Abstract
With the widespread use of endoscopy, gastrointestinal submucosal lesions are now more commonly discovered. Although endoscopic ultrasound (EUS) is superior to all other imaging techniques for the diagnosis of submucosal tumors (SMTs), it is still suboptimal for differentiating hypoechoic lesions arising from the fourth sonographic gastrointestinal wall layer, which encompass tumors with very different prognosis. EUS tissue acquisition has provided with the unique opportunity to obtain histological confirmation, but it is not accurate enough to evaluate the malignant potential of gastrointestinal stromal tumors (GISTs). In the last years, contrast-enhanced harmonic EUS (CH-EUS) emerged as a powerful imaging modality to assess the microperfusion patterns of pancreatic tumors. Based on the distinct microvascularity of malignant SMTs, it was hypothesized that CH-EUS might also assist in the differential diagnosis of SMTs. Preliminary experience in this field is now available and suggests CH-EUS as a performant modality to distinguish between benign SMTs and GISTs and to evaluate the malignant potential of GISTs. High expectations are also relied on CH-EUS for the monitoring of antiangiogenic treatments of GISTs and the evaluation of gastrointestinal neuroendocrine tumors (NETs).
Keywords: Contrast, endoscopic ultrasound, gastrointestinal stromal tumor, leiomyoma, neuroendocrine tumor, submucosal, tumors
INTRODUCTION
Submucosal tumors (SMTs) are most often fortuitously discovered during routine endoscopy.[1] In asymptomatic patients, management depends on the nature and location of the lesion. While esophageal SMTs are primarily benign tumors (leiomyomas, cysts, etc.), gastric SMTs include not only benign tumors, such as leiomyomas and lipomas, but also tumors with malignant potential primarily gastrointestinal stromal tumors (GISTs). Therefore, it is crucial to characterize these tumors.
Some endoscopic features, such as location and ulceration, may suggest a malignant nature but none of these features are specific.[2] Mucosal biopsies are usually insufficient for providing adequate tissue specimens. Endoscopic ultrasonography (EUS) is capable of distinguishing true SMTs from extramural compressions with 90% accuracy. EUS also provides other useful information, i.e., the wall layer from which the tumor arises, the size of the lesion, and the echogenicity.[3] However, the performance of EUS is suboptimal for differentiating hypoechoic lesions arising from the fourth sonographic gastrointestinal wall layer. These lesions encompass tumors with very different prognoses (e.g., leiomyomas, schwannomas, GISTs, etc.).[4] Tissue acquisition is frequently proposed in these cases, but the average diagnostic accuracy of EUS-guided fine-needle aspiration (EUS-FNA) of SMTs ranges from 60% to 80%.[5] EUS-guided fine-needle biopsy (EUS-FNB) is not superior to EUS-FNA, but the combination of techniques improves the diagnostic yield.[6,7] However, the malignant potential of GISTs, which is primarily based on the mitotic index, cannot be reliably assessed with specimens obtained via EUS-guided techniques.[7]
Transabdominal contrast-enhanced ultrasound (CEUS) combines harmonic imaging techniques with signal processing and second-generation ultrasound contrast agents. CEUS is a new and radiation-free technique that has been proven to substantially influence the differential diagnoses of tumors of the liver and kidney by depicting microperfusion. The capability of CEUS is limited for gastrointestinal wall exploration due to abdominal gas and fat. Contrast-enhanced harmonic EUS (CH-EUS) overcomes these drawbacks. Examinations of SMTs with CH-EUS follow the same steps as in other indications.[8] Preliminary experience with CH-EUS is now available.[9,10,11,12]
DIFFERENTIAL DIAGNOSES OF BENIGN SUBMUCOSAL TUMORS AND GASTROINTESTINAL STROMAL TUMORS
Enhancement patterns of gastrointestinal SMTs were first described with CEUS.[13] GISTs and gastrointestinal neuroendocrine tumors (NETs) were the most studied lesions. GISTs exhibit a slow but strong enhancement beginning at the periphery with central avascular areas.[13] More than 90% of NETs are hyper-enhanced compared with the surrounding tissue and exhibit early arterial influx (<20 s) of microbubbles, rim-like contrast enhancement with a well-defined margin of the lesion at the capillary phase and a rapid decrease in enhancement (80%) within 60 s.[14] In the largest GISTs and NETs larger than 3 cm with proliferation indices >2%, unenhanced necrotic areas appear more frequently than in their smaller counterparts.
