Abstract
Current treatment methods for melanoma have some limitations such as less target-specific action, severe side effects and resistance to drugs. Significant progress has been made in exploring novel drug delivery systems based on suitable biochemical mechanisms using nanoparticles ranging from 10 to 400 nm for drug delivery and imaging, utilizing their enhanced penetration and retention properties. Poly-lactide-co-glycolide (PLGA), a copolymer of poly-lactic acid and poly-glycolic acid, provides an ideally suited performance-based design for better penetration into skin cells, thereby having a greater potential for the treatment of melanoma. Moreover, encapsulation protects the drug from deactivation by biological reactions and interactions with biomolecules, ensuring successful delivery and bioavailability for effective treatment. Controlled and sustained delivery of drugs across the skin barrier that otherwise prohibits entry of larger molecules can be successfully made with adequately stable biocompatible nanocarriers such as PLGA for taking drugs through the small cutaneous pores permitting targeted deposition and prolonged drug action. PLGA is now being extensively used in photodynamic therapy and targeted therapy through modulation of signal proteins and drug-DNA interactions. Recent advances made on these nanomedicines and their advantages in the treatment of skin melanoma are highlighted and discussed in this review.
Keywords: Anti-cancer drugs, drug delivery, melanoma, nanomedicines, poly-lactide, co-glycolide nanoparticles
Introduction
Skin melanoma is one of the most aggressive types of cancer caused by uncontrolled division of melanocytes1,2. The majority of skin cancer deaths are due to melanoma3. Approximately, two million cases of skin cancer were reported in the Unites States of America in 2012, among whom, 75,000 (3.75%) cases belonged to the melanoma type4. In fact, melanoma caused 9000 (75%) of the total 12,000 cancer deaths in 2012, despite the fact that an early-stage localized melanoma, not spreading or metastasizing to other organs, can be successfully removed by surgical treatment with a higher survival rate. A survey reported the rate of cured melanoma patients as 97-99.8 per cent after chemosurgery5. Melanoma incidence has also been observed in some tropical countries including the subcontinents. High incidence of melanoma has also been reported from Western Australia6 in 1975 and 1976. However, due to some limitations of surgery, metastasized irremovable melanomas are now also treated with other regular therapies such as chemotherapy, radiation therapy, targeted therapy and immunotherapy7.
Conventional treatments of skin melanoma and their limitations
Some conventional treatment methods that are already established for skin melanoma other than surgery are cryotherapy and chemotherapy. The nonsurgical treatments for melanomas include adjuvant therapies such as immune and photodynamic therapies (PDTs)8,9. Unfortunately, the consequences are not satisfactory and patients’ responses to most of these therapies are very poor10. Therefore, successful treatment of advanced melanoma still remains a challenge in dermato-oncology11 and emphasis is first given to prevent the sources that are believed to cause skin melanoma from coming in contact of the skin.
Preventive strategy
Since ultraviolet (UV)-induced damage is a major source of initiation of skin cancer, one of the major areas of research is concentrated on determining right strategies for protection of skin from UV-induced damage. A few major protective/preventive strategies taken in UV-induced damage are briefly mentioned below.
Sunscreen products are often recommended for use; these contain molecular complexes that are able to absorb, reflect or scatter UV photons. Certain sunscreens have been developed that repair the solar-induced skin damage or prevent them in causing skin cancer12. Sunscreens efficiently protect the skin from solar erythema and sunburns caused by the UV photons, but their effectiveness in preventing photo-ageing depends on their ability to block the harmful UV rays. Thus, sunscreens that check the local and systemic immune-suppression are significant in the inhibition of epidermal gene modifications such as mutations on p53 gene, thymine dimer formation and induction of the apoptotic event. Some ingredients of sunscreens can cause the skin to become more sensitive causing several side effects such as redness or irritation. The Skin Cancer Foundation has recommended for the use of a sunscreen with an optimum Sun Protection Factor-15 (SPF-15) or of higher strength (SPF 30) as an important part of a complete sun protection regimen12. The SPF number has great preventive impact on UV exposed effects in skin. SPF number reflects the amount of protection the sunscreen provides against harmful UVB rays, one of the main causes of sunburn. Higher the SPF number more is the protection against UVB. For example, when one applies an SPF 30 sunscreen, he/she gets the equivalent of one min of UVB rays for each half an hour spent in the sun. Hence, remaining in the sun for one hour wearing SPF 30 sunscreen is equivalent to spending two min totally unprotected. Sunscreens with SPFs as high as 100 are available. SPF 15 sunscreens filter out approximately 93 per cent of UVB rays, while SPF 30 sunscreens filter out around 97 per cent, SPF 50 sunscreens about 98 and SPF 100 around 99 per cent. Sunscreens with an SPF lower than 15 must now include a cautionary stating that the product can only help to prevent sunburn, but not skin cancer or early ageing of the skin12. Other preventive measures such as seeking a shade, covering the exposed body with clothing and wearing sunglasses for eye protection are also recommended for total protection against UV rays.
Major therapeutic strategies
The major therapeutic strategies in conventional skin cancer treatment are as follows:
Surgical treatment: This offers one of the best ways when the cancer is defined in a particular area and has a lower chance of spreading.
Radiotherapy: This is also a well-known effective treatment that uses high-energy radiation such as gamma rays and X-rays to damage/kill the cancer cells.
Chemotherapy: This strategy uses one or more anti-neoplastic chemical drugs. Either a single drug is used at a time or a combination of several drugs simultaneously. Zelboraf (vemurafenib) is Food and Drug Administration (FDA)-approved for late-stage melanoma which is inoperable and gives a positive test for BRAF (v-raf murine sarcoma viral oncogene homolog B) mutation, a genetic alteration that appears to be responsible for melanoma13. Some physicians have used temozolomide and dacarbazine in advanced melanoma patients also14. Yervoy (ipilimumab) is approved by the FDA for advanced melanoma treatment, alone, or in combination with dacarbazine15. Other drugs include aldesleukin, ipilimumab and zelboraf (vemurafenib). Even when chemotherapy contracts the cancer cells, the effect is temporary, for about 3-6 months before the cancer starts growing again. Only in very rare cases, these work for longer periods of time.
Immunotherapy: This is a comparatively new field of cancer treatment that attempts to specifically target and kill cancer cells by manipulating the body's immune system7,9.
Photodynamic therapy: Photodynamic therapy (PDT) uses a photosensitizing (PS) agent and a specific type of light. When the photosensitizers are exposed to a particular wavelength of light, these generate free radicals and kill the nearby abnormal cells. Although originally, it was used to treat certain malignancies, currently it is also applied in the treatment of some forms of macular degeneration and several severe skin conditions including basal cell carcinomas and squamous cell carcinomas (SCCs), psoriasis, cutaneous T-cell lymphoma, acne and photorejuvenation of wrinkles16,17.
