Abstract
BACKGROUND:
The systemic impact of intra-abdominal pressure (IAP) and/or changes in carbon dioxide (CO2) during laparoscopy are not yet well defined. Changes in brain oxygenation have been reported as a possible cause of cerebral hypotension and perfusion. The side effects of anaesthesia could also be involved in these changes, especially in children. To date, no data have been reported on brain oxygenation during routine laparoscopy in paediatric patients.
PATIENTS AND METHODS:
Brain and peripheral oxygenation were investigated in 10 children (8 male, 2 female) who underwent elective minimally invasive surgery for inguinal hernia repair. Intraoperative transcranial near-infrared spectroscopy to assess regional cerebral oxygen saturation (rScO2), peripheral oxygen saturation using pulse oximetry and heart rate (HR) were monitored at five surgical intervals: Induction of anaesthesia (baseline T1); before CO2 insufflation induced pneumoperitoneum (PP) (T2); CO2 PP insufflation (T3); cessation of CO2 PP (T4); before extubation (T5).
RESULTS:
rScO2 decreases were recorded immediately after T1 and became significant after insufflation (P = 0.006; rScO2 decreased 3.6 ± 0.38%); restoration of rScO2 was achieved after PP cessation (P = 0.007). The changes in rScO2 were primarily due to IAP increases (P = 0.06). The HR changes were correlated to PP pressure (P < 0.001) and CO2 flow rate (P = 0.001). No significant peripheral effects were noted.
CONCLUSIONS:
The increase in IAP is a critical determinant in cerebral oxygenation stability during laparoscopic procedures. However, the impact of anaesthesia on adaptive changes should not be underestimated. Close monitoring and close collaboration between the members of the multidisciplinary paediatric team are essential to guarantee the patient's safety during minimally invasive surgical procedures.
Key words: Brain oxygenation, children, laparoscopy, near-infrared spectroscopy
INTRODUCTION
The successful application of laparoscopic surgery in adults has led to its increased use in the paediatric age, due to its minimally invasive nature.[1,2,3,4] The main advantages of laparoscopic surgery are a less post-operative pain, reduced wound complications, minimal scarring, a shorter hospital stay and an earlier return to normal activities including feeding and bowel movements, and return to school, when compared to traditional open surgery.[1,2,3,4] However, the procedure requires insufflation of the peritoneal cavity with an inert gas; the most commonly carbon dioxide (CO2). Although CO2 is generally accepted as the optimal gas for insufflation, increases in PaCO2 and resultant alterations in cerebral blood flow (CBF) may occur.[3,5,6,7] In addition, peritoneal insufflation and the associated increase in intra-abdominal pressure (IAP) result in increased systemic vascular resistance and decreased cardiac output.[5,6,7,8,9,10] These systemic haemodynamic alterations may result in changes in end-organ blood flow and oxygen delivery. To date, data regarding alterations in cerebral oxygenation during laparoscopic procedures in children are limited.[11,12,13,14,15]
This preliminary study uses near-infrared spectroscopy (NIRS) to test whether changes in cerebral oxygenation during the laparoscopic routine intervention for abdominal procedures in paediatric patients are primarily due to the increase in IAP or to changes in CO2 or both, and whether this has a specific effect on the brain or also the systemic circulation. We anticipated that the prevalent effect of the reduction in venous return would negatively affect cerebral oxygenation and should be a function of the insufflation pressure, whereas the increase in CO2 would have a minor role, due to its vasodilating effect on cerebral circulation. In addition, by comparing the effect of insufflation on cerebral and peripheral (finger) tissue oxygenation, we can assess whether the consequences of CO2 insufflation are essentially cerebral or systemic. Finally, the impact of the anaesthesia-induced hypotension on brain perfusion was also considered.
PATIENTS AND METHODS
Patients
We investigated brain and peripheral oxygenation in ten children who underwent elective laparoscopic abdominal surgery to treat of inguinal hernia. Surgery was performed, by an expert surgeon, under general anaesthesia, at the Paediatric Surgery Unit. Patients were recruited between April 01, 2015, and July 31, 2015. Written consent was obtained from the parents of the children before the scheduled surgical procedure. The study was performed according to the Declaration of Helsinki and with the approval of the Institutional Review Board.
