it acts to block neuronal apoptosis (for review see 13). In the retina, EPO can protect both photoreceptors and retinal ganglion cells from induced or inherited degeneration.14-24 In addition to blocking apoptosis, EPO also increases expression of antioxidant enzymes through activation of the antioxidant response element, and can limit glial reactivity (for review see 13). This, in combination with the ongoing Phase III clinical trials of EPO for the treatment of TBI,25, 26 led us to hypothesize that EPO may limit the spread of oxidative stress and neuroinflammation after an eye-directed blast thus preventing retinal cell death and optic nerve degeneration.
We first wanted to determine if short-term treatment with EPO soon after blast would be sufficient to protect the retina. Surprisingly, we show that short-term treatment with EPO exacerbates retinal cell death after trauma potentially as a result of an increase in oxidative stress from elevated erythropoiesis and retinal iron levels. However, at one week after trauma, despite no additional injections of EPO, less cell death was detected as compared to controls. We next determined if long-term treatment with EPO in the absence of increased erythropoiesis, and/or treatment with EPO beginning after the early increase in oxidative stress from blast might be more effective. In order to provide long-term sustained delivery we used adeno-associated virus (rAAV). To avoid the large increase in erythropoiesis that is caused by sustained production of EPO we instead delivered a form of EPO with attenuated erythropoietic activity (EpoR76E).19-22 We injected mice with rAAV.EpoR76E either before or one day after blast in order to compare the efficacy of treatment (elevated EPO-R76E) at blast to treatment initiated at 3 weeks after blast, long after the early rise in oxidative stress. Overall our results suggest that delayed treatment with EPO-R76E is more effective than early treatment.
METHODS
Animals
Three-month old DBA/2J or Balb/c mice (The Jackson Laboratory, Bar Harbor, ME) were maintained on a 12h light/dark cycle and provided access to food and water ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Vanderbilt University, according to AALAC guidelines. The DBA/2J mouse is susceptible to developing glaucoma beginning at about 6 months of age; therefore, all mice (and tissues) were collected at 3-3.5 months of age to avoid glaucoma-related complications.27 These mice have reactive microglia in the retina at 3-months of age, indicating a heightened neuroinflammatory state even in the absence of trauma.28 Therefore, age-matched controls were used throughout the study. For histological analyses mice were perfused with 4% paraformaldehyde (PFA; Electron Microscopy Sciences, Hatfield, PA) and phosphate buffered saline (PBS), enucleated, and the eyes were stored in 4% PFA. For RNA collection mice were euthanized, corneas were bisected, and forceps were used to separate the retina from the posterior globe. The vitreous was removed from the isolated retina, which was then frozen on dry ice and stored at −80°C.
Ocular Blast Injury
Blast was performed as previously described.29 Briefly, isofluorane anesthetized mice were secured and padded within a housing chamber that was placed within a larger tube, which shielded the body and head of the mouse from blast. The left eye of the mouse was positioned against a hole in the tube and was aligned with the barrel of the blast device. An overpressure air-wave with a peak pressure of 26psi was produced by a modified paintball marker (Empire Paintball, Sewell, NJ).
Erythropoietin Therapy – DBA/2J
Mice were given three intraperitoneal injections of 5,000U/kg EPO (Procrit, Ortho Biotech, Bridgewater, NJ) at 24h intervals. Control mice received Ringer's buffer. There were four treatment groups based on the timing of EPO delivery after blast and timing of tissue collection: 1) EPO delivered at 0, 24, and 48h post-blast, tissue collected at 7-days post-blast; 2) EPO delivered at 6, 30, and 54h post-blast, tissue collected 7-days post-blast; 3) EPO delivered at 24, 48, and 72h post-blast, tissue collected at 7-days post-blast; and 4) EPO delivered at 24, 48, and 72h post-blast, tissue collected at 3-days post-blast. The 3-day collection time point was chosen because that is the peak of cell death after blast in the DBA/2J mouse. The 7-day collection time-point was chosen as the shortest time point to potentially allow sufficient time for changes in gene expression to overcome the elevation in erythropoiesis. Since EPO has a short half-life frequent re-injection was needed so the shortest duration was optimal in order to limit the number of re-injections necessary.
Gene Therapy – Balb/c
Mice were given a single intramuscular injection of recombinant adeno-associated virus (rAAV) carrying either enhanced green fluorescent protein (rAAV2/8.CMV.eGFP) or a mutated form of EPO with attenuated erythropoietic activity (rAAV2/8.CMV.EpoR76E) at 1×109gc into the quadriceps. We chose to deliver EpoR76E rather than wild-type EPO for two reasons: 1) to avoid a dangerous rise in hematocrit that is caused by systemic gene delivery of EPO, and 2) because based on the results of the DBA/2J EPO protein experiments we hypothesized that an increase in erythropoiesis as would be induced by sustained expression of wild-type EPO would increase oxidative stress and thus cell death in the retina. Vectors were produced, purified, and titred at the University of Pennsylvania Vector Core (Philadelphia, PA). In the pre-blast group mice were injected 1-month prior to blast and collected at 1-month post-blast. In the post-blast group mice were injected 1-day post-blast and collected at 1-month post-blast. The rAAV2/8 serotype reaches peak gene expression levels three weeks after intramuscular injection.30 The collection time point was chosen based on the amount of time necessary for gene expression from rAAV and our previous data showing persistence of cell death at this time point. This paradigm allowed us to test if delayed delivery of EPO-R76E was therapeutic.
Immunohistochemistry
Eyes were embedded in Tissue Freezing Medium (Electron Microscopy Sciences) and 10μm thick sections were collected in-round on 12 slides such that each slide contained a representation of all areas of the eye/retina. Slides were rinsed with phosphate buffered saline (PBS) and incubated at room temperature in normal donkey serum at 1:20 in 0.1 M phosphate buffer with 0.5% bovine serum albumin and 0.1% Triton X 100 (PBT) for 2 hours. The slides were incubated overnight at 4°C in anti-nitrotyrosine (1:500, Millipore, Billerica, MA), anti-glial fibrillary acidic protein (GFAP, 1:400, DAKO, Carpinteria, CA), or anti-H ferritin (1:100, Abcam, Cambridge, MA) in PBT, rinsed with PBS and incubated with a secondary antibody (Life Technologies, Grand Island, NY) for 2 hours at room temperature. Slides were rinsed with PBS and mounted in Vectashield Mounting medium with 4’,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) for imaging on a Nikon Eclipse epifluorescence microscope (Nikon, Melville, NY).
Quantification of Fluorescence Intensity
Non-overlapping, but adjacent images of all areas of all retinal sections were collected using the same gain and exposure settings. Fluorescence was quantified within a rectangle of a set width and a height equivalent to the height of the retinal layer of interest using Image J according to previously published methods.31
Quantification of GFAP-positive Processes
Non-overlapping, but adjacent images of all areas of all retinal sections, with the exception of the far periphery, were collected at low magnification and montages were assembled in Photoshop (Adobe). Using Image J, the total length of retina and the length of retina containing GFAP-positive processes were measured. The percent of retina containing GFAP-positive Müller cell processes was calculated by dividing the length of GFAP-positive retina by the total length of retina and multiplying by 100.
