Abstract
This review addresses the effectiveness and safety of human papillomavirus (HPV) vaccines, the current status of its introduction in the National Immunization Programmes (NIPs) and its relevance to India, which contributes a fifth of the global burden of cervical cancer. The vast literature on efficacy, acceptability and safety of HPV vaccination and its impact after population level introduction was reviewed and discussed. The efficacy of HPV vaccines in preventing high-grade precancerous lesions caused by vaccine-targeted HPV infections was 90 per cent or higher in HPV naïve women in randomized clinical trials. Two doses at 6 or 12 months apart are recommended for 9-14 yr old girls and three doses over six months to one year period for those aged above 15 yr. More than 80 countries or territories have introduced HPV vaccination in their NIPs, of which 33 are low- and middle-income countries (LMICs); in addition, 25 LMICs have introduced pilot programmes before a phased national expansion. Significant reductions in the frequency of HPV 16 and 18 infections, genital warts and cervical premalignant lesions in vaccinated cohorts and herd immunity in general populations have been reported from countries that introduced vaccination in NIPs as early as 2007. More than 280 million doses of HPV vaccines have been administered worldwide with the excellent safety profile with no serious adverse events linked to it. The high burden of cervical cancer and the high efficacy and safety of HPV vaccination justify its introduction in the Indian NIP at the earliest possibility to substantially reduce the cervical cancer burden in future.
Keywords: Cervix cancer, control, efficacy, human papillomavirus vaccination, India, national programmes, prevention, safety
Human papillomavirus-related cervical cancer burden
Cervical cancer is the fourth leading cancer in women globally, but its major burden is felt in the low- and middle-income countries (LMICs) with very limited resources to introduce and sustain effective population-based cervical cancer screening programmes. In most Asian countries including India, cervical cancer is the second most common cancer in women1. Cervical cancer accounts for 528,000 cases in the world (445,000 cases in LMICs); it causes 265,700 estimated deaths annually globally, with 230,200 (86.6%) deaths in the LMICs2. The age-standardized incidence rate of cervical cancer varies between 5.6 and 24.3 per 100,000 women in different regions of India3. India accounts for an estimated 122,800 new cases and 67,500 deaths annually due to cervical cancer2. Although a declining trend in the cervical cancer incidence, with annual percentage change ranging between -1.1 and -3.4, has been observed in different regions of India over the last two decades, the rates remain significantly higher than in other countries in Asia and the absolute numbers of cervical cancer cases and deaths are on the increase due to population growth and advanced clinical stages at presentation3,4.
The knowledge that persistent infection with one of the oncogenic, high-risk types of human papillomaviruses (HPVs) is the ‘necessary’ cause of cervical cancer, implying that the infection is obligatory to initiate the carcinogenic process, has opened up an exciting and effective means of primary prevention using vaccination5,6. HPV is the most common infection of the genital tract epithelium, with the highest risk of acquiring the infection after sexual debut. Most of the infections are cleared over six months to two years. In a small proportion (5-10%) of infected women, the infections persist for reasons not yet well understood, and they are at high-risk of developing cervical precancerous lesions and cancer. More than 200 types of HPV have been identified and are classified into low- and high-risk types based on their potential to cause malignancy. The high-risk (carcinogenic) types include HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59. Types 66, 68 and 73 are considered as probably carcinogenic. Among these types, HPV types 16 and 18 are the most carcinogenic and are responsible for approximately 70 per cent of the cervical malignancies; HPV types 31, 33, 35, 45, 52 and 58 account for an additional 20 per cent of the cervical cancers worldwide7,8,9. In India, 80 per cent of cervical cancers and 63 per cent of high-grade cervical precancerous lesions are attributed to HPV types 16 and 18; an effective vaccine targeting these two types with high coverage of the population will have a huge impact in the country10,11,12. High-risk HPV infection is responsible for 43 per cent of the vulvar cancers and 70 per cent of vaginal cancers in women, 50 per cent of penile cancers in men and 30 per cent of oropharyngeal cancers and 88 per cent of anal cancers in both sexes13. The low-risk HPV types 6 and 11 are responsible for more than 90 per cent of genital warts14, a distressing condition in young men and women.
Human papillomavirus vaccines – Principles and mechanism of action
Globally, three types of HPV vaccines are currently available – bivalent vaccine (Cervarix™; GSK Biologicals, Belgium) targeting HPV types 16 and 18; quadrivalent vaccine (Gardasil™, Merck, USA) targeting HPV 16, 18, 6 and 11; and 9-valent vaccine (Gardasil 9™; Merck, USA) targeting HPV 31, 33, 45, 52 and 58 in addition to HPV 16, 18, 6 and 11. The first two are available in India. The L1 surface proteins of the targeted HPV types are used as the antigen. The L1 protein undergoes conformal changes to self-assemble into ‘virus-like’ particles (VLPs) in artificial production systems. The VLPs are non-infective and non-pathogenic as these are devoid of the viral DNA essential to initiate the carcinogenic process. Adjuvants used to ensure robust and long-lasting immunogenicity include aluminium phosphate and monophosphoryl lipid A combination (ASO4) in the bivalent vaccine and aluminium hydroxyl-phosphate sulphate in the quadrivalent and the 9-valent vaccine. Two intramuscular doses at six months interval are recommended for girls below 15 yr of age. For those 15 yr and above and for immune-compromised girls/women, three doses over a 6-month period are recommended15.
The HPV vaccines are highly immunogenic leading to seroconversion in more than 99 per cent of the vaccinated girls and women16,17,18,19. Following vaccination, L1 proteins are recognized by the immune system in the regional lymph nodes to generate strong antibody response (IgG), and its concentration is 1-4 logs higher than the antibody levels induced by the natural HPV infections. The IgG is exuded at the possible sites of infection (mucosa of genital tract, oral cavity, etc.), neutralizes the virus and prevents its entry into the cells. The vaccine-induced immune memory in the form of circulating plasma cells and memory B-cells allows generation and exudation of protecting IgG each time the body is challenged by exposure to HPV infection.