Two recent studies described the characteristics of SMTs that were enhanced by CH-EUS with SonoVue injection. Kannengiesser et al. performed CH-EUS of 17 gastric and esophageal SMTs, and the perfusion patterns were correlated with the cytological and histological results of EUS-FNA and the surgical specimens.[9] Eight lesions were hyper-enhanced and were histologically identified as GISTs. Nine SMTs were hypo-enhanced and corresponded to four lipomas and five leiomyomas. The time-intensity curve revealed that the echo intensity of the GISTs was significantly greater than that of the other SMTs. No significant differences were found between the lipomas and leiomyomas. The contrast agent arrival and peak times had no diagnostic value. The sensitivity and specificity for the correct discrimination between GISTs and benign SMTs were both 100%.
In the second study by Fusaroli et al., 51 SMTs were explored via CH-EUS, and the final pathological diagnoses yielded 19 GISTs, 8 NETs, 18 leiomyomas, 4 lipomas, and 2 ectopic pancreases.[10] The GISTs and NETs were almost always hyper-enhanced (95% and 87%, respectively; Figures 1 and 2) whereas 78% of the leiomyomas [Figure 3] and all of the lipomas were hypo-enhanced. A significant difference between the contrast uptake of the GISTs and leiomyomas was observed (P = 0.0007). Only a few of the SMTs were heterogeneously enhanced (some GISTs and leiomyomas). The washout of the contrast agent was predominantly slow in the GISTs and NETs and fast in the majority of the lipomas and leiomyomas.
Figure 1.
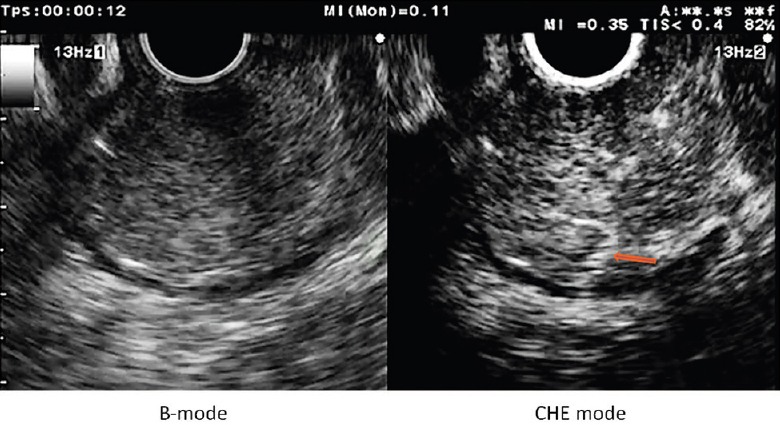
Gastric low grade dysplasia GIST, showing an homogeneous enhancement and thin regular intratumoral vessels (arrow)
Figure 2.
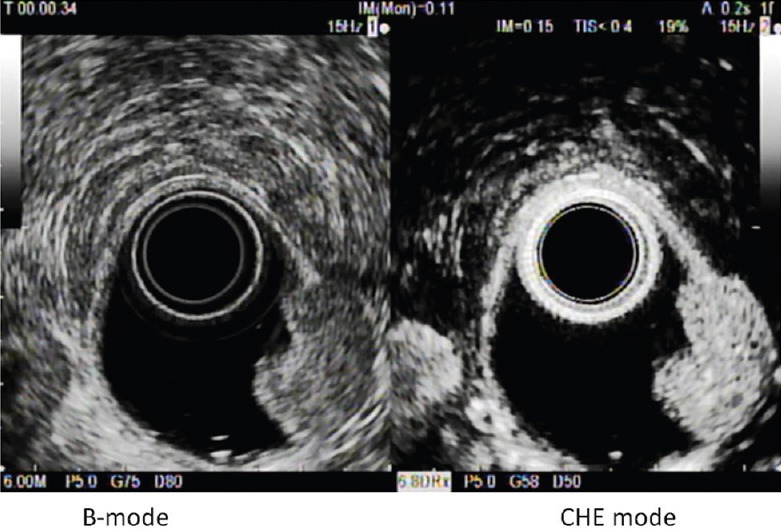
G1 duodenal NET showing an homogeneous hyper-enhancement
Figure 3.