Surgery
All surgery was performed in the same operating theatre with a stable temperature of 22 ± 1°C. Patients were placed in the supine position on a heated operating table (36 ± 1°C). Laparoscopic herniorrhaphy was accomplished, following the standard protocol, through the transabdominal approach using one 3 mm telescope and two, 3 mm or 2 mm operative instruments, placed into the lower abdomen. The pneumoperitoneum (PP) was created through the 3 mm infraumbilical camera-trocar placed via an open approach. The PP CO2 pressure which ranged from 8 to 12 mmHg (8 mmHg in children weighing <15 kg; 10 mmHg: 15–40 kg; 12 mmHg: >40 kg) was achieved with a CO2 insufflation flow rate of 1–5 L/min.
A 4-0/5-0 polypropylene monofilament suture was inserted directly through the abdominal wall.
A double N suture of the internal inguinal ring was performed with intracorporeal knots. Intraoperative diagnosis and surgical repair of contralateral hernias were performed in case of pre-operative undiagnosed contralateral hernia. All operations were performed on a day hospital basis. Patients were followed for 1 week.
Data acquisition
Intraoperative transcranial NIRS to assess regional cerebral oxygen saturation (rScO2), pulse oximetry to measure peripheral oxygen saturation (SpO2) using and heart rate (HR) were monitored continuously during the entire procedure.
Changes in rScO2 were obtained using a near-infrared spectrometer. Prior to anaesthesia induction, a transducer was placed on the frontal side of the child's head and attached with an elastic bandage to prevent displacement. The oximeter sensor was positioned on the middle finger of the left hand. HR was recorded during scanning also using pulse oximetry for heart timing and an index of pulse amplitude.
Indirect measurement of IAP during surgery was performed by the transvesical method; measurements were recorded with Medical Measurement Systems®.
The surgeon and anaesthetist were blinded to the readings to prevent intraoperative alterations in patient management.
The following parameters were analysed in five intervals (for 5 min):
Induction of anaesthesia (baseline T1);
Before CO2 PP insufflation (T2);
During CO2 PP insufflation (T3);
Cessation of the CO2 PP (T4);
Before extubation (T5).
The operative time was recorded, including the data acquisition and the anaesthesia duration (interval from beginning of induction to cessation of sevoflurane inhalation).
Anaesthesia protocol
All of the children were in good physical condition and received standardised anaesthesia, which included propofol (2–2.5 mg/kg) as a sedative-hypnotic agent and fentanyl (1–1.5 mcg/kg) as the analgesic. After tracheal tube positioning, patients underwent volume-controlled mechanical ventilation with an inspired mixture of air and oxygen using a closed breathing system (fresh gas flow of 0.75 L/min oxygen and 1.5 L/min air during anaesthesia) adjusted to achieve an end-tidal CO2 level of 32–35 mmHg. Anaesthesia was maintained via administration of sevoflurane gas (0.75–1.25 minimum alveolar concentration range). Hypotension was not prevented with fluid expansion or inotropes. Twenty minutes before the end of the intervention, all patients received paracetamol 15 mg/kg, as an analgesic.
Statistical analysis
Quantitative variables were described as the mean (standard deviation) and compared between the different time intervals with population averaged mixed multilevel models to take into account the clustered nature of the data.
P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistical package (SPSS Inc., Chicago, IL, USA) and Stata 8.0.
RESULTS
The study included 10 children (8 males and 2 females) ranging in age from 1.6 to 15.8 years (8.36 ± 5.79 years) and in weight from 10 to 65 kg (34.0 ± 22.39 kg). The mean operating time was 60.0 ± 27.8 min. The mean CO2 PP pressure was 10.6 ± 1.56 mmHg, and CO2 flow rate was 3.40 ± 1.95 L/min. The IAPs at the different surgical intervals are reported in Table 1. No intraoperative complications occurred.
Table 1.
Intra-abdominal pressure, at different surgical intervals

Regional cerebral oxygen saturation
After anaesthesia induction and before CO2 PP insufflation an initial decrease in SaO2 was observed (P = 0.15). A significant rScO2 decrease was reached at T3 compared to baseline values (P = 0.006; decrease at T3–3.6 ± 0.38%), Figure 1. Oxygen saturation restoration was achieved at T5 after CO2 PP cessation (P = 0.006).
Figure 1.
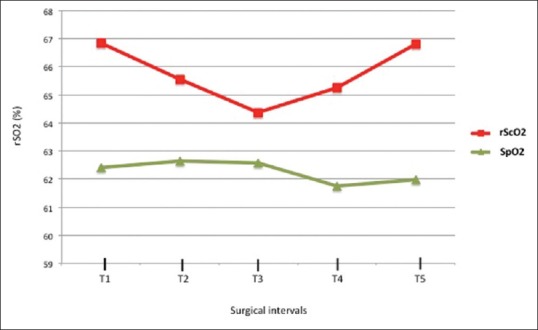
Cerebral and peripheral oxygen saturation at different surgical intervals
The changes in rScO2 strongly correlated with the IAP (P = 0.007), Figure 2. Correlation between rScO2 and CO2 PP pressure was also noted, however without reaching a significant value (P = 0.06). rScO2 changes did not correlate with CO2 flow rate.
Figure 2.
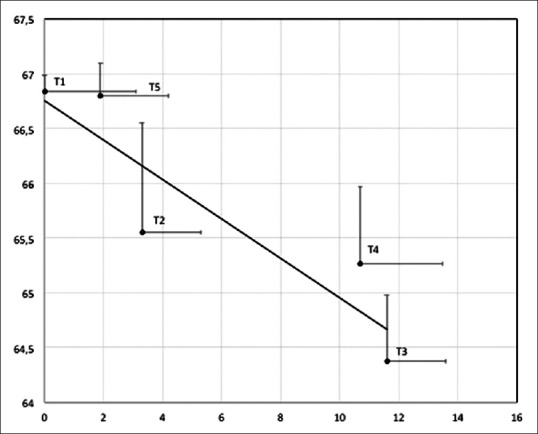
Correlation between regional cerebral oxygen saturation and intra-abdominal pressure at different surgical intervals (P = 0.007). Data are expressed as mean ± standard error
Peripheral oxygen saturation
The SpO2 values were not statistically different during the laparoscopic surgical intervals (P = 0.3), Figure 1. No significant associations between SpO2 and CO2 PP pressure (P = 0.15) or CO2 flow rate (P = 0.06) or IAP (P = 0.9) were reported.
Heart rate
Increased in HR were noted in T3, at the CO2 PP insufflation [Figure 3], but were not statistically significant (P = 0.5). The HR changes correlated with the PP pressure (P < 0.001) and CO2 flow rate (P = 0.001).
Figure 3.
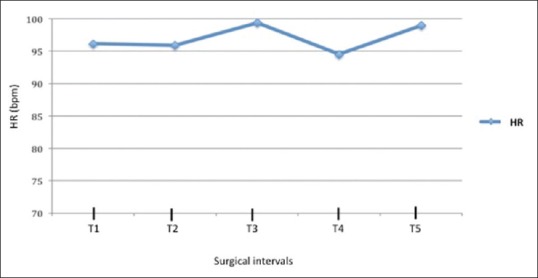
Heart rate changes at different surgical intervals
DISCUSSION
In this preliminary study, we showed a significant change in cerebral oxygenation during traditional abdominal laparoscopic inguinal hernia repair in paediatric patients. IAP seems to primarily induce changes in rScO2, with no significant peripheral impact. A significant decrease in cerebral oxygen saturation was reached after insufflation and the restoration of oxygen saturation were achieved after cessation of PP. The impact of anaesthesia on brain oxygenation changes was also evidenced.
Laparoscopy has is becoming a standard operative procedure in paediatrics while it requires further technical advances and anaesthesiologic innovations in very small children and infants. As in adults, the most important advantages it offers, include less tissue trauma, decreased post-operative pain, as well as a shorter hospital stay with minimised postoperative morbidity and mortality.[1,2,3,4] CO2 is the most commonly used gas to induce PP, because of its high diffusibility and rapid rate of absorption and excretion. However, peritoneal gas insufflation during laparoscopy may lead to possibly harmful cardiovascular and respiratory alterations that could possibly be detrimental for the patient.[5,6,7,8,9,10,13,14,16] PP exerts its effects on organ systems primarily via physical pressure on those systems and secondly through the systemic absorption of CO2 across the peritoneum and into the bloodstream.
The cerebrovascular system is known to undergo adaptive changes during laparoscopic surgery.[3] The effects of CO2 PP on cerebral haemodynamics have been investigated in adults[17,18,19] and animals,[20,21,22] but not exhaustively in children. The studies focusing on the relationship between laparoscopic surgery and regional effects on cerebral oxygenation in children are limited in number with non-homogenous data. Tsypin et al.[11] reported a 3% average of reduction in regional cerebral tissue saturation in children during gynaecological laparoscopic interventions; Tuna et al.,[15] showed that CO2 insufflation during PP in paediatric patients may not influence cerebral oxygenation under laparoscopic surgery; Tytgat et al.,[12] described that regional brain oxygenation remained unaltered during surgical treatment of hypertrophic pyloric stenosis in young infants; Bishay et al.[14] showed a significative decrease in cerebral oxygenation in infants who underwent thorascopic surgery for the treatment of esophageal atresia and congenital diaphragmatic hernia.
In this report, in paediatric patients, an average of 3.5% reduction in cerebral oxygenation during laparoscopic routine interventions for abdominal procedures was observed at T3. The changes in rScO2 were strongly correlated to the IAP. These data confirm that IAP is a critical determinant of cardiovascular stability during laparoscopy. IAP supports splanchnic vasoconstriction and reduction in blood flow through the inferior cava vein, renal and portal vein, which all result in decreased venous flow to the heart. In the paediatric age, an IAP of more than 15 mmHg leads to a decrease in cardiac output, even if the mean arterial pressure is maintained by increasing systemic vascular resistance. IAPs between 8 and 10 mmHg are usually recommended to keep physiological changes to a minimum in children. In our patients, the mean recorded insufflation was 10.6 mmHg (±1.5). During insufflation, haemodynamic redistribution caused HR variations to occur. The prevalent effect of IAP on brain oxygenation, the correlation between HR changes and CO2 flow rate indicate that a neurohumoral CO2 effect is also involved. CO2 stimulates the sympathoadrenal system causing a significant release of catecholamines and cortisol and thus increased HR. The absorption of CO2 during positive pressure PP may lead to an increased CO2 load, and CO2 elimination is related to ages with younger children eliminating more CO2 than older children; there may also be a different CO2 reabsorption between infants and children because of the different characteristics in their peritoneal surface.[23] These findings suggest that infants and small children (weighing <10–15 kg) warrant close monitoring during laparoscopy and the immediate post-operative period.
The impact of CO2 insufflation was essentially cerebral and did not affect peripheral oxygenation in our patients. The increased IAP caused by the CO2 PP did not decrease peripheral oxygenation at an acceptable IAP with regard peripheral perfusion.