Some of the drugs include acarbazine, DTIC-Dome (dacarbazine), ipilimumab, proleukin (aldesleukin), vemurafenib, yervoy (ipilimumab) and zelboraf (vemurafenib). These drugs act by directly targeting the signal protein molecules and modulating these in a targeted pathway.
Side effects of orthodox treatments
All these conventional treatments have been found to cause severe side effects such as inflammation, pain and unappealing scars5. Surgery is the most primary treatment method for melanoma because these tumours can be resistant to traditional chemo- and radio-therapies which again may cause undesirable side effects. People having skin cancer once are at risk for getting it again. These treatment methods do not offer any guarantee that the cancer will not recur. According to recent reports18,19,20, Vemurafenib causes side effects which include joint pain, tiredness, hair loss, rash or itching, nausea, skin growths or increased sensitivity of eyes to light. Some of the patients treated with this drug also develop SCC. Yervoy administration has been found to cause inflammation of the intestines (which can cause colitis), liver (which can cause liver failure), nerves (which can cause paralysis), glands and severe skin reaction. Along with these, aldesleukin causes severe side effects such as fever and chills or flu-like symptoms. Generalized redness of the face and body, or skin rash, nausea or vomiting and lowered blood pressure may also occur. Even PDT has been demonstrated to have certain limitations21. The persistent skin photosensitivity that lasts for months has restricted its use. All these side effects can be overcome by combinatorial treatment of several drugs. However, the inefficient drug delivery systems (DDSs), higher doses and lower bioavailability of the drugs still remain to be a major concern19. It has been reported that the major drugs used as anti-cancer agents for skin cancer have low solubility in water, the administration of which, can lead to clumping and eventually blockage of small blood vessels20. The search for new drug delivery approaches and newer modes of action is one of the most interesting areas that may provide major development in improving therapeutic index, biodegradability and bioavailability with a site-specific action. The new system of drug delivery may overcome solubility problems and provide protection of the drug from external environment such as photodegradation and pH alterations, at a reduced dosage by controlling the release profile. The majority of these formulations are designed for oral administration, ocular insertion and transdermal application.
Advent of novel drug delivery systems
Innovative DDSs including those based on physico-chemical mechanisms have recently been introduced in the field of medical science. Physical mechanisms are referred to as controlled and sustained DDSs, which include osmosis, diffusion, erosion, dissolution and transport of electrons. Biochemical mechanisms including the use of monoclonal antibodies, gene therapy, drug adducts and liposomes have also made their way into the medical arena. Therapeutic benefits of some new DDSs include optimising the time span of drug action, decreasing dosage frequency, controlled release at specific site and maintaining consistent drug levels. Thus, the field of nanotechnology has emerged as a new avenue for searching clinical applications against sunburns and skin cancer. Some drug delivery vehicles have shown the ability to encapsulate a variety of therapeutic agents such as hydrophilic and/or hydrophobic molecules, peptides and nucleic acids22. By encapsulating these molecules inside a biopolymer, the solubility and stability of these molecules can be improved, thus providing a chance to reconsider those potential drugs which were of limited use earlier due to their poor pharmacokinetics23. The goal of nanotechnology is to develop a highly functional system of nano-sized molecular ‘switches’ and even tissue analogues for growing several organs of the body. Most of the biological processes including cancer occur initially on a nanometer scale. Therefore, the major application of nanotechnology lies in cancer diagnosis and treatment of other diseases at early stages.
Nanoparticulate drug delivery
Extensive research into the optimal delivery and site-specific targeting of pharmaceutical and therapeutic agents is at the forefront of developments in nanomedicine24,25. A drug can be encapsulated in a nanocarrier molecule or allowed to tag the surfaces of capsules. Side effects are due to severe cytotoxicities in normal cells because the therapies are not selective. In the field of dermatology, nanoparticles (NPs) are studied for decades and some formulations are already commercially available. Several DDSs have already been used successfully for topical applications, including liposomes, solid lipid nanoparticles (SLN) and biodegradable polyester NPs such as poly-lactic acid (PLA), PLGA and poly-ε-caprolactone (PCL). Nanoparticulate systems can be administered into organisms by almost all routes including transdermal route which offers various advantages over other delivery systems26. The purpose of using these nanocarriers is to obtain a sustained and controlled release, thus maintaining therapeutic drug levels over a specified time period while reducing systemic absorption.
Some examples of drugs delivered across the skin by using NPs are minoxidil, triptolide, nucleic acids, triamcinolone acetonide acetate, dexamethasone phosphate, cyclosporin A, flufenamic acid, testosterone, caffeine, fluorouracil (FU), arthemeter, chlorhexidine, econazole nitrate, insulin, coenzyme Q1 and triclosan27. Some examples of nanocarriers that have been used for drug delivery have been enlisted in Fig. 1.
Fig. 1.
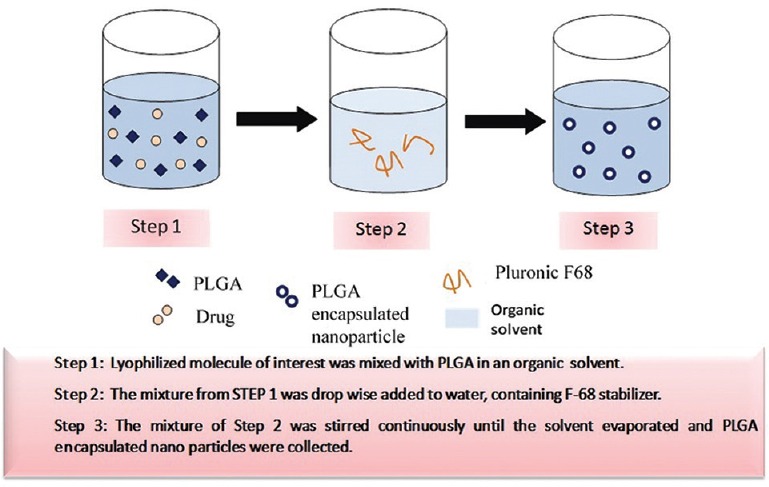
Method of poly-lactide-co-glycolide (PLGA) encapsulation of drugs for drug delivery, using solvent displacement technique under optimal conditions.
