Abstract
BACKGROUND:
In patients undergoing robot-assisted radical prostatectomy (RARP), pneumoperitoneum, intraoperative fluid restriction and prolonged Trendelenburg position may cause rhabdomyolysis (RM) due to hypoperfusion in gluteal muscles and lower extremities. In this study, it was aimed to assess effects of body mass index (BMI), comorbidities, intra-operative positioning, fluid restriction and length of surgery on the development of RM in RARP patients during the perioperative period.
SUBJECTS AND METHODS:
The study included 52 American Society of Anesthesiologists I–II patients aged 50–80 years with BMI >25 kg/m2, who underwent RARP. Fluid therapy with normal saline (1 ml/kg/h) and 6% hydroxyethyl starch 200/05 (1 ml/kg/h) was given during the surgery. Charlson comorbidity index (CCI), operation time (OT) and Trendelenburg time (TT) were recorded. Blood samples for creatine phosphokinase (CPK), blood urea nitrogen, creatinine (Cr), aspartate aminotransferase (AST), alanine transferase (ALT), lactate dehydrogenase (LDH), creatinine kinase-MB, cardiac troponin I and arterial blood gases were drawn at baseline and on 6, 12, 24 and 48 h. RM was defined by serum CPK level exceeding 5000 IU/L.
RESULTS:
Seven patients met predefined criteria for RM. There were positive correlations among serum CPK and Cr, AST, ALT and LDH levels. However, there was no significant difference in BMI, OT and TT between patients with or without RM (P > 0.05). CCI scores were higher in patients with RM than those without (3.00 ± 0.58 vs. 2.07 ± 0.62; P < 0.01). No renal impairment was detected among patients with RM at the post-operative period.
CONCLUSIONS:
It was found that comorbid conditions are more important in the development of RM during RARP rather than BMI, OT or TT. Patients with higher comorbidity are at risk for RM development and that this should be kept in mind at follow-up and when informing patients.
Key words: Acute renal failure, comorbidity, minimally invasive surgery, prostatectomy, rhabdomyolysis, Trendelenburg position
INTRODUCTION
With the advent of robotic surgery, the surgeons are faced with new surgical positioning, namely, 'steep Trendelenburg position' (STP) which more frequently demands attention to ensure patient safety.
In the surgeries at prolonged, or exaggerated lithotomy position, such as robot-assisted radical prostatectomy (RARP), intra-operative fluid restriction for urine-free surgical field to provide better visualisation and prolonged STP have been shown to expose patients to the risk of rhabdomyolysis (RM) due to hypoperfusion of gluteal muscles.[1]
RM is a rare but lethal clinical condition characterised by leakage of muscular content into blood and extracellular tissues due to acute injury of skeletal muscles.[2] Post-operative RM can be observed after vascular, neurosurgical and more commonly urological interventions. Risk factors include age (40–50 years of age), male gender, high body mass index (BMI),[3] perioperative overhydration, prolonged surgery[4] and surgical positioning (flexed lateral decubitus position and STP).[5,6] Renal failure (RF) is the most serious RM-related complication with an incidence of 15–46% and mortality of 3.4%.[7]
In this study, we aimed to assess effects of BMI, comorbidities, intra-operative positioning, fluid restriction and length of surgery on the development of RM and renal impairment in RARP patients during perioperative periods.
SUBJECTS AND METHODS
This prospective study was conducted in a reference academic centre and approved by the Institutional Review Board. All patients gave written informed consent before participation.
Fifty-two American Society of Anesthesiologists (ASA) I–II patients aged 50–80 years, BMI > 25 kg/m2 scheduled for an elective RARP were enrolled in the study.
Patients with ASA III–IV risk status, those with comorbid diseases that can cause increased muscular activity such as severe dystonia or status asthmaticus, those with renal or hepatic failure and patients on a statin or steroid therapy, were excluded from the study. It was planned to exclude patients in case of conversion to laparotomy during surgery.
