Abstract
BACKGROUND:
The aim of this study is to evaluate perioperative complications related to robotic-assisted laparoscopic surgery for management of gynaecologic disorders.
MATERIALS AND METHODS:
Eight hundred and fifty-one women who underwent robotic procedures between December 2011 and April 2015 were retrospectively included for analysis. Patient demographics, surgical outcomes and complications were evaluated.
RESULTS:
The overall complication rate was 5.5%, whereas the rate of complications for oncologic cases was 8.4%. Intra-operative complications (n = 7, 0.8%) consisted of five cases of bowel lacerations, one case of ureter laceration and one case of bladder injury. Early and late post-operative complications were 4.0% (n = 34) and 0.8% (n = 6), respectively. Six patients (0.7%) experienced Grade III complications based on the Clavien-Dindo classification and required further surgical intervention.
CONCLUSION:
Robotic-assisted laparoscopic surgery is a feasible approach for management of gynaecologic disorders; the complication rates for this type of procedure are acceptable.
Key words: Da Vinci surgical system, perioperative complications, robotic-assisted surgery
INTRODUCTION
Since the da Vinci surgical system was initially approved by the US Food and Drug Administration in 2005 for the treatment of gynaecologic disorders, the use of minimally invasive surgery with robotic assistance has grown exponentially.[1] Similar to conventional laparoscopic surgery, robotic surgery has been demonstrated to be associated with improved perioperative outcomes, shorter length of hospital stays and better quality of life as compared to laparotomy.[2,3,4,5] The robotic surgical approach addresses several restrictions that are associated with the conventional laparoscopic approach, including two-dimensional views, camera instability, a limited range of mobility for laparoscopic instruments, poor ergonomic positioning for the surgeon and steep learning curves. Indeed, the robotic surgical approach offers some advantages, such as improved three-dimensional visualisation, wristed instruments for improvement of dexterity, tremor elimination and shorter learning curves. All these improvements make using the robotic-assisted approach to perform surgical tasks more precise and safer for patients.[6,7] However, several shortcomings of using a robotic-assisted surgical platform remain, such as high cost, lack of tactile feedback and larger port size than in the laparoscopic approach. Some of these disadvantages might lead to an increase in operative complications.
Numerous studies have addressed the complications related to the conventional laparoscopic approach in the management of gynaecological disorders;[8,9] however, reports regarding complications that have occurred during robotic-assisted surgical procedures are still lacking. Because the application of a robotic platform in gynaecology is rapidly growing, accurate evaluations of the complication rates of robotic-assisted surgeries are crucial because they can help to determine whether using a robotic platform is truly feasible for treating gynaecological disorders, especially when oncologic conditions are present. Accordingly, the aim of this study was to evaluate overall and specific complications associated with robotic-assisted surgeries performed using three robotic arms by a single surgeon at a single institute.
MATERIALS AND METHODS
Between December 2011 and April 2015, 851 patients who received robotic-assisted surgery for management of gynaecologic disorders by a single surgeon at the Department of Obstetrics and Gynecology, at Taipei Medical University Hospital were retrospectively included in this study. Pathological diagnosis of all enroled cases was confirmed before operation. All robotic-assisted surgeries were performed using the da Vinci Si system (Intuitive Surgical, Inc., Sunnyvale, CA, USA). Patient demographics, including age, body mass index (BMI), disease stage for oncologic cases and prior surgical histories, were examined. Perioperative parameters including the volume of blood loss, operative time, length of hospital stay and type of complications, were evaluated. This study was conducted with the protocol approved by the Joint Institutional Review Board of Taipei Medical University.
All robotic-assisted procedures were performed with the patient in the lithotomy position under general anaesthesia. A uterine manipulator was routinely placed for adequate pelvic exposure, and a pneumoperitoneum was obtained. For the robotic-assisted surgeries, a 12-mm camera port was set 6 cm above the umbilicus, and two 8-mm robotic side ports were placed at 8–10 cm caudal-lateral to the scope for the side arms at each side of the patient, respectively. An auxiliary trocar which was set at 6–8 cm caudal-lateral to the left arm was required for complicated or oncologic cases.