Drug delivery in melanoma
A notable feature that makes NPs interesting for dermatological applications is their ability to penetrate and accumulate in the hair follicle orifices28. Drugs applied topically in classical galenic preparations may enter the skin both through the stratum corneum (SC) as well as the hair follicles in the same manner. Drug targeting by NPs or nanocapsules offer enormous advantages29,30 such as it reduces the dose, minimizes side effects, protects against biodegradation and increases stability of drugs31. NPs have the advantage of penetrating through small capillaries and are readily taken up by cells, thus ensuring targeted drug delivery. NPs allow the controlled release of drugs at target sites over a time span of several days or weeks. Loading of drugs in nanoencapsulated form allows decrease in the trans-epidermal pathway and the concentration of the drug increases in the hair follicles. This specific accumulation improves the therapeutic index for drugs in skin therapy. Several approaches have been developed by utilizing nanotechnology as a basic and applied tool in therapeutic applications of melanoma32. Earlier studies have demonstrated that topically applied NPs permeate much faster and reach in higher concentrations in skin having follicles in comparison with skin having no follicles or having selectively blocked follicles33,34. Although the presence of a relatively tight barrier formed by the inter-follicular epidermis and the epithelium of the acro infundibulum hinders drug delivery to some extent, but the presence of a horny layer in the lower follicular tract makes the barrier rather incomplete, the corneocytes in this region being reduced and not completely differentiated35. Therefore, NPs-loaded drugs put directly into the follicular duct can enhance the penetration of drugs into the viable epidermis. Further, NPs of smaller size can penetrate to greater depths36. The use of suitable particle size of nano range allows selective targeting of specific structures and cells within the skin follicles37. Moreover, particles can be temporarily accumulated in the hair follicles to create high local concentrations of drugs38. The accumulated particles can provide a source from which a prolonged drug release can be continued; this can be advantageous because it would effectively reduce the applied dose and the frequency of applications. The translocation capacity of smaller particles across intact skin is not clearly understood39,40. The NPs of 320 nm in size were found to effectively penetrate deeper into the hair follicles when compared with the non-nanoparticluate forms. These could be found in the follicles upto 10 days, whereas the non-nanoparticulate forms were not found after four days41. The state of skin also influences penetration. However, further safety measures should be taken, because of earlier reports42 which suggested that NPs could translocate across the hair follicle epithelium, especially in disrupted barriers or pre-damaged skin. It is essential that the toxicological profile of particles and their constituents as well as their biodegradability properties are carefully analysed before designing any innovative therapeutic system.
Use of PLGA in therapeutic delivery in melanoma
Topical administration of anti-cancer drugs is a new alternative for increasing drug targeting specificity and therapeutic benefits in melanoma and non-melanoma skin cancers. Efficient penetration of the anti-cancer drug in adequate quantity to annihilate tumour cells43 is considered a major challenge for treatment. Several attempts have been made by using chemical enhancers such as dimethyl sulphoxide, propylene glycol, ethanol and application of electric field, so that the drugs could overcome skin barriers successfully to reach malignancy sites deep into the skin at the cost of temporary and reversible disruption of the SC located in the deeper layers of the epidermis. For increasing penetration of anti-cancer drugs, chemical penetration enhancers are being used. However, interest has now been shifted toward use of NPs-loaded delivery system that also protects anti-cancer drugs against degradation and helps in significantly increasing tumour penetrating ability of the drugs. Further, most anti-cancer drugs show hydrophilic properties and thus have a low oil/water partition coefficient, high molecular weights and ionic characters44 that make them rather unsuitable for penetration through SC. Therefore, researchers look forward to a wide range of biodegradable and synthetic polymers such as solid-lipid NPs and polymeric NPs (PLA, PLGA and PCL) for the purpose45. PLGA has attained special importance for encapsulation of a wide variety of drugs as it is easily degradable, biocompatible and shows a controlled release profile of the incorporated entity. It is also non-toxic in nature. PLGA is synthesized by co-polymerization of two different monomers which are the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Tin (II) 2-ethylhexanoate and tin (II) alkoxides or aluminium isopropoxide acts as the most common catalyst in their preparation. The monomeric units (of glycolic or lactic acid) are linked together by ester linkages in PLGA during polymerization, which forms a linear, amorphous aliphatic polyester product. Some examples of PLGA encapsulation of drugs have been shown in Fig. 1.
PLGA forms can be identified by the ratio of monomers used. For example, PLGA 50:50 identifies a copolymer composed of 50 per cent lactic acid and 50 per cent glycolic acid. It seems that PLGA encapsulated NPs can closely contact the superficial junctions of corneocyte clusters and furrows, possibly favouring drug accumulation for several hours. This would allow for the sustained release of anti-cancer drugs. There were controversies regarding the ideal mean diameter, flexibility and superficial charge of NPs to optimize skin penetration. We have nanoencapsulated a flavone apigenin in PLGA and have studied their anti-proliferative actions against skin melanoma cell lines32. Encouraging results in the treatment of skin SCC have been reported in A431 cells (derived from human epidermoid SCC), by using PLGA coated 5-aminolevulinic acid46. The size of the spherical NPs being 65.6 nm and the encapsulation efficiency being 65.8 per cent, these NPs could be successfully taken up by the SCC cells. The choice of PLGA is also supported by the fact that it can be used to form stable NPs and has already been approved for the use in humans by the US FDA47. One kind of method used for PLGA encapsulation by solvent displacement technique is shown in Fig. 2.
Fig. 2.
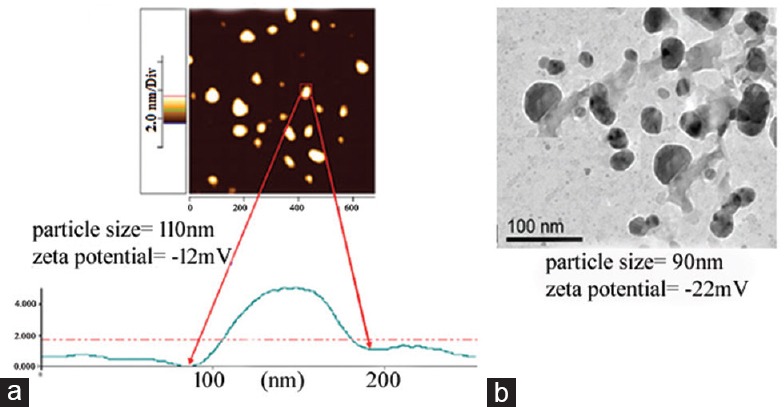
(a) Example showing atomic force microscopic (AFM) image of poly-lactide-co-glycolide encapsulated flavonoid compound (apigenin) showing the particle size to be 110 nm. (b) Transmission electron microscope (TEM) image of poly-lactide-co-glycolide encapsulated silver nanoparticles. The Figure shows that the shape and size of the PGLA coated nanoparticles were fairly uniform.
