Summary
Inositol hexakisphosphate (IP6) and inositol both regulate insulin secretion, but their combined use in the management of diabetes deserves investigation. The combined effects of IP6 and inositol supplementation were investigated in streptozotocin‐induced type 2 diabetic rats. The following groups of rats were studied for 8 weeks: non‐diabetic control, non‐diabetic high‐fat diet control, diabetic untreated, diabetic rats treated with the combination of IP6 and inositol (650 mg/kg bw) and diabetic rats treated with glibenclamide (10 mg/kg bw). High‐fat diet and streptozotocin were used to induce type 2 diabetes mellitus in Sprague–Dawley rats. Body weight, blood glucose, glycated haemoglobin, insulin, serum leptin, HOMA‐insulin resistance scores, intestinal amylase activity, serum and faecal lipids and food and fluid consumption were measured. Treatment with the combination significantly reduced blood glucose (306 ± 53 mg/dl) and insulin resistance score (1.93 ± 0.45) compared with diabetic controls (522 ± 24 mg/dl and 5.1 ± 0.69 respectively). Serum leptin (2.8 ± 0.6 ng/dl) and faecal triglycerides (108 ± 8 mg/dl) were significantly increased in rats treated with the combination compared with the diabetic control (1.8 ± 0.06 ng/dl and 86 ± 4 mg/dl). Serum triglyceride (47 ± 5.1 mg/dl), total cholesterol (98 ± 3.2 mg/dl) and food intake (26 ± 0.3 g) were significantly reduced by 45%, 25% and 25%, respectively, in rats treated with the combination compared with the diabetic control. Inositol and IP6 combined supplementation may be effective in the management of type 2 diabetes mellitus and related metabolic disorders by regulating some aspects of lipid and carbohydrate metabolism.
Keywords: diabetes mellitus, inositol hexakisphosphate, leptin, lipid, phytic acid
Diabetes mellitus is a group of endocrine or metabolic diseases characterized by impaired glucose utilization that leads to chronic hyperglycaemia which is a direct result of a relative or absolute deficiency of insulin. It is characterized by the disruption of carbohydrate, protein and fat metabolism. The metabolic disorders associated with diabetes mellitus development involve several pathogenic processes which are the basis of secondary pathophysiologic changes in multiple organs. Type 2 diabetes mellitus is the most prominent form of diabetes, affecting approximately 90% of individuals diagnosed with the disease, and is often asymptomatic (World Health Organization [WHO] 2014). Obesity and physical inactivity are the main contributing factors associated with the development of type 2 diabetes mellitus, and deaths associated with the disease are very high globally (WHO 2014). This high rate of morbidity and mortality among patients with type 2 diabetes is a result of abnormalities in blood lipid profile which contribute to the development of cardiovascular complications (Ginsberg 2000).
Inositol, also known as myo‐inositol, is a pseudovitamin, with a hexacarbon carbohydrate structure. It is found in mammalian cell membranes attached to lipids, as phosphatidylinositol (a signal transduction molecule). Inositol hexakisphosphate (IP6 or InsP6), also known as phytic acid, is a polyphosphorylated inositol derivative. It exists as a phosphorylated carbohydrate in plant and mammalian cells. Inositol and its derivatives are also common constituents of legumes and whole grains (Vucenik & Shamsuddin 2003). Shamsuddin et al. (1989) demonstrated that when IP6 and inositol are combined in the appropriate ratio, it produces two inositol 1,4,5‐trisphosphate (Ins(1,4,5)P3 or IP3) signalling molecules, an important cellular regulator.
Inositol compounds play significant roles in the regulation of insulin secretion from pancreatic beta cells (Barker et al. 2002, 2009; Vanderlinden & Vucenik 2004; Berggren & Barker 2008). IP6 is shown to function by regulating the mobilization of Ca2+ into the cell via the voltage‐dependent Ca2+ channels (Yang & Berggren 2005). IP6 enhances Ca2+‐gated channel activity by increasing beta cell phosphorylation state (Larsson et al. 1997). This occurs through deactivation of serine/threonine protein phosphatases as well as the activation of serine/threonine kinases. Studies of hippocampal neurons demonstrated that IP6 indirectly activates protein kinase A (PKA) by activating adenylyl cyclase which resulted in increased cyclic adenosine monophosphate (cAMP) production (Yang et al. 2001). Other studies demonstrated that protein kinase C (PKC) is directly activated by IP6 in the pancreatic beta cells (Efanov et al. 1997; Høy et al. 2003). PKC and PKA promote Ca2+‐induced exocytosis through serine/threonine phosphorylation. The final steps of insulin exocytosis are driven by Ca2+ influx (Berggren et al. 1993). According to Høy et al. (2002), the mode of action of IP6 does involve not only exocytosis but also endocytosis where a more significant effect is seen. The latter is achieved through the activation of calcineurin which dephosphorylates dynamin 1 and accounts for both the activation of PKC and inhibition of the phosphoinositide phosphatase synaptojanin and ultimately phosphatidylinositol 4,5‐bisphosphate production (Høy et al. 2002).
Shamsuddin et al. (1988) hypothesized that IP6 in cells dephosphorylates to form lower inositol phosphates (IP1–IP5). Preliminary studies have shown that IP6 is transported into the gastrointestinal epithelial cells intact where it is dephosphorylated (Sakamoto et al. 1993). Other studies have shown that intracellular concentration of lower inositol phosphates increased with IP6 treatment (Vucenik & Shamsuddin 1994; Grases et al. 2002). We propose that one of the several ways in which the combination works is by acting as precursors for the formation of lower inositol phosphates and phosphatidylinositols, thereby enhancing its antidiabetic effects. Treatment with the IP6/inositol combination can therefore result in increased inositol IP3 cellular concentration. IP3 plays vital regulatory roles in Ca2+ mobilization (Berridge & Irvine 1984; Irvine & Schell 2001; Irvine 2005) and therefore insulin secretion.
The safety of IP6 consumption as well as how efficiently it is absorbed from the digestive tract is still under debate among researchers (Sakamoto et al. 1993; Shamsuddin & Vucenik 1999; Grases et al. 2001). Ullah and Shamsuddin (1990) reported that IP6 displays no apparent toxicity and is thus deemed safe for consumption, even with long‐term administration. The debate continues, however, with recent research suggesting that IP6 may not be beneficial to humans as it is not thought to be found in mammalian tissue (Wilson et al. 2015). This is compounded by the theory that chronic use may be ill‐advised owing to risk of trivalent cation deficiency in individuals who are prone to mineral deficiencies (Wilson et al. 2015). Nevertheless, important multicellular functions including cell proliferation, signal transduction and differentiation are believed to be regulated by IP6 (Vucenik & Shamsuddin 2006). Studies have reported antidiabetic properties of IP6 in diabetic rats (Lee et al. 2006; Kuppusamy et al. 2011). These studies have provided valuable information to date; however, the use of an IP6/inositol combination in the treatment of type 2 diabetes has not been studied. It is theorized that the combined IP6/inositol product may be more effective in regulating serum lipids and carbohydrates via various cellular regulatory mechanisms compared with IP6 alone. Studies of this nature are important, as there is no cure for diabetes mellitus and current drug therapies for the management of the disease are costly and have undesirable side effects. As a result, there is emphasis on potential natural alternative antihyperglycaemic remedies with minimal or no side effects. We hypothesized that consumption of an IP6 and inositol combined supplement in a predetermined ratio will be effective in the management of some metabolic disturbances associated with type 2 diabetes mellitus.