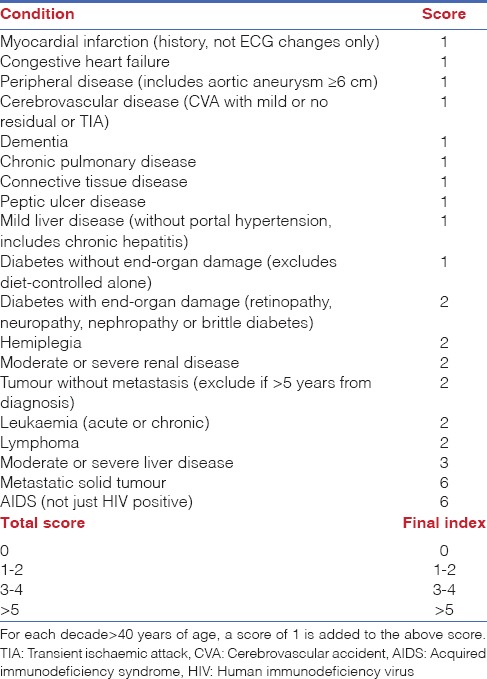
In all patients, pre-anaesthetic evaluations including laboratory tests and Charlson comorbidity index (CCI)[8,9] were performed 1 week before surgery in anaesthesia clinic. Comorbid diseases were rated based on CCI. A 4-point scale was used to rate comorbid conditions [1 mild; 4 severe]. Comorbidity grading was performed by adding scores given for each comorbid disease. Based on the grading, patients were stratified into four groups as follows: Grade 0, 1–2, 3–4 and ≥5 [Table 1].
Table 1.
Charlson comorbidity index
All patients underwent bowel preparation on the night before surgery. Low-molecular-weight heparin was initiated on the day before surgery and administered daily until discharge.
In the operation room, intubation was performed after standard anaesthesia induction with fentanyl, thiopental sodium and rocuronium bromide. Anaesthesia was maintained using 1 minimum alveolar concentration (MAC) sevoflurane in 50–50% O2-air mixture and remifentanil infusion (0.05–0.01 µg/kg/min). Sevoflurane end-tidal concentration was maintained to be 1 MAC (adjusted to age) throughout general anaesthesia.
For surgery, patients were placed in low lithotomy position. All patients were placed on a soft sponge mattress, and soft padding gel pads were provided above the shoulders. The patients were placed in a 30° STP after achieving pneumoperitoneum at an intra-abdominal pressure level of 15 mmHg. After placing patient to desired position (T0), blood samples were drawn for measurements of arterial blood gas, Na, Cl, Ca, K, blood urea nitrogen (BUN), creatinine (Cr), aspartate aminotransferase (AST), alanine transferase (ALT), lactate dehydrogenase (LDH), cardiac troponin I (cTpI), creatinine kinase (CK)-MB and creatine phosphokinase (CPK).
During the operation, normal saline (1 ml/kg/h) and 6% hydroxyethyl starch (HES) 200/05 (1 ml/kg/h) infusions were applied. Operation time (OT) and Trendelenburg time (TT) were recorded for all patients.
Blood samples were repeated on 6 h (T6), 12 h (T12) and 24 h (T24) after beginning of surgery. Hydration with 2000 ml crystalloid solution was given until 24 h after surgery. In all patients, urine output was monitored.
RM was defined as post-operative serum CPK level exceeding 5000 IU/L. It was planned to manage these patients with hypervolemic therapy, correction of acidosis using intravenous (IV) sodium bicarbonate and stimulation of diuresis by IV furosemide with a goal of maintaining minimal diuresis of 60 ml/h at pH level of 7.
Post-operative RF was defined as an increase in serum Cr of 1 mg/dL/day (or 90 mmol/L/day) for 2 consecutive days beyond the baseline.
Patients were discharged with control laboratory tests, including the same parameters, on the 48 h (T48) post-operatively.
Statistical analyses were performed using IBM SPSS version 22.0 (IBM SPSS, Turkey). Data were assessed using descriptive statistics (mean, standard deviation, median, frequency and rate). Repeated measures analysis of variance was used to assess variables with normal distribution followed by post hoc assessment with Bonferroni adjustment for multiple comparisons. Friedman's test and Wilcoxon signed rank test were used for intra-group comparisons of variables with skewed distribution. Mann–Whitney U-test was used in assessments according to RM status. Spearman's Rho correlation analysis was used to evaluate the relationship between serum CPK levels and other parameters. A P < 0.05 was considered statistically significant.