Tdt dUTP Nick End Labeling (TUNEL) Quantification
Retina sections were labeled with the TUNEL Apoptosis Detection Kit according to manufacturer's protocol (Millipore, Billerica, MA) and mounted with Vectashield Mounting Medium with DAPI. Each slide contained 24 representative sections from all retinal regions. All sections were imaged and used in the quantification resulting in the analysis of an average of 57mm retina/group. The length of retina assessed in each group was not statistically different from the other groups with the following exceptions. The 3-day EPO and pre-blast therapy groups had less overall length of retina imaged and the 6hr EPO 7d post-blast group had slightly more retina imaged (p<0.05). A TUNEL-positive cluster was defined as an 85μm2 area of retina containing five or more TUNEL-positive cells. Clusters within the inner retina (INL and GCL) and outer retina (ONL) were counted separately. The total number of TUNEL positive cells within each defined cluster was also quantified. Measurements were performed using NIS Elements Advanced Research software (Nikon, Melville, NY). The number of 3-day retinas analyzed was 3 for buffer-injected and 7 for EPO-injected. The number of 7-day post-blast retinas assessed per group was 17 for Buffer treated, 13 for 0h EPO, 9 for Group 6h EPO, and 9 for 24h EPO. The number of Balb/c mouse retinas quantified was: 9 rAAV.eGFP, 7 pre-blast rAAV.EpoR76E, and 9 post-blast rAAV.EpoR76E.
Optic Nerve Histology
Optic nerves were post-fixed in 4% paraformaldehyde and 1% glutaraldehyde and subsequently placed in 1% osmium tetroxide in 0.1 M cacodylate buffer, dehydrated in a graded ethanol series and embedded in Spurr's resin (Electron Microscopy Sciences). Starting from the proximal end of the optic nerve, 1 μm-thick sections were collected using a Reichert-Jung Ultracute E microtome and stained with 1% p-phenylenediamine in 50% methanol (Sigma-Aldrich). Sections were imaged on an Olympus Provis AX70 microscope using a 100x oil immersion objective lens. Axons were manually counted using ImageJ software by sampling 20% of the total nerve cross-sectional area using a fixed grid overlay to estimate axon density in the nerve (axons/mm2). Total number of surviving axons was estimated as the product of mean axon density and nerve cross-sectional area, as described previously.32, 33 Imaging and quantification were performed in a masked fashion.
Oxidative Stress PCR Array
The Qiagen oxidative stress RT2 profiler mouse oxidative stress kit was used according to manufacturers’ protocol. Five retinas were pooled for each plate. Data was normalized to the housekeeping gene, monogalactosyldiacylglycerol.
Statistical Analysis
All statistical analyses were calculated using Graphpad Prism software (San Diego, CA). The 7-day DBA/2J analyses were performed using ANOVA and a Tukey post-hoc test. All other data-sets were pair-wise comparisons and thus the Student's t-test was performed. The means ± SEM were calculated and presented for each data set.
RESULTS
Timing of EPO Therapy Affects Decrease in Cell Death after Blast
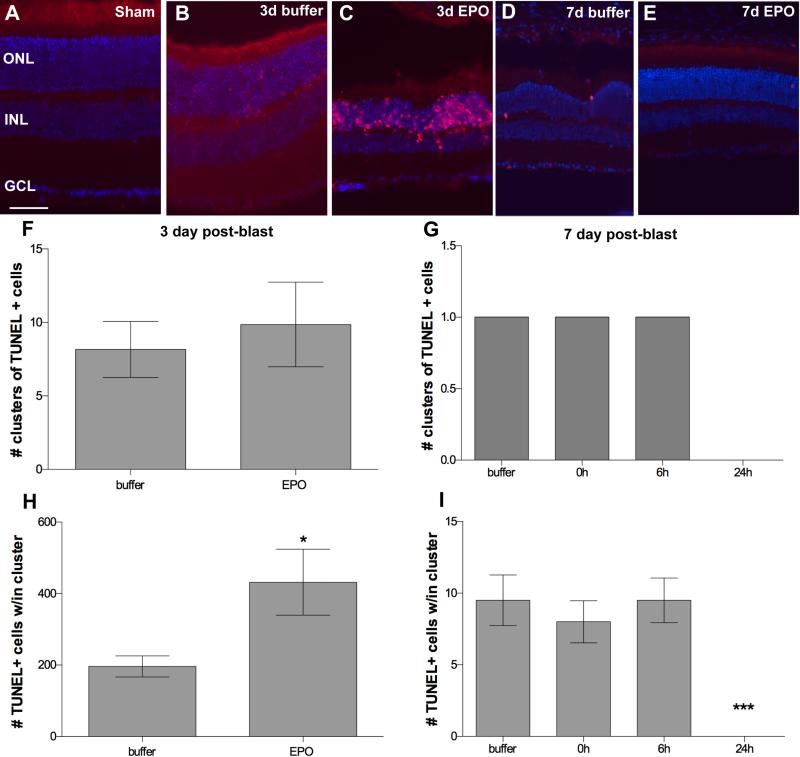
No TUNEL-positive cells were detected in sham blast retinas (Figure 1A). In the buffer-injected DBA/2J mice, 85% contained small clusters of TUNEL positive cells in the retina at 3 and 7-days post-blast, consistent with our previously published findings (Figure 1B, D).8 We have defined these clusters as an 85μm2 area of retina containing 5 or more TUNEL-positive cells. Clusters of TUNEL-positive cells were also present in 3-day post-blast retinas from mice that received EPO (Figure 1C). Occasional TUNEL-positive cells were detected in the EPO-treated 7-day post-blast retinas, but no clusters were found (Figure 1E). There was no quantitative difference in the number of TUNEL-positive clusters between groups at 3 or 7-days post-blast (Figure 1F, G). However, there was an increase in the density of TUNEL-positive cells within the clusters in the 3-day post-blast retinas from EPO-treated mice, p<0.05 (Figure 1H). In contrast, there were no TUNEL-positive cells detected in the 24h EPO treated, 7-day post-blast retinas as compared to an average of 9.8 ± 3.7 dying cells in the other groups, p<0.001 (Figure 1I).
Figure 1.
Treatment with EPO beginning at 24h post-blast decreases cell death at 7, but not 3-days post-blast. (A-E) Representative fluorescence micrographs of clusters of TUNEL-positive cells in retinas from: (A) sham blast, (B) buffer-injected 3-day post-blast, (C) EPO-injected 3-day post-blast, (D) buffer-injected 7-day post-blast, and (E) EPO-injected 7-day post-blast DBA/2J mice. TUNEL (red), DAPI (blue). Scale bar represents 50μm. (F, G) Bar graphs of clusters of TUNEL-positive cells at 3-days post-blast (F) and 7-days post-blast (G). (H, I) Bar graphs of the density of TUNEL-positive cells within each cluster at 3-days (H) and 7 days post-blast (I). Only mice injected with EPO at 24h post-blast and assessed at 7 days post-blast lacked TUNEL positive cells, ***p<0.001. Note that (G) only shows results from retinas that contained clusters of TUNEL cells, with the exception of the 24h group which contains data from all retinas since all were completely TUNEL negative. Only 18% of the buffer, 0h, and 6h 7-day post-blast retinas contained TUNEL-positive cells.