Efficacy of vaccines in randomized clinical trials (RCTs)
The HPV vaccines have been evaluated in randomized, double-blind, placebo-controlled RCTs to assess their efficacy to protect against persistent vaccine-targeted HPV infections and high-grade cervical intraepithelial neoplasia [cervical intraepithelial neoplasia grade 2 (CIN2), CIN3 and adenocarcinoma in situ (AIS)] in young adult women. An advisory committee of the World Health Organization (WHO) recommended in 2003 that the prevention of high-grade precancerous lesions would be adequate evidence of the efficacy of the vaccines to protect against invasive cervical cancers20. The pre-licensure Phase III RCT for the bivalent vaccine was the PApilloma TRIal against Cancer In young Adults (PATRICIA) trial21,22,23 and the Costa Rica HPV Vaccine Trial was the first publically funded RCT24,25. The pre-licensure Phase III RCTs to evaluate the quadrivalent vaccine were Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I and FUTURE II trials26,27. The characteristics of the participants of the Phase III trials and the vaccine efficacies observed are described in Table I. The bivalent vaccine was administered on days 1, 30 and 180 while the quadrivalent vaccine was administered on days 1, 60 and 180. Both vaccines were highly efficacious in preventing CIN2, CIN3 or AIS caused by the vaccine-targeted HPV types in young women who were not infected with the respective HPV types either in the past (seronegative to the HPV types) or at the time of enrollment (cervical sample negative for HPV DNA) and received all three doses of the vaccine (according to protocol: ATP cohort). The intention to treat (ITT) cohort included all the randomized women receiving at least a single dose irrespective of the HPV infection status. The efficacy of both vaccines in preventing high-grade CIN caused by vaccine-targeted HPV infections was 90 per cent or higher in the ATP cohorts (Table I). The efficacy of both vaccines was demonstrated in the ITT cohort also though considerably less than that observed in the ATP cohort. This signifies that the vaccines will have maximum benefit in the young sexually naïve girls who resemble the population in the ATP cohort. In addition, the quadrivalent vaccine was highly protective against genital warts caused by types 6 and 11 with vaccine efficacy of 98.9 per cent [95% confidence interval (CI) 96.1-99.9] in the ATP cohort and 79.3 per cent (95% CI 72.7-84.5) in the ITT cohort26.
Table I.
Characteristics of the participants and the key vaccine efficacy results from the randomized clinical trials
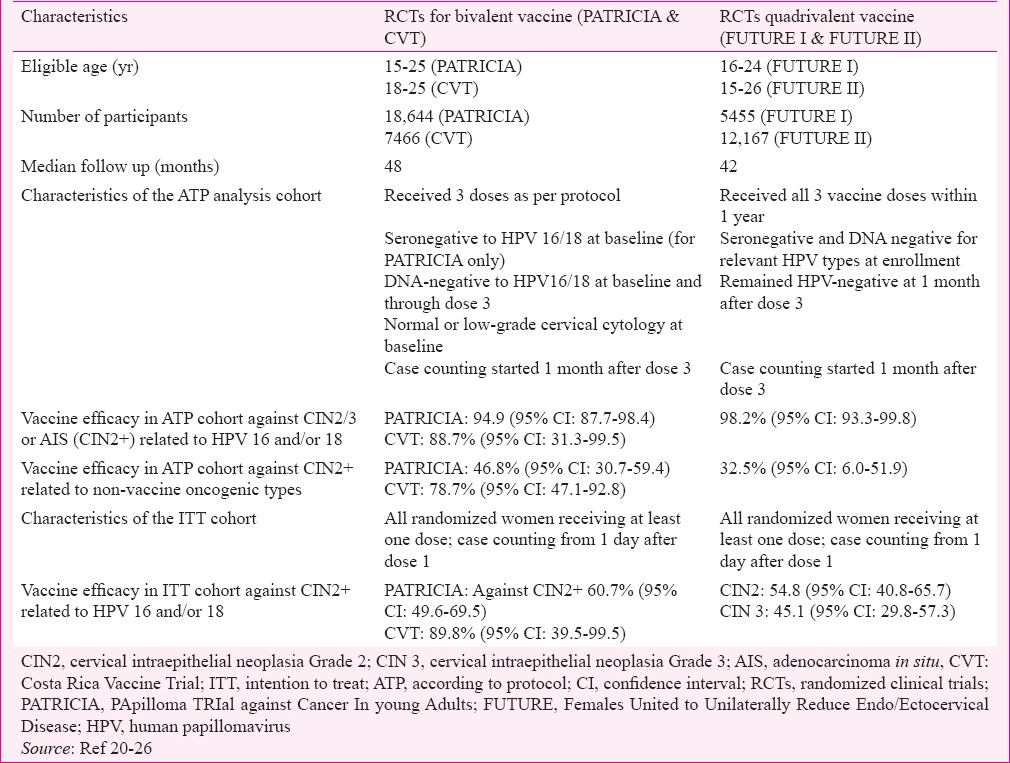
Since pre-adolescents were not included in the efficacy trials, immunobridging studies were used to extend the use of currently licensed vaccines to pre-adolescents. The protective roles of the vaccines in 9-14 yr old girls were confirmed through these studies as efficacy studies are not feasible and are unethical in this age group. In such studies the same vaccine dosage schedule was used as in the efficacy trials and induction of non-inferior serum antibody titres was used as the outcome measure. Both vaccines demonstrated high immunogenicity in girls aged 9-14 yr, and the post-vaccination antibody titres in the young girls were 1.7-2.0-fold higher than that in the 15-26 yr old women in whom the protective efficacy of the vaccine against infection and disease were already established28.