Physicochemical properties utilized in enhancing biological action in PLGA engineered therapy
PLGA-loaded NPs have been under attention for developing nanotherapeutic drugs with the properties of controlled/suspended drug and have their inherent advantages over conventional devices. Further, PLGA has various other applications, for example, in tissue engineering, for repairing/healing of bone defects and also in vaccines.
The methodology of formation of drug loaded PLGA NPs by solvent displacement technique has been shown in Fig. 1. PLGA has different physicochemical properties that make their use advantageous and commercially available; the release profile can be manipulated by selecting PLGA-loaded appropriate nanomedicines, with the suitable molecular weight and the desired lactide:gycolide ratio. The time interval of the drug release varies from several hours to months. Co-polymerization of PLGA with other biopolymers further extends the possibility of controlling the drug release profile. Many active pharmaceutical ingredients have been coated in PLGA-based delivery systems and have undergone in vivo trials, or have been released in sufficient concentrations for therapeutic effect; small interfering RNA (siRNA), proteins, peptides, anti-cancer drugs, analgesics, antibiotics and vaccines48 are some of the examples of such therapeutic use. PLGA NPs can be introduced through intravenous injection and targeted delivery can be achieved by conjugating an antibody or another molecule that has an affinity for a specific target on the surfaces, for example, tumour targeting49,50,51,52. Active cellular uptake of NPs is also possible, enabling intracellular drug delivery.
Size, surface charge and hydrophobicity
Particle size is a crucial factor in the biodistribution of long-circulating NPs and in attaining therapeutic efficacy. Earlier studies revealed that the kinetics of cellular uptake and the saturation concentration differed with the various sized NPs; 50 nm size particles showed the most efficient cellular uptake indicating it as the optimal size for efficient uptake of NPs into cells53. Also, similar sized spherical particles were taken up more efficiently than did the rod-shaped particles; this could be because of greater membrane wrapping time required for the elongated particles. Also, smaller the size of NPs, higher was their uptake in cells. Along with size, both surface charge and polymer hydrophobicity are also important features for targeted drug delivery. Although positive charge is known to improve the efficacy of gene transfer and drug delivery, yet a higher cytotoxic effect of such constructs has also been advocated. Jung et al54 showed the role of certain parameters such as surface charge, size and hydrophobicity which are important features of nano formulations. PLGA being a copolymer of glycolic and lactic acids gives an option to modify the ratio of these two components, which in turn determines its stability and degradability. A change in the ratio can also induce amphiphilicity. PLGA biodegrades in water by means of hydrolysis of the ester linkages. The methyl side groups in PLA make it more hydrophobic. Therefore, lactide rich PLGA copolymers are less hydrophilic, absorb less water and subsequently have a slower degradation rate. The change in properties of PLGA at the time of polymer biodegradation can influence the release as well as degradation rates of incorporated drug molecules efficiently. Therefore, the amphipathic charge of PLGA aids in optimized hydrophobicity, surface area and surface charge; these can influence not only the capacity to produce reactive oxygen species (ROS), but also can determine binding sites for receptors and influence dispersion and aggregation of the NPs55. Some other factors, for example, molecular weight, lactide/glycolide ratio, crystallinity and geometric regularity of individual chains also play significant roles in affecting the mechanical strength of the polymer. Chain lengths of PLGA can also be made to vary to provide optimal oral delivery.
Endolysosomal escape of PLGA nanoparticles
PLGA is much more advantageous in the use of cytoplasmic delivery than any other copolymers. Panyam et al56 demonstrated that PLGA NPs were internalized by an endocytic process because once internalized, NPs could be seen in the endolysosomal compartments. Contrary to cationic lipids or polymers, PLGA NPs can be cationic only in the endosomal compartment; these do not destabilize lysosomes. For this reason, the chance of toxicity that is associated with the use of cationic lipids and cationic polymers57 is considerably reduced.
Drug release profile
Sustained release of a drug aims at delivering the drug at a pre-determined amount for an increased period of time. The sustained release can maintain the concentration of the drug at a constant level. Studies on release kinetics of drugs encapsulated in PLGA have shown that monophasic release from PLGA-based DDSs of PLGA encapsulated drugs is generally rare58. PLGA encapsulated drugs have been found to show a biphasic release in skin melanoma cells. The release of apigenin from PLGA encapsulated forms has been analysed in our laboratory, which shows a biphasic release profile with an initial burst release (% from PLGA) up to 16 h followed by a much controlled release for up to three days (72 h)32. In our study, the equilibrium was attained within two days (48 h), as revealed from the plateau observed in the release profile between 48 and 72 h. Large particles often show triphasic release profile as a result of heterogeneous degradation. Of the classic triphasic release profile, the Phase I is usually described as the burst release phase; this phase is attributed to nonencapsulated drug particles on the surface or drug molecules close to the surface, making them easily accessible for hydration59. Other reasons that can be attributed to burst release may be for the formation of cracks and the disintegration of particles60. Phase II is often found to be a slow release phase and during this phase, the drug diffuses slowly, either through the relatively dense polymer or through a few existing pores, while polymer degradation and hydration proceed. Phase III generally exhibits a period of faster release, often attributed to the onset of erosion. This phase is sometimes referred to as the second burst phase. However, this may be mentioned that all release profiles do not follow the traditional triphasic characteristics. If the second phase is rapid, a slower phase at the end of the release period, not the typical burst release phase may be encountered.
Major applications of PLGA nanoparticles for conventional therapies of melanoma
Photodynamic therapy
PDT is used for the purpose of many cancers such as lung, gall bladder and melanoma. It is a two-stage procedure that initially administers a PS agent followed by illuminating the tumour with visible light, which is generally laser generated61,62. PS absorbs a photon of a particular wavelength to form an excited triplet state. The excited molecule thereafter, transfers energy to the (triplet) ground state of molecular oxygen which produces cytotoxic singlet oxygen (1O2) - type II reaction or undergoes electron transfer (type I reaction) by ultimately forming superoxide anion radical (O2 •–) and hydroxyl radical (OH•). Drug-based PLGA NPs have been shown to be effective for PDT in melanoma treatment63. NPs containing zinc (II) phthalocyanine (ZnPc) were synthesised by an emulsification diffusion method utilizing PLGA. Ricci-Junior and Marchetti63 studied PLGA NPs loaded with ZnPc, which, as a hydrophobic molecule, requires a hydrophobic delivery system. Despite their hydrophobic characteristics, no aggregation of ZnPc-loaded NPs could be observed after encapsulation. PLGA-based NPs for topical delivery of protoporphyrin IX (PpIX) were also proposed in PDT of skin cancers. These finding indicated a localized effect of PpIX-NPs in the epidermis plus dermis - a site of action for topical PDT suggesting a potential use of PpIX-NPs in PDT associated to skin cancer treatments64.