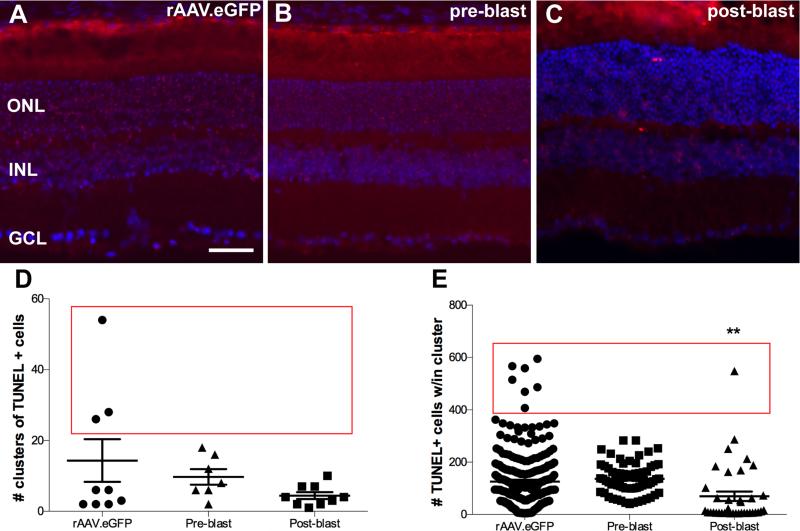
Clusters of TUNEL-positive cells were present in retinas from 1-month post-blast Balb/c mice treated with rAAV.eGFP (Figure 2A) or rAAV.EpoR76E (Figure 2B,C) regardless of treatment timing. The average number of clusters of TUNEL positive cells was similar across groups (Figure 2D). However, a subset of retinas from rAAV.eGFP treated mice had as many as 25-55 clusters, whereas the rAAV.EpoR76E treated mice never had more than 20 clusters of TUNEL-positive cells per retina (Figure 2D, red box; Bartlett's test of variance, p<0.0001). The density of TUNEL-positive cells within each cluster was comparable between the rAAV.eGFP and pre-blast rAAV.EpoR76E mice (Figure 2E). In contrast, there were fewer TUNEL-positive cells per cluster in the retinas of Balb/c mice treated with rAAV.EpoR76E post-blast, p<0.01 (Figure 2E). In addition, there was a statistically significant difference in density variance between these groups, p<0.0001 (Figure 2E, red box). With the exception of one cluster in the post-blast treatment group, the TUNEL-positive cell densities within the clusters were less than 300 cells in the rAAV.EpoR76E groups (Figure 2E) while the rAAV.eGFP group had several clusters with densities between 400-600 cells.
Figure 2.
Treatment with rAAV.EpoR76E after blast decreased the number of TUNEL-positive cells at 1-month post-blast in Balb/c mice. (A-C) Representative fluorescence micrographs of clusters of TUNEL-positive cells in retinas from mice injected with: (A) rAAV.eGFP, (B) rAAV.EpoR76E 1-month prior to blast (pre-blast), or (C) rAAV.EpoR76E 1-day after blast (post-blast). TUNEL (red), DAPI (blue). Scale bar represents 50μm. (D, E) Scatter plots of the number of clusters of TUNEL-positive cells (D) and the density of TUNEL-positive cells within each cluster (E). Mice treated with rAAV.EpoR76E post-blast had fewer TUNEL-positive cells within each cluster, **p<0.01. Red boxes indicate statistically significant difference in variance between rAAV.eGFP and both rAAV.EpoR76E treatment groups.
Timing of EPO Therapy Affects Optic Nerve Axons after Blast
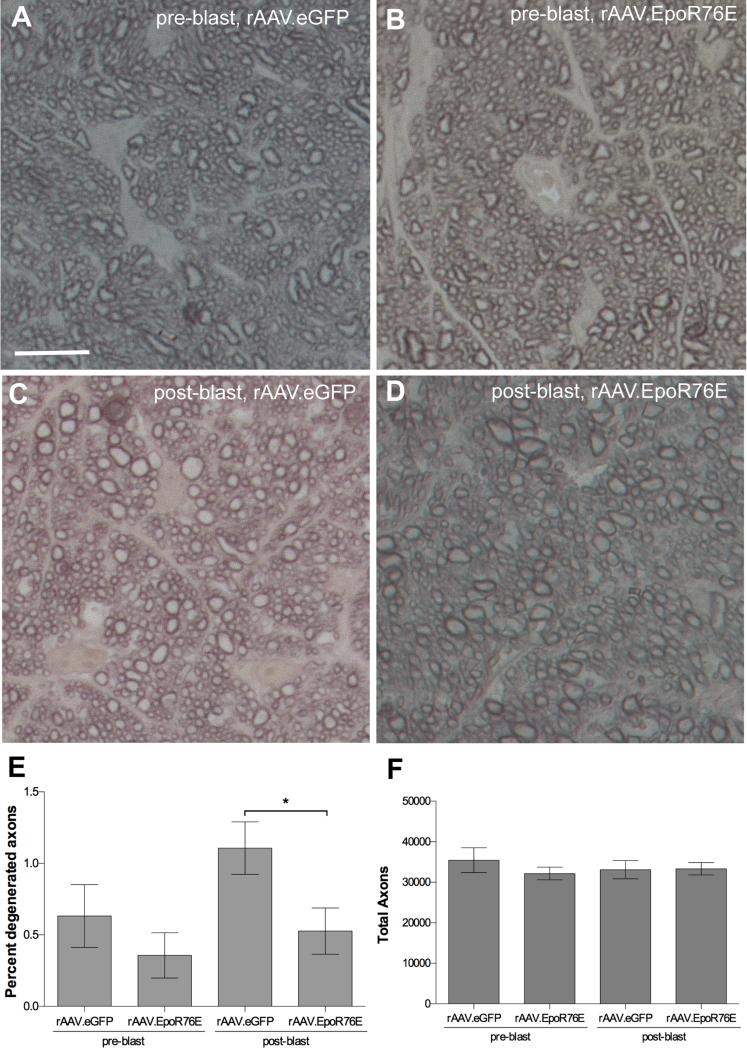
A small number of degenerated axons were present at 1-month post-blast in the Balb/c mouse injected with rAAV.eGFP as determined by dark profiles from collapsed myelin, or loose myelin (Figure 3A, C). Rare degenerating axons were also detected in the optic nerves of mice treated with rAAV.EpoR76E (Figure 3B,D). Upon quantification, a decrease in degenerating axons was detected in the mice injected with rAAV.EpoR76E after, but not in those injected prior, to blast (Figure 3E). The total number of axons was similar in all groups, likely due to the small number of degenerating axons (Figure 3F).
Figure 3.
Treatment with rAAV.EpoR76E decreased axon degeneration in the Balb/c optic nerve at 1-month post-blast. A-D) Representative brightfield micrographs of optic nerve cross-sections from mice given: (A) rAAV.eGFP pre-blast, (B) rAAV.EpoR76E pre-blast, (C) rAAV.eGFP post-blast, or D) rAAV.EpoR76E post-blast. Scale bar represents 10μm and applies to all images. (E, F) Bar graphs of the percent degenerating axons (E) and the total number of axons (F). Fewer degenerating axons were present in the post-blast rAAV.EpoR76E treatment group, *p<0.05.