The immunogenicity of both vaccines remains unaltered when administered concomitantly with diphtheria, tetanus, and acellular pertussis (Tdap) vaccine, meningococcal conjugate vaccine, hepatitis A vaccine, hepatitis B vaccine and combined hepatitis A and B vaccine and inactivated poliovirus vaccine; there was no increase in the frequency of adverse events29.
Neither of the HPV vaccines had any impact on the clearance or progression of infection in women who were infected with HPV 16 or 18 (HPV DNA positive) at enrollment, implying the absence of any therapeutic effect of the vaccines on existing HPV infections21,22,23,24,25,26,30.
Studies have evaluated the efficacy of less than three doses of the vaccine, efficacy of a new vaccine similar to a licensed vaccine or efficacy of a novel vaccine developed from the L2 viral capsid protein to induce neutralizing antibodies. A polyvalent vaccine has been developed by including VLPs for additional HPV types (9-valent vaccine by Merck) and has been evaluated. Recently, a Working Group at the International Agency for Research on Cancer (IARC) and United States National Cancer Institute (NCI) recommended that a virological end-point such as persistent HPV infection of six months or longer, rather than a disease end-point such as CIN2 or worse lesions, might be used as the primary end-point for future clinical efficacy trials31. The reduction in disease can be verified by post-licensure monitoring31. The Working Group recommended HPV 16/18-positive high-grade vulvar intraepithelial neoplasia/vaginal intraepithelial neoplasia as a disease end-point for vulvar/vaginal protection, and a persistent HPV 16/18 infection end-point for evaluating oral/oropharyngeal infection. The Working Group recommended immunobridging studies with immunological non-inferiority as the sufficient end-point for extending licensure to other population groups (e.g., those aged <16 yr), once a vaccine is shown to be effective in other population group (e.g., individuals aged 16-26 yr), with reduction in disease being verified by post-licensure monitoring31.
In a randomized, double-blind, Phase IIb-III study of the 9-valent HPV vaccine in 14,215 women aged 16-26 yr, the 9-valent vaccine generated a non-inferior antibody response to HPV-6, 11, 16 and 18 to that by the quadrivalent HPV vaccine. In the ATP cohort (receiving all three doses, naïve to relevant HPV types at enrollment and DNA negative for the relevant types till 1 month post-dose 3), the efficacy of the 9-valent vaccine in preventing the HPV 31-, 33-, 45-, 52- and 58-related CIN2 or worse disease was 96.3 per cent32. The 9-valent vaccine was not effective in preventing infection and disease related to non-vaccine targeted HPV types.
Efficacy of less than three doses of HPV vaccination
Less than three doses of HPV vaccine, if found effective, could substantially reduce costs, improve compliance, ease logistics and facilitate scale up in the national immunization programmes (NIPs). The high immunogenicity of HPV vaccines in adolescent girls prompted randomized trials comparing two doses of the vaccine (administered at 6 months interval) in girls below 15 yr of age to three doses in young women 15 yr or above33,34. These trials and a few non-randomized studies19,35,36,37,38 confirmed that two doses of both bivalent and quadrivalent HPV vaccines, administered at an interval of six months or more between doses, are immunologically non-inferior to three doses. Non-randomized comparisons of less than three doses of bivalent HPV vaccine in the Costa Rica Vaccine and PATRICIA trials37,38 and of quadrivalent HPV vaccine in an Indian study19 indicated that one or two doses by default were as protective as three doses in preventing persistent HPV 16 or HPV 18 infections, although one dose was less immunogenic than three doses. However, one dose generated detectable titres of neutralizing antibodies and one-dose antibodies were as avid as three-dose antibodies19,37,38. The above observations and the fact that there is no known minimum threshold concentration of antibodies that is protective prompted the investigators to recommend that one-dose merits further assessment19,37,38.
The WHO after reviewing the available evidence on less than three-doses recommended a two-dose schedule for girls (at an interval of 6 months, which may be extended to 12 months to facilitate vaccination) if vaccination is initiated prior to 15 yr of age and a three-dose schedule (at 0, 1-2 and 6 months) if vaccination is initiated after 15th birthday and for immunocompromised individuals, including those infected with HIV15.
Countries implementing HPV vaccination as part of the national immunization programmes
The HPV vaccines have been licensed almost in all the countries across the globe, with the notable exception being People's Republic of China. More than 80 countries have introduced HPV vaccine in the NIPs, of which 33 are LMICs; in addition, 25 LMICs have introduced HPV vaccination in pilot demonstration programmes as a prelude to national scaling up in NIPs (Table II)39,40. Many Pacific Island nations have implemented HPV vaccination41. In most programmes, a school-based approach is predominantly used to deliver the vaccine to the targeted adolescents with additional efforts using field clinics, and primary health centres to cover girls who missed vaccination and do not attend schools. Almost all pilot demonstration programmes are supported by Gavi - The Vaccine Alliance and use a two-dose schedule39. With efforts by Gavi the procurement price of both vaccines is reduced to around US$5, the prospects of introducing HPV vaccination in the low-income Gavi-eligible countries has substantially improved.
Table II.
