Abstract
Endoscopic ultrasound (EUS) plays an important role in imaging of the mediastinum and abdominal organs. Since the introduction of US contrast agents (UCA) for transabdominal US, attempts have been made to apply contrast-enhanced US techniques also to EUS. Since 2003, specific contrast-enhanced imaging was possible using EUS. Important studies have been published regarding contrast-enhanced EUS and the characterization of focal pancreatic lesions, lymph nodes, and subepithelial tumors. In this manuscript, we describe the relevant UCA, their application, and specific image acquisition as well as the principles of image tissue characterization using contrast-enhanced EUS. Safety issues, potential future developments, and EUS-specific issues are reviewed.
Keywords: Definity, endosonography, Levovist, Optison, SonoVue, ultrasound
INTRODUCTION
Endoscopic ultrasound (EUS) plays an important role in imaging of the mediastinum and abdominal organs including the esophagus, stomach, duodenum, rectum, pancreas, biliary tract, peritoneal cavity, and retroperitoneum.[1,2,3,4] It has proven value in lesion characterization and intervention guidance.[5,6,7,8,9,10,11]
Since the introduction of US contrast agents (UCA) for transabdominal US, attempts have been made to apply this technique to EUS but contrast-specific imaging was not technically possible using endoscopic probes. Therefore, UCA were displayed using conventional Doppler techniques, leading to adequate results in certain indications.[12,13,14,15] In 2005, Dietrich et al. published their experience with a prototype software used since 2003,[16] but it lasted until 2008 when specific contrast-enhanced EUS using harmonics imaging became commercially available. Nowadays, a solid body of research has been published concerning contrast-enhanced EUS in pancreatic lesions, lymph nodes, submucosal tumors of the gastrointestinal (GI) tract, and less common applications including the biliary tract, vascular indications, and characterization of epithelial tumors of the GI tract.
In this manuscript, we describe the composition of the UCA in general and refer to their approved indications. We also describe contrast-specific imaging and safety issues. Finally, we discuss reasons when and why contrast-enhanced EUS should be applied, including future perspectives.
ULTRASOUND CONTRAST AGENTS
Ultrasound contrast agents composition
The lifetime of air bubbles is short. In 1968, Gramiak and Shah reported observations of clouds of bubbles after intra-aortic catheter injection of saline.[17] Further investigations reported on UCA consisting of saline, indocyanine green, hydrogen peroxide, dextrose, and renografin.[18] Another approach was autologous blood injections at rapid rates which produced more stable bubbles.[19] Neither gelatin nor agarose gel proved to be useful to stabilize bubbles.[20] Synthetic polymers consisted of cyanoacrylate and air were marketed under the name of Sonovist® (Schering, Berlin, Germany). Those bubbles lasted more than 10 min and were taken up by the reticuloendothelial system.[21,22] Other tested materials included poly(D, L-lactide-co-glycolide)[23,24] and poly (vinyl-alcohol).[25]
One of the first goals in producing effective UCAs around 1980 was to obtain stability long enough to reach the right heart. Since lung capillaries are efficient filters, it was not until the 1990s when left heart contrast became possible. Contrast-enhancing agents with improved stability to effectively enhance the blood pool appeared in 1995. The next objective was to produce bubbles enabling real-time imaging. This goal was reached by replacing air with poorly soluble gases, e.g., perfluorocarbons, which improved bubble durability, along with sophisticated acoustic parameters enabling the development of software algorithms which could efficiently differentiate UCA from tissue signals.
All currently commercially available UCA consist of an inert gas encapsulated by a shell. The shell mainly influences the viscoelastic properties, i.e., stability and durability,[26] while the gas determines solubility and the majority of the bubbles’ acoustic properties. As true blood pool agents, perfluorocarbons bubbles range from 1 to 10 μm in size, permitting passage through the pulmonary vascular system, which is essential for access to the systemic circulation.[27] Soft shell materials consist of phospholipids or other surfactants and demonstrate improved nonlinear oscillations.[28] Protein-shelled microbubbles are also available (e.g., Optison®) consisting of an albumin shell around perfluoropropane gas.
The terms “ first and second generation UCA” are sometimes used to differentiate agents with air from those with low soluble gases. Although this overly simplifies the distinctions mentioned above, it is sufficient in clinical practice since development of second generation UCA leads to near complete disappearance of first generation agents due to the ease of use and effectiveness of the former.