Timing of EPO Therapy Affects Glial Reactivity after Blast
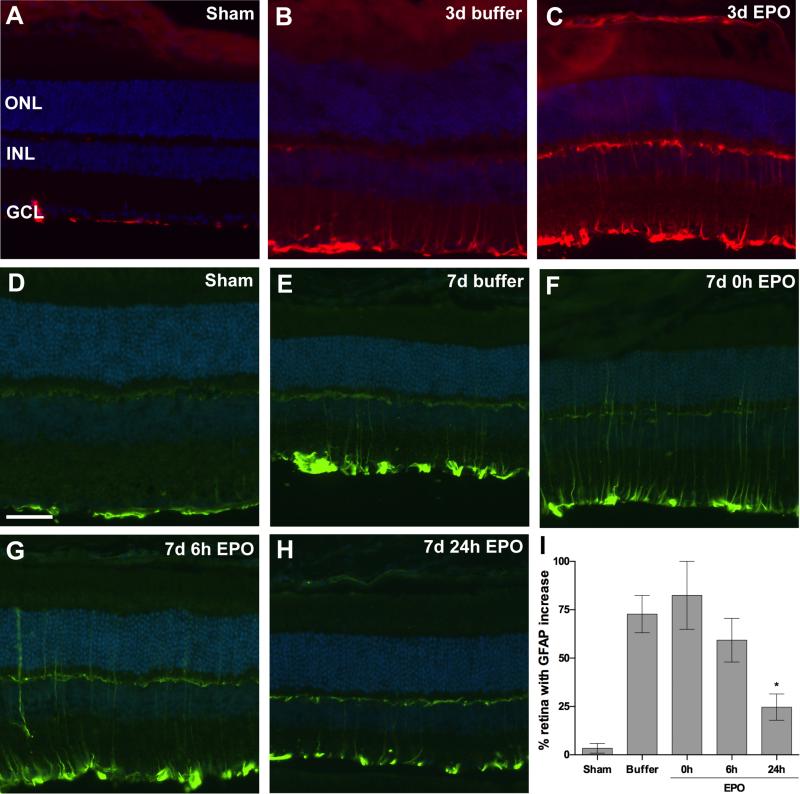
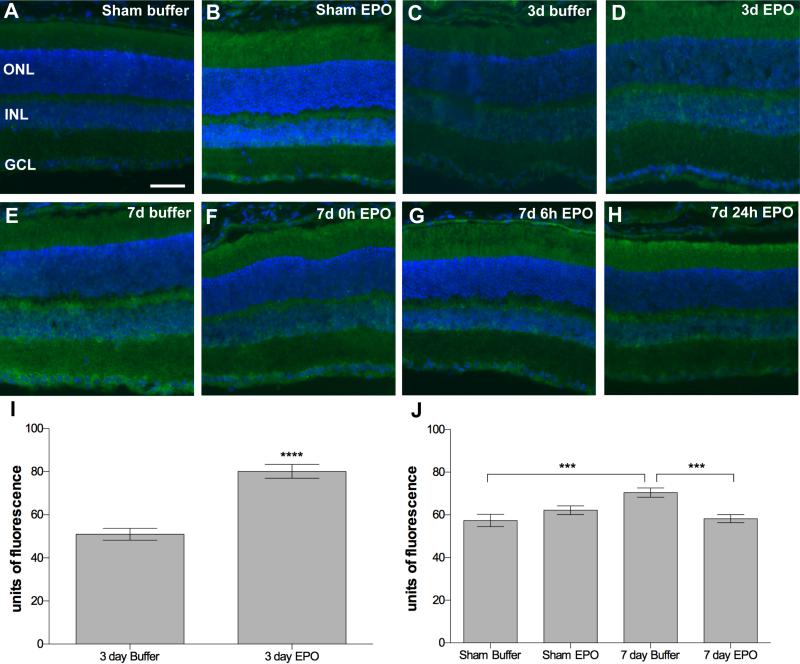
In retinas from sham blast mice GFAP immunolabeling was restricted to astrocytes and Müller cell endfeet (Figure 4A, D). An eye-directed blast causes focal increases in GFAP immunolabeling in the Müller cells of DBA/2J mice at both 3 and 7-days post-blast (Figure 4B, E; 8). The regions with GFAP-positive Müller cell processes are typically within the mid-peripheral retina. The micrographs are from these pockets and thus the specific retinal region varies, resulting in different retinal thickness. GFAP-positive Müller cell processes were also present in EPO-treated post-blast retinas, although to a lesser extent in the 7-day post-blast group that received EPO beginning at 24h after blast (Figure 4C, F-H). The decrease in GFAP labeling in the latter group was statistically significant when compared to the buffer treated group, p<0.05 (Figure 4I). The extent of GFAP immunolabeling in the 3-day post-blast mice was not quantified since the labeling appeared equivalent between the buffer and EPO-treated mice.
Figure 4.
Treatment with EPO beginning at 24h post-blast decreases glial reactivity at 7, but not 3, days post-blast. (A-C) Representative fluorescence micrographs of GFAP immunolabeling in retinas from sham blast mice (A), or 3 day post-blast mice injected with buffer (B) or EPO (C). GFAP (red), DAPI (blue). (D-H) Representative fluorescence micrographs of GFAP immunolabeling in retinas from sham blast mice (D), or 7 day post-blast mice injected with: E) buffer or (F-H) EPO beginning at 0hr (F), 6h (G), or 24h (H) after blast. GFAP (green), DAPI (blue). Scale bar in D represents 50μm and applies to all images. (I) Bar graph of the percent of retina containing GFAP-positive Müller cell processes. Mice treated with EPO beginning at 24h post-blast had a significantly smaller amount of retina containing reactive Muller cells as compared to the buffer injected blast-exposed mice, *p<0.05.
In sham blast Balb/c mice, GFAP immunolabeling was also restricted to astrocytes and Müller cell endfeet (data not shown). After blast, GFAP immunolabeling extends into the Müller cell processes regardless of treatment (data not shown). Quantification confirmed no difference in the extent of retina containing GFAP-positive Müller cell processes between groups.
Nitrosative Stress Post-Blast is Affected by EPO Therapy
The other early marker we have previously demonstrated to be increased after blast is anti-nitrotyrosine, an indicator of peroxynitrite activity.8 Consistent with our previous reports we detected low levels of immunolabeling in sham blast DBA/2J mouse retinas (Figure 5A) and bright immunolabeling for nitrotyrosine in focal areas of the retina primarily localized to the inner retina after blast (Figure 5C,E). Immunolabeling was also present, primarily within the inner retina, in all EPO-treated retinas regardless of blast exposure (Figure 5B,D,F-H). In the 3-day post-blast DBA/2J retinas there was a large increase in nitrotyrosine immunofluorescence in retinas from EPO-treated mice as compared to buffer-treated mice, p<0.0001 (Figure 5I). In the 7-day post-blast cohort nitrotyrosine levels were also increased as compared to sham controls, p<0.001 (Figure 5J). This increase was blunted by treatment with EPO, p<0.001.
Figure 5.
Treatment with EPO beginning at 24h post-blast decreases oxidative stress at 7 days, but not 3 days, post-blast in the DBA/2J mouse. (A-H) Representative fluorescence micrographs of nitrotyrosine immunolabeling in retinas from: (A, B) sham blast mice injected with buffer (A) or EPO (B); (C, D) 3 day post-blast mice treated with buffer (C) or EPO (D); and E-H) 7 day post-blast mice treated with buffer (E) or EPO beginning at 0h (F), 6h (G), or 24h (H) post-blast. Nitrotyrosine (green), DAPI (blue). Scale bar represents 50μm and applies to all images. (I, J) Bar graphs of quantification of the mean anti-nitrotyrosine immunofluorescence intensity in the inner retina of: (I) 3-day post-blast DBA/2J mice and (J) 7 day sham and post-blast DBA/2J mice treated with buffer or EPO at 24, 48, and 72h after blast. ***p<0.001, ****p<0.0001.