RESULTS
All patients were male in the study population. Mean age was 61.92 ± 6.99 years (range: 50–78) whereas mean BMI was 29.83 ± 5.54 kg/m2 (range: 23–56). Mean OT was 215.48 ± 28.65 min (range: 180–300) and mean TT was 184.23 ± 21.43 min (140–240). Mean CCI value was 2.19 ± 0.69 regarding perioperative risks.
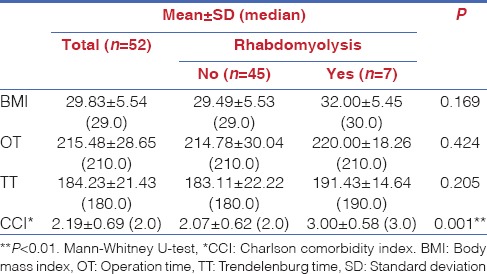
There were seven patients with post-operative serum CPK level > 5000 IU/L, meeting predefined RM criteria. No significant differences were detected in BMI, OT and TT between patients with or without RM (P > 0.05). Mean CCI value was significantly higher in patients with RM when compared to those without (3.00 ± 0.58 vs. 2.07 ± 0.62; P = 0.001, P < 0.01) [Table 2].
Table 2.
Assessment of patients with body mass index, operation time, Trendelenburg time and Charlson comorbidity index
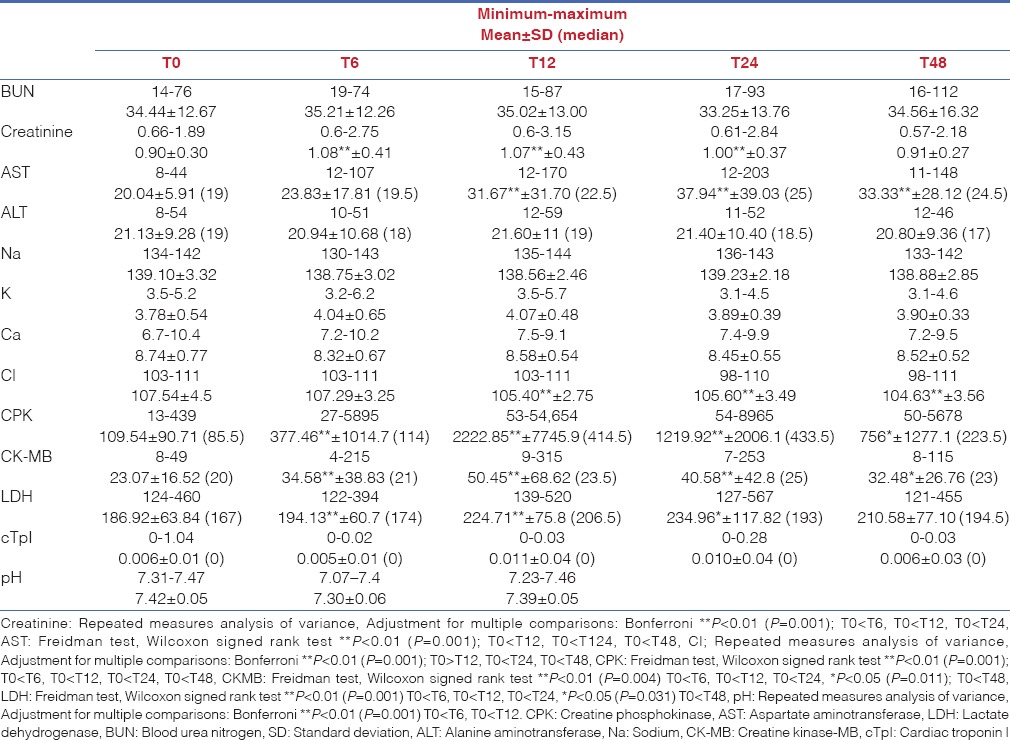
In all patients, all mean serum values were within normal limits before surgery [Table 3]. Median serum CPK level was 85.5 IU/L (range: 13–449) at baseline. All patients showed a significant increase in median serum CPK level from 114 IU/L (range: 27–5985) on 6 h to 414.5 IU/L (range: 53–54,554 IU/L) on 12 h with a peak value of 433 IU/L (range: 54–8964) on 24 h (P = 0.001, P < 0.01).