Materials and methods
Animal care
Healthy adult male Sprague–Dawley rats (168 ± 5.9 g) were procured from Harlan Laboratories Inc. (Indianapolis, IN, USA). The rats were housed in polypropylene cages with a solid floor and bedding materials. The room was maintained at controlled temperature (22 ± 2°C), with humidity (45 ± 5%) and 12/12‐hour light/dark cycle. The animals had free access to standard rat laboratory diet (PicoLab® Rodent Diet 20; 5053) or high‐fat diet consisting 45% fat as a percentage of total kcal (D12451; Research Diets Inc. New Brunswick, NJ, USA). They were also given clean drinking water ad libitum.
Ethical approval statement
All animal experiments were in accordance with and approved in advance by the Institutional Animal Care and Use Committee (IACUC) of the Institute of Biosciences and Technology, Texas A&M Health Sciences Center, Houston, USA (protocol number 011645).
Induction of type 2 diabetes
Induction of type 2 diabetes was carried out according to a modified version of previously used protocols (Danda et al. 2005; Srinivasan et al. 2005). The rats were fed 45% high‐fat diet for 4 weeks. At the end of week 2, type 2 diabetes was induced by a single intraperitoneal (i.p.) administration of 35 mg/kg body weight of streptozotocin (Sigma‐Aldrich, St. Louis, MO, USA) dissolved in 0.1 M of cold citrate buffer, pH 4.5. This solution was freshly prepared, shielded from light and administered within 5 min of preparation. Control rats were only injected with an equivalent volume of citrate buffer. One week later, the non‐fasting blood glucose level was measured and animals with a blood glucose ≥300 mg/dl were considered diabetic. To further confirm type 2 diabetes, an antidiabetic drug response test was carried out (briefly described below). The rats were classified as being type 2 DM based on a positive response to glibenclamide.
Experimental design
This animal trial was an 8‐week study. For the first 4 weeks of the experiment, six rats were fed a normal basal diet (PicoLab® Rodent Diet 20; 5053) and 24 rats were fed 45% high‐fat diet. Diabetes was induced in 18 of the rats on the high‐fat diet. At the end of week 4, the rats were assigned the following five groups (six rats per group): normal control (NC; non‐diabetic rats fed basal diets), high‐fat control (HFC; high‐fat diet and non‐diabetic), diabetic untreated control (DC), diabetic rats treated with combined IP6 and inositol supplement (hereafter referred to as the combination; 650 mg/kg body weight/day) and glibenclamide positive control group (10 mg/kg body weight/day). During the treatment period, all the rats were fed basal diet along with their respective treatment regime as outlined above. The experiment was designed to administer a dosage of 1% IP6 and inositol combination which is equivalent to 650 mg/kg body weight at a ratio of 220:800. The IP6 and inositol used were extracted from rice and supplied by Vita‐Tech International Inc. (Tustin, CA, USA). Glibenclamide (pharmaceutical grade) and the combined IP6 and inositol were dissolved in 1% sodium carboxymethyl cellulose (Na‐CMC) and were administered once daily via oral gavage. The control groups (NC, HFC and DC) received Na‐CMC daily. Glibenclamide is slightly soluble in water, so to improve the solubility and ultimately the bioavailability of this drug, Na‐CMC was used as the transport medium.
Antidiabetic drug response test
To confirm the validity and suitability of the type 2 diabetes animal models, its response to the conventional antidiabetic drug (glibenclamide) was tested. Fasting blood glucose concentrations of the diabetic rats were measured. Following this, 10 mg/kg body weight of glibenclamide was administrated orally using intubation needles in 1% sodium carboxymethyl cellulose (Na‐CMC) at a dose of 0.5 ml/kg body weight. The blood glucose concentration of each rat was then measured 120 min after the administration of glibenclamide.
Blood glucose concentration and body weight measurements
Non‐fasting blood glucose levels of each group of rats were measured with the ReliOn blood glucose monitoring system on the following days: 14, 21, 28, 42, 49 and 56. At the start of the experiment, initial body weights were recorded. Rats were weighed once weekly throughout the study.
Fluid and food intake
The amount of food consumed and that of fluid consumed were calculated from the daily unconsumed rat chow and deionized water.
Intestinal analysis
The intestine of each rat was excised, weighed and sectioned into proximal (duodenum) and distal (jejunum and ileum) portions. Sodium chloride (0.9%) solution was used to flush the intestinal lumen several times. The scraped mucosa was homogenized and centrifuged (5000 g), and the supernatant was frozen until required for assays (Percival & Schneeman 1979). Wotton (1964) and Bradford (1976) methods were used to determine intestinal amylase activity and the protein concentration of the intestinal homogenate respectively.
Faecal lipid profile analyses
The Folch et al. (1957) method was used for lipid extraction from the faecal samples. The faecal samples were collected, dried for 1 h at 70°C, weighed and homogenized with a chloroform–methanol mixture (2:1, v/v) (20 ml per gram of faeces). The mixtures were incubated for 60 min at 60°C with constant agitation in an orbital shaking incubator. The homogenates were then centrifuged for 5 min at 3000 g to recover the supernatants. The supernatants were thoroughly washed with 0.2 ml of its volume of 0.9% sodium chloride solution by vortexing. The mixtures were allowed to separate into two phases by low‐speed centrifugation (1000 g for 10 min). The upper phases were removed without disturbing the lower chloroform phases using a pipette. The upper aqueous layers were discarded, while the lower layers containing the purified lipids were retained, and the lipid contents were determined. The Zlatkis et al. (1953) method was used to determine the cholesterol levels, while the method of Gottfried and Rosenberg (1973) was used to determine triglyceride levels.
Biochemical analysis
The rats were fasted overnight and euthanized by decapitation at the end of the 8‐week period. Blood samples were collected and stored in the appropriate vacutainer tubes. The insulin and glycohaemoglobin (HbA1c) levels were measured using the Mercodia Rat Insulin Elisa kit (Mercodia, Uppsala, Sweden) and the Stanbio Glycohaemoglobin kit (Stanbio Laboratory, Boerne, TX, USA) respectively. The protocols were carried out according to the manufacturers' instructions. Total cholesterol, triglyceride and HDL‐cholesterol levels were determined using the Stanbio Sirrus Clinical Chemistry Analyzer. Insulin resistance was estimated using the Matthews et al. (1985) homoeostasis model assessment (HOMA‐IR) equation:
Insulin resistance score (HOMA‐IR) = [Insulin (U/l) × Fasting blood glucose (mmol/l)]/22.5
Blood glucose: 1 mmol/l = 18 mg/dl.
Statistical analysis
All data were analysed using the Statistical Package for the Social Sciences (spss) version 20 software (SPSS Inc., Chicago, IL, USA). Variation among test groups was evaluated using the one‐way anova. Post hoc analysis was carried out using Duncan's multiple‐range test to assess the significant difference among the means (P < 0.05). For tables and figures, different superscript letters indicate significant difference among test groups. Paired Student's t‐test was used to determine the significant difference in blood glucose levels before and after the administration of glibenclamide (P < 0.05). The results were expressed as mean ± SEM.