Low- and middle-income countries with ongoing human papillomavirus vaccination programmes in public health services
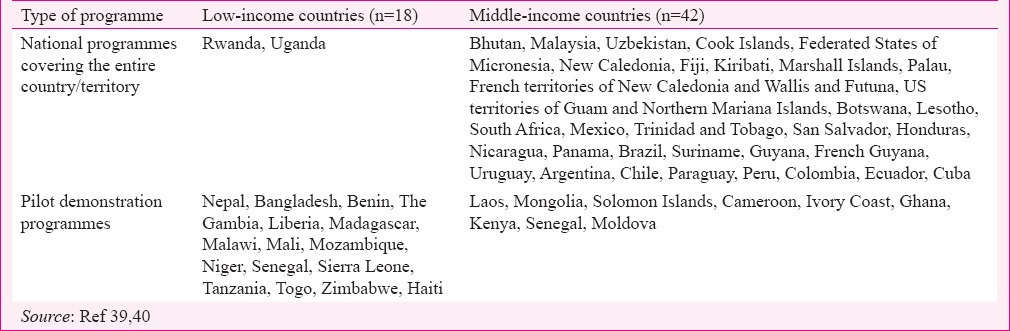
While Australia, Denmark, USA and Canada were the first high-income countries to introduce HPV vaccination in NIPs in 2007, Panama (2008) in Latin America, Bhutan (2009) in Asia and Rwanda (2010) in Africa were the first LMICs that introduced HPV vaccination42,43,44. Currently, eight of 10 girls aged 9-13 yr in the Latin American region have access to HPV vaccination through NIP thanks to the foresight, wisdom and commitment on the part of the national governments and the pooled, bulk, negotiated purchase of the vaccines on behalf of these countries by the revolving fund of the Pan American Health Organization. The largest HPV vaccination programme in any LMIC is in Brazil that targets five million 11-12 yr old girls annually with two doses; Uganda targets 850,000 (11 yr old) girls and Malaysia targets 250,000 (12 yr old) girls annually with two doses45,46. Most LMICs including Brazil, Mexico, Malaysia and South Africa currently use a two dose schedule. HPV vaccination coverage in most LMIC programmes and demonstration projects exceed 80 per cent, and thus participation in LMIC programmes is much higher than in most high-income countries. The governments in countries that have implemented programmes are closely monitoring the implementation and safety profile of the vaccine; for instance, Malaysia, since 2010 has administered more than four million doses of HPV vaccines with the excellent safety profile and the Malaysian Government have defended the safety of HPV vaccines from its own experience47.
Effectiveness of HPV vaccine in the national immunization programmes
The early protection offered by the vaccine at the population level against vaccine-targeted HPV infections, genital warts and cervical premalignant lesions has been reported from countries that introduced the vaccine between 2007 and 201048,49,50,51,52,53,54,55. In Denmark, the atypia or worse and CIN2 or worse lesions increased in all age groups during 2000-2010, the incidence of such lesions decreased significantly in women younger than 18 yr [estimated annual percentage change (EAPC) - 33.4%; 95% CI -49.6; -12.0] and in 18-20 yr old women (EAPC - 12.6%; 95% CI -19.3; -5.3), after the introduction of HPV vaccination programme; however, no significant decrease was seen in older age groups50.
In a pooled analysis of 20 eligible studies involving 140 million person years of follow up after vaccination, HPV 16 and 18 infections decreased significantly between the pre- and post-vaccination periods in high-income countries with 50 per cent or more HPV vaccination coverage by 68 per cent [relative risk (RR) 0.32, 95% CI 0.19-0.52]; anogenital warts decreased significantly by 61 per cent (RR 0.39, 0.22-0.71) in girls 13-19 yr of age; significant reductions in warts were observed in boys younger than 20 yr of age (RR 0.66, 95% CI 0.47-0.91) and in women 20-39 yr of age (RR 0.68, 95% CI 0.51-0.89), suggesting significant herd immunity offered by vaccination53. Significant reductions were also recorded in HPV types 31, 33 and 45 in girls (RR 0.72, 95% CI 0.54-0.96), suggesting some level of cross-protection. In countries where female vaccination coverage was lower than 50 per cent, significant reductions in HPV types 16 and 18 infection (RR 0.50, 95% CI 0.34-0.74) and in warts (RR 0.86, 95% CI 0.79-0.94) occurred in girls <20 yr of age. Sentinel surveillance to monitor the impact of HPV vaccination in the US indicated a 72 per cent decline in CIN2 or worse lesions in women who had vaccination four or more years before the screening test that led to the diagnosis of CIN2+ disease54.
An ecological analysis in British Columbia, Canada, indicated 86 per cent (95% CI 53-96) reduction in CIN2 or worse lesions in young women aged 15-17 yr after the introduction of the HPV vaccination; however, no reduction was found in 18-22 yr old women during the same period55. The above results are promising for the long-term effects of HPV vaccination in reducing the cervical cancer burden.
Human papillomavirus vaccine safety
Extensive data on the safety of HPV vaccines are available from clinical trials and the population programmes. Globally, more than 270 million doses have been administered with no serious adverse event linked to the HPV vaccine and with an excellent safety profile. The adverse events reported after the HPV vaccine administration were generally mild in intensity and were similar to those expected after any vaccination. These included vaccination site pain, tenderness, swelling, fever, headache, myalgia and gastrointestinal symptoms. The adverse events are considered as ‘serious’ if the events occurring at any point of time lead to hospitalization, prolongation of an existing hospitalization, permanent disability, life-threatening illness or death. A meta-analysis of the vaccine trials concluded that the frequency of serious adverse events [odds ratio (OR) 0.99; 95% CI 0.87-1.14] and death (OR 0.91, 95% CI 0.39-2.14) were similar in the vaccinated and control groups56. The majority of deaths reported were accidental in nature, and none was attributable to the vaccines. A study from India reported no serious adverse event attributable to the vaccine after administering 34,856 doses of the quadrivalent vaccine to 10-18 yr old girls and following them over four years19.