Table 3.
Laboratory test values of the patients
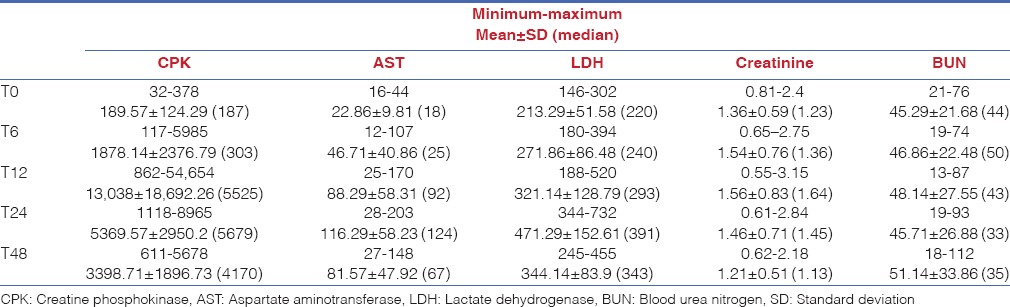
In patients with RM, there were significant increases in serum CPK, Cr, AST, LDH and BUN values on 6, 12, 24 and 48 h when compared to baseline. In seven patients with RM (13.5%), mean serum CPK levels was 1878.14 ± 2376.79 IU/L (range: 117–5985) whereas 13,038.00 ± 18,693.26 (range: 1118–8956) on 12 h. Similarly, Cr values reached to peak value between 6 and 12 h while AST and LDH were increased to peak value on 24 h. However, BUN reached to peak value on 48 h [Table 4].
Table 4.
Laboratory values of the patients with rhabdomyolysis
Serum CK-MB, Cr and LDH levels were increased on 6 h. CK-MB reached to peak value on 12 h whereas LDH and AST on 24 h. No significant difference was detected in BUN, ALT, cTpI, Na, K, Ca and urine density.
On 6, 12, 24 and 48 h, serum CPK value was positively correlated to serum Cr (r: 0.331, P: 0.017*; r: 0.428, P: 0.002**; r: 0.318, P: 0.021*; and r: 0.344, P: 0.012*), LDH (r: 0.312, P: 0.024*; r: 0.519, P: 0.001**; r: 0.609, P: 0.001*; and r: 0.585, P: 0.001*) while CPK values was positively correlated to serum AST on the 12, 24 and 48 h (r: 0.461, P: 0.001*; r: 0.737, P: 0.001**; and r: 0.692, P: 0.001*). Again, serum CPK value was positively correlated to ALT values on 24 and 48 h (r: 0.535, P: 0.001*; r: 0.327, P: 0.002**; and r: 0.018). There was a significant negative correlation between serum CPK and pH values on 12 h (r: −0.317, P: 0.022*).
No significant correlation was detected between CPK values and BMI, OT, TT and amount of intra-operative fluid but positive correlations were detected between CPK and CCI values on 12 h (r: 0.333, P: 0.016*), 24 (r: 0.411, P: 0.002**) and 48 (r: 0.379, P: 0.006*). (Spearman's Rho correlation analysis: *P < 0.05, **P < 0.01).
No patients with RM showed signs of RF.
DISCUSSION
Robotic technology is having a considerable impact on urological practice and with wide utilisation of this approach, there are a number of questions raised about the safety and efficacy. Positioning injuries in the perioperative period are one of the inherent risks of surgery, particularly in robot-assisted urologic surgery and often result in unrecognised morbidity.[10]
RM can be defined as leakage of intracellular components into blood or extracellular space due to either reversible or irreversible degeneration of cell membrane or sarcolemma of muscle cells. Hypoxia, reperfusion injury or direct injury of cell membrane can result in the efflux of intracellular proteins to extracellular space. As a result of the leakage, an increase can occur in CPK, LDH, myoglobin, aldolase, phosphate and potassium levels.[11]
RM can be seen in drug or alcohol abuse, connective tissue disorders, in patients using 3-hydroxy-3-methylglutaryl-coenzyme A reductase, in genetic disorders or as a result of excessive exercises.[1,2] RM can also be seen as a result of compression of muscular tissue due to surgical positioning in prolonged laparoscopic or robotic surgeries.