Uptake of microbubbles by Kupffer cells has been described in the liver and by macrophages outside of the liver. This mechanism depends on shell composition, size, and surface properties and cannot be predicted simply by the shell material.[29] The mechanism is understood for Levovist®, Sonazoid®, and Optison®.[29] The extended late phase in Sonazoid® is termed the “post-vascular phase” where it may persist for several hours in the liver and spleen.[30,31]
So-called nanobubbles have inferior oscillation behavior relative to microbubbles but are of interest in therapeutic approaches; nanobubbles have a size of 400–800 nm and can extravasate into tumor tissue.[26] The accumulation of nanobubbles in tumors is referred to as passive targeting.[32]
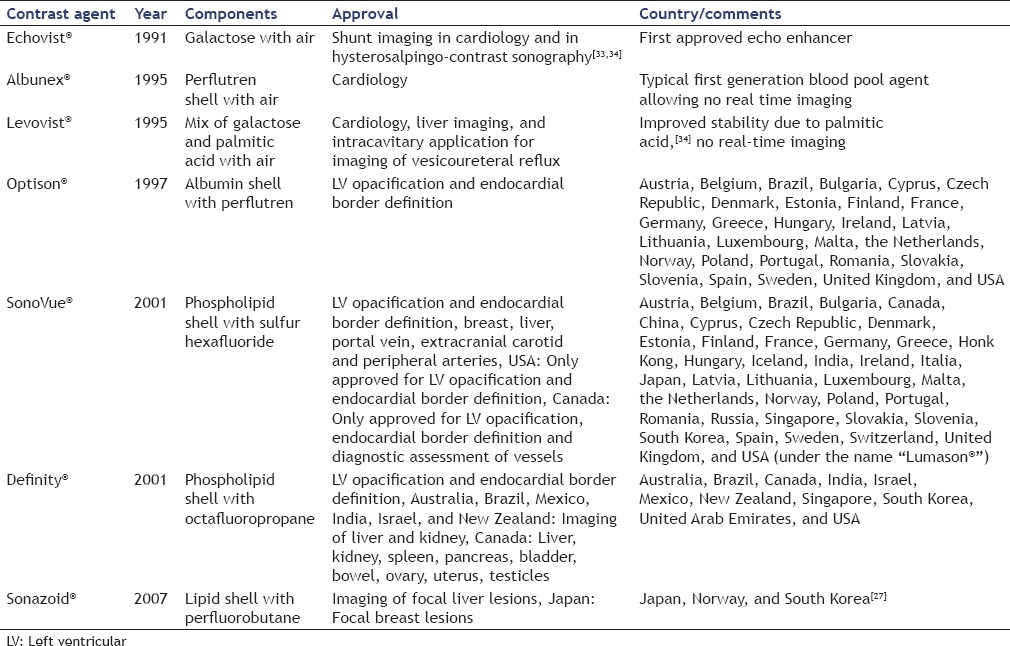
The relevant UCA are summarized in Table 1.
Table 1.
Relevant ultrasound contrast agents and their relevant features
ULTRASOUND CONTRAST AGENTS IMAGING
From the bubble to the ultrasound signal
UCA provide significant alterations in the reflection pattern. First, they increase the backscattered signal dramatically.[35] When acoustic pressure is applied, UCA resonate in a linear manner. With increasing acoustic pressure, nonlinear vibrational patterns appear.[27] Tissue produces harmonic resonances only at higher mechanical index (MI), thus differentiation of signal origin whether tissue or UCA is possible. Using filter systems, multiples of the natural frequencies are received, allowing a certain amount of background (non-UCA) signal suppression. High-pressure levels disrupt microbubbles producing powerful signals and signals of different qualities. Nonlinear patterns[27] are of importance and are described below.
Bolus injection versus infusion
In most indications, UCA are simply injected as a bolus. Intravenous infusion is of interest in cardiac imaging and other indications. Wash-in and wash-out kinetics are produced with controlled destruction using US pulses with high MI. Higher rates of adverse events such as premature ventricular contractions in patients with coronary diseases have been reported after continuous injection.
UCA have been also used in physiological and nonphysiological (extravascular) body cavities.[36] This is an off-label use except the application of Levovist® into the urinary bladder for evaluation of vesicoureteral reflux. Extravascular applications with SonoVue®, mentioned in the European Federation of Societies for US in Medicine and Biology guideline,[37] include injection of contrast for assessment of hysterosalpingo-contrast-sonography, ascites to evaluate hepatic hydrothorax, bile ducts via percutaneous transhepatic cholangiography and drainage,[38,39] endoscopic retrograde cholangiography or surgically placed T-tube, sialography, perianal fistula, abscesses,[40] pseudocysts, gastroesophageal reflux, Zenker's diverticulum,[41] enema, and nephrostomy tubes.[1,2,6,37]
How can we use ultrasound contrast agents imaging?
In cardiology, UCA are used to improve difficult echocardiograms. The frequency of difficult echocardiograms is given as approximately 30%. UCA imaging can improve these in a significant percentage.[42,43,44,45,46] Cardiologic guidelines recommend UCA use in the following cases:
If two contiguous segments of the left ventricular (LV) cavum are not observed
To improve Doppler evaluations if the initial spectrum signals are inadequate[27,47]
If serial assessment of ejection fraction is required since UCA decrease variability
If apical hypertrophic cardiomyopathy and noncompaction is suspected
In the case of intracavitary thrombi, LV aneurysms, Takotsubo myopathy.[48]
In most other indications, it is necessary to discriminate UCA from tissue signal as completely as possible.
Doppler-based methods
In high MI imaging, stimulated acoustic emission is used with color or power Doppler. The microbubbles are destroyed using a high MI US impulse and the received signal is a complex US wave mix resulting in a Doppler shift.[49] It is particularly useful in the late phase of a UCA with tissue specificity, e.g., Levovist®.[50] In EUS, color Doppler imaging has been used and it could be demonstrated that due to the small size of the image window real-time imaging was possible although spatial resolution was poor.[51]
Vascular recognition imaging represents a method in which advanced low MI contrast-specific imaging is mixed with a Doppler technique adding direction information in larger vessels.[52,53,54]
Low mechanical index imaging and contrast-specific imaging
The optimal contrast-enhanced imaging method should provide high-resolution real-time imaging over a long period with B-mode information side by side or as an overlay to the UCA signal. Current imaging methods come close to that aim. Drawbacks are a varying degree of bubble destruction and a low quality of the fundamental (tissue) image and reduced local contrast resolution. Low MI techniques have two effects: First, bubbles do not burst and second, they elicit harmonic US waves. Reduced MI can lead to problems in depth penetration.[55,56] For providing specific UCA imaging, initially, a low-pass filter was used to remove the fundamental waves. The next evolution generating higher spatial resolution was the use of phase inversion modes with which the complete bandwidth of the transducer can be utilized. Here, phase inverted pulses are sent simultaneously which results in information = 0 when echoes are linear, and summing of information ≠ 0 when nonlinear echoes, such as UCA echoes reflect.