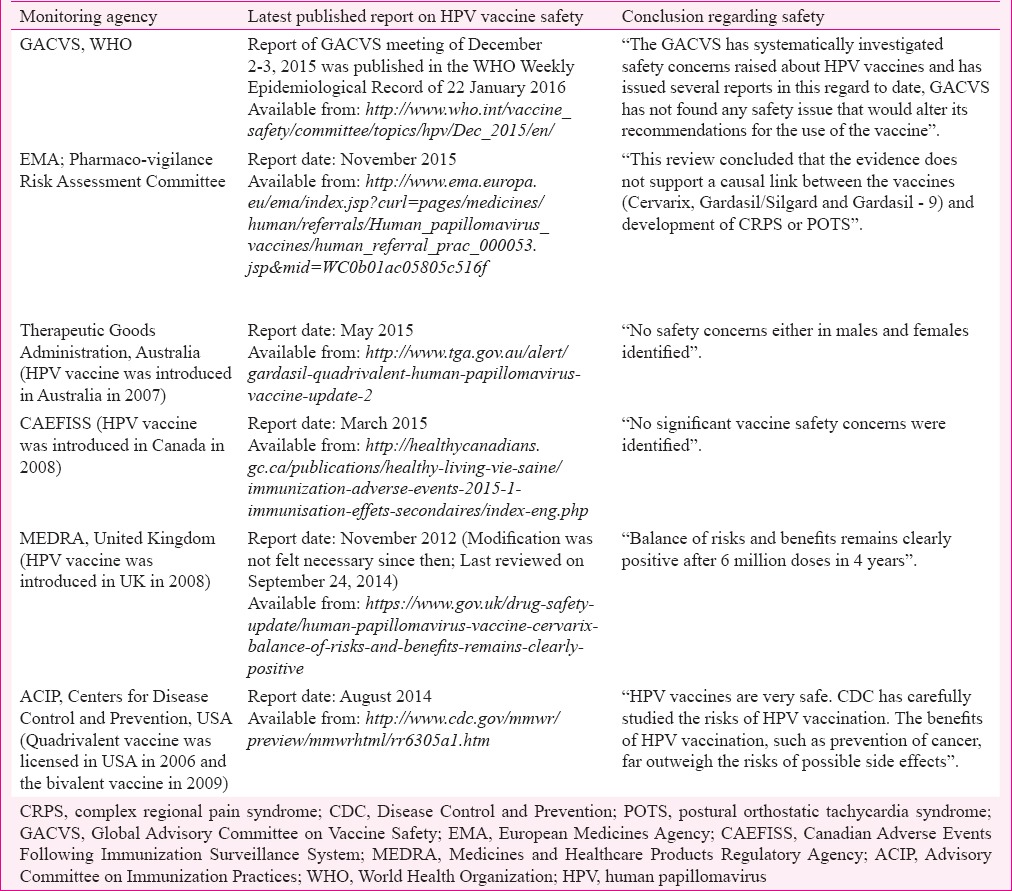
The estimated 200 million doses of the quadrivalent and 80 million doses of the bivalent vaccines have been administered globally till June 201557. The vaccine surveillance systems in countries regularly monitor and report the serious and the non-serious adverse events after HPV vaccination. Some of the national monitoring authorities such as the Therapeutic Goods Administration, Australia, Adverse Events Following Immunization Surveillance System (Canadian Adverse Events Following Immunization Surveillance System, CAEFISS), Canada, Medicines and Healthcare Products Regulatory Agency, United Kingdom and the United States (US) Centers for Disease Control and Prevention (CDC) have robust system of recording and reviewing all the post-vaccination adverse events. The mandates of these organizations include continuous reviewing of the safety of the vaccines and informing healthcare professionals as well as the public of the latest safety updates. The International monitoring agencies such as the Global Advisory Committee on Vaccine Safety, the WHO and the European Medicines Agency are also monitoring the vaccine safety and critically evaluating all the serious adverse events. The details of the latest published reports on HPV vaccine safety by these agencies are provided in Table III. All these have concluded that the vaccines are safe, there are no undue safety concerns to withhold or stop the vaccination and the benefits far outweigh the risks.
Table III.
Latest position documents of the international and selected national agencies monitoring the human papillomavirus vaccine safety in the national immunization programmes
Syncope or fainting attack within 15 min of vaccination has been associated with the HPV vaccination though the event is related to the vaccination process rather than the vaccine itself. Adolescent girls are more prone to such fainting attacks after injections, and similar attacks are also seen after administration of other vaccines such as meningococcal vaccine (fourth dose) and Tdap vaccines to the adolescent girls. The girls should be vaccinated in a sitting or supine position to prevent such fainting attacks and related injuries and should be observed for 15-30 min after vaccination.
Recently, concerns have been expressed regarding two conditions reported after HPV vaccination: the complex regional pain syndrome, chronic pain affecting the limbs; and the postural orthostatic tachycardia syndrome, a condition where the heart rate increases abnormally after sitting or standing up, causing dizziness, weakness, palpitation, etc. The review by the WHO Vaccine Advisory Committee found that the overall rates of these syndromes in vaccinated girls were not different from the expected rates in these age groups, even after taking into account possible underreporting58.
Possible autoimmune disorders including the neurological diseases such as Guillain-Barré syndrome (GBS) and demyelinating disorders have been reported among the recipients of HPV vaccine. The surveillance of 9-26 yr old women in USA receiving 1,490,428 doses of quadrivalent vaccine (from 2006 till 2012) by CDC using the Vaccine Safety Datalink did not observe any increased risk of GBS. Not a single incident case of GBS was detected in the medical records within 42 days following vaccine administration59. A health register-based linkage study in Sweden and Denmark for 23 different autoimmune and five neurologic conditions among quadrivalent vaccine recipients (n = 296,826) aged 10-17 yr did not find any consistent evidence of causal association of these diseases with the vaccine60. Recently, a retrospective cohort study by the French National Agency for Medicines and Health Products Safety involving more than two million girls observed similar frequency of all autoimmune conditions in vaccinated and unvaccinated girls except the GBS; however, the risk of GBS within three months of vaccination was very small (1/100,000 vaccinated girls) though comparatively higher than the unvaccinated girls61. GBS is more commonly observed in the adolescent girls and young adult women, the target age for the HPV vaccines. Till date, there is no evidence that the incidence of GBS following HPV vaccination is higher than the background rate observed in age- and sex-matched unvaccinated populations. There was no elevated risk of thromboembolism in a large national register based study in Denmark involving 500,345 vaccinated women of 10-44 yr of age62,63,64.