Surgical staging procedures for management of endometrial cancer, included ascites cytology, total hysterectomy, bilateral salpingo-oophorectomy (BSO), bilateral pelvic lymph node dissection (BPLND) and para-aortic lymph node dissection (PALND), as described in our previous report.[10] For management of ovarian cancer, surgical procedures, including ascites cytology, total hysterectomy, BSO, BPLND, PALND, omentectomy and appendectomy were performed.[11] The surgeries performed for cervical cancer management included radical hysterectomy and BSO with the removal of bilateral pelvic lymph nodes as described previously.[12] For total hysterectomy with or without adnexectomy, bilateral uterine artery ligation was performed as previously described,[13] prior to the main procedure. For patients with large uteruses or myomas, we routinely removed the large uterus piece by piece using a scalpel through the vaginal opening, and immediately sutured the vaginal cuff transvaginally by hand.[14] For myomectomy and oophorectomy or cystectomy, detached tissue was placed into synthetic endobags and minced by morcellation to avoid the spread of cellular material from the morcellated tissue.[15]
The operation time was defined as the time from skin incision to closure, minus the docking time. The total volume of the fluids collected by suction constituted the volume of blood loss. Patients who were suffering from malignant cancers and benign conditions were admitted 2 days and 1 day before the operation day for colon preparation, respectively. Pre-operative computed tomography and magnetic resonance imaging examinations were completed before admission. The length of hospital stay was, therefore, defined as the number of post-operative days until the patient was discharged. Complications were categorised as being either intra-operative or post-operative events. The post-operative complications were further categorised as being either early (before 42 days post-operative) or late stage (more than 42 days post-operative) complications. Clavien-Dindo classification standards were also used to stratify complications into five grades based on the therapeutic and clinical effects.[16]
RESULTS
A total of 857 patients who were scheduled to receive robotic-assisted surgery were retrospectively reviewed for this study. However, six patients (0.7%) who were diagnosed with uterine leiomyomas or adenomyosis, and had initially planned to undergo robotic-assisted hysterectomy, were converted to conventional laparotomy due to severe adhesions. As a result, 851 patients were included in the study. The patient demographics are listed in Table 1. The median age and BMI were 46 years (range 22–89 years) and 22.2 kg/m2 (range 16.2–44.2 kg/m2), respectively. A total of 165 patients (19.3%) had undergone at least one peri-or abdominal-pelvic surgery. Surgical modalities included 109 staging surgeries for endometrial cancer (12.8%), 64 staging surgeries for ovarian cancer (7.5%), 42 radical hysterectomies for cervical cancer (4.9%), 370 total hysterectomies with or without adnexectomy for benign disorders (43.5), 144 oophorectomies or ovarian cystectomies for endometriosis (16.9%) and 122 myomectomies (14.3%).
Table 1.
Baseline characteristics of the enroled patients
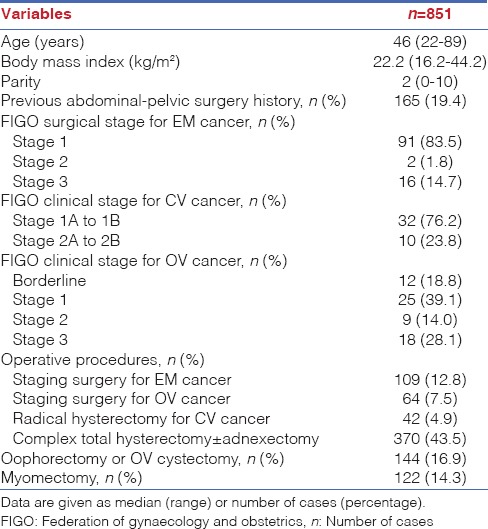
In Table 2, the surgical outcomes of all enroled cases are listed. The overall mean operative time and estimated blood loss were 149.7 ± 42.5 min and 133.6 ± 181.2 mL, respectively. The longest operative time and highest estimated blood loss were recorded after staging surgery for ovarian cancer and myomectomy, with a mean operative time of 179 min and blood loss at 204 mL, respectively. The mean length of hospital stay was 3.2 ± 3.1 days. The longest length of hospital stays was for those receiving staging surgeries for treatment of ovarian cancer, with an average length of hospital stay of 5.7 ± 8.7 days. The average weight of the uterus was 281.7 ± 233.9 g.
Table 2.