Why do we use ultrasound contrast agents imaging?
The described methods lead to separately displayed tissue and contrast signals. In general, the following questions can be answered.
Vital versus avital
The easiest way to use UCA is to differentiate enhancement versus nonenhancement. Due to the blood pool character and the high specificity of the signal, this is possible with high reliability.
In some clinical questions, neoplastic lesions and avital structures must be differentiated:
Liver abscess, since pus frequently is not anechoic[40]
Intraductal papillary mucinous neoplasia with focal mucus accumulations which mimics neoplastic nodules[57]
Gallbladder stones without calcification which could be misdiagnosed as polyps
Cardiac lesions mimicking thrombus but which are in fact neoplasms (unpublished data).
Detection of nonvascularized areas provides important information in characterizing many lesions. For example, focal nodular hyperplasia shows a central scar in about 2/3 of patients[58] while GI stromal tumors typically show necrotic areas in contrast to lipoma, schwannoma, and leiomyoma.[59,60,61]
General enhancement
Typically, the enhancement intensity of a lesion is compared with the surrounding reference tissue. Typical pancreatic ductal adenocarcinomas show a markedly lower degree of UCA uptake compared to surrounding pancreatic tissue in more than 90% of cases. In comparison, other entities, e.g., neuroendocrine tumors, lymphoma, metastases, and the “pseudosolid” entity “serous microcystic adenoma,” typically show hyperenhancement. In daily practice, this discrimination from ductal adenocarcinoma is highly valuable due to the different approach in therapy and prognosis.[51,62] In liver cirrhosis, regenerative nodules typically show enhancement similar to the surrounding liver tissue whereas hepatocellular carcinoma are hypervascular and, therefore, hyperenhancing in more than 90% of cases.[31,63]
To differentiate atelectasis from lung neoplasia, the earliest appearance of UCA is significant. Early enhancement is indicative of atelectasis supplied by vasa communes, which lack the oxygen-rich blood demanded by neoplasms. Since vasa communes derive from the right ventricle, they enhance earlier than 7 s after injection while the vasa privata enhance later. This later enhancement pattern has a high probability for neoplasia but caution is required as infarcted lung can appear similarly.[64,65,66,67]
Early phase – late phase
When UCA are injected as a bolus, the wash-in and wash-out kinetics can be evaluated. Analysis of the liver late phase allows reliable differentiation between benign and malignant focal liver lesions due to the hepatic dual blood supply. Lesions without portal veins show a shorter contrast enhancement resulting in a relative hypoenhancement about 30 s after injection. Such focal liver lesions are mostly malignant.[55,58,63,68,69,70,71,72,73]
In the spleen, late phase hypoenhancement has a lower positive predictive value though for lesions with late phase enhancement similar to the surrounding tissue malignancy can virtually be ruled out. This principle is advantageous in daily routine when deciding which patients should be offered biopsy.[74,75,76]
Special patterns
Specific patterns are shown by some tumor entities, e.g., centrifugal pattern of hepatic focal nodular hyperplasia and peripheral nodular enhancement in hemangioma.[58,68,72,77]
Quantification
Enhancement kinetics are described elsewhere in this special issue of EUS.
ULTRASOUND CONTRAST AGENTS SAFETY
In October 2007, the Food and Drug Administration (FDA) issued a new product labeling for UCA due to four patient deaths and about 190 serious adverse events with unclear causation but association with UCA use.[78] A “black box” warning regarding multiple disease state contraindications to UCA use was mandated, including acute myocardial infarction, decompensated heart failure, ventricular arrhythmias, or patients with high risk for the latter, respiratory failure, emphysema, conditions that may cause pulmonary hypertension and so on. Critics claimed that there was no proof for more than temporal relation, and cited the higher rates of incidents associated with alternative procedures after acute cardiac events. In 2008, the FDA downgraded the contraindications to warnings. Safety studies in around 200,000 patients receiving Optison® and Definity® demonstrated a very low adverse event rate.[79,80,81] For Optison® and Definity®, there was an approximately 1:10,000 incidence of acute anaphylactoid reaction immediately after UCA injection.[82] In 2006, Piscaglia et al. published an analysis of 23,188 investigations using SonoVue® for abdominal indications. Twenty-eight Italian centers provided data with no fatal events recorded. There were 29 adverse events, of which 2 were serious, resulting in a rate of 0.0086%. Four adverse events required treatment.[83] SonoVue® has additionally been tested in patients with chronic obstructive pulmonary disease without relevant problems.[84]
FUTURE PERSPECTIVES
Therapeutic applications of UCA include targeted thrombolysis and substance delivery. Examples are inducing thrombus dissolution in acute ST elevation myocardial infarction and intracranial thrombolysis. This has led to the development of micro- and nano-bubbles. Another example is sonoporation-induced drug delivery in patients with pancreatic cancer. An interesting contrast development is silica shell particles, which are not actually bubbles, but be shown on high MI and could be useful as a therapeutic vehicle.[26]
Targeting
Passive targeting refers to the tendency of microbubbles to accumulate in malignant lesions due to leaky vasculature and lack of lymph vessels draining the tissue. Active targeting requires surface modification, and the target must be presented on the luminal side of endothelial cells due to the blood pool character of microbubbles. In vitro studies and animal studies have been reported for models in thrombosis detection, atherogenesis,[85,86,87] and transplant rejection.[88] The diagnosis of tumors has been successfully shown in animals.[89] Nevertheless, clinical studies have not been performed so far.