The CDC and the US Food and Drug Administration have extensively reviewed the 96 reports of deaths in vaccinated girls/women between 2006 and 2014. A detailed review of each death by these agencies did not identify any pattern of occurrence of death with respect to time after vaccination, vaccine doses or diagnosis at death to suggest any causal association with the HPV vaccine65.
The HPV vaccines are not recommended in pregnancy. However, the analysis of the outcomes of the inadvertent administration of the vaccine in pregnant women in the trials did not document any higher rates of spontaneous abortions, preterm births, birth defects or other adverse consequences. No intervention other than stopping further vaccinations is recommended if a woman becomes pregnant after receiving incomplete doses of the vaccine or receives the vaccine while pregnant66,67.
Is human papillomavirus vaccination relevant to India?
India is the largest contributor to the global burden of cervical cancer. The slow decline in cervical cancer incidence rates seen in different population-based cancer registries in the country is very much debated about and has been mainly attributed to the changing reproductive profile with fewer child births and increasing age at marriage and first childbirth in addition to improving socio-economic conditions and women's empowerment. The declining incidence rates have prompted some experts to question the need for HPV vaccination as a primary prevention measure in the Indian NIP and they instead suggest screening with visual inspection with acetic acid (VIA) as the major intervention for cervical cancer control in India68. Some experts even went to the extent of suggesting that efforts to improve basic hygiene and sanitation are enough to tackle the burden of cervical cancer in the country69. Unsubstantiated claims about the lack of vaccine safety from some have led to further debates as well.
However, several regions of India still have rates higher than most Asian countries, and the absolute number of cases is on the increase due to population growth. Moreover, the falling incidence rates seem to be reaching a plateau and unlikely to decline further unless specific interventions are put in place. Fewer than five per cent of the eligible women in India have ever been screened and there are almost no government-sponsored population-based cervix screening programmes in the country, except in the State of Tamil Nadu where one round of VIA screening has been offered to women through the government health services1. The impact of this programme on the cervical cancer burden is not clear. Screening typically requires repeated interventions at least every five years with high coverage of targeted women and involves a number of steps such as quality-assured testing, diagnosis, treatment and follow up care for it to be effective. Introducing such efficiently organized population-based cervical cancer screening programmes will require substantial resources and could be a challenging task. Cervix screening programmes in many Latin American countries did not have any impact on cervical cancer burden despite several rounds of intervention since the 1970s and many of them have recently reorganized their screening programmes70,71. Even in high-resourced settings, a screening programme takes a minimum of 10-15 yr to evolve. However, the preventive potential of HPV vaccination is substantially augmented by accompanying effective screening programmes, and these are, therefore, complementary strategies.
An HPV vaccination programme targeting a single year cohort of 9-13 yr old girls is a two time intervention at 6 or 12 months apart and will build up a cohort of women at very low risk of HPV16 and 18 infection and, consequently, at a low risk for cervical cancer. The direct impact of HPV vaccination and the herd effects will prevent a substantial proportion (>70%) of cervical cancers in these cohorts of women. In such cohorts, if resources permit, introducing even a low-intensity screening programme such as one round of HPV screening when they reach 30 or 35 yr of age will contribute to further augmented protection by detecting and treating any lesion caused by vaccine non-targeted HPV types and lesions in those who missed vaccination. The high burden of cervical cancer and the high efficacy and safety of HPV vaccination justify its introduction in the Indian NIP at the earliest possibility to substantially reduce the cervical cancer burden in future. Initial resistance against any new vaccination has not been uncommon in India, and only strong government efforts have led to sustained vaccination programmes with such important achievements such as small pox eradication and polio elimination in the country. A study conducted in 201272 indicated that routine vaccination of 12 yr old girls in Brazil could prevent approximately 118,825 cases of cervical cancer, 33,700 deaths from cervical cancer, 1.8 million cases of CIN2/3 and 9.5 million cases of genital warts in the next 50 years, and the Brazilian Government has initiated HPV vaccination targeting 12 yr old girls since 2014. The efforts by the New Delhi State government to launch HPV vaccination in the National Capital Territory is a welcome development in this context73.
To conclude, the HPV vaccines with their well-established efficacies in preventing high grade cervical premalignant lesions in the clinical trials as well as the real life programmes have created a great opportunity for India to reduce the huge burden of cervical cancers. Cervical cancer screening implemented through population-based organized approach is also an effective strategy to tackle the disease; though logistically highly complex, resource intensive and is non-existent or at its nascent stage in India. The policy makers and the public health administrators in India need to be aware of the complementary roles of the two strategies and initiate measures to implement both to reduce the burden of a preventable cancer.
Footnotes
Conflicts of Interest: None.
References
- 1.Bruni L, Barrionuevo-Rosas L, Albero G, Aldea M, Serrano B, Valencia S, et al. ICO (Institute Català d’Oncologia) Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases Report- World. Summary Report. 2015. [accessed on September 21, 2016]. Available from: http://www.hpvcentre.net/statistics/reports/XWX.pdf .