Surgical outcomes of the enroled patients for six different modalities
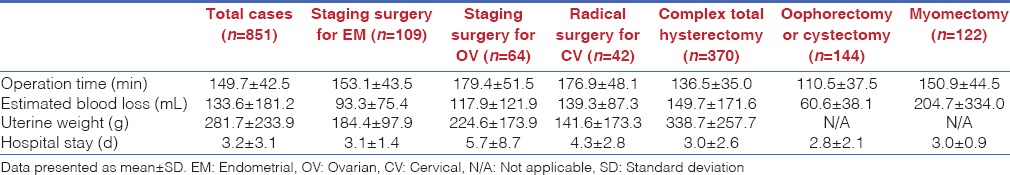
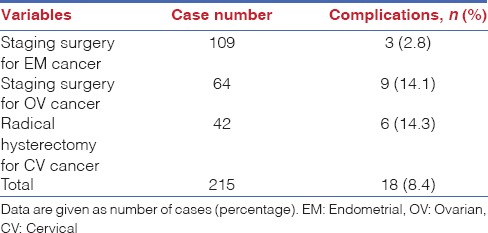
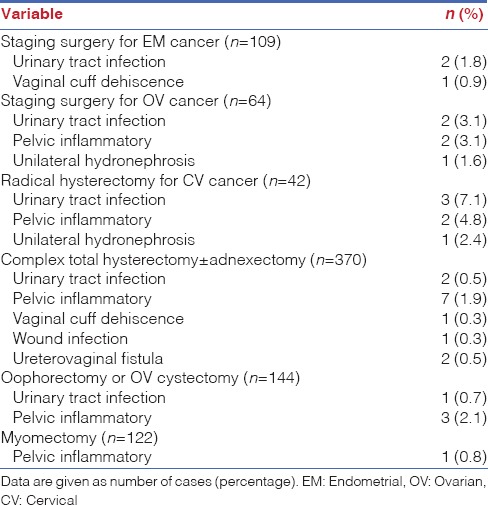
The complication data for both intra-operative and post-operative issues, of all enroled cases from each surgical modality are shown in Table 3. Six patients (0.7%) scheduled for total hysterectomies were converted to conventional laparotomy due to severe adhesions. The overall complication rate was 5.5%, including intra-operative and post-operative complications. The complication rate for management of oncologic cases was 8.4% [Table 4]. Intra-operative complications including one case of ureter laceration and one case of bladder injury occurred during total hysterectomy. Five cases of bowel laceration occurred during total hysterectomy (n = 3) and the staging surgery for management of ovarian cancer (n = 2). However, these injuries were managed by a robotic surgery system, without conversion to any other surgical approach. Early post-operative complications (<42 days post-operative) were observed in 34 patients. Included were instances of febrile morbidity (n = 2), urinary tract infection (n = 10), ileus (n = 6), pelvic inflammatory disease (n = 15) and vaginal cuff dehiscence (n = 1). Regarding late post-operative complications (after 42 days post-operative), six patients were examined, including 1 with vaginal cuff dehiscence, 2 with hydronephrosis, 1 with wound infection and 2 with ureterovaginal fistula.
Table 3.
Intra- and post-operative complications
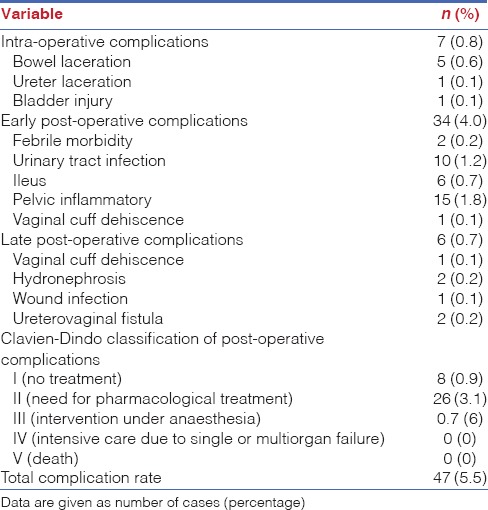
Table 4.