Targeted therapy
Resistance to therapy in malignant melanoma with high rate of mortality is an important factor that poses an enormous challenge. Therefore, novel therapeutic strategies such as immunotherapy and targeted therapy for melanoma are urgently needed. A new active targeting DDS combining chemotherapy and active specific-immunotherapy have been designed. It has been demonstrated that dacarbazine (DTIC)- loaded PLA conjugated to TRAIL-receptor-2 (DR5) monoclonal antibody (DTIC-NPs-DR5 mAb) provides an active targeting DDS that has the ability to specifically target DR5-overexpressing malignant melanoma cells and thus become efficiently internalized65. It has also been reported that cucurbitacin (Cuc)-loaded PLGA particles of different sizes (about 50, 5 μm and 270 nm, respectively) can act as sustained-release system for intratumoural injection and their physicochemical properties like in vitro cytotoxicity, particles-cells interactions, pharmacokinetics and pharmacodynamics can be systemically followed66. It was also possible to engineer NPs from a unique hexadentate-PLGA polymer chemically conjugated to PD98059 that acts as a selective MAPK (mitogen-activated protein kinase) inhibitor67. NP-mediated targeting of MAPK can inhibit the proliferation of melanoma and lung carcinoma cells and triggers apoptosis in vitro. We observed that nano-apigenin could directly target nuclear DNA to reduce DNA damage and trigger apoptotic signalling responses by generating ROS and mitochondria mediated pathway32. Earlier, we also formulated nanoencapsulation of a naturally occurring coumarin-scopoletin 7-hydroxy-6-methoxy coumarin (HMC) that was isolated from plant Gelsemium sempervirens, which is known to possess anti-cancer potentials. We encapsulated the drug with PLGA and examined whether its cellular uptake, bioavailability and apoptotic (anti-cancer) potentials could be increased vis-a-vis the unencapsulated HMC68. Study of mRNA expressions of some signal molecules revealed that normal human mesangial cells downregulated expression of cyclin-D1, proliferating cell nuclear antigen, survivin and Stat-3 and upregulated expression of p53 and caspase-3 that consecutively induced the apoptotic event vis-a-vis unencapsulated HMC. The trans-[Ru(NO)Cl(cyclam)](PF6)2 and [Ru(NO)(Hedta)] encapsulated in PLGA NPs for successful delivery of nitric oxide in melanoma cells showed pronounced cytotoxicity and phototoxicity69. Localized application of therapies has also been found to yield good results and, therefore, recommended for occasional use. The release patterns of the particles not only affect the biological efficiency at the in vitro system but also can equally affect in the animal models70,71. This has been concluded from cytotoxicity and pharmacokinetic/pharmacodynamic studies. An oil-in-oil emulsion/solvent evaporation method was used in a previous study71 to fabricate albumin/drug loaded magnetic nanocomposite spheres and these nanospheres were tested to evaluate the efficacy of the DDS on skin cancer. Human serum albumin, PLGA, 5-FU, magnetic NPs (10 nm) and fluorescent labelling molecules (diphenylhexatriene) were utilized to develop this delivery system.
Targeting DNA
In earlier investigations, [epigallocatechin-3-gallate (EGCG)], a polyphenolic constituent of black [theaflavin (TF)] and green tea were entrapped in PLGA NPs to check any preventive potential against 7,12-dimethylbenzanthracene (DMBA)-induced DNA damage in mouse skin72. Pre-treatment (topically) with either TF or EGCG exhibited protection against DMBA-induced DNA damage. TF or EGCG-loaded PLGA-NPs showed a better ability to prevent DMBA-induced DNA damage at much lower concentrations, which opened up a new dimension in chemoprevention research72. We entrapped synthetic coumarin with PLGA NPs (Nano Coumarin: NC) and tested for its anti-cancer potentials68. NC demonstrated greater uptake of drug and showed anti-cancer potentials in melanoma cell line A375. The data obtained from circular dichroism spectra (CD) and melting temperature profile (Tm) of calf thymus DNA treated with NC could be analysed to determine if the drug had actively interacted with the target DNA. According to the results, a dose dependent interaction of NC with calf thymus DNA, which could effectively change the structure and conformation with increased stability, was observed70.
Use of PLGA in some other delivery systems
Although extensive innovation has been made over the past decade, there still remains a need for integrated, easily adaptable drug delivery and imaging modalities. Extensive research has now made it known that polymeric NPs serve as a versatile medium for the delivery and monitoring of highly toxic compounds in vivo, which could be attributed to their enhanced drug loading capacity, biological stability and extended in vivo circulation and could account for more than 80 per cent of the available therapeutics in clinical use.
siRNA polyplexes
Synthesis of active and safe siRNAs has significantly been one of the active areas of research for targeted delivery of drugs (Fig. 1). STAT3 (signal transducer and activator of transcription 3) silencing in the dendritic cells has been found to be favourable for cancer immunotherapy73. Findings suggest that STAT3 knockdown in murine melanoma by polyethylenimine (PEI) siRNA polyplexes or their stearic acid derivative (PEI-StA), could induce cell death in vitro and in vivo73. Studies confirmed the efficient drug uptake in the dendritic cells and subsequent endosomal localization of both NP types. STAT3 activity that had been silenced by PLGA-P (PLGA NPs containing siRNA polyplexes of PEI) could be restored by the application of PLGA-PS (PLGA NPs containing siRNA polyplexes of PEI with stearic acid derivative) of STAT3 siRNA having the ability to induce dendritic cell maturation and functionality. This fact has been experimentally evidenced by the upregulation of CD86 expression, high secretion of tumour necrosis factor – α (TNF-α) and allogenic T cell proliferation at a significant level74. We formulated NPs-based encapsulation of siRNA (NsiRNA) with PEI and PLGA. Results revealed PEI-PLGA to be a promising carrier for the delivery of siRNA targeting STAT3 expression, which can be utilized as an effective strategy for cancer therapy75. The basis of adding PEI in PLGA–PEI complex is also to avoid aggregation of NPs and to maintain the surface charge reversal of nanocomplex at the acidic pH (<5) found in the lysosomes. The introduction of PEI dramatically changed the surface charge of STAT3 siRNA NPs from negative to positive, facilitating adherence of the NPs to the negatively charged cellular membranes. The encapsulation efficiency of the formulated NPs was 81.7 per cent. Liu et al76 demonstrated the release profile of PLGA-PEG siRNA NPs. The in vitro release of siRNA was suggested to be pH dependent. But while one study inferred that the release was faster at pH 4.0 (500 U lipase) than in pH 7, another study77 opposed this finding and suggested that the electrostatic-bound DNA/RNA could be released from the NP complexes at alkaline pH (>10) or by the presence of high salt concentrations. DNA protection through binding to amino-modified silica NPs could be due to positive charges that keep Mg2+ away from the positively charged NPs on the amino group of the NPs and/or by conformational change(s) of DNA structure when these are embedded onto the surface of NPs. The small size of the NPs may force the RNA to become bound in such a way that cleavage is either impossible or at least strikingly slowed on the NPs surface. However, this hypothesis still needs to be checked for correctness.