Results
Weekly non‐fasting blood glucose levels
Figure 1 shows the non‐fasting blood glucose (NFBG) concentrations in diabetic rats treated with the combination or glibenclamide. Before induction of type 2 diabetes, there were no significant differences in NFBG concentration among the groups (P = 0.76). However, 1 week after induction, a significant increase in the NFBG level was noted in the STZ‐induced diabetic groups when compared with the non‐diabetic groups (P < 0.05). Following the treatment of the diabetic groups, there was a significant reduction in the NFBG concentration in the combination or glibenclamide‐treated group when compared with the diabetic control group (P < 0.05).
Figure 1.
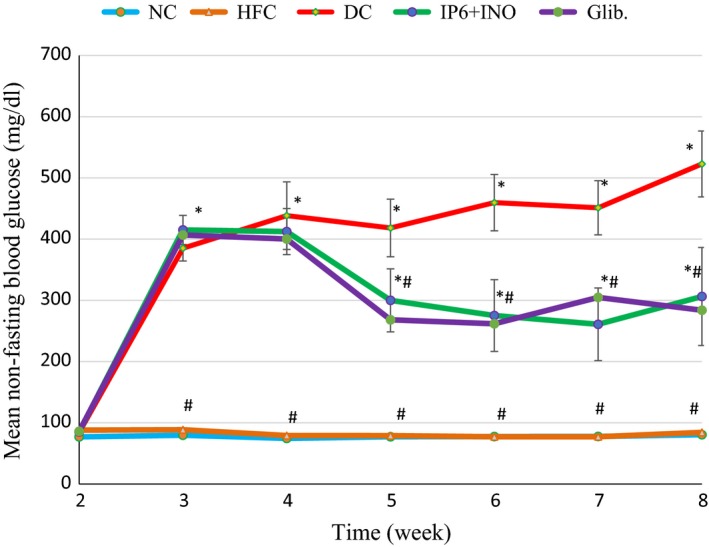
Non‐fasting blood glucose concentration in diabetic rats treated with combined IP6 & inositol supplement or glibenclamide. *indicates a significant difference at p < 0.05 compared with NC at the same time. # indicates a significant difference at p < 0.05 compared with DC at the same time period.
Change in body weight
Figure 2 shows the change in body weight in diabetic rats treated with the combination or glibenclamide. For the first two weeks of the experiment, there was no significant difference in body weight among the groups. The untreated diabetic group (DC) gained less weight compared with the other groups. Although diabetic rats treated with the combination showed an increase in body weight throughout the feeding trial, the gain in body weight was significantly less than that of the diabetic rats treated with glibenclamide (P < 0.05).
Figure 2.
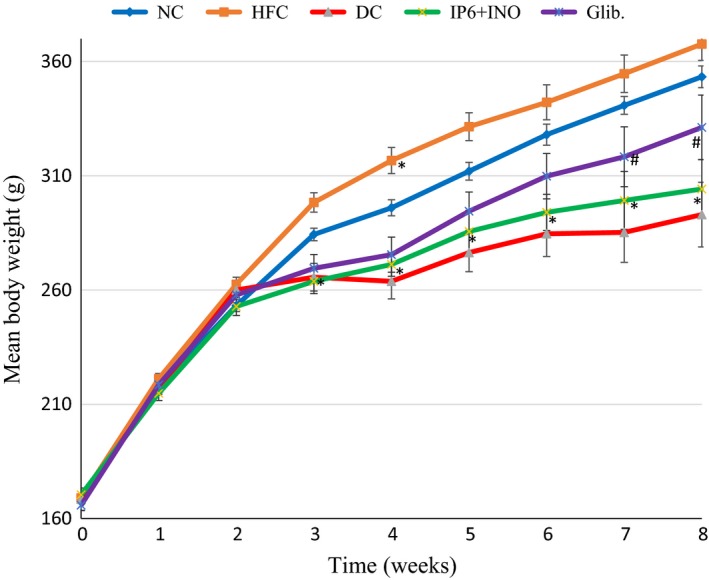
Mean body weight changes in diabetic rats treated with combined IP6 and inositol supplement or glibenclamide. * indicates a significant difference at p < 0.05 compared with NC at the same time. # indicates a significant difference at p < 0.05 compared with DC at the same time period.
Fluid intake
Table 1 shows the mean daily fluid consumption in diabetic rats treated with combination or glibenclamide. The untreated diabetic group consumed more fluid when compared with the other groups. There was no significant difference in fluid consumption between diabetic rats treated with the combination or glibenclamide at weeks 5 (P = 0.74) and 6 (P = 0.98) of the experiment. However, it was noted in the final two weeks of the experiment that there was a significant reduction in fluid consumption in the combination‐treated group when compared with the glibenclamide‐treated group (P < 0.05).
Table 1.
Mean daily fluid intake for type 2 diabetic rats treated with combined IP6 and inositol supplement or glibenclamide
| Time (week) | Fluid intake per day (ml/day) | ||||
|---|---|---|---|---|---|
| NC | HFC | DC | IP6 + INO | Glib. | |
| 5 | 34.6 ± 0.5a | 33.3 ± 1.3a | 147.6 ± 4.2b | 81.2 ± 3.4c | 70.6 ± 5.5c |
| 6 | 39 ± 2.3a | 34 ± 3.0a | 150 ± 4.1b | 83 ± 9.3c | 83 ± 10c |
| 7 | 32.8 ± 0.3a | 33.1 ± 0.7a | 156.4 ± 8.7b | 72.3 ± 7.8c | 90.1 ± 4.7d |
| 8 | 29.6 ± 2.1a | 30 ± 0.1a | 147.2 ± 0.12b | 70.4 ± 8.9c | 82.5 ± 4.6d |
Data are shown as mean ± SEM. The figures with different superscript letters are significantly different at P < 0.05.
NC, normal control; HFC, high‐fat control; DC, diabetic untreated control; IP6+INO, combined IP6 and inositol.
Food intake
Table 2 shows the mean daily food consumed over the last four weeks of treatment. There were no significant differences observed in food consumption between the normal and HFC groups throughout the last 4 weeks of the experiment (week 5: P = 1.00; week 6: P = 0.41; week 7: P = 0.26; week 8; P = 0.38). The diabetic control group consumed significantly more food at weeks 6, 7 and 8 than all the treatment groups (P < 0.05). There was no significant difference in food consumption among the groups treated with the combination or glibenclamide by weeks 5 (P = 0.55) and 6 (P = 0.89) of the feeding trial. However, food consumption was significantly lower in the combination‐treated group compared with the glibenclamide‐treated group (P < 0.05).
Table 2.
Mean daily food intake for type 2 diabetic rats treated with combined IP6 and inositol supplement or glibenclamide
| Time (week) | Food intake per day (g/d) | ||||
|---|---|---|---|---|---|
| NC | HFC | DC | IP6 + INO | Glib. | |
| 5 | 19.7 ± 0.3a | 19.6 ± 0.2a | 24.4 ± 2.5b | 25 ± 1.1b | 25.7 ± 1.7b |
| 6 | 17 ± 0.4a | 17.6 ± 0.9a | 34.4 ± 4c | 22.6 ± 4b | 24 ± 0.3b |
| 7 | 20.5 ± 0.3a | 19.3 ± 0.1a | 33.7 ± 1.6b | 23.8 ± 0.3c | 28.8 ± 0.7d |
| 8 | 21.1 ± 0.1a | 20.6 ± 0.02a | 35.9 ± 0.8b | 26 ± 0.3c | 28.5 ± 0.2d |
Data are shown as mean ± SEM. The figures with different superscript letters are significantly different at P < 0.05.
NC, normal control; HFC, high‐fat control; DC, diabetic untreated control; IP6+INO, combined IP6 and inositol.