Therapeutic approaches
The concept of utilizing UCA to enhance the vibratory effects generated by US pulses has gained much attention. The research group around Tachibana demonstrated in 1995 that US thrombolysis is more effective in the presence of bubbles, which is explained by cavitation and other effects.[90] Molina et al.[91] demonstrated the rate of arterial recanalization in stroke patients to be higher in patients with additional microbubble injection compared to injection of tissue plasminogen activator alone. Petit et al.[92] found similar effects.
US triggered substance delivery is based on the principle of destroying contrast bubbles by applying high energy US, which additionally increases capillary and cell membrane permeability.[93]
Microbubbles may also be used for gene therapy, but even with specially designed UCA, their effectiveness remains inferior to viral transfection modalities. Nevertheless, side effects of viral transfection are relevant and local control of UCA-controlled gene therapy is better.[94,95] The principle of US-mediated drug release seems to be promising. Here, also sonoporation helps open the blood–brain barrier.[96] Anti-tumor drugs could be delivered using UCA, but studies on animals have not reached the point where clinical trials seem to be close.
ENDOSCOPIC ULTRASOUND APPLICATION
This manuscript provides not only an overview of UCA but also of the meaning of UCA in US imaging. UCA applications already play an important role in daily routine of many sonographers but remain novel to EUS specialists. Several manuscripts on the use of contrast-enhanced EUS have been published. The following issues have been covered: Characterization of solid and cystic pancreatic masses, GI subepithelial lesions, biliary tract diseases, lymph node, and tumor staging. The mentioned applications will be covered by the other manuscripts in this volume of EUS.
EUS is different to percutaneous US in several respects:
The patient is under sedation, thus time-consuming methods must be critically considered;
The benefit of contrast-enhanced EUS must be high enough to justify prolongation of sedation;
Currently available UCA, contrast imaging software, and transducer bandwidth are less optimal than the transcutaneous approach resulting in reduced depth penetration and near field bubble destruction;
Full doses of SonoVue® are typically necessary with the current equipment unlike percutaneous techniques where half or quarter dosages are generally used;
Breath-hold maneuvers are less practical.
EUS should be performed considering these factors. The investigator should try to optimize the native image as much as possible using machine adjustments and probe position. Contrast-enhanced EUS is unlikely to provide additional information when B-mode is insufficient. Short real-time imaging intervals should be interrupted using intermittent contrast-enhanced imaging to reduce bubble destruction to a minimum. The investigator should focus on a specific question during contrast-enhanced EUS (e.g., the early phase in pancreatic cancer, uptake or no uptake in solid nodules of cystic pancreas lesions, necrotic areas in subepithelial upper GI tract lesions, or late phase in the liver) and concentrate on the indication and specific question. The method is valuable, but the ease of use is less proven than in percutaneous studies. Comparative studies between Sonazoid® and SonoVue® have not been published to date.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jenssen C, Hocke M, Fusaroli P, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part IV – EUS-guided interventions: General aspects and EUS-guided sampling (Long Version) Ultraschall Med. 2016;37:E33–76. doi: 10.1055/s-0035-1553785. [DOI] [PubMed] [Google Scholar]
- 2.Fusaroli P, Jenssen C, Hocke M, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part V. Ultraschall Med. 2015 doi: 10.1055/s-0035-1553462. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Dietrich CF, Jenssen C. Evidence based endoscopic ultrasound. Z Gastroenterol. 2011;49:599–621. doi: 10.1055/s-0029-1246021. [DOI] [PubMed] [Google Scholar]
- 4.Dietrich CF, Hocke M, Jenssen C. Interventional endosonography. Ultraschall Med. 2011;32:8–22. doi: 10.1055/s-0029-1246017. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich CF, Lorentzen T, Appelbaum L, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part III – Abdominal treatment procedures (Long Version) Ultraschall Med. 2016;37:E1–32. doi: 10.1055/s-0035-1553917. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich CF, Lorentzen T, Appelbaum L, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part III – Abdominal treatment procedures (Short Version) Ultraschall Med. 2016;37:27–45. doi: 10.1055/s-0035-1553965. [DOI] [PubMed] [Google Scholar]
- 7.Lorentzen T, Nolsøe CP, Ewertsen C, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part I. General aspects (long Version) Ultraschall Med. 2015;36:E1–14. doi: 10.1055/s-0035-1553593. [DOI] [PubMed] [Google Scholar]