One method suggested to protect the patient against RM is to provide better perfusion at compressed regions by permissive hypertension. However, in robotic surgeries, normo-tension or controlled hypotension is preferred to achieve blood-free surgical site. In addition, STP and normo-hydration can complicate anastomosis by causing urine flow during ureterovesical anastomosis.[12] Thus, fluid restriction (normal saline [1 ml/kg/h] and 6% HES 200/05 [1 ml/kg/h] infusions), as we also applied in our study, is recommended RAPR in procedures in particular. However, this can cause relative hypovolaemia and decreased perfusion in compressed tissues in robotic prostatectomy procedures, resulting in increased risk for RM. In our study, hydration with 2000 ml crystalloid solution was also provided until 24 h after surgery.
Besides, it was reported that re-positioning by 2-h intervals during surgery could be helpful to prevent RM development although we could not use re-positioning due to the inability of operation table used in our study.[13]
In addition, robotic surgery by itself might have an impact on RM. Recently Gelpi-Hammerschmidt et al. performed a study to compare the outcomes of robotic, laparoscopic and open surgery regarding RM. They found that robotic surgery associated with higher risk of post-operative RM. The authors postulated that the lack of tactile feedback may cause excess tension or muscular compression from instruments attached to the surgical robot, a factor not present in open surgery or pure laparoscopy.[14]
In RM, stable haemodynamic is of importance, since acidosis and profound hypotension can worsen RM.[15] In our study, it was found that serum CPK levels were increased by enhancing acidosis and that there was a weak negative correlation between CPK and pH (r = −0.317, P = 0.022).
Diagnostic criteria for RM include severe muscular pain at lumbar, pelvic and gluteal region immediately after laparoscopic surgery, 5-fold increase than normal serum CPK levels in the absence of myocardial ischaemia (CPK > 1000 IU/L) and the presence of myoglobinuria.[5,12] In general, serum CPK, LDH, AST and ALT values are used in the follow-up.[16] Molecular mechanism linking hepatic dysfunction to RM has not been fully elucidated and it is considered to be multifactorial. However, serum cTpI elevation can be seen in some cases despite lacking go cardiac ischaemia. Punukollu et al. found serum cTpI elevation (>0.6) in 21% of the cases with RM in the absence of electrocardiogram changes and findings of acute coronary syndrome.[17] It was suggested that this could be due to myocardial injury, acidosis and hypoperfusion resulting from the release of reactive oxygen species and cytokines into circulation due to inflammatory process induced by recruitment of granulocytes to the damaged muscle tissue. In our study, serum elevation or acute coronary syndrome findings were not observed during or after surgery.
In our study, there was gluteal pain in all cases with RM; however, similar complaints were also observed in the patients without RM. Again, elevated serum CPK levels were detected in all patients due to traumatic effect of surgical procedure, but serum CPK levels reached up to a level that was diagnostic for RM. In agreement with literature, mild elevation was detected in serum AST, CK-MB and LDH levels. It was found that serum CPK level was positively correlated to serum Cr, AST and LDH values while there was a weak negative correlation between serum CPK level and pH value. In patients with RM, serum CPK, LDH, AST and Cr levels were increased when compared to baseline values. These findings suggest that monitorisation of serum CPK levels can be important in patients and surgical interventions with risk for RM and should not be omitted.
The presence of myoglobinuria is a diagnostic criterion for RM; however, myoglobinuria can be observed when plasma myoglobin value reaches to 0.5–1.5 mg/dL. In some cases, there may be an intermittent increase in myoglobin and it can be failed to detect myoglobin in urine. The absence of myoglobin in either serum or urine cannot exclude RM since it returns to normal level 6 h after muscular injury.[5] There is a typical reddish-brown discoloration in urine in the presence of myoglobinuria. However, it was impossible to assess characteristic urinary discoloration related to myoglobinuria in our cases since all patients included underwent radical prostatectomy in our study.