Intestinal amylase activity
Figure 3 shows the proximal and distal intestinal amylase activity in diabetic rats treated with the combination or glibenclamide. There was no significant difference in the distal intestinal amylase activities among the groups. However, there was a significant reduction in the proximal intestinal amylase activity in the group treated with the combination compared with the group treated with glibenclamide (P < 0.05).
Figure 3.
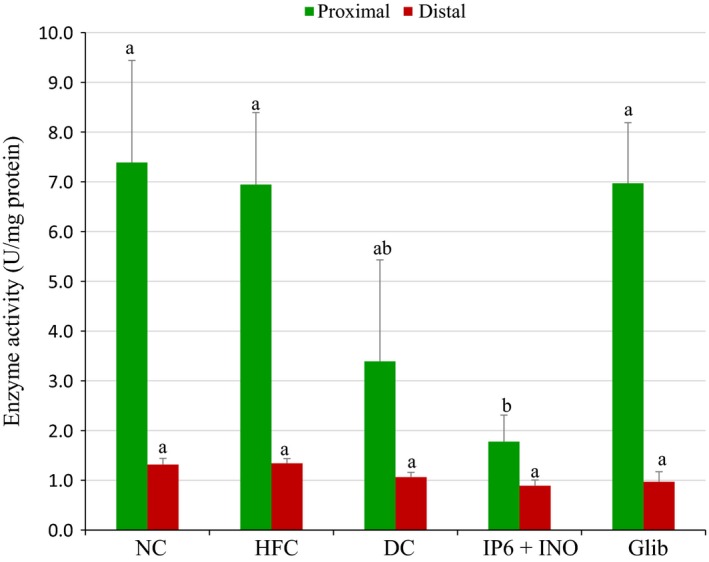
Amylase activity in the proximal and distal intestinal mucosa of diabetic rats treated with combined IP6 and inositol supplement or glibenclamide. Data are shown as mean ± S.E.M. The figures with different superscript letters are significantly different at p < 0.05
Faecal lipid levels
Figure 4 shows faecal total cholesterol and triglyceride levels in diabetic rats treated with the combination or glibenclamide. Faecal triglyceride and total cholesterol concentrations were significantly increased in diabetic rats treated with the combination compared with the NC (P < 0.05).
Figure 4.
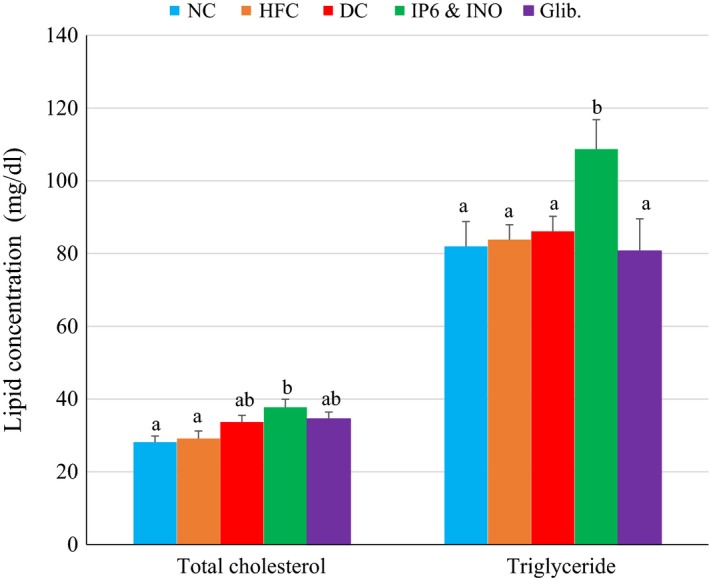
Fecal lipid profile in diabetic rats treated with combined IP6 and inositol supplement or glibenclamide. Data are shown as mean ± S.E.M. The figures with different superscript letters are significantly different at p < 0.05.
Serum leptin
Figure 5 shows the serum leptin levels in diabetic rats treated with the combination or glibenclamide. Serum leptin level was significantly increased in the combination‐treated group compared with the other groups (P < 0.05).
Figure 5.
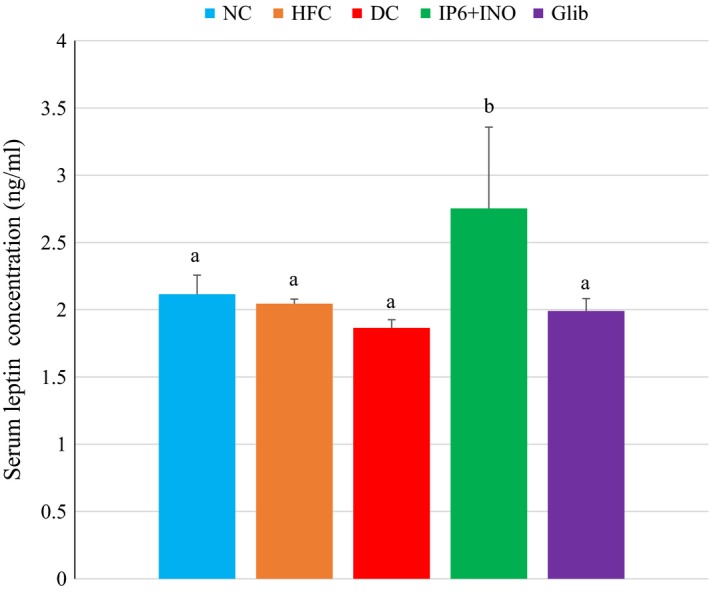
Serum leptin concentration of diabetic rats treated with combined IP6 and inositol or glibenclamide. Data are shown as mean ± S.E.M. The figures with different superscript letters are significantly different at p < 0.05.
Serum insulin, HOMA‐IR, glycated haemoglobin concentration and intestinal weight
Table 3 shows glycated haemoglobin levels, insulin concentration, intestinal weight and homoeostatic model assessment for insulin resistance (HOMA‐IR). There was a significant increase in the HbA1c % among the untreated and treated diabetic groups compared with the non‐diabetic groups (P < 0.05). There were no significant differences in the insulin levels among the groups (P = 0.5). The homoeostatic model assessment for insulin resistance (HOMA‐IR) score in the diabetic control group was significantly higher when compared with the other groups (P = 0.002). Intestinal weight was significantly elevated in the diabetic control and glibenclamide‐treated group compared with the other groups (P = 005). However, there was no significant difference in intestinal weight between the NC and the combination‐treated group.
Table 3.
Glycated haemoglobin levels, fasting insulin levels and homoeostatic model assessment for insulin resistance score and intestinal weight in diabetic rats treated with combined IP6 and inositol supplement or glibenclamide
| Parameters | NC | HFC | DC | IP6 + INO | Glib. |
|---|---|---|---|---|---|
| HbA1c (%) | 4.22 ± 0.21a | 4.41 ± 0.08a | 6.33 ± 0.15b | 5.91 ± 0.36b | 5.98 ± 0.2b |
| Fasting insulin (U/l) | 4.8 ± 0.20a | 5.0 ± 0.23a | 5.2 ± 0.15a | 4.9 ± 0.12a | 4.8 ± 0.19a |
| HOMA‐IR | 0.86 ± 0.75a | 0.89 ± 0.45a | 5.1 ± 0.69b | 1.93 ± 0.45a | 1.7 ± 0.64a |
| Intestinal wt. (g) | 7.89 ± 0.38a | 7.99 ± 0.47a | 13.9 ± 0.58b | 9.3 ± 1.16a | 12.38 ± 3.4b |
Values that share different superscript letters horizontally are significantly different (P < 0.05)
NC, normal control; HFC, high‐fat control; DC, diabetic untreated control; IP6+INO, combined IP6 and inositol.