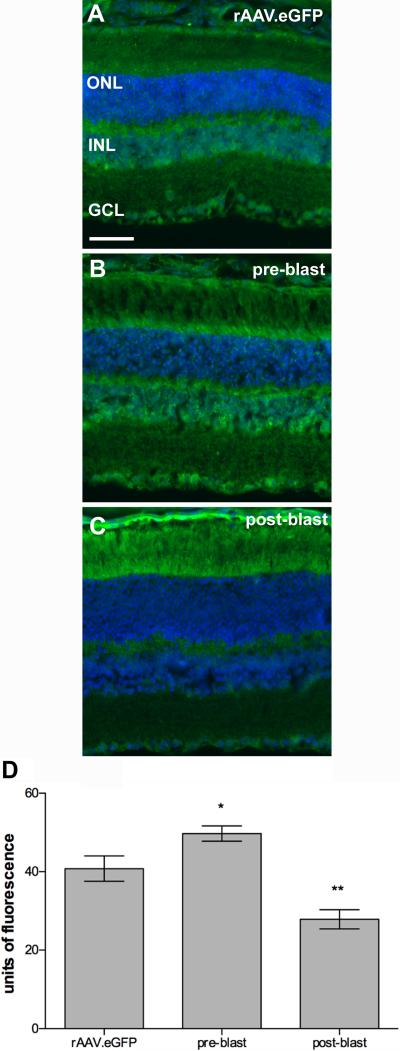
Immunolabeling for nitrotyrosine was also evident at 1-month post-blast in the Balb/c mice (Figure 6A-C). Nitrotyrosine immunofluorescence was increased in the pre-blast rAAV.EpoR76E group, p<0.05, and decreased in the post-blast rAAV.EpoR76E group, p<0.01, as compared to rAAV.eGFP injected Balb/c mice (Figure 6D).
Figure 6.
Treatment with rAAV.EpoR76E alters nitrotyrosine immunofluorescence in the inner retina of Balb/c mice after blast. A-C) Representative fluorescence micrographs of nitrotyrosine immunolabeling in retinas from mice injected with: (A) rAAV.eGFP, (B) rAAV.EpoR76E prior to blast, or (C) rAAV.EpoR76E after blast. Scale bar represents 50μm and applies to all images. (D) Bar graph of quantification of anti-nitrotyrosine immunofluorescence in the inner retina at 1-month post-blast. Pre-blast group were injected with rAAV.EpoR76E 1-month prior to blast. Post-blast group was injected with rAAV.EpoR76E 1 day after blast. There was no difference in the pre- or post-blast rAAV.eGFP groups so they were combined. *p<0.05, **p<0.01.
Retinal Ferritin Levels Increased after EPO Therapy
The decrease in cell death by EPO at 7-days, but not at 3-days post-blast was a surprise. One potential explanation could be an increase in retinal iron levels as a result of increased erythropoiesis. An increase in iron in the presence of oxygen radicals could push the Fenton reaction resulting in increased hydroxyl radical and greater retina cell death. Erythropoiesis, but not EPO directly, causes a decrease in hepcidin, which results in increased iron availability in tissues.34, 35 A sensitive marker for increased iron is the iron binding protein, ferritin, because its levels are increased when iron levels are high.36
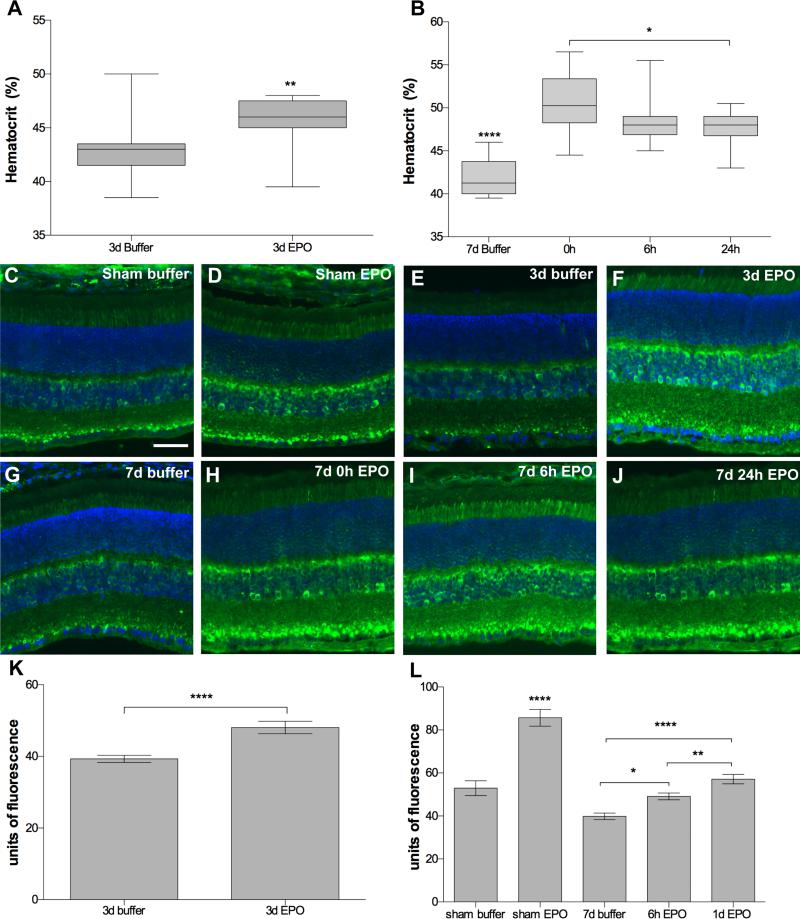
In the 3-day post-blast mice the hematocrit increased from 43 ± 3.1% (avg ± sd) in buffer-injected mice to 46 ± 2.2% in EPO-injected mice, p<0.01 (Figure 7A). In the 7-day post-blast mice, the hematocrit was increased from 42 ± 2.1% in the buffer-injected mice to 50.5 ± 3.4%, 48.5 ± 3.0%, and 47.6 ± 2.1% in the 0h, 6h, and 24h EPO-treated mice, respectively, p<0.0001 compared to buffer (Figure 7B). The hematocrit was also statistically significantly higher in the 0h EPO group as compared to the 24h EPO group, p<0.05.
Figure 7.
Evidence for increased erythropoiesis and iron delivery in the EPO-treated DBA/2J mouse. (A, B) Box plots of hematocrit level at 3-days (A) or 7-days post-blast (B) showing an increase in all EPO treated mice. Note that the 7d post-blast buffer-injected mice had a lower hematocrit than all other groups, ****p<0.0001. *p<0.05, **p<0.01. Treatment with EPO also causes an increase in H-ferritin. (C-J) Representative fluorescence micrographs of retinas labeled with anti-H-ferritin. (C, D) Sham blast mice injected with: (C) buffer or (D) EPO. (E, F) 3 day post-blast mice injected with: (E) buffer, or (F) EPO. (G-J) 7 day post-blast mice injected with: (G) buffer, or (H-J) EPO beginning at (H) 0h, (I) 6h, or (J) 24h after blast. H-ferritin (green), DAPI (blue). Scale bar represents 50μm and applies to all images. (K, L) Bar graphs of the mean anti-ferritin immunofluorescence intensity in retinas from: (K) 3 days post-blast DBA/2J mice, ****p<0.0001, and (L) sham and 7 days post-blast DBA/2J mice, showing an increase in retinal ferritin in all EPO treated mice *p<0.05, **p<0.01, ****p<0.0001.