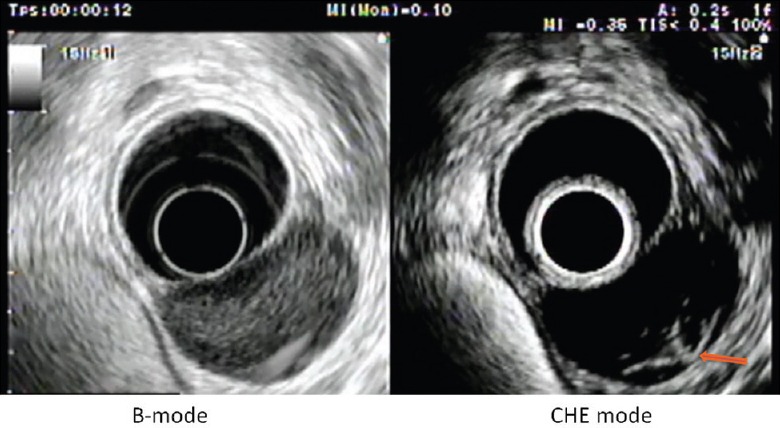
Cardial leiomyoma showing an hypo-enhancement, with rare regular and fine vessels arising from a vascular pedicle (arrow)
ASSESSMENT OF THE MALIGNANT POTENTIAL OF THE GASTROINTESTINAL STROMAL TUMORS
Predicting the prognosis of GISTs remains challenging. Current prognostic factors that are used to estimate the malignancy potential of nonmetastatic GISTs include tumor size, location, and the mitotic index. Nevertheless, metastases occasionally occur in tumors smaller than 50 mm and those with a low mitotic index. Recently, high intratumoral microvessel density and high levels of the expression of vascular endothelial growth factor (VEGF), which is considered to be the main mediator of tumor angiogenesis, have been proven to correlate with poor prognosis for this tumor type.[15] EUS provides accurate information about tumor size, but pathological specimens obtained by EUS-FNA or EUS-FNB are usually insufficient for evaluating the mitotic count.[6] Imaging of microvascularization may be a complementary technique for appreciating the prognosis of these tumors. CEUS and CH-EUS have been evaluated in three series that sought to determine the malignancy potential of GISTs.[11,12,16]
Fukuta et al.[16] compared the CEUS vascular patterns of 13 GISTs. The lesions were classified as malignant based on the histological diagnosis of the surgical specimens, tumor size, and the presence of metastases or invasion of other sites. Two types of enhancement were noticed, i.e., the “poor pattern,” which represented vessels that were only in the periphery of the tumor, and the “rich pattern,” which represented abundant vessels flowing from the periphery to the central part of the tumor. The vessel count of the histological specimens that were stained with factor VIII was significantly higher in the GISTs with rich pattern than in the GISTs with poor pattern (P < 0.01). According to the final diagnoses, a rich pattern in the CEUS was noticed in the 7 malignant GISTs and in one of the 6 benign GISTs. On the other hand, histology based on the surgical specimens resulted in the classification of only 4 tumors as malignant. Three histologically benign GISTs were found to be malignant due to the presence of metastases. The authors concluded that CEUS imaging is more closely correlated with the final diagnosis than the histological findings.