- 2.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Lyon: International Agency for Research on Cancer; 2013. [Google Scholar]
- 3.Time Trends in Cancer Incidence Rates, 1982-2010. National Cancer Registry Programme: ICMR Bangalore. 2013 [Google Scholar]
- 4.Asthana S, Chauhan S, Labani S. Breast and cervical cancer risk in India: an update. Indian J Public Health. 2014;58:5–10. doi: 10.4103/0019-557X.128150. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Human Papillomaviruses. Lyon: IARC; 2007. [Google Scholar]
- 7.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007;121:621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 8.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 9.Clifford G, Franceschi S, Diaz M, Muñoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24(Suppl 3):S3/26–34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Sankaranarayanan R, Bhatla N, Gravitt PE, Basu P, Esmy PO, Ashrafunnessa KS, et al. Human papillomavirus infection and cervical cancer prevention in India, Bangladesh, Sri Lanka and Nepal. Vaccine. 2008;26(Suppl 12):M43–52. doi: 10.1016/j.vaccine.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Gheit T, Vaccarella S, Schmitt M, Pawlita M, Franceschi S, Sankaranarayanan R, et al. Prevalence of human papillomavirus types in cervical and oral cancers in central India. Vaccine. 2009;27:636–9. doi: 10.1016/j.vaccine.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Deodhar K, Gheit T, Vaccarella S, Romao CC, Tenet V, Nene BM, et al. Prevalence of human papillomavirus types in cervical lesions from women in rural Western India. J Med Virol. 2012;84:1054–60. doi: 10.1002/jmv.23310. [DOI] [PubMed] [Google Scholar]
- 13.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012;13:607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 14.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Immunization, vaccines and biologicals: human papillomavirus (HPV) Geneva: WHO; 2016. [accessed on September 14, 2016]. Available from: http://www.who.int/immunization/diseases/hpv/en . [Google Scholar]
- 16.Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118:2135–45. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 17.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5:705–19. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 18.Zhu FC, Chen W, Hu YM, Hong Y, Li J, Zhang X, et al. Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18-25 years: Results from a randomized controlled trial. Int J Cancer. 2014;135:2612–22. doi: 10.1002/ijc.28897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sankaranarayanan R, Prabhu PR, Pawlita M, Gheit T, Bhatla N, Muwonge R, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre prospective cohort study. Lancet Oncol. 2016;17:67–77. doi: 10.1016/S1470-2045(15)00414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagliusi SR, Teresa Aguado M. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine. 2004;23:569–78. doi: 10.1016/j.vaccine.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 21.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 22.Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 23.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 24.Herrero R, Hildesheim A, Rodríguez AC, Wacholder S, Bratti C, Solomon D, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26:4795–808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hildesheim A, Wacholder S, Catteau G, Struyf F, Dubin G, Herrero R CVT Group. Efficacy of the HPV-16/18 vaccine: Final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 vaccine trial. Vaccine. 2014;32:5087–97. doi: 10.1016/j.vaccine.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 27.Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila) 2009;2:868–78. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 28.Reisinger KS, Block SL, Lazcano-Ponce E, Samakoses R, Esser MT, Erick J, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: A randomized controlled trial. Pediatr Infect Dis J. 2007;26:201–9. doi: 10.1097/01.inf.0000253970.29190.5a. [DOI] [PubMed] [Google Scholar]
- 29.Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine. 2014;32:2670–4. doi: 10.1016/j.vaccine.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(Suppl 5):F123–38. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.IARC HPV Working Group. Primary end-points for prophylactic HPV vaccine trials. IARC Working Group Reports, No. 7. Lyon: International Agency for Research on Cancer; 2014. [PubMed] [Google Scholar]
- 32.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 33.Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: A randomized clinical trial. JAMA. 2013;309:1793–802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 34.Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: Results from a randomized study. Hum Vaccin Immunother. 2014;10:1155–65. doi: 10.4161/hv.28022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013;6:1242–50. doi: 10.1158/1940-6207.CAPR-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazcano-Ponce E, Stanley M, Muñoz N, Torres L, Cruz-Valdez A, Salmerón J, et al. Overcoming barriers to HPV vaccination: Non-inferiority of antibody response to human papillomavirus 16/18 vaccine in adolescents vaccinated with a two-dose vs. a three-dose schedule at 21 months. Vaccine. 2014;32:725–32. doi: 10.1016/j.vaccine.2013.11.059. [DOI] [PubMed] [Google Scholar]
- 37.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103:1444–51. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreimer AR, Struyf F, Del Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: Combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol. 2015;16:775–86. doi: 10.1016/S1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanson CM, Eckert L, Bloem P, Cernuschi T. Gavi HPV Programs: Application to implementation. Vaccines (Basel) 2015;3:408–19. doi: 10.3390/vaccines3020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaccine in national immunization programme update. WHO. [accessed on September 21, 2016]. Available from: www.who.int/immunization/monitoring_surveillance/VaccineIntroStatus.pptx .
- 41.Obel J, McKenzie J, Buenconsejo-Lum LE, Durand AM, Ekeroma A, Souares Y, et al. Mapping HPV vaccination and cervical cancer screening practice in the pacific region-strengthening national and regional cervical cancer prevention. Asian Pac J Cancer Prev. 2015;16:3435–42. doi: 10.7314/apjcp.2015.16.8.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tshomo U, Franceschi S, Dorji D, Baussano I, Tenet V, Snijders PJ, et al. Human papillomavirus infection in Bhutan at the moment of implementation of a national HPV vaccination programme. BMC Infect Dis. 2014;14:408. doi: 10.1186/1471-2334-14-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binagwaho A, Wagner CM, Gatera M, Karema C, Nutt CT, Ngabo F. Achieving high coverage in Rwanda's national human papillomavirus vaccination programme. Bull World Health Organ. 2012;90:623–8. doi: 10.2471/BLT.11.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Binagwaho A, Ngabo F, Wagner CM, Mugeni C, Gatera M, Nutt CT, et al. Integration of comprehensive women's health programmes into health systems: Cervical cancer prevention, care and control in Rwanda. Bull World Health Organ. 2013;91:697–703. doi: 10.2471/BLT.12.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uganda launches Human Papillomavirus Vaccine. WHO. [accessed on September 21, 2016]. Available from: http://www.afro.who.int/en/uganda/pressmaterials/item/8186-uganda-launches-human-papillomavirus - vaccine.html .
- 46.Lessons learned: Building the school-based HPV program in Malaysia and opportunities for piggybacking. Ministry of Health Malaysia. [accessed on September 21, 2016]. Available from: http://adva.asia/ads/day2/Rohani-Jahis+Saidatul-Norbaya-Buang.pdf .