The sensitivity of the cerebrovascular system may vary with age,[24] moreover in all ages diminished perioperative cerebral oxygen saturation is correlated with poor neurodevelopment outcomes.[25] NIRS is a non-invasive device that uses infrared light (a technique similar to pulse oximetry) to penetrate living tissue and estimate brain tissue oxygenation by measuring the absorption of infrared light by tissue chromophores.[26] This technology can identify deficits in cerebral oxygenation, thus guiding for interventions or therapy to reverse cerebral oxygenation issues thereby and prevent long-term neurological sequelae. NIRS is used extensively during cardiac and non-cardiac surgery to monitor cerebral and somatic perfusion. Cerebral oximetry with NIRS may also be a helpful monitoring tool for detecting real-time rScO2 changes during paediatric laparoscopic procedures, in which PP is created with CO2 and to prevent any potential decrease in brain oxygenation.[3] Regional SO2 decreases from baseline or a regional SO2 <50% are associated with a high incidence of cerebral ischemia, post-operative cognitive dysfunction, and longer hospital stays.[3,15,27] We found an SO2 < 5% reduction in brain tissue saturation in children during routine laparoscopy; thus long-term neurological outcome should be evaluated in children and particularly in infants whose cerebral metabolic reserve seems to be lower and poorly tolerated by the brain.[28,29]
Cerebral autoregulation and CO2 reactivity, two homeostatic mechanisms important for the control of CBF, are impaired by inhalation anaesthesia. The effects on CBF depend on the balance between its indirect vasoconstrictive action, which is secondary to flow-metabolism coupling, and its direct vasodilatory properties. CO2 reactivity, CBF velocity, and cerebral autoregulation seem to remain intact during sevoflurane or propofol administration. However, Rhondali et al.[29] showed how the use of sevoflurane anaesthesia in children younger than 2 years had a great impact on brain oxygenation, inducing a reduction in the mean arterial pressure associated with rSO2 variations; 15 min after induction increased SO2 was observed, but this decrease was lower in children aged <6 months.[29] The timing of paediatric autoregulation is not yet defined.
In our study, a standardised anaesthetic protocol including propofol and sevoflurane was used, and data recorded at T2 suggest that the influence of the anaesthesia on cerebral circulation should be taken into account during laparoscopy. The non-significant increase in HR values after induction, and before insufflation (T1–T2, average 10 min), supports the vasodilatory property of sevoflurane on haemodynamic mechanisms in children when no preventive treatment for anaesthesia-induced hypotension is adopted. Similar side effects have already been described during general anaesthesia for non-laparoscopic procedures in paediatric surgery. Treatment with fluid expansion or inotropes to prevent hypotension, during the anaesthesia protocol, may be considered a determining factor to properly define the consequences of CO2 insufflation. The different approaches to anaesthesiological management could also explain the non-homogenous results regarding alterations in cerebral oxygenation during laparoscopic procedures in children.[11,12,13,14]
This preliminary report should be viewed in light of some limitations of the study. The sample size was limited and a larger number of children are mandatory to confirm the results, to analyse cerebral oxygenation at different ages; different types of surgery (open vs. laparoscopy), different insufflation pressures and anaesthetic agents. The timing of the cerebral autoregulation also needs to be defined. Secondly, we did not monitor PaCO2 changes during CO2 insufflation. It has been reported that end-tidal CO2 pressure may not correlate with PaCO2, therefore, arterial blood gas analysis monitoring would be useful during laparoscopic procedures.[15] In our study, the routine laparoscopic inguinal hernia repair procedure did not allow for invasive PaCO2 monitoring. Finally, no data on brain oxygenation effects were followed post-operatively.
Despite these limitations, this study points to new study directions to ameliorate our knowledge of pathophysiological age-related changes during mini-invasive surgery.[30] Paediatric laparoscopy could benefit from a new dedicated anaesthesiological approach.[30]
CONCLUSIONS
Cerebral oxymetry with NIRS may be useful to anticipate any potential decrease in brain oxygenation and protect the neurodevelopmental outcome in paediatric invasive and non-invasive surgical procedures. The increase in IAP is a critical determinant in cerebral oxygenation stability during laparoscopic surgery in paediatrics, even though the impact of anaesthetic agents on adaptive changes should be taken into consideration when the anaesthesia protocol, does not call for fluid expansion and inotropes.
Our data suggest that a dedicated paediatric anaesthesiological team, close monitoring and close collaboration between paediatric surgeons, paediatricians, anaesthesiologists are essential to guarantee the child's safety during all type of surgery and particularly during the laparoscopic approach in infants and small children, whose cerebral metabolic reserve is low. Better outcomes in mini-invasive surgery could be obtained in the near future even for the treatment of multi-step paediatric congenital pathologies in small infants.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank Dr. L. Kelly for English revision of the manuscript.
REFERENCES
- 1.Tam PK. Laparoscopic surgery in children. Arch Dis Child. 2000;82:240–3. doi: 10.1136/adc.82.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Truchon R. Anaesthetic considerations for laparoscopic surgery in neonates and infants: A practical review. Best Pract Res Clin Anaesthesiol. 2004;18:343–55. doi: 10.1016/j.bpa.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Moka E. Cerebral oximetry and laparoscopic surgery. J Minim Access Surg. 2006;2:47–8. doi: 10.4103/0972-9941.26644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta R, Singh S. Challenges in paediatric laparoscopic surgeries. Indian J Anaesth. 2009;53:560–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Hodgson C, McClelland RM, Newton JR. Some effects of the peritoneal insufflation of carbon dioxide at laparoscopy. Anaesthesia. 1970;25:382–90. doi: 10.1111/j.1365-2044.1970.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 6.Wittgen CM, Andrus CH, Fitzgerald SD, Baudendistel LJ, Dahms TE, Kaminski DL. Analysis of the hemodynamic and ventilatory effects of laparoscopic cholecystectomy. Arch Surg. 1991;126:997–1000. doi: 10.1001/archsurg.1991.01410320083011. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen NT, Wolfe BM. The physiologic effects of pneumoperitoneum in the morbidly obese. Ann Surg. 2005;241:219–26. doi: 10.1097/01.sla.0000151791.93571.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponsky JL. Complications of laparoscopic cholecystectomy. Am J Surg. 1991;161:393–5. doi: 10.1016/0002-9610(91)90605-d. [DOI] [PubMed] [Google Scholar]
- 9.Westerband A, Van De Water J, Amzallag M, Lebowitz PW, Nwasokwa ON, Chardavoyne R, et al. Cardiovascular changes during laparoscopic cholecystectomy. Surg Gynecol Obstet. 1992;175:535–8. [PubMed] [Google Scholar]
- 10.McLaughlin JG, Scheeres DE, Dean RJ, Bonnell BW. The adverse hemodynamic effects of laparoscopic cholecystectomy. Surg Endosc. 1995;9:121–4. doi: 10.1007/BF00191950. [DOI] [PubMed] [Google Scholar]
- 11.Tsypin LE, Mikhel'son VA, Chusov KP, Kazharskaia EIu, Lazarev VV, Prokop'ev GG, et al. Central and cerebral hemodynamics during gynecological laparoscopic interventions in children. Anesteziol Reanimatol. 2007;1:30–2. [PubMed] [Google Scholar]
- 12.Tytgat SH, Stolwijk LJ, Keunen K, Milstein DM, Lemmers PM, van der Zee DC. Brain oxygenation during laparoscopic correction of hypertrophic pyloric stenosis. J Laparoendosc Adv Surg Tech A. 2015;25:352–7. doi: 10.1089/lap.2014.0592. [DOI] [PubMed] [Google Scholar]
- 13.Tytgat SH, van Herwaarden MY, Stolwijk LJ, Keunen K, Benders MJ, de Graaff JC, et al. Neonatal brain oxygenation during thoracoscopic correction of esophageal atresia. Surg Endosc. 2015 doi: 10.1007/s00464-015-4559-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishay M, Giacomello L, Retrosi G, Thyoka M, Nah SA, McHoney M, et al. Decreased cerebral oxygen saturation during thoracoscopic repair of congenital diaphragmatic hernia and esophageal atresia in infants. J Pediatr Surg. 2011;46:47–51. doi: 10.1016/j.jpedsurg.2010.09.062. [DOI] [PubMed] [Google Scholar]
- 15.Tuna AT, Akkoyun I, Darcin S, Palabiyik O. Effects of carbon dioxide insufflation on regional cerebral oxygenation during laparoscopic surgery in children: A prospective study. Rev Bras Anestesiol. 2016:pii: S0034-709400038-6. doi: 10.1016/j.bjane.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Westgarth-Taylor C, de Lijster L, van Bogerijen G, Millar AJ, Karpelowsky J. A prospective assessment of renal oxygenation in children undergoing laparoscopy using near-infrared spectroscopy. Surg Endosc. 2013;27:3696–704. doi: 10.1007/s00464-013-2950-3. [DOI] [PubMed] [Google Scholar]