Complication rate for oncologic patients
Complications according to the Clavien-Dindo classification were also reported in Table 3. Many patients' early and late post-operative complications were spontaneously resolved by supporting treatment (Grade I). Patients who had urinary tract infections, pelvic inflammation or wound infections in the early or late post-operative period were discharged after conservative management with antibiotics (Grade II). For Grade III complications which were defined as cases requiring surgical intervention under anaesthesia, six patients were examined (0.7%), including those with cases of vaginal cuff dehiscence (n = 2), unilateral hydronephrosis (n = 2) and ureterovaginal fistula (n = 2). Among them, one patient with vaginal cuff dehiscence in the late post-operative period was examined after undergoing staging surgery for management of endometrial cancer. One patient with vaginal cuff dehiscence in the early post-operative stage and two with ureterovaginal fistula in the late post-operative stage were identified after they had undergone total hysterectomies. In addition, two patients experienced complications of unilateral hydronephrosis after undergoing staging surgery for treatment of cervical and ovarian cancers. In four cases, patients received a ureteral stent insertion due to ureterovaginal fistula and unilateral hydronephrosis. It was determined that no patient experienced severe complications leading to organ failure or death after receiving robotic-assisted surgery.
The classification of the complications according to surgical modality is shown in Table 5. The most frequent complications greater than or equal to Grade II among all the surgical procedures were urinary tract infections and pelvic inflammatory disease with an occurrence rate of 2.9%. These situations were resolved by supporting treatment with antibiotics.
Table 5.
Type of complications based on the treatment modality (Clavien-Dindo classification ≥2)
DISCUSSION
Robotic platforms have been well established in our institute since December 2011. During the 3½ years, over 1000 gynaecologic cases have been completed using the da Vinci Si surgical system. Among them, 857 patients who were diagnosed with gynaecological disorders, including benign and malignant tumours, have received robotic-assisted surgery by a single surgeon. Although numerous studies have demonstrated the feasibility of robotic-assisted surgery in gynaecology, the complications that occur during robotic surgery still draw physicians' attention in gynaecological and surgical research. In the current investigation, we evaluated the complication rate of robotic-assisted surgery for the treatment of gynaecologic disorders, including benign and malignant tumours.
Several studies have demonstrated the feasibility and complications of robotic-assisted surgery for management of gynaecological disorders, including benign and malignant conditions. Wechter et al. reported that the overall complication rate was 21.6% (250 out of 1155 patients) in robotic-assisted gynaecological surgery, and 31.8% (70 out of 220 patients) in oncologic cases.[17] Complications considered ≥3 according to Clavien-Dindo classification occurred in 5.2% of patients. Yim et al. also provided a peri-operative complication rate of 18.8% in 298 robotic-assisted gynaecological cases while reporting a complication rate of 16.1% (39/242) for oncologic cases.[18] The rate of complications ≥ 3 according to Clavien-Dindo standards was 1.7% (5/298). In this study, the overall complication rate was 5.5% while the rate for oncologic cases was 8.4%. Only 6 patients were reported to have Clavien-Dindo rated complications ≥ 3 (0.7%). Complication rates of gynaecological surgery for oncologic patients were anticipated to be higher than those in benign cases due to extensive dissection and the broader surgical field. In this study, the rates of complications for oncologic and benign patients were 8.4% (18/215) and 4.6% (29/636), respectively. Similar results have been found in several previous studies.[17,18,19] These results indicated that the complication rate in our cohort was acceptable as compared to other rates reported in previous studies.
In our cohort, the intra-operative complication rate was 0.8%. One case of ureter laceration, one case of bladder injury and three cases of bowel laceration occurred during complicated total hysterectomy. In addition, two cases of bowel perforation were reported in the staging surgery for the management of ovarian cancer. We found that these seven patients had prior pelvic surgical histories for the treatment of benign disorders such as myomectomy. Regarding adhesion severity, their scores were 4–6 according to an adhesion scoring system adopted from the Adhesion Scoring Group.[20] In general, severe adhesions increase surgical difficulties resulting in an elevated complication rate, a high risk of conversion to laparotomy and an increased rate of readmission[21] for endoscopic surgical approaches including robotic and laparoscopic modalities. In our experience, even though a robotic platform is feasible for the management of gynaecological conditions in patients with pelvic adhesions,[14] selection of patients with severe adhesions for treatment using a robotic platform needs to be reconsidered.