Transdermal delivery of PLGA nanoparticles
Transdermal delivery of nanocarriers has been known to be due to their ability to deliver therapeutic agents in a sustained manner with a controlled ratio. The permeability of estradiol-loaded PLGA NPs through the skin of rat has been examined earlier. After loading estradiol in NPs, the bioavailability of the drug significantly increased78. One study suggested that if iontophoresis was applied to enhance the permeability of NPs in in vivo system, the enhanced permeability effect was also manifested in an enhanced transdermal delivery of therapeutic agents79.
Microneedle-mediated intradermal nanoparticle delivery
Researchers tried to deliver a model hydrophobic dye called Nile red, into the skin by using microneedle technology80. The dye was loaded into PLGA NPs, and polymeric microneedle arrays were prepared from aqueous blends of the mucoadhesive copolymer Gantrez® AN-13981. These were tailored to finally obtain one mg of Nile red-loaded PLGA NPs. This delivery strategy could help to overcome the local delivery problem of highly hydrophobic agents, which are presently being confronted with some disadvantageous currently available delivery practices.
Transcutaneous delivery of PLGA nanoparticles
Needle-based delivery is the common practice of vaccine and therapeutic delivery82. However, a needle-based delivery system has elucidated a number of inherent risks and disadvantages. Some major concerns are the need for storage of liquid formulations at cold temperatures, requirement of trained personnel and reduced safety due to needle re-use and injuries. Vaccination and therapeutic administration through the skin could be an alternative strategy83 that could avoid the stated risks and, therefore, technologies that help to make efficient transcutaneous delivery of drugs and vaccines have now attracted serious attention of researchers84. This culminated in the discoveries related to the utility of microneedle arrays for efficient and pain-free disruption of the SC, facilitating transcutaneous delivery of a variety of bioactive materials85,86. Dried water-soluble drug formulations could be directly coated onto the surfaces of solid microneedles for an easy microneedle delivery. Earlier, researchers designed experiments to find out the extent of penetration and distribution of PLGA NPs in the human skin treated with microneedles. The quantitative results suggested that the permeation of NPs into the skin was enhanced by microneedles, but no NP could reach the receptor solution; the permeation could only be more efficient with increased NP concentration until a limit value was reached81. These experimental results confirmed that microneedles could enhance the intradermal delivery of PLGA NPs. One study87 suggested that this strategy would also prove to be useful for topical drug administration. Microneedles have also been successfully applied to enhance the skin permeability of small and large molecules and even NPs, by producing micron-sized pores in the SC layer of the skin. The feasibility of using microneedles to increase the skin permeability of 5-FU was also tested. The anti-tumour activity of a commercially available 5-FU topical cream (5%) was significantly enhanced when the cream was applied on a skin area of a mouse model with B16-F10 mouse melanoma cells implanted in the subcutaneous space that was pre-treated with microneedles, as compared to when the cream was simply applied on a skin area, underneath which the tumour cells were implanted and without pre-treatment of the skin with microneedles87. Clinical efficacy of topical application of 5-FU against tumours such as basal cell carcinoma was found to be improved by integrating microneedle technology into the therapy88.
Particles used other than PLGA in drug delivery for melanoma
DDSs other than PLGA have already been used successfully for topical applications, including modified liposomes, SLN and biodegradable polyester NPs such as PLA and PCL. Moreover, a heat sensitive liposomal taxol was formulated to be delivered in murine melanoma. This drug was prepared by using egg phosphatidylcholine and cholesterol combined with ethanol; the use of this drug produced significant reduction in tumour volume in B16F10 murine melanoma that was transplanted into C57BI/6 mice89. The efficacy of polyvinylpyrrolidone NPs containing taxol has also been examined utilizing the reverse micro-emulsion method in melanoma.
Conclusion and future perspectives
The treatment efficacy for metastatic melanoma depends on having a better understanding of the pathogenesis of the disease; early diagnosis and identification of individual molecular typing, and novel and effective DDSs are some of the key factors which need to be given due consideration for a successful treatment. Nanotechnology has offered a new perspective in the area of early diagnosis and treatment of melanoma. Further, it is now known that NP-delivered anti-cancer drugs are more target-specific as these concentrate more in tumour tissue, thus improving treatment efficacy and relatively reducing side effects. Because of the fact that polymers used in nanoencapsulation can be either biodegradable or non-biodegradable, these provide significant flexibility in design; further, these can be made synthetically or derived from natural sources. The advantages of the biodegradable polymers are that these are generally degraded into individual monomers by the metabolic processes and are removed from the body via normal metabolic pathways. The physicochemical properties of polymers are responsible for their degradation and drug release kinetics; these properties can be precisely controlled by manipulation of molecular weight, dispersity index, hydrophobicity and crystallinity. In general, drugs can be released in a controlled manner with specific knowledge of the first-order kinetics. The property of drug diffusion through the polymeric matrix can also be triggered in response to the local environment. However, in some therapeutic strategies such as PDT, the release profile of biodegradable polymer-based nanomaterials as carriers for PS agents is governed by the interactions with various compartments, and is affected by the rate of degradation of the polymer and the interactions between drug and polymer. These factors make it difficult to predict an accurate release profile for the photosensitizers molecules. It should be noted that NP-based PDT is still in its infancy and much remains to be learned. For the successful clinical application of NPs for drug delivery, one should carefully avoid the use of nanodrugs having severe side effects. Another aspect that needs critical consideration is the cytotoxicity of the drug to the normal cells and its ability to cause leaching of components. Restricted use of larger NPs is advisable as these can accumulate in the endoplasmic reticulum system. Further, one should be careful about the immunogenicity of NPs which can cause autoimmunity. Moreover, combined strategies involving judicious use of the microneedles and targeted NP techniques can provide an alternative route to deliver drugs into specific skin tissues. Targeting of specific mast cells and lymphocytes in the dermis can improve the therapeutic efficacy. However, more detailed study should be conducted with this perspective. Thus, different types of NPs should be tested, particularly for their possible side effects, because sometimes side effects caused by NPs can even be fatal. Safety issues such as transcutaneous penetration of NPs should be taken into account. In addition, multifunctional NPs capable of carrying imaging agents and delivering multiple drugs are now being developed for improved ability of detection and treatment of cancer. Further studies are also needed to avoid side effects, if any, caused by some NPs so that these can be recommended for safe clinical use without much concern. Several NPs-delivered drugs have been approved by the US FDA and some are currently in clinical trial stage. ABI-007 or NP albumin bound (nab)-paclitaxel has already been approved by the FDA for clinical use90. Zhang et al91 demonstrated that doxorubicin (DOX) loaded gold NPs, a targeted chemotherapeutic agent, was very effective against a melanoma cell line. However, more studies are needed to streamline the treatment methodologies that can screen out the adverse side effects and only the beneficial effects could be delivered to the patients. Though we are in a sphere of activities when new system of treatment of skin cancer is seen in the horizon, but we have to go many more miles for harvesting unstinted benefits of the new technologies at hand.