- 17.Kitajima T, Shinohara M, Ogata H. Cerebral oxygen metabolism measured by near-infrared laser spectroscopy during laparoscopic cholecystectomy with CO2 insufflation. Surg Laparosc Endosc. 1996;6:210–2. [PubMed] [Google Scholar]
- 18.Fujii Y, Tanaka H, Tsuruoka S, Toyooka H, Amaha K. Middle cerebral arterial blood flow velocity increases during laparoscopic cholecystectomy. Anesth Analg. 1994;78:80–3. doi: 10.1213/00000539-199401000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Kitajima T, Okuda Y, Yamaguchi S, Takanishi T, Kumagai M, Ido K. Response of cerebral oxygen metabolism in the head-up position during laparoscopic cholecystectomy. Surg Laparosc Endosc. 1998;8:449–52. [PubMed] [Google Scholar]
- 20.Josephs LG, Este-McDonald JR, Birkett DH, Hirsch EF. Diagnostic laparoscopy increases intracranial pressure. J Trauma. 1994;36:815–8. doi: 10.1097/00005373-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Este-McDonald JR, Josephs LG, Birkett DH, Hirsch EF. Changes in intracranial pressure associated with apneumic retractors. Arch Surg. 1995;130:362–5. doi: 10.1001/archsurg.1995.01430040024002. [DOI] [PubMed] [Google Scholar]
- 22.Halverson A, Buchanan R, Jacobs L, Shayani V, Hunt T, Riedel C, et al. Evaluation of mechanism of increased intracranial pressure with insufflation. Surg Endosc. 1998;12:266–9. doi: 10.1007/s004649900648. [DOI] [PubMed] [Google Scholar]
- 23.McHoney M, Corizia L, Eaton S, Kiely EM, Drake DP, Tan HL, et al. Carbon dioxide elimination during laparoscopy in children is age dependent. J Pediatr Surg. 2003;38:105–10. doi: 10.1053/jpsu.2003.50021. [DOI] [PubMed] [Google Scholar]
- 24.de Waal EE, de Vries JW, Kruitwagen CL, Kalkman CJ. The effects of low-pressure carbon dioxide pneumoperitoneum on cerebral oxygenation and cerebral blood volume in children. Anesth Analg. 2002;94:500–5. doi: 10.1097/00000539-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kussman BD, Wypij D, Laussen PC, Soul JS, Bellinger DC, DiNardo JA, et al. Relationship of intraoperative cerebral oxygen saturation to neurodevelopmental outcome and brain magnetic resonance imaging at 1 year of age in infants undergoing biventricular repair. Circulation. 2010;122:245–54. doi: 10.1161/CIRCULATIONAHA.109.902338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobias JD. Cerebral oxygenation monitoring: Near-infrared spectroscopy. Expert Rev Med Devices. 2006;3:235–43. doi: 10.1586/17434440.3.2.235. [DOI] [PubMed] [Google Scholar]
- 27.Casati A, Spreafico E, Putzu M, Fanelli G. New technology for noninvasive brain monitoring: Continuous cerebral oximetry. Minerva Anestesiol. 2006;72:605–25. [PubMed] [Google Scholar]
- 28.Rhondali O, André C, Pouyau A, Mahr A, Juhel S, De Queiroz M, et al. Sevoflurane anesthesia and brain perfusion. Paediatr Anaesth. 2015;25:180–5. doi: 10.1111/pan.12512. [DOI] [PubMed] [Google Scholar]
- 29.Rhondali O, Juhel S, Mathews S, Cellier Q, Desgranges FP, Mahr A, et al. Impact of sevoflurane anesthesia on brain oxygenation in children younger than 2 years. Paediatr Anaesth. 2014;24:734–40. doi: 10.1111/pan.12397. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed M, Nessa M, Islam MS, Siddiq AK. Effects of pneumoperitoneum during laparoscopic surgery in young children. JAFCM Bangledesh. 2009;5:18–20. [Google Scholar]