Several studies have been conducted on the feasibility and safety of robotic-assisted myomectomy.[22,23] The overall peri-operative complication rates reported for these robotic-assisted surgical procedures were 13.8–19.5%, whereas what we reported here was 0.8% (1 out of 122 patients). In addition, the complication rate of robotic-assisted simple or complex hysterectomy for treatment of gynaecological benign disorders has previously been investigated.[24,25] Statistical analysis regarding benign hysterectomy performed using four different modalities, including abdominal, vaginal, laparoscopic and robotic procedures, was conducted by Luciano et al.[24] The Premier Research Database, which collected numerous surgical outcomes of patients from 156 US hospitals, was utilised for the study. In the analysis, the overall complication rate of robotic-assisted hysterectomy was 14.8% (3077 out of 20781 patients). Moreover, Rosero et al. reported clinical outcomes of patients who underwent laparoscopic-and robotic-assisted hysterectomy for management of benign disorders using the Nationwide Inpatient Sample database.[25] The complication rate of 8.8% (685 out of 7788 patients) was found. However, the complication rate of robotic hysterectomy in our cohort was 6.5% (24 out of 370 patients), indicating that robotic-assisted surgery for management of gynaecological benign conditions was a feasible approach with adequate complication rate.
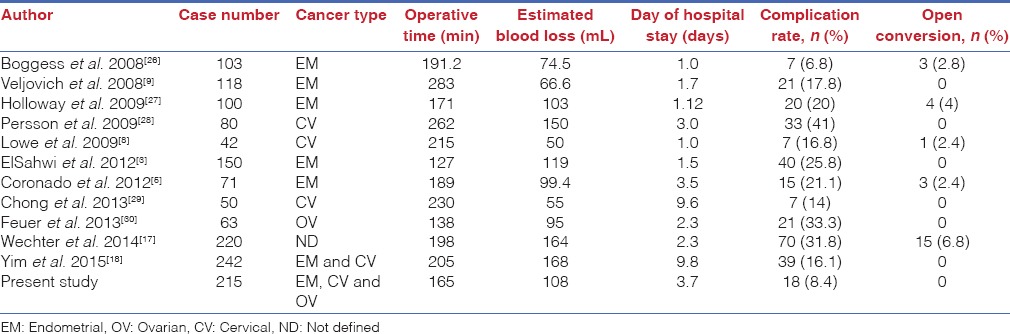
There are numerous studies demonstrating the feasibility and complications of robotic surgery for treatment of malignant conditions. Table 6 summarises the surgical outcomes from these published research studies and shows that the complication rate of our cohort was comparable to those published in previous studies. In these previous reports, the overall complication rate was reported at 6.8–41% (we reported 8.4% here). Conversion to laparotomy was reported as 0–6.8% (we reported no cases).
Table 6.
Surgical outcomes of robotic surgeries for oncologic patients
Hysterectomy is applied for treatment of gynaecological disorders, including benign and malignant tumours. For selective patients who received robotic-assisted surgery, the uterus is usually removed from the vagina. The vaginal cuff is then closed endoscopically. In our experiences, once the uterus was extracted from the vagina, the vaginal cuff was instantly closed by hand through suturing transvaginally. The advantages of performing this procedure are as follows: (1) The pneumoperitoneum does not need to be maintained, (2) In the robotic surgical system, the needle holder is not required for the surgical procedure, which may decrease the costs for patients, (3) The risk of vaginal cuff dehiscence is decreased and (4) For treatment of benign conditions, an assistant port is not required for the surgical procedure. In this study, two cases of vaginal cuff dehiscence were reported, including one case in staging surgery for endometrial cancer management and one case in complicated hysterectomy for treatment of a benign tumour. The rate of vaginal cuff dehiscence was 0.3% (2 out of 585 patients) after robotic-assisted hysterectomies. In a study by Uccella et al., the rate of vaginal cuff dehiscence was 0.3% (2/665) after laparoscopic hysterectomies with transvaginal cuff closure.[31] Uccella et al. have also reported that patients who underwent vaginal closure with endoscopic suture (20 out of 2332, 0.86%) had a higher incidence of cuff dehiscence than patients who had suture with the transvaginal approach (3 out of 1241, 0.24%) during endoscopic surgeries.[32] However, a study reported by Kho et al. indicated that the occurrence of vaginal cuff separation after hysterectomy with endoscopic cuff closure performed by the robotic approach was 4.1% (21/510).[33] Moreover, previous studies have demonstrated that 2-layer suture was associated with low incidence of vaginal cuff dehiscence.[34,35] In our cohort, vaginal cuff closure was performed using the 2-layer suture method. It may be one of the reasons why the incidence of vaginal cuff dehiscence in our report was low.
These results have led to a conclusion that the suture method may be one of the risk factors associated with vaginal cuff dehiscence. Previous research has indicated that patients who received chemotherapy after operations had a high risk of dehiscence (approximately 3%) as compared to patients who did not (0.4%).[36] However, in this study, 68 out of 215 oncologic patients (31.6%) received chemotherapy after surgeries, but only one patient experienced vaginal cuff dehiscence.
In this study, all kind of complications is commonly occurred in minimally invasive techniques. No any robotic platform-related complication, such as mechanical failure, was reported in our cohort. In a study of 298 patients who received robotic surgery, one patient who was diagnosed with cervical cancer and initially planned to receive robotic-assisted staging surgery was converted to laparoscopic-assisted surgery due to mechanical failure of the robotic system.[18] Hence, an incidence of robotic platform-related complication is as low as 0.3%. The limitation of this study is the lack of comparisons with laparoscopic surgery. Direct comparison of our data with rates of robotic and laparoscopic complications is difficult due to the fact that the number of patients who received laparoscopic surgery by the same surgeon during the same period (between December 2011 and April 2015) was limited. However, the strength of this study is to include a large cohort from single institute and to provide a greater perspective on perioperative complications that could potentially occur during robotic-assisted surgery. We collected 203 women who underwent conventional laparoscopic-assisted surgery between 2005 and 2014 for management of gynaecologic malignances, including endometrial, ovarian and cervical cancers. Our unpublished results showed that the overall complication rate for laparoscopic surgery was 6.9% (14 out of 203 patients). If comparing to an oncologic group which managed by robotic surgery in this study, there is no significant difference was found between groups (8.4% for robotic vs. 6.9% for laparoscopic).
CONCLUSION
Our results in this study demonstrate that a robotic platform is a feasible and safe approach with acceptable complication rates for management of gynaecological disorders including benign and malignant conditions.
Financial support and sponsorship
Ministry of Science and Technology (MOST 104-2320-B-038-063).
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to acknowledge the Taipei Medical University Hospital Cancer Center for the assistance in data collection. This work is granted by the Ministry of Science and Technology (MOST 104-2320-B-038-063).
REFERENCES
- 1.Sinno AK, Fader AN. Robotic-assisted surgery in gynecologic oncology. Fertil Steril. 2014;102:922–32. doi: 10.1016/j.fertnstert.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Tinelli R, Malzoni M, Cosentino F, Perone C, Fusco A, Cicinelli E, et al. Robotics versus laparoscopic radical hysterectomy with lymphadenectomy in patients with early cervical cancer: A multicenter study. Ann Surg Oncol. 2011;18:2622–8. doi: 10.1245/s10434-011-1611-9. [DOI] [PubMed] [Google Scholar]
- 3.ElSahwi KS, Hooper C, De Leon MC, Gallo TN, Ratner E, Silasi DA, et al. Comparison between 155 cases of robotic vs 150 cases of open surgical staging for endometrial cancer. Gynecol Oncol. 2012;124:260–4. doi: 10.1016/j.ygyno.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Wright JD, Burke WM, Wilde ET, Lewin SN, Charles AS, Kim JH, et al. Comparative effectiveness of robotic versus laparoscopic hysterectomy for endometrial cancer. J Clin Oncol. 2012;30:783–91. doi: 10.1200/JCO.2011.36.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronado PJ, Herraiz MA, Magrina JF, Fasero M, Vidart JA. Comparison of perioperative outcomes and cost of robotic-assisted laparoscopy, laparoscopy and laparotomy for endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2012;165:289–94. doi: 10.1016/j.ejogrb.