It is well known that CPK is not highly specific but sensitive marker for RM. Various studies considered CPK as a prognostic factor of RM. However, there is controversy in this concern. Although most studies declare a significant correlation between CPK and RM, there are a few opposite reports. In literature, most of the studies used this marker to identify RM and to show their outcomes.[18] In a more recent systemic review and meta-analysis showed a positive correlation between CPK- and RM-induced acute kidney injury.[19]
In general, serum CPK level reaches to a peak level on 12 h and begins to decrease 1–3 days after reaching peak level. The extent of elevation in serum CPK level is proportional to muscular mass involved. Serum CPK level > 16,000 IU/L has found to be associated with RF.[20]
In our study, serum CPK levels in patients with RM were increased on 6 h (1878.14 ± 2376.79), reaching to peak value on 12 h (13,038.00 ± 8629.26), and started to decrease by 24 h in agreement with literature. These patients were managed with appropriate fluid therapy, and no RF was developed.
It is well known that obese patients comprise a high-risk group for perioperative complications. In our study, mean BMI was 29 in the study population while it was 30 in patients with RM. In the literature, there are many cases series on RM development due to prolonged surgical procedures in morbid obese patients. In particular, RM rate up to 22.7% was reported in laparoscopic bariatric surgeries.[3,20]
In a study on 324 cases underwent laparoscopic bariatric surgery, Faintuch et al. detected RM in 16 patients (4.9%) and reported that RF was risk for only those with elevated serum CPK level and pain, moderate elevation in serum CPK could be tolerated well, and that it was rarely associated with serious clinical impact.[6]
In a study of 315 patients (mean age: 66 years; mean BMI: 31 kg/m2) underwent minimally invasive spine surgery at the prone position, Dakwar et al. reported that dialysis was required in 2 patients with RM. Authors found RM in 5 of 315 patients with mean OT of 420 min, and that mean serum CPK level was 25,861.[21] In our study, RM was detected in 7 of 52 patients with mean age of 61.9 years, mean BMI of 29.83 and mean OT of 215.48 min. In patients with RM, mean BMI was 220 min (P = 0.424, P > 0.05) whereas mean BMI was 32. Higher incidence of RM in our study despite shorter mean OT and lower mean age compared to the study by Dakwar et al. was attributed to laparoscopic robotic surgery, fluid restriction and positional effects of surgery.[21]
Intraperitoneal CO2 insufflation used in laparoscopic surgery also plays a role in the development of RM. Intraperitoneal CO2 insufflation increases intra-abdominal pressure up to 12–15 mmHg and can further impair poor perfusion, particularly in obese patients. It is important to use intra-operative padding, intra-operative repositioning and restriction of OT to protect these patients against RM. Once RM is identified, early diagnosis and treatment can prevent long-term complication.[2,22]
In our study, no significant differences were detected in OT, TT and BMI values between patients with or without RM; however, there was a trend towards higher BMI value in patients with RM. In addition, it was found that CPK levels did not correlate to OT, TT and BMI. In our study, silicone padding was employed in compression sites with soft surgical mattress and close haemodynamic monitorisation was performed in all patients since mean BMI was about 30 in the study population.
In a study comparing three different surgical position in neurosurgical cases, Targa et al. reported that RM development was associated to OT with no RM in cases with OT of 3.5 h, OT was longer than 5 h in cases with ARF development, and that surgeries longer than 5 h were most significant risk factor for RM.[23] In our study, RM was found in 7 patients despite mean OT of 3.5 h (215.48 min) and mean TT of 3 h (184.32 min). It is thought that this was due to STP used in surgery, comorbid diseases in this age group of patients and mean BMI reaching to the threshold value of obesity.
The impact of comorbidities on survival can vary in different cancers. The effect of comorbidities on survival is limited in rapidly progressing, aggressive, refractory cancers while comorbidities can be the primary factor predicting survival of the patients in cancer types with slow progression and higher response rates such as prostate cancer. In many studies on cancer patients, CCI was used to predict mortality related to chronic disease.[7,24] In our study, CCI score was significantly higher in patients with RM when compared to those without (3.00 vs. 2.07; P = 0.001, P < 0.01). Based on these results, we think that comorbidities may comprise a risk for RM in patients with prostate cancer.
It is thought that RF, a most serious complication of RM and incidence was reported to be 15–46% with death occurring in 3.4% of cases.[7] Thus, it is recommended to ensure diuresis of 200–300 cc/h by administering 500 cc/h crystalloid solutions in patients at risk when CPK level was increased by 2- or 3-folds. Aggressive fluid resuscitation with monitoring central venous pressure is a widely accepted treatment approach. Although alkalinisation of urine is recommended in patients with CPK level ≥6000, it should be initiated earlier in patients with acidaemia, dehydration or underlying renal disorder. It is recommended to infuse to maintain urine pH above 7.[25] In our study, urinary alkalisation with intravenous NaHCO3 infusion was employed in one patient with CKP level >56,000.