- 8.Lorentzen T, Nolsøe CP, Ewertsen C, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part I. General aspects (Short Version) Ultraschall Med. 2015;36:464–72. doi: 10.1055/s-0035-1553601. [DOI] [PubMed] [Google Scholar]
- 9.Sidhu PS, Brabrand K, Cantisani V, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part II. Ultraschall Med. 2015;36:E15–35. doi: 10.1055/s-0035-1554036. [DOI] [PubMed] [Google Scholar]
- 10.Jenssen C, Brkljacic B, Hocke M, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part VI – Ultrasound-guided vascular interventions. Ultraschall Med. 2015 doi: 10.1055/s-0035-1553450. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Dietrich CF, Lorentzen T, Sidhu PS, et al. An introduction to the EFSUMB guidelines on interventional ultrasound (INVUS) Ultraschall Med. 2015;36:460–3. doi: 10.1055/s-0035-1553462. [DOI] [PubMed] [Google Scholar]
- 12.Becker D, Strobel D, Bernatik T, et al. Echo-enhanced color-and power-Doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointest Endosc. 2001;53:784–9. doi: 10.1067/mge.2001.115007. [DOI] [PubMed] [Google Scholar]
- 13.Nomura N, Goto H, Niwa Y, et al. Usefulness of contrast-enhanced EUS in the diagnosis of upper GI tract diseases. Gastrointest Endosc. 1999;50:555–60. doi: 10.1016/s0016-5107(99)70083-0. [DOI] [PubMed] [Google Scholar]
- 14.Hirooka Y, Naitoh Y, Goto H, et al. Contrast-enhanced endoscopic ultrasonography in gallbladder diseases. Gastrointest Endosc. 1998;48:406–10. doi: 10.1016/s0016-5107(98)70012-4. [DOI] [PubMed] [Google Scholar]
- 15.Hirooka Y, Goto H, Ito A, et al. Contrast-enhanced endoscopic ultrasonography in pancreatic diseases: A preliminary study. Am J Gastroenterol. 1998;93:632–5. doi: 10.1111/j.1572-0241.1998.179_b.x. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich CF, Ignee A, Frey H. Contrast-enhanced endoscopic ultrasound with low mechanical index: A new technique. Z Gastroenterol. 2005;43:1219–23. doi: 10.1055/s-2005-858662. [DOI] [PubMed] [Google Scholar]
- 17.Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol. 1968;3:356–66. doi: 10.1097/00004424-196809000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Feinstein SB, Shah PM, Bing RJ, et al. Microbubble dynamics visualized in the intact capillary circulation. J Am Coll Cardiol. 1984;4:595–600. doi: 10.1016/s0735-1097(84)80107-2. [DOI] [PubMed] [Google Scholar]
- 19.Kremkau FW, Gramiak R, Carstensen EL, et al. Ultrasonic detection of cavitation at catheter tips. Am J Roentgenol Radium Ther Nucl Med. 1970;110:177–83. doi: 10.2214/ajr.110.1.177. [DOI] [PubMed] [Google Scholar]
- 20.D’Arrigo JS, Mano Y. Bubble production in agarose gels subjected to different decompression schedules. Undersea Biomed Res. 1979;6:93–8. [PubMed] [Google Scholar]
- 21.Forsberg F, Basude R, Liu JB, et al. Effect of filling gases on the backscatter from contrast microbubbles: Theory and in vivo measurements. Ultrasound Med Biol. 1999;25:1203–11. doi: 10.1016/s0301-5629(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 22.Fritzsch T, Schlief R. Future prospects for echo-enhancing agents. Clin Radiol. 1996;51(Suppl 1):56–8. [PubMed] [Google Scholar]
- 23.Cui W, Bei J, Wang S, et al. Preparation and evaluation of poly(L-lactide-co-glycolide) (PLGA) microbubbles as a contrast agent for myocardial contrast echocardiography. J Biomed Mater Res B Appl Biomater. 2005;73:171–8. doi: 10.1002/jbm.b.30189. [DOI] [PubMed] [Google Scholar]
- 24.Eisenbrey JR, Hsu J, Wheatley MA. Plasma sterilization of poly lactic acid ultrasound contrast agents: Surface modification and implications for drug delivery. Ultrasound Med Biol. 2009;35:1854–62. doi: 10.1016/j.ultrasmedbio.2009.06.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavalieri F, El Hamassi A, Chiessi E, et al. Stable polymeric microballoons as multifunctional device for biomedical uses: Synthesis and characterization. Langmuir. 2005;21:8758–64. doi: 10.1021/la050287j. [DOI] [PubMed] [Google Scholar]
- 26.Paefgen V, Doleschel D, Kiessling F. Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Front Pharmacol. 2015;6:197. doi: 10.3389/fphar.2015.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appis AW, Tracy MJ, Feinstein SB. Update on the safety and efficacy of commercial ultrasound contrast agents in cardiac applications. Echo Res Pract. 2015;2:R55–62. doi: 10.1530/ERP-15-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leong-Poi H, Song J, Rim SJ, et al. Influence of microbubble shell properties on ultrasound signal: Implications for low-power perfusion imaging. J Am Soc Echocardiogr. 2002;15(10 Pt 2):1269–76. doi: 10.1067/mje.2002.124516. [DOI] [PubMed] [Google Scholar]
- 29.Chen CC, Borden MA. The role of poly(ethylene glycol) brush architecture in complement activation on targeted microbubble surfaces. Biomaterials. 2011;32:6579–87. doi: 10.1016/j.biomaterials.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanagisawa K, Moriyasu F, Miyahara T, et al. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol. 2007;33:318–25. doi: 10.1016/j.ultrasmedbio.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver – Update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11–29. doi: 10.1055/s-0032-1325499. [DOI] [PubMed] [Google Scholar]
- 32.Yin T, Wang P, Zheng R, et al. Nanobubbles for enhanced ultrasound imaging of tumors. Int J Nanomedicine. 2012;7:895–904. doi: 10.2147/IJN.S28830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uzuner N, Horner S, Pichler G, et al. Right-to-left shunt assessed by contrast transcranial Doppler sonography: New insights. J Ultrasound Med. 2004;23:1475–82. doi: 10.7863/jum.2004.23.11.1475. [DOI] [PubMed] [Google Scholar]
- 34.Cosgrove D. Echo enhancers and ultrasound imaging. Eur J Radiol. 1997;26:64–76. doi: 10.1016/s0720-048x(96)01150-3. [DOI] [PubMed] [Google Scholar]
- 35.Calliada F, Campani R, Bottinelli O, et al. Ultrasound contrast agents: Basic principles. Eur J Radiol. 1998;27(Suppl 2):S157–60. doi: 10.1016/s0720-048x(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 36.Ignee A, Schuessler G, Cui XW, et al. Intracavitary contrast medium ultrasound-different applications, a review of the literature ad future prospects. Ultraschall Med. 2013;34:504–25. doi: 10.1055/s-0033-1335546. [DOI] [PubMed] [Google Scholar]
- 37.Piscaglia F, Nolsøe C, Dietrich CF, et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 38.Ignee A, Baum U, Schuessler G, et al. Contrast-enhanced ultrasound-guided percutaneous cholangiography and cholangiodrainage (CEUS-PTCD) Endoscopy. 2009;41:725–6. doi: 10.1055/s-0029-1214956. [DOI] [PubMed] [Google Scholar]
- 39.Ignee A, Cui X, Schuessler G, et al. Percutaneous transhepatic cholangiography and drainage using extravascular contrast enhanced ultrasound. Z Gastroenterol. 2015;53:385–90. doi: 10.1055/s-0034-1398796. [DOI] [PubMed] [Google Scholar]
- 40.Ignee A, Jenssen C, Cui XW, et al. Intracavitary contrast-enhanced ultrasound in abscess drainage – Feasibility and clinical value. Scand J Gastroenterol. 2016;51:41–7. doi: 10.3109/00365521.2015.1066423. [DOI] [PubMed] [Google Scholar]
- 41.Cui XW, Ignee A, Baum U, et al. Feasibility and usefulness of using swallow contrast-enhanced ultrasound to diagnose Zenker's diverticulum: Preliminary results. Ultrasound Med Biol. 2015;41:975–81. doi: 10.1016/j.ultrasmedbio.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Dolan MS, Riad K, El-Shafei A, et al. Effect of intravenous contrast for left ventricular opacification and border definition on sensitivity and specificity of dobutamine stress echocardiography compared with coronary angiography in technically difficult patients. Am Heart J. 2001;142:908–15. doi: 10.1067/mhj.2001.117608. [DOI] [PubMed] [Google Scholar]
- 43.Vlassak I, Rubin DN, Odabashian JA, et al. Contrast and harmonic imaging improves accuracy and efficiency of novice readers for dobutamine stress echocardiography. Echocardiography. 2002;19:483–8. doi: 10.1046/j.1540-8175.2002.00483.x. [DOI] [PubMed] [Google Scholar]
- 44.Cohen JL, Cheirif J, Segar DS, et al. Improved left ventricular endocardial border delineation and opacification with OPTISON (FS069), a new echocardiographic contrast agent. Results of a phase III Multicenter Trial. J Am Coll Cardiol. 1998;32:746–52. doi: 10.1016/s0735-1097(98)00311-8. [DOI] [PubMed] [Google Scholar]
- 45.Kitzman DW, Goldman ME, Gillam LD, et al. Efficacy and safety of the novel ultrasound contrast agent perflutren (definity) in patients with suboptimal baseline left ventricular echocardiographic images. Am J Cardiol. 2000;86:669–74. doi: 10.1016/s0002-9149(00)01050-x. [DOI] [PubMed] [Google Scholar]
- 46.Reilly JP, Tunick PA, Timmermans RJ, et al. Contrast echocardiography clarifies uninterpretable wall motion in intensive care unit patients. J Am Coll Cardiol. 2000;35:485–90. doi: 10.1016/s0735-1097(99)00558-6. [DOI] [PubMed] [Google Scholar]
- 47.Albrecht T, Cosgrove DO, Butler-Barnes J, et al. Comparison of bolus and infusion of the ultrasound contrast media levovist for color doppler ultrasound of renal arteries. Rofo. 2000;172:824–9. doi: 10.1055/s-2000-7892. [DOI] [PubMed] [Google Scholar]
- 48.Porter TR, Abdelmoneim S, Belcik JT, et al. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: A focused update from the American Society of Echocardiography. J Am Soc Echocardiogr. 2014;27:797–810. doi: 10.1016/j.echo.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Albrecht T, Blomley MJ, Heckemann RA, et al. Stimulated acoustic emissions with the ultrasound contrast medium levovist: A clinically useful contrast effect with liver-specific properties. Rofo. 2000;172:61–7. doi: 10.1055/s-2000-11101. [DOI] [PubMed] [Google Scholar]
- 50.Cosgrove D. Ultrasound contrast agents: An overview. Eur J Radiol. 2006;60:324–30. doi: 10.1016/j.ejrad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 51.Dietrich CF, Ignee A, Braden B, et al. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590–7.e1. doi: 10.1016/j.cgh.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 52.Lassau N, Chami L, Benatsou B, et al. Dynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumor perfusion: A new diagnostic tool to evaluate the early effects of antiangiogenic treatment. Eur Radiol. 2007;17(Suppl 6):F89–98. doi: 10.1007/s10406-007-0233-6. [DOI] [PubMed] [Google Scholar]