We then performed anti-ferritin immunolabeling and quantification of fluorescence to determine if these slight increases in hematocrit were sufficient to cause an increase in retinal iron levels. The anti-ferritin labeled the photoreceptor inner segments, inner nuclear layer, and punctate structures in the inner plexiform layer as previously reported (Figure 7).36 Ferritin immunolabeling appeared similar in buffer-treated, sham and blast-exposed mice (Figure 7C, E, G). However, the amount of labeling appeared increased in retinas of mice that received EPO (Figure 7D, F, H-J) regardless of exposure to blast. Quantification confirmed an increase in ferritin levels in the retinas from EPO treated 3-day post-blast retinas as compared to buffer-injected mice, p<0.0001 (Figure 7K). This increase was primarily due to EPO and not blast since a large increase was detected in sham blast mice treated with EPO as compared to buffer injected sham mice, p<0.0001 (Figure 7L). Similar to the 3-day post-blast result, there was also an increase in ferritin immunolabeling in the EPO treated 7-day post-blast retinas as compared to buffer-injected 7-day blast mice, p<0.0001 (Figure 7L).
Despite expressing Epo-R76E rather than wild-type EPO, the Balb/c mice exhibited a rise in hematocrit similar to what we detected in the short-term EPO protein study in the DBA/2J mice. The hematocrit in mice injected with rAAV.eGFP was 45 ± 4.0%. In contrast mice treated with rAAV.EpoR76E had a hematocrit of 50 ± 5.0% (pre-blast cohort) and 57 ± 11% (post-blast cohort), p<0.05 as compared to rAAV.eGFP mice. The ferritin immunolabeling pattern in Balb/c mouse retinas appeared similar in all post-blast groups regardless of treatment (data not shown). Quantification confirmed that there was no difference in ferritin levels between the pre-blast rAAV.eGFP and rAAV.EpoR76E groups (data not shown). In contrast, despite an elevated hematocrit, ferritin immunolabeling levels were lower in the post-blast rAAV.EpoR76E group, 53 ± 1.8 (mean fluorescence ± SEM), as compared to the post-blast rAAV.eGFP group, 69 ± 2.1, p<0.0001.
DBA/2J Oxidative Stress Gene Expression is Altered by Blast and EPO
An increase in oxidative stress due to the Fenton reaction could explain the lack of protection in the 3-day post-blast EPO and the pre-blast rAAV.EpoR76E treatment groups. However, it does not explain why protection was achieved in the 7-day post-blast group that received EPO beginning at 1-day post-blast and in the post-blast rAAV.EpoR76E treatment groups. One potential explanation is that more time is needed for EPO to induce expression of antioxidant enzymes from the antioxidant response element within the retina than is required to induce erythropoiesis and iron delivery. To assess this we performed a PCR microarray to quantify message levels of proteins associated with oxidative stress and antioxidant mechanisms. We compared buffer and EPO treated mice at both 3 and 7 days post-blast (Figure 8).
Figure 8.
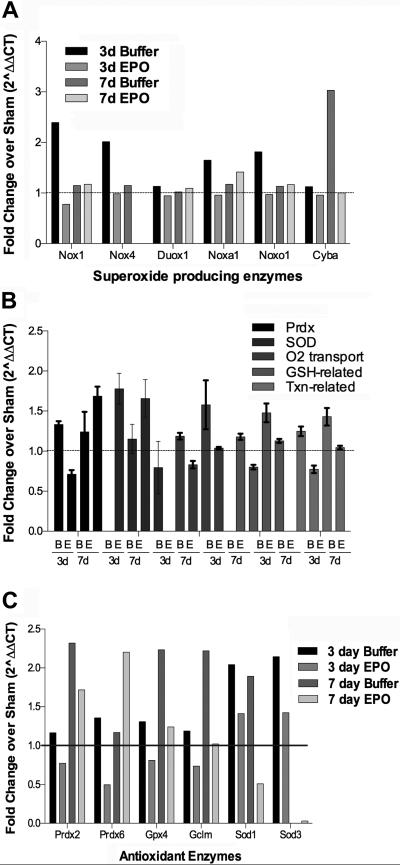
Slight changes in expression of some oxidative stress related proteins. (A) Bar graph of message levels of superoxide ion producing enzymes. (B) Bar graph of message levels of antioxidant enzymes grouped into functional families. B= buffer, E=EPO. C) Bar graph of mRNA transcripts at least 2-fold different from the sham controls.
In the 3-day post-blast, buffer-injected retinas there were increases in the mRNA levels of several NADPH oxidases, which work together to produce superoxide ion (Figure 8A).37, 38 In particular, Nox4 transcript was increased. Nox4 can act on its own to produce superoxide ion.37, 38 In addition, expression of Nox 1, Noxa1, and Noxo1 were also increased. The expression of these proteins returned to sham levels at 7-days post-blast, however, mRNA levels of cytochrome b-245 alpha (Cyba), a NOX interacting protein, were increased at that time-point. EPO did not appear to alter expression levels of any of these proteins with the exception of decreasing Cyba message levels at 7-days post-blast. Expression of these enzymes is increased in a retinal ischemia model and may contribute to the death of the retinal ganglion cells.38
The mRNAs were organized into functional groups and plotted to assess trends (Figure 8B). There was an increase in expression of peroxireductases (Prdx) and superoxide dismutases in the 3-day post-blast, buffer-injected retinas suggestive of an endogenous protective mechanism. In contrast, in all groups there was a decrease in gene expression in the EPO-treated 3-day post-blast retinas as compared to the buffer group. At 7-days post-blast gene expression levels of the antioxidant enzymes were comparable to or higher than the 3-day post-blast group. At 7-days post-blast EPO again appeared to cause a decrease in expression of most of these enzymes, although levels were higher than in the EPO-treated 3-day post-blast group. One notable exception was an increase in Prdx in the EPO-treated 7-day post-blast group. In order to further investigate this effect, the expression of individual mRNAs with changes from sham of greater than 2-fold was graphed (Figure 8C). The increase in Prdx overall is due to increases specifically in Prdx2 and Prdx6. These enzymes are expressed from the antioxidant response element, consistent with the antioxidant action of EPO (for review see 13). This individual analysis also shows increases of 2-fold or greater of Gpx4, Gclm, Sod1, and Sod3 in buffer-injected post-blast mice at 3 and/or 7 days and not in the EPO-treated 7-day post-blast retinas as we had predicted. This may be due to technical limitations of a microarray, and/or the focal and variable nature of the injury. Future studies will need to confirm these results using RT-PCR and/or Western blot analysis.
DISCUSSION
In agreement with TBI studies, we detected less cell death, optic nerve degeneration, glial reactivity, and oxidative stress by systemic injection of EPO (for review see 39). Further, as in other models, we also detected efficacy with EPO when treatment was delayed until 24h post-injury.40, 41 However, the effect was timing specific with efficacy only detected when treatment was initiated at least 1 day post-injury and assessed at 7 days or 1-month post-injury. When treatment was initiated or stopped earlier, no benefit was found. This effect was not strain-specific since we also detected protection in the Balb/c mouse when treatment was initiated 3 weeks after blast, but not when it was initiated prior to blast.