Dehydration and acidosis resulting from fluid restriction comprise additional risk for RF development. It was suggested that mannitol could be given to improve renal perfusion and loop diuretic to increase urine output after ensuring appropriate intravascular volume in such patients.[26] In advanced RF, haemodialysis should not be delayed. Haemodialysis indications include serum Cr level >1.5 mg/dL, base deficit > −4, serum CPK value >5000 and presence of myoglobin.[27] In our study, serum Cr levels were within normal range, and there was no patient with base deficit > −4. In addition, no RF was developed in 7 patients with RM and no complication was experienced in patients with elevated serum CPK levels by early hydration. All patients were discharged within 3 days.
Limitations
The limitation of the study is lack of a control group undergoing robotic surgery at same position without fluid restriction.
CONCLUSIONS
We found that RM can develop in RARP procedures although it is defined as minimally invasive surgery and that comorbid conditions are more important in the development of RM rather than BMI, OT and TT. Patients with higher comorbidity are at risk for RM development and that this should be kept in mind at follow-up and when informing patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Vijay MK, Vijay P, Kundu AK. Rhabdomyolysis and myogloginuric acute renal failure in the lithotomy/exaggerated lithotomy position of urogenital surgeries. Urol Ann. 2011;3:147–50. doi: 10.4103/0974-7796.84965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim TK, Yoon JR, Lee MH. Rhabdomyolysis after laparoscopic radical nephrectomy – A case report. Korean J Anesthesiol. 2010;59(Suppl 1):S41–4. doi: 10.4097/kjae.2010.59.S.S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leroux C, Béliard C, Theolat M, Testa S, Péan D, Moreau D, et al. Postoperative antero-external tibial compartment syndrome: Co-responsibility of the operating table. Ann Fr Anesth Reanim. 1999;18:1061–4. doi: 10.1016/s0750-7658(00)87440-7. [DOI] [PubMed] [Google Scholar]
- 4.Sukegawa I, Miyabe M, Fujii T, Hoshi T, Takahashi S, Toyooka H. Rhabdomyolysis after nephrectomy in the lateral flexed decubitus position. Masui. 2003;52:882–5. [PubMed] [Google Scholar]
- 5.Mrsic V, Nesek Adam V, Grizelj Stojcic E, Rasic Z, Smiljanic A, Turcic I. Acute rhabdomyolysis: A case report and literature review. Acta Med Croatica. 2008;62:317–22. [PubMed] [Google Scholar]
- 6.Faintuch J, de Cleva R, Pajecki D, Garrido AB, Jr, Cecconello I. Rhabdomyolysis after gastric bypass: Severity and outcome patterns. Obes Surg. 2006;16:1209–13. doi: 10.1381/096089206778392202. [DOI] [PubMed] [Google Scholar]
- 7.Reisiger KE, Landman J, Kibel A, Clayman RV. Laparoscopic renal surgery and the risk of rhabdomyolysis: Diagnosis and treatment. Urology. 2005;66(5 Suppl):29–35. doi: 10.1016/j.urology.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 10.Skarecky DW. Robotic-assisted radical prostatectomy after the first decade: Surgical evolution or new paradigm. ISRN Urol. 2013;2013:157379. doi: 10.1155/2013/157379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brede CM, Lane BR. Prevention, diagnosis and treatment of rhabdomyolysis after urological surgery. J Urol. 2016;195:245–6. doi: 10.1016/j.juro.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Karaoren G, Bakan N, Yuruk CT, Cetinkaya AO. Effects of bowel preparation and fluid restriction in robot-assisted radical prostatectomy patients. Turk J Anaesthesiol Reanim. 2015;43:100–5. doi: 10.5152/TJAR.2014.57704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galyon SW, Richards KA, Pettus JA, Bodin SG. Three-limb compartment syndrome and rhabdomyolysis after robotic cystoprostatectomy. J Clin Anesth. 2011;23:75–8. doi: 10.1016/j.jclinane.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Gelpi-Hammerschmidt F, Tinay I, Allard CB, Su LM, Preston MA, Trinh QD, et al. The contemporary incidence and sequelae of rhabdomyolysis following extirpative renal surgery: A population based analysis. J Urol. 2016;195:399–405. doi: 10.1016/j.juro.2015.08.084. [DOI] [PubMed] [Google Scholar]
- 15.Scharman EJ, Troutman WG. Prevention of kidney injury following rhabdomyolysis: A systematic review. Ann Pharmacother. 2013;47:90–105. doi: 10.1345/aph.1R215. [DOI] [PubMed] [Google Scholar]
- 16.Keene R, Froelich JM, Milbrandt JC, Idusuyi OB. Bilateral gluteal compartment syndrome following robotic-assisted prostatectomy. Orthopedics. 2010;33:852. doi: 10.3928/01477447-20100924-25. [DOI] [PubMed] [Google Scholar]
- 17.Punukollu G, Gowda RM, Khan IA, Mehta NJ, Navarro V, Vasavada BC, et al. Elevated serum cardiac troponin I in rhabdomyolysis. Int J Cardiol. 2004;96:35–40. doi: 10.1016/j.ijcard.2003.04.053. [DOI] [PubMed] [Google Scholar]
- 18.Mattei A, Di Pierro GB, Rafeld V, Konrad C, Beutler J, Danuser H. Positioning injury, rhabdomyolysis, and serum creatine kinase-concentration course in patients undergoing robot-assisted radical prostatectomy and extended pelvic lymph node dissection. J Endourol. 2013;27:45–51. doi: 10.1089/end.2012.0169. [DOI] [PubMed] [Google Scholar]
- 19.Safari S, Yousefifard M, Hashemi B, Baratloo A, Forouzanfar MM, Rahmati F, et al. The value of serum creatine kinase in predicting the risk of rhabdomyolysis-induced acute kidney injury: A systematic review and meta-analysis. Clin Exp Nephrol. 2016;20:153–61. doi: 10.1007/s10157-015-1204-1. [DOI] [PubMed] [Google Scholar]
- 20.Mognol P, Vignes S, Chosidow D, Marmuse JP. Rhabdomyolysis after laparoscopic bariatric surgery. Obes Surg. 2004;14:91–4. doi: 10.1381/096089204772787356. [DOI] [PubMed] [Google Scholar]
- 21.Dakwar E, Rifkin SI, Volcan IJ, Goodrich JA, Uribe JS. Rhabdomyolysis and acute renal failure following minimally invasive spine surgery: Report of 5 cases. J Neurosurg Spine. 2012;16:104. doi: 10.3171/2011.2.SPINE10369. [DOI] [PubMed] [Google Scholar]
- 22.Kikuno N, Urakami S, Shigeno K, Kishi H, Shiina H, Igawa M. Traumatic rhabdomyolysis resulting from continuous compression in the exaggerated lithotomy position for radical perineal prostatectomy. Int J Urol. 2002;9:521–4. doi: 10.1046/j.1442-2042.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 23.Targa L, Droghetti L, Caggese G, Zatelli R, Roccella P. Rhabdomyolysis and operating position. Anaesthesia. 1991;46:141–3. doi: 10.1111/j.1365-2044.1991.tb09362.x. [DOI] [PubMed] [Google Scholar]
- 24.Sarfati D. Review of methods used to measure comorbidity in cancer populations: No gold standard exists. J Clin Epidemiol. 2012;65:924–33. doi: 10.1016/j.jclinepi.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: Do bicarbonate and mannitol make a difference? J Trauma. 2004;56:1191–6. doi: 10.1097/01.ta.0000130761.78627.10. [DOI] [PubMed] [Google Scholar]
- 26.Hogg K. Rhabdomyolysis and the use of sodium bicarbonate and/or mannitol. Emerg Med J. 2010;27:305–8. doi: 10.1136/emj.2009.090530a. [DOI] [PubMed] [Google Scholar]
- 27.Kellum JA. Acute kidney injury. Crit Care Med. 2008;36(4 Suppl):S141–5. doi: 10.1097/CCM.0b013e318168c4a4. [DOI] [PubMed] [Google Scholar]