Serum lipids
Figure 6 shows the serum lipid profile in diabetic rats treated with the combination supplement or glibenclamide. The group treated with combination had significantly lower serum total cholesterol levels when compared with the diabetic control and glibenclamide groups (P < 0.05). The serum triglyceride levels were significantly elevated in the diabetic control and the glibenclamide‐treated group compared with the NC (P < 0.05). Serum HDL was mildly higher in the group treated with the combination, but was not significantly different compared with the other groups.
Figure 6.
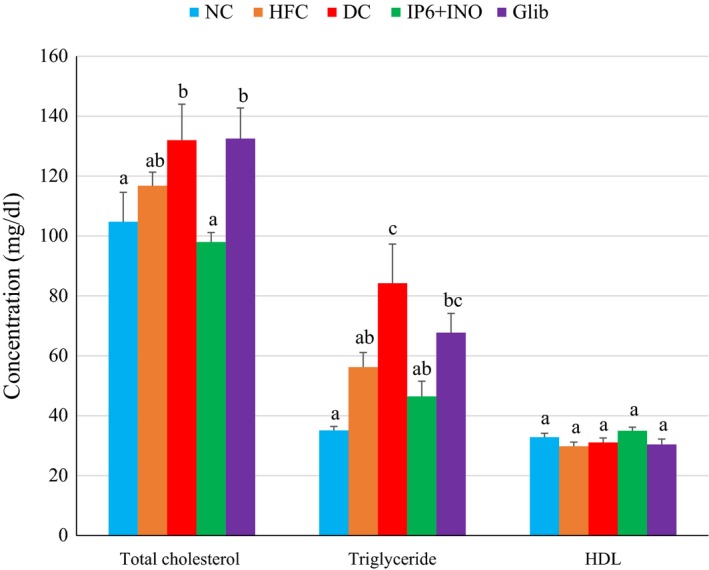
Serum lipid profile in diabetic rats treated with glibenclamide or combined IP6 and inositol supplement. Data are shown as mean ± S.E.M. The figures with different superscript letters are significantly different at p < 0.05.
Discussion
In this study, 45% high‐fat diet followed by streptozotocin administration (35 mg/kg body weight) resulted in successful induction of type 2 diabetes in rats. Previous studies have demonstrated that the consumption of high‐fat diet for 2 weeks or more may result in insulin resistance in rats without elevated blood glucose (Danda et al. 2005; Srinivasan et al. 2005; Zhang et al. 2008). Insulin resistance precedes hyperglycaemia in humans with type 2 diabetes mellitus. The administration of a low dose of STZ in rats induces a mild impairment of insulin secretion that results in a hyperglycaemic condition. To confirm whether a type 2 diabetic model was achieved in this study, the sensitivity of the diabetic rat model was evaluated with a conventional antidiabetic drug (glibenclamide). Stocks et al. (1988) reported that glibenclamide had no effect on blood glucose concentration in type 1 diabetic models of the disease. Therefore, a positive response to this test suggests that a type 2 diabetic model was achieved. There was a significant reduction in blood glucose concentration after the administration of a single oral dose of glibenclamide. The data showed no significant difference in fasting serum insulin levels between the diabetic and non‐diabetic groups, and this was reported to be characteristic of type 2 diabetes (Reed et al. 2000). Interestingly, no significant increase was observed in fasting insulin concentration of diabetic rats treated with glibenclamide or combined IP6 and inositol, although the blood glucose concentrations in these groups were significantly reduced. Studies showing the effect of glibenclamide on the beta cell function in patients with type 2 diabetes have found similar results (Shapiro et al. 1989; O'Meara et al. 1990). These and other studies have concluded that the insulin secretagogue effect of glibenclamide occurred by increasing the pancreatic beta cells' responsiveness to glucose (Shapiro et al. 1989; O'Meara et al. 1990; Patanè et al. 2000). It was proposed that this may be the predominant mechanism of action of glibenclamide and this effect may have been concealed by the fasting conditions (Shapiro et al. 1989; O'Meara et al. 1990). Studies by Lee et al. (2006) also found that phytic acid reduced the blood glucose concentration in diabetic KK mice without affecting the fasting insulin concentration. The results obtained in this study are in line with these findings and suggest that the IP6/inositol combination may function similar to glibenclamide in this respect. The calculated homoeostatic model assessment for insulin resistance (HOMA‐IR) score showed that there was insulin resistance in the diabetic control group. However, the administration of the combination or glibenclamide to the diabetic rats significantly reduced insulin resistance to normal levels.
Previous research (Dilworth et al. 2005; Lee et al. 2006; Kuppusamy et al. 2011; Omoruyi et al. 2013) has demonstrated the hypoglycaemic effects of phytic acid alone in diabetic and non‐diabetic rats. In this study however, the main focus was on the treatment of type 2 diabetic rats with an IP6/inositol combination product that we theorized may be more effective than IP6 or inositol alone. It was found that the treatment of diabetic rats with the combination or glibenclamide resulted in a significant reduction in the non‐fasting blood glucose concentration compared with the diabetic untreated group. We observed that serum glycated haemoglobin concentrations were not significantly altered among the diabetic groups. This was possibly attributed to the short‐term duration of the study. Further investigations are therefore needed to assess the long‐term effect of the combination supplementation on glycated haemoglobin levels in type 2 diabetic rats.
Intestinal weight significantly increased in the diabetic control and the glibenclamide‐treated group compared with the NC group. Several studies have reported similar increases in intestinal weight in streptozotocin‐induced diabetic rats (Jervis & Levin 1966; Schedl & Wilson 1971). It was proposed that increased intestinal weight may be due to modifications to the brush border cell enzyme activities geared at increasing intestinal monosaccharide absorption. However, in the diabetic state, increased monosaccharide absorption leads to increased blood glucose concentration which can further complicate the pathophysiology of the disease. In this study, there was significant reduction in intestinal weight in the diabetic rats treated with the combination compared with the diabetic control group. This may be as a result of the combination's role in altering the activities of some enzymes involved in carbohydrate absorption. Thompson (1988) suggested that phytic acid reduces blood glucose concentration by impairing starch digestion. Similarly, Liu et al. (2008) reported a reduction in endogenous carbohydrase activity and digestive capability in animal model fed a phytate‐supplemented diet. Similarly, Deshpande and Cheryan (1984) reported a reduction in amylase activity when minor quantities of phytate were introduced to the diet. In this study, we noted a significant reduction in the proximal intestinal amylase activity in diabetic rats treated with the combination compared with the diabetic group treated with glibenclamide and the non‐diabetic control group. This suggests that administration of the combination may lead to a reduction in the rate of intestinal carbohydrate digestion. This in turn leads to reduced blood glucose concentration. As mentioned above, reduced carbohydrase activity (in this case amylase) could be one of the factors contributing to the decreased intestinal weight in the diabetic rats treated with the combination.