Acknowledgment
Authors acknowledge the University Grants Commission (UGC), New Delhi, India, for awarding Emeritus Fellowship to the second author (ARK-B).
Footnotes
Conflicts of Interest: None.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Jerant AF, Johnson JT, Sheridan CD, Caffrey TJ. Early detection and treatment of skin cancer. Am Fam Physician. 2000;62:357–68. 375-6, 381-2. [PubMed] [Google Scholar]
- 4.American Cancer Society. Skin cancer facts. [accessed on June 05, 2013]. Available from: http://www.cancer.org/cancer/cancercauses/sunanduvexposure/skin-cancer-facts .
- 5.Zitelli JA, Mohs FE, Larson P, Snow S. Mohs micrographic surgery for melanoma. Dermatol Clin. 1989;7:833–43. [PubMed] [Google Scholar]
- 6.Baade P, Meng X, Youlden D, Aitken J, Youl P. Time trends and latitudinal differences in melanoma thickness distribution in Australia, 1990-2006. Int J Cancer. 2012;130:170–8. doi: 10.1002/ijc.25996. [DOI] [PubMed] [Google Scholar]
- 7.Boyle GM. Therapy for metastatic melanoma: An overview and update. Expert Rev Anticancer Ther. 2011;11:725–37. doi: 10.1586/era.11.25. [DOI] [PubMed] [Google Scholar]
- 8.Lopez RF, Lange N, Guy R, Bentley MV. Photodynamic therapy of skin cancer: controlled drug delivery of 5-ALA and its esters. Adv Drug Deliv Rev. 2004;56:77–94. doi: 10.1016/j.addr.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Martinez JC, Otley CC. The management of melanoma and nonmelanoma skin cancer: A review for the primary care physician. Mayo Clin Proc. 2001;76:1253–65. doi: 10.4065/76.12.1253. [DOI] [PubMed] [Google Scholar]
- 10.Nagore E, Oliver V, Botella-Estrada R, Moreno-Picot S, Guillén C, Fortea JM. Clinicopathological analysis of 1571 cutaneous malignant melanomas in Valencia, Spain: Factors related to tumour thickness. Acta Derm Venereol. 2006;86:50–6. doi: 10.2340/00015555-0004. [DOI] [PubMed] [Google Scholar]
- 11.Bundscherer A, Hafner C, Maisch T, Becker B, Landthaler M, Vogt T. Antiproliferative and proapoptotic effects of rapamycin and celecoxib in malignant melanoma cell lines. Oncol Rep. 2008;19:547–53. [PubMed] [Google Scholar]
- 12.Berwick M. Counterpoint: Sunscreen use is a safe and effective approach to skin cancer prevention. Cancer Epidemiol Biomarkers Prev. 2007;16:1923–4. doi: 10.1158/1055-9965.EPI-07-0391. [DOI] [PubMed] [Google Scholar]
- 13.Holderfield M, Deuker MM, McCormick F, McMahon M. Targeting RAF kinases for cancer therapy: BRAF mutated melanoma and beyond. Nat Rev Cancer. 2014;14:455–67. doi: 10.1038/nrc3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribas A, Hauschild A, Kefford R, Punt CJ, Haanen JB, Marmol M, et al. Phase III, open-label, randomized, comparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide [TMZ] or dacarbazine [DTIC]) in patients with advanced melanoma. 2008 ASCO Annual Meeting Proceedings (Post-Meeting Edition) J Clin Oncol. 2008;26(15S):LBA9011. [Google Scholar]
- 15.Fellner C. Ipilimumab (yervoy) prolongs survival in advanced melanoma: Serious side effects and a hefty price tag may limit its use. PT. 2012;37:503–30. [PMC free article] [PubMed] [Google Scholar]
- 16.Davids LM, Kleemann B. Combating melanoma: The use of photodynamic therapy as a novel, adjuvant therapeutic tool. Cancer Treat Rev. 2011;37:465–75. doi: 10.1016/j.ctrv.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Zuazaga J, Cooper KD, Baron ED. Photodynamic therapy in dermatology: Current concepts in the treatment of skin cancer. Expert Rev Anticancer Ther. 2005;5:791–800. doi: 10.1586/14737140.5.5.791. [DOI] [PubMed] [Google Scholar]
- 18.Hagen B, Trinh VA. Managing side effects of vemurafenib therapy for advanced melanoma. J Adv Pract Oncol. 2014;5:400–10. [PMC free article] [PubMed] [Google Scholar]
- 19.Chadha R, Kapoor VK, Thakur D, Kaur R, Arora P, Jain DVS. Drug carrier systems for anticancer agents: A review. J Sci Ind Res. 2008;67:185–97. [Google Scholar]
- 20.Liang XJ, Chen C, Zhao Y, Wang PC. Circumventing tumor resistance to chemotherapy by nanotechnology. Methods Mol Biol. 2010;596:467–88. doi: 10.1007/978-1-60761-416-6_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrouenraets MB, Visser GW, Snow GB, van Dongen GA. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003;23:505–22. [PubMed] [Google Scholar]
- 22.Sharma H, Sahu G, Sahu S. Recent approaches of drug delivery for skin cancer. World J Pharm Pharm Sci. 2014;3:300–15. [Google Scholar]
- 23.Fortina P, Kricka LJ, Surrey S, Grodzinski P. Nanobiotechnology: The promise and reality of new approaches to molecular recognition. Trends Biotechnol. 2005;23:168–73. doi: 10.1016/j.tibtech.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: Current status and future prospects. FASEB J. 2005;19:311–30. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 25.Thompson M. Nanomedicine – A tremendous research opportunity for analytical chemists. Analyst. 2004;129:6712. doi: 10.1039/b409906k. [DOI] [PubMed] [Google Scholar]
- 26.Desai P, Patlolla RR, Singh M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol Membr Biol. 2010;27:247–59. doi: 10.3109/09687688.2010.522203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escobar-Chavez JJ, Díaz-Torres R, Rodríguez-Cruz IM, Domínguez-Delgado CL, Morales RS, Angeles-Anguiano E, et al. Nanocarriers for transdermal drug delivery. Res Rep Transdermal Drug Deliv. 2012;1:3–17. [Google Scholar]