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Magrina JF, Kho RM, Weaver AL, Montero RP, Magtibay PM. Robotic radical hysterectomy: Comparison with laparoscopy and laparotomy. Gynecol Oncol. 2008;109:86–91. doi: 10.1016/j.ygyno.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Fanning J, Fenton B, Purohit M. Robotic radical hysterectomy. Am J Obstet Gynecol. 2008;198:649.e1–4. doi: 10.1016/j.ajog.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Lowe MP, Chamberlain DH, Kamelle SA, Johnson PR, Tillmanns TD. A multi-institutional experience with robotic-assisted radical hysterectomy for early stage cervical cancer. Gynecol Oncol. 2009;113:191–4. doi: 10.1016/j.ygyno.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Veljovich DS, Paley PJ, Drescher CW, Everett EN, Shah C, Peters WA., 3rd Robotic surgery in gynecologic oncology: Program initiation and outcomes after the first year with comparison with laparotomy for endometrial cancer staging. Am J Obstet Gynecol. 2008;198:679.e1–9. doi: 10.1016/j.ajog.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Chiou HY, Chiu LH, Chen CH, Yen YK, Chang CW, Liu WM. Comparing robotic surgery with laparoscopy and laparotomy for endometrial cancer management: A cohort study. Int J Surg. 2015;13:17–22. doi: 10.1016/j.ijsu.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Chen CH, Chiu LH, Chen HH, Chan C, Liu WM. Comparison of robotic approach, laparoscopic approach and laparotomy in treating epithelial ovarian cancer. Int J Med Robot. 2015 doi: 10.1002/rcs.1655. doi: 10.1002/rcs.1655. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Chiu LH, Chang CW, Yen YK, Huang YH, Liu WM. Comparing robotic surgery with conventional laparoscopy and laparotomy for cervical cancer management. Int J Gynecol Cancer. 2014;24:1105–11. doi: 10.1097/IGC.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 13.Liu WM, Wang PH, Chou CS, Tang WL, Wang IT, Tzeng CR. Efficacy of combined laparoscopic uterine artery occlusion and myomectomy via minilaparotomy in the treatment of recurrent uterine myomas. Fertil Steril. 2007;87:356–61. doi: 10.1016/j.fertnstert.2006.07.1497. [DOI] [PubMed] [Google Scholar]
- 14.Chiu LH, Chen CH, Tu PC, Chang CW, Yen YK, Liu WM. Comparison of robotic surgery and laparoscopy to perform total hysterectomy with pelvic adhesions or large uterus. J Minim Access Surg. 2015;11:87–93. doi: 10.4103/0972-9941.147718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cucinella G, Granese R, Calagna G, Somigliana E, Perino A. Parasitic myomas after laparoscopic surgery: An emerging complication in the use of morcellator? Description of four cases. Fertil Steril. 2011;96:e90–6. doi: 10.1016/j.fertnstert.2011.05.095. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wechter ME, Mohd J, Magrina JF, Cornella JL, Magtibay PM, Wilson JR, et al. Complications in robotic-assisted gynecologic surgery according to case type: A 6-year retrospective cohort study using Clavien-Dindo classification. J Minim Invasive Gynecol. 2014;21:844–50. doi: 10.1016/j.jmig.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Yim GW, Kim SW, Nam EJ, Kim S, Kim YT. Perioperative complications of robot-assisted laparoscopic surgery using three robotic arms at a single institution. Yonsei Med J. 2015;56:474–81. doi: 10.3349/ymj.2015.56.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannisto P, Harter P, Heitz F, Traut A, du Bois A, Kurzeder C. Implementation of robot-assisted gynecologic surgery for patients with low and high BMI in a German gynecological cancer center. Arch Gynecol Obstet. 2014;290:143–8. doi: 10.1007/s00404-014-3169-9. [DOI] [PubMed] [Google Scholar]
- 20.Improvement of interobserver reproducibility of adhesion scoring systems. Adhesion Scoring Group. Fertil Steril. 1994;62:984–8. [PubMed] [Google Scholar]
- 21.Lower AM, Hawthorn RJ, Ellis H, O'Brien F, Buchan S, Crowe AM. The impact of adhesions on hospital readmissions over ten years after 8849 open gynaecological operations: An assessment from the surgical and clinical adhesions research study. BJOG. 2000;107:855–62. doi: 10.1111/j.1471-0528.2000.tb11083.x. [DOI] [PubMed] [Google Scholar]