- 47.HPV vaccine safe, says health ministry. [accessed on September 21, 2016]. Available from: http://www.freemalaysiatoday.com/category/nation/2015/01/24/hpvvaccine-safe-says-health-ministry/
- 48.Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Cummins E, Liu B, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645–51. doi: 10.1093/infdis/jis590. [DOI] [PubMed] [Google Scholar]
- 49.Kavanagh K, Pollock KG, Potts A, Love J, Cuschieri K, Cubie H, et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer. 2014;110:2804–11. doi: 10.1038/bjc.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia – Nationwide follow-up of young Danish women. J Natl Cancer Inst. 2014;106:djt460. doi: 10.1093/jnci/djt460. [DOI] [PubMed] [Google Scholar]
- 51.Pollock KG, Kavanagh K, Potts A, Love J, Cuschieri K, Cubie H, et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer. 2014;111:1824–30. doi: 10.1038/bjc.2014.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garland SM. The Australian experience with the human papillomavirus vaccine. Clin Ther. 2014;36:17–23. doi: 10.1016/j.clinthera.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Drolet M, Bénard É, Boily MC, Ali H, Baandrup L, Bauer H, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect Dis. 2015;15:565–80. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hariri S, Bennett NM, Niccolai LM, Schafer S, Park IU, Bloch KC, et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States – 2008-2012. Vaccine. 2015;33:1608–13. doi: 10.1016/j.vaccine.2015.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogilvie GS, Naus M, Money DM, Dobson SR, Miller D, Krajden M, et al. Reduction in cervical intraepithelial neoplasia in young women in British Columbia after introduction of the HPV vaccine: An ecological analysis. Int J Cancer. 2015;137:1931–7. doi: 10.1002/ijc.29508. [DOI] [PubMed] [Google Scholar]
- 56.Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: A systematic review of randomized controlled trials. CMAJ. 2007;177:469–79. doi: 10.1503/cmaj.070948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Australian Government – Department of Health. Gradasil (Quadrivalent Human Papillomavirus Vaccine), Update 2. Australian Government. 2015. [accessed on April 6, 2016]. Available from: http://www.tga.gov.au/alert/gardasil-quadrivalent-human-papillomavirusvaccine-update-2 .
- 58.World Health Organization. Global Advisory Committee on Vaccine Safety, 2-3 December 2015. Wkly Epidemiol Rec. 2016;3:21–31. [Google Scholar]
- 59.Sukumaran L. Human Papillomavirus (HPV) Vaccine Safety Update. Advisory Committee on Immunization Practices, Atlanta. 2015 [Google Scholar]
- 60.Arnheim-Dahlström L, Pasternak B, Svanström H, Sparén P, Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: Cohort study. BMJ. 2013;347:f5906. doi: 10.1136/bmj.f5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.National Security Agency of Medicines and Health Products. HPV vaccines and auto-immune diseases: Pharmacoepidemiological Study. ANSM. 2015 [Google Scholar]
- 62.Chao C, Klein NP, Velicer CM, Sy LS, Slezak JM, Takhar H, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193–203. doi: 10.1111/j.1365-2796.2011.02467.x. [DOI] [PubMed] [Google Scholar]
- 63.Gee J, Naleway A, Shui I, Baggs J, Yin R, Li R, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: Findings from the Vaccine Safety Datalink. Vaccine. 2011;29:8279–84. doi: 10.1016/j.vaccine.2011.08.106. [DOI] [PubMed] [Google Scholar]
- 64.Scheller NM, Pasternak B, Svanström H, Hviid A. Quadrivalent human papillomavirus vaccine and the risk of venous thromboembolism. JAMA. 2014;312:187–8. doi: 10.1001/jama.2014.2198. [DOI] [PubMed] [Google Scholar]
- 65.Centers for Disease Control and Prevention. Frequently Asked Questions about HPV Vaccines Safety. Atlanta: CDC; 2016. [Google Scholar]
- 66.Wacholder S, Chen BE, Wilcox A, Macones G, Gonzalez P, Befano B, et al. Risk of miscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: Pooled analysis of two randomised controlled trials. BMJ. 2010;340:c712. doi: 10.1136/bmj.c712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garland SM, Ault KA, Gall SA, Paavonen J, Sings HL, Ciprero KL, et al. Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18 vaccine: A combined analysis of five randomized controlled trials. Obstet Gynecol. 2009;114:1179–88. doi: 10.1097/AOG.0b013e3181c2ca21. [DOI] [PubMed] [Google Scholar]
- 68.Need for an HPV vaccine. The Hindu. August 28, 2016. [accessed on September 21, 2016]. Available from: http://www.thehindu.com/opinion/op-ed/need-for-an-hpv-vaccine/article9040754.ece .
- 69.Gupta S, Kerkar RA, Dikshit R, Badwe RA. Is human papillomavirus vaccination likely to be a useful strategy in India? South Asian J Cancer. 2013;2:193–7. doi: 10.4103/2278-330X.119887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lazcano-Ponce EC, Moss S, Alonso de Ruiz P, Salmeron Castro J, Hernand Avila M. Cervical cancer screening in developing countries: why is it ineffective? The case of Mexico. Arch Med Res. 1999;30:240–50. doi: 10.1016/s0188-0128(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 71.Murillo R, Almonte M, Pereira A, Ferrer E, Gamboa OA, Jerünimo J, et al. Cervical cancer screening programs in Latin America and the Caribbean. Vaccine. 2008;26(Suppl 11):L37–48. doi: 10.1016/j.vaccine.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 72.Kawai K, de Araujo GT, Fonseca M, Pillsbury M, Singhal PK. Estimated health and economic impact of quadrivalent HPV (types 6/11/16/18) vaccination in Brazil using a transmission dynamic model. BMC Infect Dis. 2012;12:250. doi: 10.1186/1471-2334-12-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delhi first state to launch HPV vaccine as public health programme in schools. March 1, 2016. The Indian Express. [accessed on September 21, 2016]. Available from: http://indianexpress.com/article/cities/delhi/delhi-first-state-to-launch-hpv-vaccine-as-public-health-programme-in-schools .