- 53.Lassau N, Chami L, Péronneau P. Current events about echography in 2006: Position of the ultrasound functional imaging for the early evaluation of targeted therapeutics. Bull Cancer. 2006;93:1207–11. [PubMed] [Google Scholar]
- 54.Lassau N, Lamuraglia M, Chami L, et al. Gastrointestinal stromal tumors treated with imatinib: Monitoring response with contrast-enhanced sonography. AJR Am J Roentgenol. 2006;187:1267–73. doi: 10.2214/AJR.05.1192. [DOI] [PubMed] [Google Scholar]
- 55.Dietrich CF, Ignee A, Greis C, et al. Artifacts and pitfalls in contrast-enhanced ultrasound of the liver. Ultraschall Med. 2014;35:108–25. doi: 10.1055/s-0033-1355872. [DOI] [PubMed] [Google Scholar]
- 56.Dietrich CF, Ignee A, Hocke M, et al. Pitfalls and artefacts using contrast enhanced ultrasound. Z Gastroenterol. 2011;49:350–6. doi: 10.1055/s-0029-1245851. [DOI] [PubMed] [Google Scholar]
- 57.Fusaroli P, Serrani M, De Giorgio R, et al. Contrast harmonic-endoscopic ultrasound is useful to identify neoplastic features of pancreatic cysts (With Videos) Pancreas. 2016;45:265–8. doi: 10.1097/MPA.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 58.Dietrich CF, Schuessler G, Trojan J, et al. Differentiation of focal nodular hyperplasia and hepatocellular adenoma by contrast-enhanced ultrasound. Br J Radiol. 2005;78:704–7. doi: 10.1259/bjr/88181612. [DOI] [PubMed] [Google Scholar]
- 59.Hocke M, Ignee A, Dietrich CF. Advanced endosonographic diagnostic tools for discrimination of focal chronic pancreatitis and pancreatic carcinoma – Elastography, contrast enhanced high mechanical index (CEHMI) and low mechanical index (CELMI) endosonography in direct comparison. Z Gastroenterol. 2012;50:199–203. doi: 10.1055/s-0031-1281824. [DOI] [PubMed] [Google Scholar]
- 60.Jenssen C, Barreiros AP, Will U, et al. German survey on EUS-guided diagnosis and management of gastrointestinal stromal tumors (GISTs) – Evidence or “gut-feeling”? Ultraschall Med. 2015;36:494–500. doi: 10.1055/s-0034-1398970. [DOI] [PubMed] [Google Scholar]
- 61.Jenssen C, Dietrich CF. Endoscopic ultrasound of gastrointestinal subepithelial lesions. Ultraschall Med. 2008;29:236–56. doi: 10.1055/s-2008-1027388. [DOI] [PubMed] [Google Scholar]
- 62.Dietrich CF, Jenssen C, Allescher HD, et al. Differential diagnosis of pancreatic lesions using endoscopic ultrasound. Z Gastroenterol. 2008;46:601–17. doi: 10.1055/s-2008-1027523. [DOI] [PubMed] [Google Scholar]
- 63.Ignee A, Weiper D, Schuessler G, et al. Sonographic characterisation of hepatocellular carcinoma at time of diagnosis. Z Gastroenterol. 2005;43:289–94. doi: 10.1055/s-2004-813815. [DOI] [PubMed] [Google Scholar]
- 64.Görg C, Bert T, Kring R, et al. Transcutaneous contrast enhanced sonography of the chest for evaluation of pleural based pulmonary lesions: Experience in 137 patients. Ultraschall Med. 2006;27:437–44. doi: 10.1055/s-2006-927021. [DOI] [PubMed] [Google Scholar]
- 65.Caremani M, Benci A, Lapini L, et al. Contrast enhanced ultrasonography (CEUS) in peripheral lung lesions: A study of 60 cases. J Ultrasound. 2008;11:89–96. doi: 10.1016/j.jus.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Linde HN, Holland A, Greene BH, et al. Contrast-enhancend sonography (CEUS) in pneumonia: Typical patterns and clinical value – A retrospective study on n=50 patients. Ultraschall Med. 2012;33:146–51. doi: 10.1055/s-0031-1273280. [DOI] [PubMed] [Google Scholar]
- 67.Sartori S, Postorivo S, Vece FD, et al. Contrast-enhanced ultrasonography in peripheral lung consolidations: What's its actual role? World J Radiol. 2013;5:372–80. doi: 10.4329/wjr.v5.i10.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strobel D, Seitz K, Blank W, et al. Contrast-enhanced ultrasound for the characterization of focal liver lesions – Diagnostic accuracy in clinical practice (DEGUM multicenter trial) Ultraschall Med. 2008;29:499–505. doi: 10.1055/s-2008-1027806. [DOI] [PubMed] [Google Scholar]
- 69.Schuessler G, Ignee A, Hirche T, et al. Improved detection and characterisation of liver tumors with echo-enhanced ultrasound. Z Gastroenterol. 2003;41:1167–76. doi: 10.1055/s-2003-45274. [DOI] [PubMed] [Google Scholar]
- 70.Ignee A, Piscaglia F, Ott M, et al. A benign tumour of the liver mimicking malignant liver disease – Cholangiocellular adenoma. Scand J Gastroenterol. 2009;44:633–6. doi: 10.1080/00365520802538229. [DOI] [PubMed] [Google Scholar]
- 71.Dietrich CF, Schreiber-Dietrich D, Schuessler G, et al. Contrast enhanced ultrasound of the liver – State of the art. Dtsch Med Wochenschr. 2007;132:1225–31. doi: 10.1055/s-2007-979403. [DOI] [PubMed] [Google Scholar]
- 72.Dietrich CF, Mertens JC, Braden B, et al. Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology. 2007;45:1139–45. doi: 10.1002/hep.21615. [DOI] [PubMed] [Google Scholar]
- 73.Dietrich CF, Maddalena ME, Cui XW, et al. Liver tumor characterization – Review of the literature. Ultraschall Med. 2012;33(Suppl 1):S3–10. doi: 10.1055/s-0032-1312897. [DOI] [PubMed] [Google Scholar]
- 74.Cui XW, Ignee A, De Molo C, et al. Littoral cell angioma of the spleen. Z Gastroenterol. 2013;51:209–12. doi: 10.1055/s-0032-1325556. [DOI] [PubMed] [Google Scholar]