A surprise finding was the lack of protection in 7-day post-blast retinas treated with EPO beginning at 0 or 6h post-blast. In these groups the last EPO injection was performed at or before 54h post-injury. Erythropoiesis was increased in all EPO-treated mice, causing a correlative increase in retina ferritin levels. The increase in iron could promote the Fenton reaction, converting hydrogen peroxide to the much more damaging hydroxyl radical and causing greater cell death. The peak of cell death after an eye-directed blast in this strain is 3 days post-blast.8 EPO has a rapid systemic half-life of approximately 2.5h in rodents.12, 42 Thus, in addition to having elevated tissue iron levels from an increased hematocrit, these mice likely did not have sufficient levels of EPO in the retina during the peak of cell death to induce expression of antioxidant enzymes and counteract the ongoing oxidative stress. EPO can activate Nrf2 within neuronal tissue to induce transcription from the antioxidant response element resulting in increased levels of many antioxidant enzymes (for review see 13). In agreement with this analysis, the oxidative stress microarray showed low levels of all tested anti-oxidant enzymes in the EPO-treated 3-day post-blast retinas. This lack of increase in anti-oxidant enzymes likely also explains the lack of benefit, and in fact, the increase in cell death, in the EPO-treated retinas at 3-days post-blast (the peak of cell death after blast-induced trauma in this strain).8 These results suggest that the benefit of EPO at 7-days post-blast was in spite of greater damage at 3-days post-blast.
Based on these results, greater therapeutic benefit would be expected by treatment with EPO if increased erythropoiesis is avoided completely. This can be achieved either through local delivery of EPO 18, 23, 24, 43 or by systemic delivery of forms of EPO that have attenuated erythropoietic activity and yet maintain their neuroprotective function.20-22, 44, 45 To test this we treated mice with rAAV.EpoR76E, and, similar to the EPO studies, we detected less cell death when therapy was initiated after, but not before, blast. EPO-R76E does induce a slight rise in hematocrit that is comparable, or lower, than what was induced by EPO treatment in the DBA/2J mice. Retinal ferritin levels were not increased in the pre-blast treatment group, and were surprisingly decreased in the post-blast group. The greater benefit in the post-blast group demonstrates that providing therapeutic levels of EPO as late as 3-weeks after blast is still effective.
In terms of clinical translation, these results suggest that: 1) oxidative stress is an important early component of retinal damage after blast trauma, 2) rapid treatment is not critical, 3) intraocular treatment with EPO maybe more effective than systemic therapy as it would avoid induction of erythropoiesis, and 4) EPO is protective to retinal neurons and axons in the optic nerve after eye trauma.
ACKNOWLEDGMENTS
This research was funded by Department of Defense grants W81XW-10-1-0528 (TSR), W81XWH-13-1-0048 (TSR), W81XWH-15-1-0096 (TSR), NIH grants R01 EY022349 (TSR) and P30 EY008126 (David Calkins), Fight for Sight Summer Undergraduate Fellowship (LDS) and Research to Prevent Blindness Unrestricted Funds (Paul Sternberg, Jr.).
Footnotes
The authors report no conflicts of interest.
The views, opinions and/or findings contained in this research paper are those of the authors and do not necessarily reflect the views of the Department of Defense and should not be construed as an official DoD/Army position, policy or decision unless so designated by other documentation. No official endorsement should be made.
REFERENCES
- 1.World Health Organization (WHO) [November 5, 2015];World Health Organization Priority Eye Disease: Corneal Opacities. Available at: http://www.who.int/blindness/causes/priority/en/index8.html.
- 2.U.S. Centers for Disease Control and Prevention (CDC) Morbidity and Mortality Weekly Reports (MMWR). Quickstats: Average Annual Rate of Eye-related Emergency Department Visits for Injuries and Medical Conditions,* by Age GroupUnited States, 2007–2010. [November 5, 2015];MMWR. 2013 62:374. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6218a9.htm. [Google Scholar]
- 3.Hilber DJ. Eye injuries, active component, U.S. Armed Forces, 2000-2010. MSMR. 2011;18:2–7. [PubMed] [Google Scholar]
- 4.Cockerham GC, Goodrich GL, Weichel ED, Orcutt JC, Rizzo JF, Bower KS, Schuchard RA. Eye and visual function in traumatic brain injury. J Rehab Res Develop. 2009;46:811–8. doi: 10.1682/jrrd.2008.08.0109. [DOI] [PubMed] [Google Scholar]
- 5.Cockerham GC, Rice TA, Hewes EH, Cockerham KP, Lemke S, Wang G, Lin RC, Glynn-Milley C, Zumhagen L. Closed-eye ocular injuries in the Iraq and Afghanistan wars. New Eng J Med. 2011;364:2172–3. doi: 10.1056/NEJMc1010683. [DOI] [PubMed] [Google Scholar]
- 6.Scott R. The injured eye. Philos Trans R Soc Lond B Biol Sci. 2011;366:251–60. doi: 10.1098/rstb.2010.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weichel E, Colyer M, Bautista C, Bower KS, French L. Traumatic brain injury associated with combat ocular trauma. J Head Trauma Rehab. 2009;24:41–50. doi: 10.1097/HTR.0b013e3181956ffd. [DOI] [PubMed] [Google Scholar]
- 8.Bricker-Anthony C, Hines-Beard J, D'Surney L, Rex TS. Exacerbation of blast-induced ocular trauma by an immune response. J Neuroinflammation. 2014;11:192. doi: 10.1186/s12974-014-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bricker-Anthony C, Hines-Beard J, Rex TS. Molecular changes and vision loss in a mouse model of closed-globe blast trauma. Invest Ophthalmol Vis Sci. 2014;55:4853–62. doi: 10.1167/iovs.14-14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hines-Beard J, Marchetta J, Gordon S, Chaum E, Geisert EE, Rex TS. A mouse model of ocular blast injury that induces closed globe anterior and posterior pole damage. Exp Eye Res. 2012;99:63–70. doi: 10.1016/j.exer.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bricker-Anthony C, Hines-Beard J, Rex TS. Eye-directed overpressure airwave-induced trauma causes lasting damage to the anterior and posterior globe: a model for testing cell-based therapies. J Ocul Pharmacol Ther. 2016 Mar 16; doi: 10.1089/jop.2015.0104. epub ahead of print:doi 10.1089/jop.2015.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood) 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 13.Bond WS, Rex TS. Evidence that erythropoietin modulates neuroinflammation through differential action on neurons, astrocytes, and microglia. Front Immunol. 2014;5:523. doi: 10.3389/fimmu.2014.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Reme CE. Hif-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–24. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- 15.Grimm C, Wenzel A, Stanescu D, Samardzija M, Hotop S, Groszer M, Naash M, Gassmann M, Reme C. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J Neurosci. 2004;24:5651–8. doi: 10.1523/JNEUROSCI.1288-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junk A, Mammis A, Savitz S, Singh M, Roth S, Malhotra S, Rosenbaum PS, Cerami A, Brines M, Rosenbaum DM. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Nat Acad Sci USA. 2002;99:10659–64. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilic U, Kilic E, Soliz J, Bassetti C, Gassmann M, Hermann D. Erythropoietin protects from axotomy-induced degeneration of retinal ganglion cells by activating erk-1/-2. FASEB J. 2005;19:249–51. doi: 10.1096/fj.04-2493fje. [DOI] [PubMed] [Google Scholar]