Sakamoto et al. evaluated the yield of CH-EUS, CE multidetector computed tomography (CT), power Doppler EUS (PD-EUS), and EUS-FNA compared with the pathology of resected specimen in 29 patients.[11] The tumor size ranged from 1 to 12 cm. Based on the mitotic index, 16 cases were classified as high-grade malignancies and 13 were classified as low-grade malignancies. Two different CH-EUS image patterns were defined, i.e. type I with regular vessels and homogeneous enhancement [Figure 1] and type II with irregular vessels and heterogeneous enhancement [Figure 4]. All of the type I tumors and 5 of the 21 type II tumors exhibited low-grade malignancy. The type II pattern determined high-grade malignancy with 100% sensitivity whereas the CE multidetector CT, PD-EUS, and EUS-FNA sensitivities were 31%, 63%, and 63%, respectively. Similar results were obtained even in the smaller tumors. In the series of Yamashita et al., 13 patients with GISTs underwent presurgical CH-EUS.[12] The analysis of the intratumoral vessels on the CH-EUS was compared with the histological degrees of angiogenesis and the VEGF expressions of the surgical specimens. On pathological examination, all of the intermediate high-risk GISTs expressed high levels of VEGF whereas all of the low-risk GISTs were VEGF-negative. Moreover, the vessels were larger than 500 μm in diameter and lacked of elastic fibers only in the intermediate high-risk GISTs. Upon CH-EUS, all of the tumors without intratumoral vessels were found to exhibit low-grade malignancy. Five of the 6 tumors with intratumoral vessels were classified as intermediate high-risk. A statistically significant association was observed between the presence of intratumoral vessels and an increased risk of malignancy (P < 0.005). These results confirmed that vascularity may be a predictor of malignancy in GISTs and that CH-EUS has the potential to analyze these criteria even if in some cases, it overvalues the malignancy potential.
Figure 4.
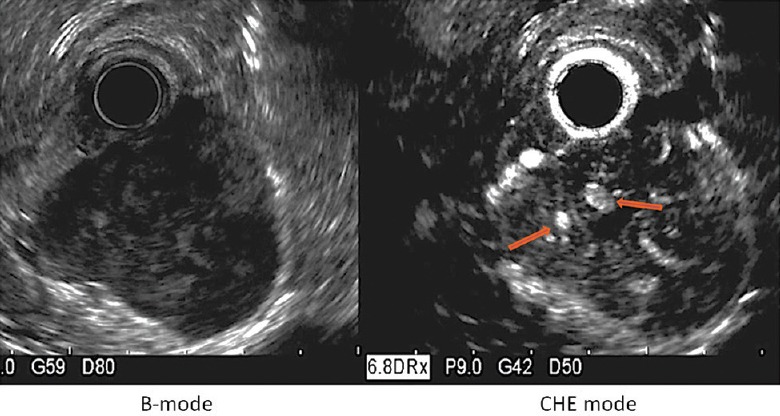
Gastric high grade malignancy GIST, showing an inhomogeneous enhancement large irregular intratumoral vessels (arrows)
FUTURE APPLICATIONS
Predicting responses to antiangiogenic treatments of gastrointestinal stromal tumors
No study has been performed with CH-EUS to assess the early response of GISTs subjected to targeted therapy with antiangiogenic agents. Nevertheless, many series have proven the value of CEUS for this purpose. These studies have led to the inclusion of CEUS in the European and International Guidelines for monitoring antiangiogenic treatments and the European Society for Medical Oncology Guidelines for GIST management.[17,18] Based on the preliminary results of CH-EUS in the assessment of the malignancy potential of GISTs, this field should be further explored.
Assessment of the malignancy potential and monitoring of the therapeutic response of neuroendocrine tumors
NETs typically possess well-developed capillary networks. In contrast to GISTs, the NETs with the lowest malignancy potential are the most vascularized ones and express the highest levels of VEGF.[19] The role of Doppler CE-EUS, which uses power or color Doppler but not dedicated contrast harmonic, has only been evaluated in pancreatic NETs.[20] The results were very promising; the sensitivity, specificity, and accuracy of CE-EUS for the diagnosis of malignant NETs are 91%, 90%, and 90%, respectively. Another study has evaluated the CEUS variations in perfusion parameters for some liver metastatic NETs that were treated with targeted therapies. Early changes at day 30 were correlated with tumor responses at 6 months.[21] Because the biologic properties of NETs are the same in all of the abdominal locations,[20] studies testing the performance of CH-EUS in the assessment of the malignancy potential of NETs and the monitoring of therapeutic response should be performed in the near future.
CONCLUSION
The preliminary results indicate that CH-EUS can successfully visualize the microvascularity of SMTs. This ability may aid in the characterization of submucosal lesions and the prediction of the malignancy potential of GISTs. Further studies are required to obtain more objective and comparable criteria. Future research will determine the potential of CH-EUS for the monitoring of targeted treatments for GISTs and the evaluation of gastrointestinal NETs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20–3. doi: 10.1007/BF00591381. [DOI] [PubMed] [Google Scholar]
- 2.Nishida T, Kawai N, Yamaguchi S, et al. Submucosal tumors: Comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013;25:479–89. doi: 10.1111/den.12149. [DOI] [PubMed] [Google Scholar]
- 3.Hwang JH, Saunders MD, Rulyak SJ, et al. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202–8. doi: 10.1016/s0016-5107(05)01567-1. [DOI] [PubMed] [Google Scholar]
- 4.Kim GH, Park DY, Kim S, et al. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J Gastroenterol. 2009;15:3376–81. doi: 10.3748/wjg.15.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913–9. doi: 10.1016/j.gie.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Fernández-Esparrach G, Sendino O, Solé M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: A randomized crossover study. Endoscopy. 2010;42:292–9. doi: 10.1055/s-0029-1244074. [DOI] [PubMed] [Google Scholar]
- 7.Polkowski M, Gerke W, Jarosz D, et al. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: A prospective study. Endoscopy. 2009;41:329–34. doi: 10.1055/s-0029-1214447. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Sánchez MV, Napoléon B. Contrast-enhanced harmonic endoscopic ultrasound imaging: Basic principles, present situation and future perspectives. World J Gastroenterol. 2014;20:15549–63. doi: 10.3748/wjg.v20.i42.15549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannengiesser K, Mahlke R, Petersen F, et al. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand J Gastroenterol. 2012;47:1515–20. doi: 10.3109/00365521.2012.729082. [DOI] [PubMed] [Google Scholar]
- 10.Fusaroli P, D’Ercole MC, Geroni L, et al. Contrast enhanced-endoscopic ultrasonography in the differential diagnosis of gastrointestinal submucosal tumors. Gastrointest Endosc. 2013;77(5 Suppl):AB398. [Google Scholar]
- 11.Sakamoto H, Kitano M, Matsui S, et al. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos) Gastrointest Endosc. 2011;73:227–37. doi: 10.1016/j.gie.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita Y, Kato J, Ueda K, et al. Contrast-enhanced endoscopic ultrasonography can predict a higher malignant potential of gastrointestinal stromal tumors by visualizing large newly formed vessels. J Clin Ultrasound. 2015;43:89–97. doi: 10.1002/jcu.22195. [DOI] [PubMed] [Google Scholar]
- 13.Ignee A, Schuessler G, Dietrich CF. Gastrointestinal stromal tumours of the upper gastrointestinal tract – Imaging with contrast enhanced ultrasound. Ultraschall Med. 2007;28:V_5_15. [Google Scholar]
- 14.Dörffel Y, Wermke W. Neuroendocrine tumors: Characterization with contrast-enhanced ultrasonography. Ultraschall Med. 2008;29:506–14. doi: 10.1055/s-2008-1027555. [DOI] [PubMed] [Google Scholar]
- 15.Basilio-de-Oliveira RP, Pannain VL. Prognostic angiogenic markers (endoglin, VEGF, CD31) and tumor cell proliferation (Ki67) for gastrointestinal stromal tumors. World J Gastroenterol. 2015;21:6924–30. doi: 10.3748/wjg.v21.i22.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuta N, Kitano M, Maekawa K, et al. Estimation of the malignant potential of gastrointestinal stromal tumors: The value of contrast-enhanced coded phase-inversion harmonics US. J Gastroenterol. 2005;40:247–55. doi: 10.1007/s00535-004-1531-6. [DOI] [PubMed] [Google Scholar]
- 17.Piscaglia F, Nolsøe C, Dietrich CF, et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 18.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver - Update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Marion-Audibert AM, Barel C, Gouysse G, et al. Low microvessel density is an unfavorable histoprognostic factor in pancreatic endocrine tumors. Gastroenterology. 2003;125:1094–104. doi: 10.1016/s0016-5085(03)01198-3. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa T, Itoh A, Kawashima H, et al. Usefulness of EUS combined with contrast-enhancement in the differential diagnosis of malignant versus benign and preoperative localization of pancreatic endocrine tumors. Gastrointest Endosc. 2010;71:951–9. doi: 10.1016/j.gie.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 21.Lassau N, Vilgrain V, Taieb S, et al. Evaluation with DCE-US of antiangiogenic treatments in 539 patients allowing the selection of one surrogate marker correlated to overall survival. J Clin Oncol. 2012;30(Suppl):A4618. [Google Scholar]