- 75.von Herbay A, Barreiros AP, Ignee A, et al. Contrast-enhanced ultrasonography with SonoVue: Differentiation between benign and malignant lesions of the spleen. J Ultrasound Med. 2009;28:421–34. doi: 10.7863/jum.2009.28.4.421. [DOI] [PubMed] [Google Scholar]
- 76.Ignee A, Cui X, Hirche T, et al. Percutaneous biopsies of splenic lesions – A clinical and contrast enhanced ultrasound based algorithm. Clin Hemorheol Microcirc. 2014;58:529–41.t. doi: 10.3233/CH-141813. [DOI] [PubMed] [Google Scholar]
- 77.Dietrich CF, Ignee A, Trojan J, et al. Improved characterisation of histologically proven liver tumours by contrast enhanced ultrasonography during the portal venous and specific late phase of SHU 508A. Gut. 2004;53:401–5. doi: 10.1136/gut.2003.026260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Main ML. Ultrasound contrast agent safety: From anecdote to evidence. JACC Cardiovasc Imaging. 2009;2:1057–9. doi: 10.1016/j.jcmg.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 79.Dolan MS, Gala SS, Dodla S, et al. Safety and efficacy of commercially available ultrasound contrast agents for rest and stress echocardiography a multicenter experience. J Am Coll Cardiol. 2009;53:32–8. doi: 10.1016/j.jacc.2008.08.066. [DOI] [PubMed] [Google Scholar]
- 80.Aggeli C, Giannopoulos G, Roussakis G, et al. Safety of myocardial flash-contrast echocardiography in combination with dobutamine stress testing for the detection of ischaemia in 5250 studies. Heart. 2008;94:1571–7. doi: 10.1136/hrt.2007.135145. [DOI] [PubMed] [Google Scholar]
- 81.Main ML, Ryan AC, Davis TE, et al. Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent (multicenter registry results in 4,300,966 consecutive patients) Am J Cardiol. 2008;102:1742–6. doi: 10.1016/j.amjcard.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 82.Szebeni J. Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology. 2005;216:106–21. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 83.Piscaglia F, Bolondi L. Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents. The safety of Sonovue in abdominal applications: Retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–75. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 84.Bokor D, Chambers JB, Rees PJ, et al. Clinical safety of SonoVue, a new contrast agent for ultrasound imaging, in healthy volunteers and in patients with chronic obstructive pulmonary disease. Invest Radiol. 2001;36:104–9. doi: 10.1097/00004424-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Villanueva FS, Jankowski RJ, Klibanov S, et al. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation. 1998;98:1–5. doi: 10.1161/01.cir.98.1.1. [DOI] [PubMed] [Google Scholar]
- 86.Unger E, Metzger P, 3rd, Krupinski E, et al. The use of a thrombus-specific ultrasound contrast agent to detect thrombus in arteriovenous fistulae. Invest Radiol. 2000;35:86–9. doi: 10.1097/00004424-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 87.Unger EC, McCreery TP, Sweitzer RH, et al. Acoustically active lipospheres containing paclitaxel: A new therapeutic ultrasound contrast agent. Invest Radiol. 1998;33:886–92. doi: 10.1097/00004424-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 88.Weller GE, Lu E, Csikari MM, et al. Ultrasound imaging of acute cardiac transplant rejection with microbubbles targeted to intercellular adhesion molecule-1. Circulation. 2003;108:218–24. doi: 10.1161/01.CIR.0000080287.74762.60. [DOI] [PubMed] [Google Scholar]
- 89.Fokong S, Fragoso A, Rix A, et al. Ultrasound molecular imaging of E-selectin in tumor vessels using poly n-butyl cyanoacrylate microbubbles covalently coupled to a short targeting peptide. Invest Radiol. 2013;48:843–50. doi: 10.1097/RLI.0b013e31829d03ec. [DOI] [PubMed] [Google Scholar]
- 90.Tachibana K, Tachibana S. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation. 1995;92:1148–50. doi: 10.1161/01.cir.92.5.1148. [DOI] [PubMed] [Google Scholar]
- 91.Molina CA, Ribo M, Rubiera M, et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–9. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 92.Petit B, Bohren Y, Gaud E, et al. Sonothrombolysis: The contribution of stable and inertial cavitation to clot lysis. Ultrasound Med Biol. 2015;41:1402–10. doi: 10.1016/j.ultrasmedbio.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 93.Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1153–66. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dewitte H, Van Lint S, Heirman C, et al. The potential of antigen and TriMix sonoporation using mRNA-loaded microbubbles for ultrasound-triggered cancer immunotherapy. J Control Release. 2014;194:28–36. doi: 10.1016/j.jconrel.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 95.Sun RR, Noble ML, Sun SS, et al. Development of therapeutic microbubbles for enhancing ultrasound-mediated gene delivery. J Control Release. 2014;182:111–20. doi: 10.1016/j.jconrel.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lammers T, Koczera P, Fokong S, et al. Theranostic USPIO-loaded microbubbles for mediating and monitoring blood-brain barrier permeation. Adv Funct Mater. 2015;25:36–43. doi: 10.1002/adfm.201401199. [DOI] [PMC free article] [PubMed] [Google Scholar]