- 22.Bedient CE, Magrina JF, Noble BN, Kho RM. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol. 2009;201:566.e1–5. doi: 10.1016/j.ajog.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 23.Advincula AP, Xu X, Goudeau S, th, Ransom SB. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: A comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698–705. doi: 10.1016/j.jmig.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Luciano AA, Luciano DE, Gabbert J, Seshadri-Kreaden U. The impact of robotics on the mode of benign hysterectomy and clinical outcomes. Int J Med Robot. 2016;12:114–24. doi: 10.1002/rcs.1648. [DOI] [PubMed] [Google Scholar]
- 25.Rosero EB, Kho KA, Joshi GP, Giesecke M, Schaffer JI. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122:778–86. doi: 10.1097/AOG.0b013e3182a4ee4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, et al. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: Robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol. 2008;199:360.e1–9. doi: 10.1016/j.ajog.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Holloway RW, Ahmad S, DeNardis SA, Peterson LB, Sultana N, Bigsby GE, th, et al. Robotic-assisted laparoscopic hysterectomy and lymphadenectomy for endometrial cancer: Analysis of surgical performance. Gynecol Oncol. 2009;115:447–52. doi: 10.1016/j.ygyno.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Persson J, Reynisson P, Borgfeldt C, Kannisto P, Lindahl B, Bossmar T. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185–90. doi: 10.1016/j.ygyno.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Chong GO, Lee YH, Hong DG, Cho YL, Park IS, Lee YS. Robot versus laparoscopic nerve-sparing radical hysterectomy for cervical cancer: A comparison of the intraoperative and perioperative results of a single surgeon's initial experience. Int J Gynecol Cancer. 2013;23:1145–9. doi: 10.1097/IGC.0b013e31829a5db0. [DOI] [PubMed] [Google Scholar]
- 30.Feuer GA, Lakhi N, Barker J, Salmieri S, Burrell M. Perioperative and clinical outcomes in the management of epithelial ovarian cancer using a robotic or abdominal approach. Gynecol Oncol. 2013;131:520–4. doi: 10.1016/j.ygyno.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Uccella S, Ghezzi F, Mariani A, Cromi A, Bogani G, Serati M, et al. Vaginal cuff closure after minimally invasive hysterectomy: Our experience and systematic review of the literature. Am J Obstet Gynecol. 2011;205:119.e1–12. doi: 10.1016/j.ajog.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Uccella S, Ceccaroni M, Cromi A, Malzoni M, Berretta R, De Iaco P, et al. Vaginal cuff dehiscence in a series of 12,398 hysterectomies: Effect of different types of colpotomy and vaginal closure. Obstet Gynecol. 2012;120:516–23. doi: 10.1097/AOG.0b013e318264f848. [DOI] [PubMed] [Google Scholar]
- 33.Kho RM, Akl MN, Cornella JL, Magtibay PM, Wechter ME, Magrina JF. Incidence and characteristics of patients with vaginal cuff dehiscence after robotic procedures. Obstet Gynecol. 2009;114(2 Pt 1):231–5. doi: 10.1097/AOG.0b013e3181af36e3. [DOI] [PubMed] [Google Scholar]
- 34.Shen CC, Hsu TY, Huang FJ, Roan CJ, Weng HH, Chang HW, et al. Comparison of one- and two-layer vaginal cuff closure and open vaginal cuff during laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 2002;9:474–80. doi: 10.1016/s1074-3804(05)60522-1. [DOI] [PubMed] [Google Scholar]
- 35.Jeung IC, Baek JM, Park EK, Lee HN, Kim CJ, Park TC, et al. A prospective comparison of vaginal stump suturing techniques during total laparoscopic hysterectomy. Arch Gynecol Obstet. 2010;282:631–8. doi: 10.1007/s00404-009-1300-0. [DOI] [PubMed] [Google Scholar]
- 36.Drudi L, Press JZ, Lau S, Gotlieb R, How J, Eniu I, et al. Vaginal vault dehiscence after robotic hysterectomy for gynecologic cancers: Search for risk factors and literature review. Int J Gynecol Cancer. 2013;23:943–50. doi: 10.1097/IGC.0b013e31828f38e1. [DOI] [PubMed] [Google Scholar]