- 28.Hadgraft J. Modulation of the barrier function of the skin. Skin Pharmacol Appl Skin Physiol. 2001;14:72–81. doi: 10.1159/000056393. [DOI] [PubMed] [Google Scholar]
- 29.Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 2003;8:1112–20. doi: 10.1016/s1359-6446(03)02903-9. [DOI] [PubMed] [Google Scholar]
- 30.Fahmy TM, Samstein RM, Harness CC, Mark Saltzman WM. Surface modification of biodegradable polyesters with fatty acid conjugates for improved drug targeting. Biomaterials. 2005;26:5727–36. doi: 10.1016/j.biomaterials.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Couvreur P, Barratt G, Fattal E, Legrand P, Vauthier C. Nanocapsule technology: A review. Crit Rev Ther Drug Carrier Syst. 2002;19:99–134. doi: 10.1615/critrevtherdrugcarriersyst.v19.i2.10. [DOI] [PubMed] [Google Scholar]
- 32.Das S, Das J, Samadder A, Paul A, Khuda-Bukhsh AR. Strategic formulation of apigenin-loaded PLGA nanoparticles for intracellular trafficking, DNA targeting and improved therapeutic effects in skin melanoma in vitro. Toxicol Lett. 2013;223:124–38. doi: 10.1016/j.toxlet.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Michel M, L’Heureux L, Pouliot R, Xu W, Auger FA, Germain L. Characterization of a new tissue-engineered human skin equivalent with hair. In Vitro Cell Dev Biol Anim. 1999;35:318–26. doi: 10.1007/s11626-999-0081-x. [DOI] [PubMed] [Google Scholar]
- 34.Teichmann A, Otberg N, Jacobi U, Sterry W, Lademann J. Follicular penetration: Development of a method to block the follicles selectively against the penetration of topically applied substances. Skin Pharmacol Physiol. 2006;19:216–23. doi: 10.1159/000093117. [DOI] [PubMed] [Google Scholar]
- 35.Vogt A, Blume-Peytavi U. Biology of the human hair follicle. New knowledge and the clinical significance. Hautarzt. 2003;54:692–8. doi: 10.1007/s00105-003-0562-x. [DOI] [PubMed] [Google Scholar]
- 36.Toll R, Jacobi U, Richter H, Lademann J, Schaefer H, Blume-Peytavi U. Penetration profile of microspheres in follicular targeting of terminal hair follicles. J Invest Dermatol. 2004;123:168–76. doi: 10.1111/j.0022-202X.2004.22717.x. [DOI] [PubMed] [Google Scholar]
- 37.Lademann J, Richter H, Teichmann A, Otberg N, Blume-Peytavi U, Luengo J, et al. Nanoparticles - An efficient carrier for drug delivery into the hair follicles. Eur J Pharm Biopharm. 2007;66:159–64. doi: 10.1016/j.ejpb.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Lademann J, Richter H, Schaefer H, Blume-Peytavi U, Teichmann A, Otberg N, et al. Hair follicles – A long-term reservoir for drug delivery. Skin Pharmacol Physiol. 2006;19:232–6. doi: 10.1159/000093119. [DOI] [PubMed] [Google Scholar]
- 39.Cross SE, Innes B, Roberts MS, Tsuzuki T, Robertson TA, McCormick P. Human skin penetration of sunscreen nanoparticles: in-vitro assessment of a novel micronized zinc oxide formulation. Skin Pharmacol Physiol. 2007;20:148–54. doi: 10.1159/000098701. [DOI] [PubMed] [Google Scholar]
- 40.Baroli B, Ennas MG, Loffredo F, Isola M, Pinna R, López-Quintela MA. Penetration of metallic nanoparticles in human full-thickness skin. J Invest Dermatol. 2007;127:1701–12. doi: 10.1038/sj.jid.5700733. [DOI] [PubMed] [Google Scholar]
- 41.Weissenböck A, Wirth M, Gabor F. WGA-grafted PLGA-nanospheres: Preparation and association with Caco-2 single cells. J Control Release. 2004;99:383–92. doi: 10.1016/j.jconrel.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Wang L, Fan Y, Feng Q, Cui FZ. Biocompatibility and toxicity of nanoparticles and nanotubes. J Nanomater. 2012;2012:1–19. [Google Scholar]
- 43.DeLouise LA. Applications of nanotechnology in dermatology. J Invest Dermatol. 2012;132:964–75. doi: 10.1038/jid.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souza JG, Gelfuso GM, Simão PS, Borges AC, Lopez RFV. Iontophoretic transport of zinc phthalocyanine tetrasulfonic acid as a tool to improve drug topical delivery. Anticancer Drugs. 2011;22:783–93. doi: 10.1097/CAD.0b013e3283468979. [DOI] [PubMed] [Google Scholar]
- 45.Marquele-Oliveira F, de Almeida Santana DC, Taveira SF, Vermeulen DM, de Oliveira AR, da Silva RS, et al. Development of nitrosyl ruthenium complex-loaded lipid carriers for topical administration: Improvement in skin stability and in nitric oxide release by visible light irradiation. J Pharm Biomed Anal. 2010;53:843–51. doi: 10.1016/j.jpba.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci. 2010;351:19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–90. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 48.Fonseca C, Simões S, Gaspar R. Paclitaxel-loaded PLGA nanoparticles: Preparation, physicochemical characterization and in vitro anti-tumoral activity. J Control Release. 2002;83:273–86. doi: 10.1016/s0168-3659(02)00212-2. [DOI] [PubMed] [Google Scholar]
- 49.Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems – A review. Int J Pharm. 2011;415:34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 50.Yoo HS, Lee KH, Oh JE, Park TG. In vitro and in vivo anti-tumor activities of nanoparticles based on doxorubicin-PLGA conjugates. J Control Release. 2000;68:419–31. doi: 10.1016/s0168-3659(00)00280-7. [DOI] [PubMed] [Google Scholar]
- 51.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, et al. Polymeric nanoparticle-encapsulated curcumin (“Nanocurcumin”): A novel strategy for human cancer therapy. J Nanobiotechnol. 2007;5:1–18. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gómez-Gaete C, Tsapis N, