Abstract
Background and Objectives:
There have been few studies to date evaluating the effectiveness of contrast-enhanced endoscopic ultrasound (CE-EUS) for detecting mural nodules in patients with branch duct-type intraductal papillary mucinous neoplasm (BD-IPMN) of the pancreas. We aim to evaluate the effectiveness of CE-EUS for detecting mural nodules in BD-IPMN.
Patients and Methods:
Of the 427 BD-IPMN patients, 21 patients (4.9%) in whom the presence of mural nodules was suggested by CE computed tomography (CT) or magnetic resonance imaging (MRI), or in whom the presence of nodule-like lesions as shown by fundamental EUS, were examined by CE-EUS.
Results:
The mean diameter of cystic lesions was 29.8 ± 12.8 mm. The mean diameter of mural nodules was 9.5 ± 5.7 mm. BD-IPMN was detected in the pancreatic head in 16 cases, pancreatic body in 2 cases, and pancreatic tail in 3 cases. The mean follow-up period was 17.2 ± 11.9 months. The detection rates of mural nodule-like lesions in BD-IPMN patients on CT, MRI, and fundamental EUS were 36.8%, 63.2%, and 100%, respectively. The detection rates of true mural nodules in BD-IPMN patients on CT, MRI, and fundamental EUS were 85.7%, 71.4%, and 100%, respectively. The echo levels of mural nodule-like lesions on fundamental EUS were hyperechoic in 6 patients, isoechoic in 9 patients, and hypoechoic in 6 patients. The final diagnosis was mucus lumps in 14 patients and mural nodules in 7 patients. The contrast patterns observed were avascular, isovascular, and hypervascular in 14, 3, and 4 patients, respectively. No patients showed a hypovascular pattern. Fourteen patients showing an avascular pattern were diagnosed as having mucus lumps, and they were able to avoid surgical resection. Of the 7 patients who were diagnosed as having mural nodules, 5 underwent surgical resection. The pathological findings were adenocarcinoma in 2 patients and adenoma in 3 patients. Of the 3 adenoma patients, fundamental EUS demonstrated a hypoechoic area in 1 patient and an isoechoic area in 2 patients. Of the 2 adenocarcinoma patients, 1 each showed a hypoechoic area and a hyperechoic area. It was difficult to distinguish between patients with adenoma and patients with adenocarcinoma using the echo levels obtained from fundamental EUS.
Conclusions:
CE-EUS may be useful for avoiding the overdiagnosis of BD-IPMN with mural nodule-like lesions. However, it has difficulty in distinguishing between clearly benign and malignant lesions in BD-IPMN.
Keywords: Contrast-enhanced endoscopic ultrasound, intraductal papillary mucinous neoplasm, mucus lump, mural nodule, sonazoid
INTRODUCTION
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas shows a wide spectrum of histological presentations ranging from adenoma with mild atypia to adenocarcinoma. IPMN is divided into two types, namely, main duct-type and branch duct-type (BD). BD-IPMN develops slowly and has a favorable prognosis.[1] A revised version of the consensus guidelines for the management of IPMN was published in 2012,[2] and an international common medical consensus for the management of IPMN was consequently established. The surgical indications and high-risk stigmata for BD-IPMN include obstructive jaundice in patients with cystic lesions in the pancreatic head that show an enhanced solid component. The presence of mural nodules is also included in the surgical indications for BD-IPMN.
Mural nodules in IPMN are occasionally very small. Fundamental endoscopic ultrasound (EUS) has a higher spatial resolution owing to its higher frequency (5–20 MHz) and enables better observation from a point closer to the pancreas than transabdominal US. Therefore, B-mode EUS is effective for detecting mural nodules in BD-IPMN. Although typical mucus lumps are relatively easy to diagnose on fundamental EUS, atypical mucus lumps may be difficult to distinguish from mural nodules on fundamental EUS.[3]
Contrast-enhanced EUS (CE-EUS) has recently been developed to improve the qualitative diagnostic performance of fundamental EUS. However, there are very few reports in the literature regarding the effectiveness of CE-EUS for the diagnosis of BD-IPMN.
This study aimed to retrospectively assess the discrimination between mucus lumps and mural nodules of BD-IPMN by CE-EUS.
PATIENTS AND METHODS
Patients
The records of 427 IPMN patients who underwent EUS at Tokyo Medical University between April 2010 and April 2014 were reviewed. The final diagnosis of mural nodules was obtained by surgical specimen analysis, whereas that of mucus lumps was obtained by CE-EUS. Of the 427 IPMN patients, 21 patients (4.9%) were suspected to have mural nodule-like lesions on CE computed tomography (CE-CT) or magnetic resonance imaging (MRI), or by incidental detection of nodule-like lesions on fundamental EUS. All patients without exclusion adaptations and who underwent all examinations, namely, CE-CT, MRI, fundamental EUS, and CE-EUS, were enrolled in this study. Patients were excluded if they were below 18 years of age or did not provide informed consent. Patients with egg allergy were also excluded as this is a contraindication with sonazoid. This study was approved by the Institutional Review Board of Tokyo Medical University.
Contrast-enhanced endoscopic ultrasound procedure
CE-EUS was performed using HI VISION900 or HI VISION Avius (Hitachi Aloka Medical, Inc.,) and the EG3870UTK endoscope (Pentax Co., Ltd.), or using ProSound SSDα-10 (Hitachi Aloka Medical, Inc.,) and the GF-UE260-AL5 endoscope (Olympus Corp.).
The second-generation contrast agent sonazoid was used to analyze the perfusion characteristics of microvessels. The recommended volume of sonazoid administration is 0.015 mL/kg body weight (i.e., 0.9 mL should be administered to an adult weighing 60 kg). If tissue harmonic imaging with high sensitivity is used, favorable imaging results can be obtained using half of the recommended volume.[4] Therefore, the sonazoid volume used in our patients was 0.5 mL, regardless of their body weight. A previous study reported that the reduction rate of the echo intensity at 1 min from the peak was the greatest in pancreatic cancer, followed by benign diseases such as mass-forming pancreatitis and neuroendocrine tumor (P < 0.05).[5] In the present study, we observed sonazoid washout in the lesions for 120 s.
Evaluation
The detection rates and echo levels of mural nodule-like lesions were evaluated by fundamental EUS, and vascularity was assessed from the level of the washout of the contrast agent in the lesions on CE-EUS.
A typical mucus lump on fundamental EUS has characteristics of a globular hyperechoic lesion with a globular anechoic area in the lesion [Figure 1]. The echo levels on fundamental EUS were classified into three levels: Hypoechoic, isoechoic, and hyperechoic. The evaluation of echo levels was used for the comparison of the mural nodule-like lesions and pancreatic parenchyma. The types of vascularization observed on CE-EUS were classified into four patterns: Avascular, hypovascular, isovascular, and hypervascular. Washout on CE-EUS indicates the rapid disappearance of the injected contrast agent in the lesion during the observation period.
Figure 1.
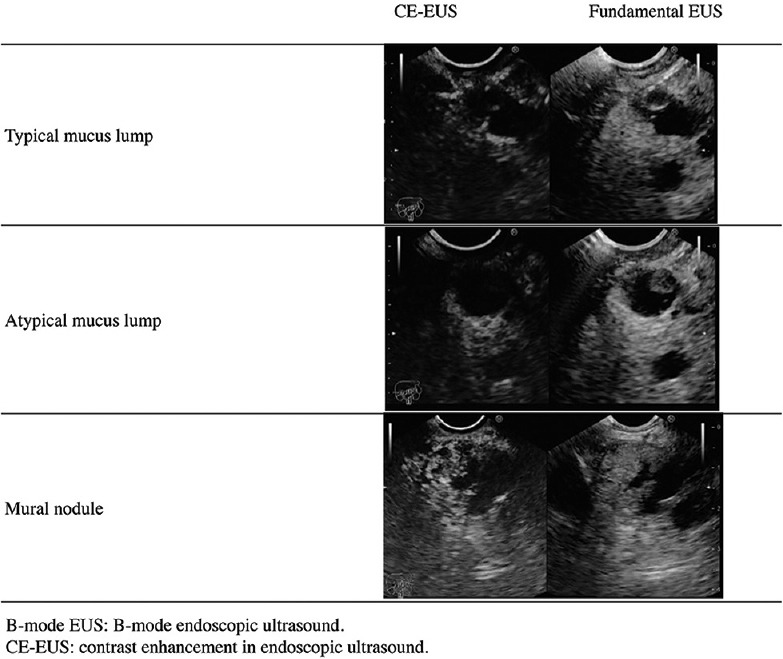
Top panels: A typical mucus lump. The lesion appeared as a globular hyperechoic area with a central anechoic area in the cyst on fundamental endoscopic ultrasound and was not enhanced by sonazoid on contrast-enhanced endoscopic ultrasound. Middle panels: An atypical mucus lump. The lesion appeared as a papillary hyperechoic area in the cyst on fundamental endoscopic ultrasound and was not enhanced by sonazoid on contrast-enhanced endoscopic ultrasound. Bottom panels: A mural nodule. The lesion appeared as a papillary hyperechoic area in the cyst on fundamental endoscopic ultrasound and was enhanced by sonazoid on contrast-enhanced endoscopic ultrasound
Patients who had mural nodule-like lesions that were considered to be benign mucus lumps on CE-EUS underwent follow-up B-mode US and CE-EUS after 6 months. If the mural nodule-like lesions were unchanged, a final diagnosis of benign mucus lump was made. However, a limitation of this study is that all lesions shown to be avascular on CE-EUS were considered to be mucus lumps as the final diagnosis at this stage without any confirmatory pathological findings.
Statistical analyses
Normally distributed data were expressed as mean ± standard deviation statistical significance was determined using the Chi-square test, Fisher exact probability test, or Aspin–Welch t-test. A P < 0.05 was considered to indicate a statistically significant difference between two groups. Statistical analysis was performed using SPSS (ATMS Co., Ltd., Tokyo, Japan).
RESULTS
Baseline characteristics of the patients
Twenty-one BD-IPMN patients (men/women: 16/5) who underwent CE-EUS were included in the study. The mean age of the patients was 65.6 ± 11.5 years. The mean diameter of the cystic lesions was 29.8 ± 12.8 mm. The mean diameter of the main pancreatic duct was 3.4 ± 2.1 mm. The mean diameter of the mural nodules was 9.5 ± 5.7 mm. BD-IPMN was detected in the pancreatic head in 16 cases, pancreatic body in 2 cases, and pancreatic tail in 3 cases. The mean follow-up period was 17.2 ± 10.9 months [Table 1].
Table 1.
Baseline characteristics of patients (n=21)

Detection rates of mural nodules
All patients enrolled in this study underwent CE-CT, MRI, and fundamental EUS. All mural nodules were suspected to be isovascular by CE-CT. A mural nodule like-lesion was detected as the only defect on a heavy T2-weighted image on MRI. The detection rates of mural nodule-like lesions in BD-IPMN patients on CT, MRI, and fundamental EUS were 36.8%, 63.2%, and 100%, respectively. There was no significant difference in the detection rate of mural nodule-like lesions in the BD-IPMN patients. However, the detection rate of mural nodule-like lesions in the BD-IPMN patients on fundamental EUS was higher than that on CE-CT and MRI [Table 2]. Suspected mural nodules were detected in 7 of 21 cases by CT, 12 of 21 cases by MRI, and 21 of 21 cases by fundamental EUS. The detection ratio of mural nodules by CE-CT was 71.4% (5/7), whereas that of mucus lumps by CE-CT was 50.0% (7/14). There was no significant difference in the detection ratios of mural nodules and mucus lumps by CE-CT (P = 0.640).
Table 2.
The detected rate of mural nodules by each imaging technique
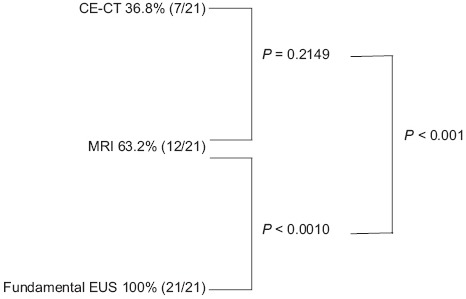
The detection ratio of mural nodules by MRI was 71.4% (5/7), whereas that of mucus lumps by MRI was 50.0% (7/14). There was no significant difference in the detection ratios of mural nodules and mucus lumps by MRI (P = 0.640). CT and MRI showed similar detectable proportions of mural nodules and mucus lumps.
Details of mural nodule-like lesions detected in branch duct-type intraductal papillary mucinous neoplasm patients on fundamental endoscopic ultrasound and contrast-enhanced endoscopic ultrasound
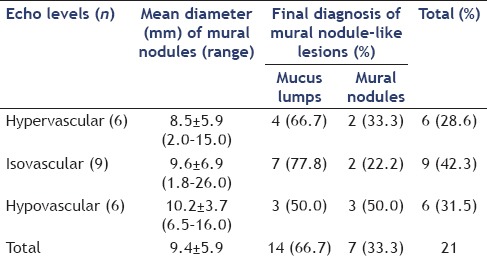
Mural nodule-like lesions were detected on fundamental EUS as hyperechoic areas in 6 cases (typical mucus-like lesions in 3 cases), isoechoic areas in 9 cases, and hypoechoic areas in 6 cases. The final diagnosis was mucus lumps in 14 cases and mural nodules in 7 cases. It was difficult to distinguish between mucus lumps and mural nodules in BD-IPMN patients on fundamental EUS [Table 3].
Table 3.
Details of the mural nodules detected on fundamental endoscopic ultrasound
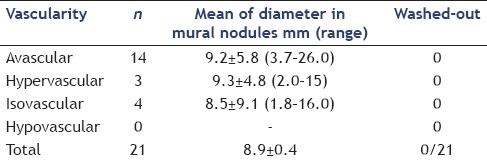
The types of vascularization observed on CE-EUS were classified into 4 patterns: Avascular, hypovascular, isovascular, and hypervascular. The numbers of patients with avascular, isovascular, and hypervascular patterns on CE-EUS were 14, 3, and 4, respectively. There were no patients with a hypovascular pattern in this present study. All 14 patients with an avascular pattern were diagnosed as having mucus lumps and were able to avoid surgical resection [Table 4]. Of the 7 patients with mural nodules, surgical resection was performed in 5 patients.
Table 4.
Details of the mural nodules on contrast-enhanced-endoscopic ultrasound
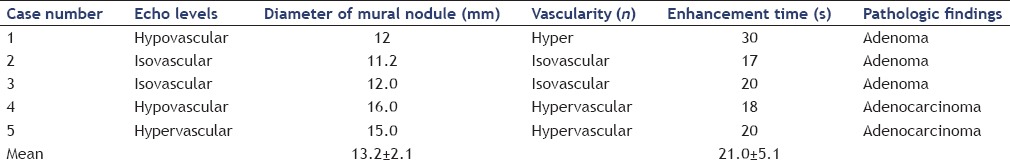
The pathological findings of the surgically resected specimens were adenocarcinoma in 2 patients and adenoma in 3 patients. In the 3 patients with adenoma, the mural nodule-like lesions were hypoechoic on fundamental EUS in 1 patient and isoechoic in 2 patients. In the 2 patients with adenocarcinoma, the mural nodule-like lesions were hypoechoic on B-mode EUS in 1 patient and hyperechoic in the other patient. It was difficult to distinguish between adenoma and adenocarcinoma from the echo levels on fundamental EUS. In the 3 patients with adenoma, the mural nodule-like lesions observed on CE-EUS showed a hypervascular pattern in 1 patient and an isovascular pattern in 2 patients. In the 2 patients with adenocarcinoma, the mural nodule-like lesions observed on CE-EUS showed a hypovascular pattern in 1 patient and a hypervascular pattern in the other patient. The mean time from injecting the contrast medium to the start of enhancement of the vessels in the mural nodules was 21.0 ± 5.1 s in the 5 patients who underwent surgical resection. There was no significant difference in the time to the enhancement of the mural nodules between adenoma and adenocarcinoma. There were no cases showing washout of the contrast agent in the mural nodules during the observation periods. Moreover, there were no cases of irregular appearance of arterial vessels over a short distance after contrast agent injection. It was difficult to distinguish between adenoma and adenocarcinoma in BD-IPMN patients on fundamental EUS and CE-EUS [Table 5].
Table 5.
Details of the mural nodules on B-mode ultrasound and contrast-enhanced-endoscopic ultrasound in resection cases
DISCUSSION
Malignant transformation in BD-IPMN is very rare and has been reported to occur in only 2%-3% of patients per year.[6,7] Furthermore, BD-IPMN is recognized as slow growing, and invasive BD-IPMN has a comparatively better prognosis than pancreatic invasive ductal carcinoma.[8,9,10] However, no systemic chemotherapies for invasive BD-IPMN have been established to date. Therefore, early detection of malignant transformation in BD-IPMN is very important.
Many studies have reported that the presence of mural nodules in BD-IPMN suggests malignant transformation.[11,12,13] Thus, the presence of mural nodules is a very important factor for deciding therapeutic strategies for BD-IPMN.
Visualization of mural nodules in BD-IPMN is often difficult on CE-CT and MRI. On the other hand, fundamental EUS has a higher sensitivity than CE-CT and MRI for detecting small pancreatic lesions. In the present study, the mean diameter of mural nodule-like lesions was 9.5 ± 5.7 mm. The detection rates of mural nodules on CE-CT, MRI, and fundamental EUS were 36.8%, 63.2%, and 100%, respectively. It was previously reported that the sensitivity of EUS in detecting small pancreatic neoplasms of 3 cm diameter or less (99%) was higher than that of CT (77%).[14] Another study showed that the sensitivity of EUS in detecting small pancreatic neoplasms of 3 cm diameter or less (94%) was higher than those of CT (69%) and MRI (83%).[15] Yasuda et al. detected pancreatic masses of <10 mm diameter in 3 out of 132 patients by EUS; however, these masses could not be detected by CE-CT.[16] In the present study, fundamental EUS could detect all mural nodule-like lesions that were detected by CE-CT or MRI. The detection rate of these mural nodule-like lesions in BD-IPMN patients was higher on fundamental EUS than on CE-CT or MRI. Most of the mural nodules detected in BD-IPMN patients were very small. Thus, fundamental EUS is valuable for the diagnosis of mural nodules in BD-IPMN patients.
Fundamental EUS has an excellent lesion detection rate. However, in 80.9% (17/21) of the patients in this study who did not have typical mucus lumps, it was difficult to distinguish between mucus lumps and mural nodules. Fundamental EUS was based on the B-mode US pattern; therefore, there were limitations in qualitative diagnosis, particularly in the discrimination between benign and malignant pancreatic lesions.
To compensate for this weak point, CE-EUS has been developed. Thus far, many studies have reported the effectiveness of CE-EUS for detecting pancreatic lesions. However, there are very few reports on the effectiveness of CE-CT in distinguishing between mural nodules and mucus lumps in BD-IPMN.
Information on the presence of mural nodules in BD-IPMN is very important for deciding the therapeutic strategies. In the present study, 66.7% (14/21) of the BD-IPMN patients were diagnosed as having mucus lumps by CE-EUS, and they could avoid surgical resection owing to overdiagnosis. Some studies have reported that CE-US could distinguish between mural nodules and mucus lumps.[17,18] CE-EUS, which has a higher spatial resolution than CE-US, may have a higher diagnostic performance for IPMN. Thus, CE-EUS may be able to more specifically distinguish between mural nodules and mucus lumps than CE-US.
Although the presence of mural nodules in IPMN is an indication for surgical resection, many studies have indicated that adenomas are often included in BD-IPMN with mural nodules.[19,20,21,22] In recent years, some studies have reported that it is possible to distinguish between benign and malignant pancreatic solid lesions using CE-EUS. Hocke et al. have evaluated the differentiation of inflammation from pancreatic carcinoma in 86 cases by CE-CT using the second-generation contrast agent SonoVue based on the perfusion characteristics of microvessels.[23] The sensitivity and specificity of CE-EUS for detecting pancreatic cancer were 91.1% and 93.3%, respectively. The criteria for malignancy in the present study were as follows: Absence of detectable vascularization before contrast agent injection, irregular appearance of arterial vessels over a short distance after contrast agent injection, and no detection of venous vessels in the lesion. A malignant lesion was assumed if all the criteria were met. However, it was not possible to distinguish between benign BD-IPMN and malignant BD-IPMN in the present study using this method. Although we were able to diagnose malignant BD-IPMN in 2 patients based on the pathological findings following surgical resection, there were no lesions with irregular arterial vessels over a short distance after the contrast agent injection. It is believed that even if cancer is small, its metabolism can be maintained at a low level or even without tumor vessels. Furthermore, IPMNs are believed to undergo a transition from adenoma to carcinoma. As the mural nodules observed in the present study were very small, the growth of the tumor vessels may have been insufficient for detection. Therefore, the detection of extremely small malignant BD-IPMN on CE-EUS may have been difficult under these circumstances.
As for study limitation, all avascular lesions on CE-EUS were given a final diagnosis of mucus lumps without any confirmatory pathological findings or long follow-up.
CONCLUSIONS
CE-EUS may be a useful technique for avoiding the overdiagnosis of BD-IPMN with mural nodule-like lesions. However, it is difficult to clearly distinguish between benign and malignant lesions in BD-IPMN using the current CE-EUS technique.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank Dr. Edward Barroga (http://www.orcid.org/0000-0002-8920-2607), Associate Professor and Senior Editor from the Department of International Medical Communications of Tokyo Medical University for editing the manuscript.
REFERENCES
- 1.Ikeuchi N, Itoi T, Sofuni A, et al. Prognosis of cancer with branch duct type IPMN of the pancreas. World J Gastroenterol. 2010;16:1890–5. doi: 10.3748/wjg.v16.i15.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Xu C, Li Z, Wallace M. Contrast-enhanced harmonic endoscopic ultrasonography in pancreatic diseases. Diagn Ther Endosc 2012. 2012 doi: 10.1155/2012/786239. 786239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sofuni A, Itoi T, Tuji S, et al. New advances in contrast-enhanced ultrasonography for pancreatic disease-usefulness of the new generation contrast agent and contrast-enhanced ultrasonographic imaging method. J Gastroenterol Hepatol Res. 2012;10:233–40. [Google Scholar]
- 5.Matsubara H, Itoh A, Kawashima H, et al. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas. 2011;40:1073–9. doi: 10.1097/MPA.0b013e31821f57b7. [DOI] [PubMed] [Google Scholar]
- 6.Kang MJ, Jang JY, Kim SJ, et al. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2011;9:87–93. doi: 10.1016/j.cgh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Lévy P, Jouannaud V, O’Toole D, et al. Natural history of intraductal papillary mucinous tumors of the pancreas: Actuarial risk of malignancy. Clin Gastroenterol Hepatol. 2006;4:460–8. doi: 10.1016/j.cgh.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Maire F, Hammel P, Terris B, et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717–22. doi: 10.1136/gut.51.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo SM, Ryu JK, Lee SH, et al. Survival and prognosis of invasive intraductal papillary mucinous neoplasms of the pancreas: Comparison with pancreatic ductal adenocarcinoma. Pancreas. 2008;36:50–5. doi: 10.1097/MPA.0b013e31812575df. [DOI] [PubMed] [Google Scholar]
- 10.Schnelldorfer T, Sarr MG, Nagorney DM, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639–46. doi: 10.1001/archsurg.143.7.639. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244–9. doi: 10.1002/bjs.4265. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto T, Aramaki M, Yada K, et al. Optimal management of the branch duct type intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol. 2003;36:261–5. doi: 10.1097/00004836-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: A multicenter study in Japan. Pancreas. 2011;40:364–70. doi: 10.1097/MPA.0b013e31820a5975. [DOI] [PubMed] [Google Scholar]
- 14.Rösch T, Lorenz R, Braig C, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347–52. doi: 10.1016/s0016-5107(91)70729-3. [DOI] [PubMed] [Google Scholar]
- 15.Müller MF, Meyenberger C, Bertschinger P, et al. Pancreatic tumors: Evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994;190:745–51. doi: 10.1148/radiology.190.3.8115622. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda I, Iwashita T, Doi S, et al. Role of EUS in the early detection of small pancreatic cancer. Dig Endosc. 2011;23(Suppl 1):22–5. doi: 10.1111/j.1443-1661.2011.01113.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji S, Sofuni A, Moriyasu F, et al. Contrast-enhanced ultrasonography in the diagnosis of gallbladder disease. Hepatogastroenterology. 2012;59:336–40. doi: 10.5754/hge11447. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita Y, Ueda K, Itonaga M, et al. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: A single-center prospective study. J Ultrasound Med. 2013;32:61–8. doi: 10.7863/jum.2013.32.1.61. [DOI] [PubMed] [Google Scholar]
- 19.Hwang DW, Jang JY, Lee SE, et al. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: A 15-year experience at a single academic institution. Langenbecks Arch Surg. 2012;397:93–102. doi: 10.1007/s00423-010-0674-6. [DOI] [PubMed] [Google Scholar]
- 20.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: An updated experience. Ann Surg. 2004;239:788–97. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadakari Y, Ienaga J, Kobayashi K, et al. Cyst size indicates malignant transformation in branch duct intraductal papillary mucinous neoplasm of the pancreas without mural nodules. Pancreas. 2010;39:232–6. doi: 10.1097/MPA.0b013e3181bab60e. [DOI] [PubMed] [Google Scholar]
- 22.Kanno A, Satoh K, Hirota M, et al. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol. 2010;45:952–9. doi: 10.1007/s00535-010-0238-0. [DOI] [PubMed] [Google Scholar]
- 23.Hocke M, Schulze E, Gottschalk P, et al. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246–50. doi: 10.3748/wjg.v12.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]