The data on body weight gain showed that the treatment of type 2 diabetes with glibenclamide may provide fewer benefits due to the significant increase in body weight observed in this group compared with the combination‐treated group. Leptin is a hormone that plays significant roles in the regulation of body weight and food intake (Klok et al. 2006). Activation of the leptin receptor initiates various signalling cascades that result in alterations in food consumption (Schwartz 2001; Sahu 2004). Any reduction in leptin action results in an increased drive for food, reduced satiety and energy utilization (Ahima et al. 1996). Reduced leptin action has also been reported to diminish liver insulin sensitivity, while increasing liver glucose production, decreasing glucose uptake into muscle and reducing the overall metabolic rate (Myers & Olson 2012; Schwartz et al. 2013). Leptin released from the adipose tissue conveys information to the brain about the body's energy status with the resultant decrease in food intake and subsequent increase in energy utilization to maintain body fat stores (Halaas et al. 1995; Jørgensen et al. 1998; Farooqi et al. 2001; Jeon et al. 2003). Obese patients have been reported to be leptin‐resistant; hence, treatment must be geared towards overcoming leptin insensitivity. In this study, a significant increase in the serum leptin levels of the diabetic rats treated with the combination was noted. This increase in serum leptin could be a possible mechanism by which food intake and body weight gain were significantly reduced in the combination‐treated group. However, there are conflicting reports on the role of leptin in diabetes in humans and animal models. Previous reports indicate that leptin increases insulin sensitivity, reduces hepatic glucose production and decreases glucagon levels, while insulin is known to upregulate leptin production and secretion in adipose tissue (Seufert 2004). Further studies showed that leptin is able to ameliorate hyperglycaemia by increasing tissue glucose uptake independent of insulin and by suppressing hepatic glucose production (German et al. 2011). On the contrary, Söderberg et al. (2007) reported that high leptin levels are associated with the future development of diabetes in Mauritian men. However, other research indicates that leptin therapy improves hyperglycaemia and insulin resistance in a mouse model of type 2 diabetes (Toyoshima et al. 2005). Leptin gene therapy has been reported to improve type 1 and type 2 diabetes and diet‐induced obesity in animal models (Yaspelkis et al. 2001; Yu et al. 2008; Fujikawa et al. 2010). This is the first study highlighting the effect of IP6/inositol combination on serum leptin concentration in diabetic animals. We hypothesized that the observed significant increase in the serum leptin level may increase hepatic insulin sensitivity. This in addition to the observed significant decrease in insulin resistance and reduced food intake may account for the hypoglycaemic activity of the combined IP6 and inositol supplement treatment. It is theorized that IP6 and inositol consumption may be beneficial to diabetics in regard to reducing the risks associated with obesity and other downstream pathological complications. The link between type 2 diabetes and obesity has been well established for decades, and the main basis for this connection is insulin resistance. Studies have found that IP6 and myo‐inositol reduced insulin resistance through the activation of peroxisome proliferator‐activated nuclear receptors (PPARs) in a dose‐dependent manner thereby inhibiting lipolysis, increasing glucose uptake and increasing lipid storage in differentiated adipocytes (Kim et al. 2014; Malarvizhi et al. 2016). The reduction in serum lipids along with reduced blood glucose and enhanced insulin sensitivity noted in this study is consistent with these in vitro findings, suggesting that the mechanism of action for these outcomes may be mediated through activation of PPARs. In this regard, treatment with the combined IP6 and inositol supplement may be effective in controlling dyslipidaemia and obesity which are common features of type 2 diabetes mellitus.
There was a significant increase in serum triglyceride and total cholesterol concentrations in the diabetic control group compared with the NC group. However, treatment with the combination significantly reduced serum total cholesterol and triglyceride levels compared with the diabetic control group. This may be due to the reported increase in intestinal lipase activity in rats treated with phytic acid (Dilworth et al. 2015). There were no significant changes observed in serum lipids in diabetic rats treated with glibenclamide compared with the untreated diabetic control group. Mughal et al. (1999) reported that patients with type 2 diabetes treated with glibenclamide showed no significant changes in plasma lipoprotein and lipids. Sulphonylureas stimulate insulin production and can result in excess insulin, which causes increased carbohydrate and fat storage with resultant weight gain. Increased weight gain was noted in the diabetic group treated with glibenclamide compared with the other diabetic groups. Increased faecal cholesterol and triglyceride concentrations were also noted in the diabetic group treated with the combination. This may account for the observed reductions in serum cholesterol and triglyceride concentrations in these animals. Omoruyi et al. (2013) highlighted a positive association between decreased body weight and increased HDL in diabetic rats fed phytic acid. It is however not known whether it is increased lipid uptake or increased utilization that may be affecting serum HDL in these animals. In the present study, similar trends were noted in the group treated with the combined IP6 and inositol supplement. However, the observed reduction in weight gain in the diabetic control did not result in elevated serum HDL‐cholesterol. Overall, reduced cholesterol and triglycerides, along with increased HDL‐cholesterol levels, are desirable in the effective management of type 2 diabetes mellitus. Hence, treatment with the combined IP6 and inositol supplement may be effective in controlling dyslipidaemia which is a common feature of type 2 diabetes mellitus.
Conclusion
This study showed that consumption of an IP6 and inositol combination by diabetic animals resulted in reduced serum amylase, increased insulin sensitivity and increased serum leptin activity. These may be possible mechanisms for the significant reduction in blood glucose concentration observed in these animals. Further studies regarding the molecular mechanisms underlying these observations are needed. Total cholesterol, triglycerides and food and fluid intake were also significantly reduced in diabetic rats treated with the combination. These are factors proven to be beneficial in the management of type 2 diabetes mellitus. Data from this study indicate that treatment of type 2 diabetic rats with the combination may be more effective in curtailing some indices of diabetes including polyphagia, polydipsia and dyslipidaemia, compared with the glibenclamide treatment. Further studies are needed on the effects of the combination on leptin and ghrelin regulation as well as its effects on adipogenesis and dyslipidaemia in the diabetic state.
Conflict of Interest
The authors report no declarations of interest.
Funding source
Financial support was provided by Office of Graduate Studies & Research and the New Initiative Grant towards successful execution of this project.
Acknowledgements
This study was funded by the Principal's New Initiative Grant and Postgraduate Research and Publications Grants provided by The University of the West Indies, Mona Campus.