- 18.King CE, Rodger J, Bartlett C, Esmaili T, Dunlop SA, Beazley LD. Erythropoietin is both neuroprotective and neuroregenerative following optic nerve transection. Exp Neurol. 2007;205:48–55. doi: 10.1016/j.expneurol.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Rex TS, Allocca M, Domenici L, Surace EM, Maguire AM, Lyubarsky A, Cellerino A, Bennett J, Auricchio A. Systemic but not intraocular epo gene transfer protects the retina from light-and genetic-induced degeneration. Mol Ther. 2004;10:855–61. doi: 10.1016/j.ymthe.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan TA, Geisert EE, Hines-Beard J, Rex TS. Systemic aav-mediated gene therapy preserves retinal ganglion cells and visual function in dba/2j glaucomatous mice. Hum Gene Ther. 2011;22:1191–200. doi: 10.1089/hum.2011.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan TA, Geisert EE, Templeton JP, Rex TS. Dose-dependent treatment of optic nerve crush by exogenous systemic mutant erythropoietin. Exp Eye Res. 2012;96:36–41. doi: 10.1016/j.exer.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan T, Rex TS. Systemic gene delivery protects the photoreceptors in the retinal degeneration slow mouse. Neurochem Res. 2011;36:613–8. doi: 10.1007/s11064-010-0272-6. [DOI] [PubMed] [Google Scholar]
- 23.Tsai J, Wu L, Worgul B, Forbes M, Cao J. Intravitreal administration of erythropoietin and preservation of retinal ganglion cells in an experimental rat model of glaucoma. Curr Eye Res. 2005;30:1025–31. doi: 10.1080/02713680500320729. [DOI] [PubMed] [Google Scholar]
- 24.Weishaupt J, Rohde G, Polking E, Siren A-L, Ehrenreich H, Bahr M. Effect of erythropoietin on axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:1514–22. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- 25.Presneill J, Little L, Nichol A, French C, Cooper DJ, Haddad S, Duranteau J, Huet O, Skrifvars M, Arabi Y, et al. Statistical analysis plan for the erythropoietin in traumatic brain injury trial: A randomised controlled trial of erythropoietin versus placebo in moderate and severe traumatic brain injury. Trials. 2014;15:501. doi: 10.1186/1745-6215-15-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichol A, French C, Little L, Presneill J, Cooper DJ, Haddad S, Duranteau J, Huet O, Skrifvars M, Arabi Y, et al. Erythropoietin in traumatic brain injury: Study protocol for a randomized controlled trial. Trials. 2015;16:39. doi: 10.1186/s13063-014-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in dba/2j mice. Invest Ophthalmol Vis Sci. 1998;39:951–62. [PubMed] [Google Scholar]
- 28.Bosco A, Steele MR, Vetter ML. Early microglia activation in a mouse model of chronic glaucoma. J Compar Neurol. 2011;519:599–620. doi: 10.1002/cne.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hines-Beard J, Marchetta J, Gordon S, Chaum E, Geisert EE, Rex TS. A mouse model of ocular blast injury that induces closed globe anterior and posterior pole damage. Exper Eye Res. 2012;99:63–70. doi: 10.1016/j.exer.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louboutin JP, Wang L, Wilson JM. Gene transfer into skeletal muscle using novel aav serotypes. J Gene Med. 2005;7:442–51. doi: 10.1002/jgm.686. [DOI] [PubMed] [Google Scholar]
- 31.Weitlauf C, Ward NJ, Lambert WS, Sidorova TN, Ho KW, Sappington RM, Calkins DJ. Short-term increases in transient receptor potential vanilloid-1 mediate stress-induced enhancement of neuronal excitation. J Neurosci. 2014;34:15369–81. doi: 10.1523/JNEUROSCI.3424-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bond WS, Hines-Beard J, GoldenMerry YL, Davis M, Farooque A, Sappington RM, Calkins DJ, Rex TS. Virus-mediated EpoR76E Therapy Slows Optic Nerve Axonopathy in Experimental Glaucoma. Mol Ther. 2016;24:230–9. doi: 10.1038/mt.2015.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inman DM, Sappington RM, Horner PJ, Calkins DJ. Quantitative correlation of optic nerve pathology with ocular pressure and corneal thickness in the dba/2 mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:986–96. doi: 10.1167/iovs.05-0925. [DOI] [PubMed] [Google Scholar]
- 34.Kim A, Nemeth E. New insights into iron regulation and erythropoiesis. Curr Opin Hematol. 2015;22:199–205. doi: 10.1097/MOH.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gammella E, Diaz V, Recalcati S, Buratti P, Samaja M, Dey S, Noguchi CT, Gassmann M, Cairo G. Erythropoietin's inhibiting impact on hepcidin expression occurs indirectly. Am J Physiol Regul Integr Comp Physiol. 2015;308:R330–5. doi: 10.1152/ajpregu.00410.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He X, Hahn P, Iacovelli J, Wong R, King CE, Bhisitkul R, Massaro-Giordano M, Dunaief JL. Iron homeostasis and toxicity in retinal degeneration. Prog Ret Eye Res. 2007;26:649–73. doi: 10.1016/j.preteyeres.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 38.Dvoriantchikova G, Grant J, Santos AR, Hernandez E, Ivanov D. Neuronal nad(p)h oxidases contribute to ros production and mediate rgc death after ischemia. Invest Ophthalmol Vis Sci. 2012;53:2823–30. doi: 10.1167/iovs.12-9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng W, Xing Z, Yang J, Wang Y, Wang W, Huang W. The efficacy of erythropoietin in treating experimental traumatic brain injury: a systematic review of controlled trials in animal models. J Neurosurg. 2014;121:653–64. doi: 10.3171/2014.6.JNS132577. [DOI] [PubMed] [Google Scholar]
- 40.Gawad AE, Schlichting L, Strauß O, Zeitz O. Antiapoptotic properties of erythropoietin: Novel strategies for protection of retinal pigment epithelial cells. Eye. 2009;23:2245–50. doi: 10.1038/eye.2008.398. [DOI] [PubMed] [Google Scholar]
- 41.Xiong Y, Mahmood A, Meng Y, Zhang Y, Qu C, Schallert T, Chopp M. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: Comparison of treatment with single and triple dose. J Neurosurg. 2010;113:598–608. doi: 10.3171/2009.9.JNS09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee DE, Son W, Ha BJ, Oh MS, Yoo OJ. The prolonged half-lives of new erythropoietin derivatives via peptide addition. Biochem Biophys Res Commun. 2006;339:380–5. doi: 10.1016/j.bbrc.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 43.Rex TS, Wong Y, Kodali K, Merry S. Neuroprotection of photoreceptors by direct delivery of erythropoietin to the retina of the retinal degeneration slow mouse. Exp Eye Res. 2009;89:735–40. doi: 10.1016/j.exer.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–42. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 45.Dumont F, Bischoff P. Non-erythropoietic tissue-protective peptides derived from erythropoietin: WO2009094172. Expert Opin Ther Pat. 2010;20:715–23. doi: 10.1517/13543771003627464. [DOI] [PubMed] [Google Scholar]