References
- Ahima R.S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Maratos‐Flier E., Flier J.S. (1996) Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252. [DOI] [PubMed] [Google Scholar]
- Barker C.J., Leibiger I.B., Leibiger B. & Berggren P.O. (2002) Phosphorylated inositol compounds in beta‐cell stimulus‐response coupling. Am. J. Physiol. Endocrinol. Metzrab. 283, E1113–E1122. [DOI] [PubMed] [Google Scholar]
- Barker C.J., Illies C., Fiume R., Gaboardi G.C., Yu J., Berggren P.O. (2009) Diphosphoinositol pentakisphosphate as a novel mediator of insulin exocytosis. Adv. Enzyme Regul. 49, 168–173. [DOI] [PubMed] [Google Scholar]
- Berggren P.O. & Barker C.J. (2008) A key role for phosphorylated inositol compounds in pancreatic beta‐cell stimulus‐secretion coupling. Adv. Enzyme Regul. 48, 276–294. [DOI] [PubMed] [Google Scholar]
- Berggren P.O., Arkhammar P., Islam M.S., et al (1993) Regulation of cytoplasmic free Ca2+ in insulin‐secreting cells. Adv. Exp. Med. Biol. 334, 25–45. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. & Irvine R.F. (1984) Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312, 315–321. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Danda R.S., Habiba N.M., Rincon‐Choles H., Bhandari B.K., Barnes J.L., Abboud H.E. & Pergola P.E. (2005) Kidney involvement in a nongenetic rat model of type 2 diabetes. Kidney Int. 68, 2562–2571. [DOI] [PubMed] [Google Scholar]
- Deshpande S.S. & Cheryan M. (1984) Effect of phytic acid, divalent cations and their interactions on alpha amylase activity. J. Food Sci. 47, 2080–2081. [Google Scholar]
- Dilworth L.L., Omoruyi F.O., Simon O.R., Morrison E.Y.S.A. & Asemota H.N. (2005) The effect of phytic acid on the levels of blood glucose and some enzymes of carbohydrate and lipid metabolism. West Indian Med. J. 54, 102–106. [DOI] [PubMed] [Google Scholar]
- Dilworth L.L., Omoruyi F.O. & Asemota H.N., (2015) Effects of IP6 and sweet potato (Ipomoea batatas) phytate on serum, liver and faecal lipids in rats. Int. J. Food Sci. Nutr. Eng. 5, 53–58. [Google Scholar]
- Efanov A.M., Zaitsev S.V. & Berggren P.O. (1997) Inositol hexakisphosphate stimulates no Ca2+‐mediated and primes Ca2+‐mediated exocytosis of insulin by activation of protein kinase C. Proc. Natl Acad. Sci. USA 94, 4435–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi I.S., Keogh J.M., Kamath S. et al (2001) Partial leptin deficiency and human adiposity. Nature 414, 34–35. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M. & Sloane Stanley G.H. (1957) A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509. [PubMed] [Google Scholar]
- Fujikawa T., Chuang J.C., Sakata I., Ramadori G. & Coppari R. (2010) Leptin therapy improves insulin‐deficient type 1 diabetes by CNS‐dependent mechanisms in mice. Proc. Natl Acad. Sci. USA 107, 17391–17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German J.P., Thaler J.P., Wisse B.E. et al (2011) Leptin activates a novel CNS mechanism for insulin‐independent normalization of severe diabetic hyperglycemia. Endocrinology 152, 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H.N. (2000) Insulin resistance and cardiovascular disease. J. Clin. Invest. 106, 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried S. & Rosenberg B. (1973) Improved manual spectrophotometric procedure for determination of serum triglycerides. Clin. Chem. 1078, 1077–1078. [PubMed] [Google Scholar]
- Grases F., Simonet B.M., Vucenik I., Prieto R.M., Costa‐Bauzá A., March J.G. & Shamsuddin A.M. (2001) Absorption and excretion of orally administered inositol hexaphosphate (IP6 or phytate) in humans. BioFactors 15, 53–61. [DOI] [PubMed] [Google Scholar]
- Grases F., Simonet B.M., Vucenik I., Perellóa J., Prietoa R.M. & Shamsuddin A.M. (2002) Effects of exogenous inositol hexakisphosphate (InsP6) on the levels of InsP6 and of inositol trisphosphate (InsP3) in malignant cells, tissues and biological fluids. Life Sci. 71, 1535–1546. [DOI] [PubMed] [Google Scholar]
- Halaas J.L., Gajiwala K.S. & Maffei M. (1995) Weight‐reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546. [DOI] [PubMed] [Google Scholar]
- Høy M., Efanov A.M., Bertorello A.M. et al (2002) Inositol hexakisphosphate promotes dynamin I‐mediated endocytosis. Proc. Natl Acad. Sci. USA 99, 6773–6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høy M., Berggren P.O. & Gromada J. (2003) Involvement of protein kinase C‐epsilon in inositol hexakisphosphate‐induced exocytosis in mouse pancreatic beta‐cells. J. Biol. Chem. 278, 35168–35171. [DOI] [PubMed] [Google Scholar]
- Irvine R.F. (2005) Inositide evolution – towards turtle domination? J. Physiol. 566, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R.F. & Schell M.J. (2001) Back in the water: the return of the inositol phosphates. Nat. Rev. Mol. Cell Biol. 2, 327–338. [DOI] [PubMed] [Google Scholar]
- Jeon J.Y., Steadward R.D., Wheeler G.D., Bell G., McCargar L., Harber V. (2003) Intact sympathetic nervous system is required for leptin effects on resting metabolic rate in people with spinal cord injury. J. Clin. Endocrinol. Metab. 88, 402–407. [DOI] [PubMed] [Google Scholar]
- Jervis E.L. & Levin R.J. (1966) Anatomic adaptation of the alimentary tract of the rat to the hyperphagia of chronic alloxan‐diabetes. Nature 210, 139–145. [DOI] [PubMed] [Google Scholar]
- Jørgensen J.O., Vahl N., Dall R. & Christiansen J.S. (1998) Resting metabolic rate in healthy adults: relation to growth hormone status and leptin levels. Metabolism 47, 1134–1139. [DOI] [PubMed] [Google Scholar]
- Kim J.N., Han S.N. & Kim H.K. (2014) Phytic acid and myo‐inositol support adipocyte differentiation and improve insulin sensitivity in 3T3‐L1 cells. Nutr. Res. 34, 723–731. [DOI] [PubMed] [Google Scholar]
- Klok M.D., Jakobsdottir S. & Drent M.L. (2006) The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes. Rev. 8, 21–34. [DOI] [PubMed] [Google Scholar]
- Kuppusamy A., Muthusamy U., Thirumalaisamy S.A. et al (2011) In vitro (α‐glucosidase and α‐amylase inhibition) and in vivo antidiabetic property of phytic acid (IP6) in streptozotocin‐nicotinamide‐induced type 2 diabetes mellitus (NIDDM) in rats. J. Comp. Integr. Med. 8, 1553–3840. [DOI] [PubMed] [Google Scholar]
- Larsson O., Barker C.J. Sjöholm A. et al (1997) Inhibition of phosphatases and increased Ca2+ channel activity by inositol hexakisphosphate. Science 278, 471–474. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Park H.J., Chun H.K., Cho S.Y., Cho S.M. & Lillehoj H.S. (2006) Dietary phytic acid lowers the blood glucose level in diabetic KK mice. Nutr. Res. 26, 474–479. [Google Scholar]
- Liu N., Ru Y.J., Li F.D. & Cowieson A.J. (2008) Effect of diet containing phytate and phytase on the activity and messenger ribonucleic acid expression of carbohydrases and transporter in chickens. J. Anim. Sci. 86, 3432–3439. [DOI] [PubMed] [Google Scholar]
- Malarvizhi R., Sali V.K., Kumari M. & Vasanthi H.R. (2016) Adipogenesis in obesity is modulated by IP6 in peanuts through activation of the nuclear receptors (PPARs). J. Obes. Overweight 2, 103. [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F. & Turner R.C. (1985) Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. [DOI] [PubMed] [Google Scholar]
- Mughal M.A., Maheri W.M., Aamir K., Jan M. & Ali M. (1999) The effects of glibenclamide on serum lipids and lipoproteins in type II non‐insulin dependent diabetes mellitus. J. Pak. Med. Assoc. 49, 89–92. [PubMed] [Google Scholar]
- Myers M.G. Jr & Olson D.P. (2012) Central nervous system control of metabolism. Nature 491, 357–363. [DOI] [PubMed] [Google Scholar]
- O'Meara N.M., Shapiro E.T., Van Cauter E.C. & Polonsky K.S. (1990) Effect of glyburide on beta‐cell responsiveness to glucose in non‐insulin‐dependent diabetes mellitus. Am. J. Med. 89, S11–S16. [DOI] [PubMed] [Google Scholar]
- Omoruyi F.O., Budiaman A., Eng Y., Olumese F.E., Hoesel J.L., Ejilemele A. & Okorodudu A.O. (2013) The potential benefits and adverse effects of phytic acid supplement in streptozotocin‐induced diabetic rats. Adv. Pharmacol. Sci. 2013, Article ID 172494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanè G., Piro S., Anello M., Rabuazzo A.M., Vigneri R. & Purrello F. (2000) Exposure to glibenclamide increases rat beta‐cells sensitivity to glucose. Br. J. Pharmacol. 129, 887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival S.S. & Schneeman B.O. (1979) Long‐term pancreatic response to feeding heat damaged casein in rats. J. Food Sci. 43, 634–636. [DOI] [PubMed] [Google Scholar]
- Reed M.J., Meszaros K., Entes L.J., Claypool M.D., Pinkett J.G., Gadbois T.M. & Reaven G.M. (2000) A new rat model of type 2 diabetes: the fat‐fed, streptozotocin‐treated rat. Metab. Clin. Exp. 49, 1390–1394. [DOI] [PubMed] [Google Scholar]
- Sahu A. (2004) Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front. Neuroendocrinol. 24, 225–253. [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Vucenik I. & Shamsuddin A.M. (1993) [3H] phytic acid (inositol hexaphosphate) is absorbed and distributed to various tissues in rats. J. Nutr. 123, 713–720. [DOI] [PubMed] [Google Scholar]
- Schedl H.P. & Wilson H.D. (1971) Effects of diabetes on intestinal growth in the rat. J. Exp. Zool. 176, 487–495. [DOI] [PubMed] [Google Scholar]
- Schwartz M.W. (2001) Brain pathways controlling food intake and body weight. Exp. Biol. Med. 226, 978–981. [DOI] [PubMed] [Google Scholar]
- Schwartz M.W., Seeley R.J., Tschöp M.H., Woods S.C., Morton G.J., Myers M.G. & D'Alessio D. (2013) Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert J. (2004) Leptin effects on pancreatic beta‐cell gene expression and function. Diabetes 53, S152–S158. [DOI] [PubMed] [Google Scholar]
- Shamsuddin A.M. & Vucenik I. (1999) Mammary tumor inhibition by IP6: a review. Anticancer Res. 19, 3671–3674. [PubMed] [Google Scholar]
- Shamsuddin A.M., Elsayed A. & Ullah A. (1988) Suppression of large intestinal cancer in F344 rats by inositol hexaphosphate. Carcinogenesis 9, 577–580. [DOI] [PubMed] [Google Scholar]
- Shamsuddin A.M., Ullah A. & Chakravarthy A.K. (1989) Inositol and inositol hexaphosphate suppress cell proliferation and tumor formation in CD‐1 mice. Carcinogenesis 10, 1461–1463. [DOI] [PubMed] [Google Scholar]
- Shapiro E.T., Van Cauter E., Tillil H. et al (1989) Glyburide enhances the responsiveness of the β‐cell to glucose but does not correct the abnormal patterns of insulin secretion in noninsulin‐dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 69, 571–576. [DOI] [PubMed] [Google Scholar]
- Söderberg S., Zimmet P., Tuomilehto J., Chitson P., Gareeboom H., Alberti K.G. & Shaw J.E. (2007) Leptin predicts the development of diabetes in Mauritian men, but not women: a population‐based study. Int. J. Obes. (Lond) 31, 1126–1133. [DOI] [PubMed] [Google Scholar]
- Srinivasan K., Viswanad B., Asrat L., Kaul C.L. & Ramarao P. (2005) Combination of high‐fat diet‐fed and low‐dose streptozotocin‐treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 52, 313–320. [DOI] [PubMed] [Google Scholar]
- Stocks A.E., Ma A., Howlett V. & Cameron D.P. (1988) Lack of effect of glibenclamide on insulin requirements and diabetic control in persons with insulin‐dependent diabetes. Med. J. Aust. 149, 472–473. [DOI] [PubMed] [Google Scholar]
- Thompson L.U. (1988) Antinutrients and blood glucose. Food Technol. 42, 123–132. [Google Scholar]
- Toyoshima Y., Gavrilova O., Yakar S. et al (2005) Leptin improves insulin resistance and hyperglycemia in a mouse model of type 2 diabetes. Endocrinology 146, 4024–4035. [DOI] [PubMed] [Google Scholar]
- Ullah A. & Shamsuddin A.M. (1990) Dose‐dependent inhibition of large intestinal cancer by inositol hexaphosphate in F344 rats. Carcinogenesis 11, 2219–2222. [DOI] [PubMed] [Google Scholar]
- Vanderlinden N.D. & Vucenik I. (2004) Too Good to be True?. Mississauga, ON: Bearing Marketing Communications Ltd. [Google Scholar]
- Vucenik I. & Shamsuddin A.M. (1994) [3H]‐Inositol hexaphosphate (phytic acid) is rapidly absorbed and metabolized by murine and human malignant cells in vitro . J. Nutr. 124, 861–868. [DOI] [PubMed] [Google Scholar]
- Vucenik I. & Shamsuddin A.M. (2003) Cancer inhibition by inositol hexaphosphate (IP6) and inositol: from laboratory to clinic. J. Nutr. 133, 3778S–3784S. [DOI] [PubMed] [Google Scholar]
- Vucenik I. & Shamsuddin A.M. (2006) Protection against cancer by dietary IP6 and inositol. Nutr. Cancer 55, 109–125. [DOI] [PubMed] [Google Scholar]
- Wilson M.S.C., Bulley S.J., Pisani F., Irvine R.F. & Saiardi A. (2015) A novel method for the purification of inositol phosphates from biological samples reveals that no phytate is present in human plasma or urine. Open Biol. 5, 150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2014) Global Status Report on Noncommunicable Diseases. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Wotten I.D.P. (1964) Micro‐Analysis in Medical Biochemistry London: J and A Churchill, pp. 110–115. [Google Scholar]
- Yang S.N. & Berggren P.O. (2005) Beta‐cell CaV channel regulation in physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 288, E16–E28. [DOI] [PubMed] [Google Scholar]
- Yang S.N., Yu J., Mayr G.W., Hofmann F., Larsson O. & Berggren P.O. (2001) Inositol hexakisphosphate increases L‐type Ca2+ channel activity by stimulation of adenylyl cyclase. FASEB J. 15, 1753–1763. [DOI] [PubMed] [Google Scholar]
- Yaspelkis B.B., Davis J.R., Saberi M. et al (2001) Leptin administration improves skeletal muscle insulin responsiveness in diet induced insulin‐resistant rats. Am. J. Physiol. Endocrinol. Metab. 280, E130–E142. [DOI] [PubMed] [Google Scholar]
- Yu X., Park B.H., Wang M.Y., Wang Z.V. & Unger R.H. (2008) Making insulin‐deficient type 1 diabetic rodents thrive without insulin. Proc. Natl Acad. Sci. USA 105, 14070–14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Lv X.Y., Li J., Xu Z.G. & Chen L. (2008) The characterization of high‐fat diet and multiple low‐dose streptozotocin induced type 2 diabetes rat model. Exp. Diabetes Res. 2008, 704045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatkis A., Zak B. & Boyle A.J. (1953) A new method for the direct determination of serum cholesterol. J. Lab. Clin. Med. 41, 486–492. [PubMed] [Google Scholar]


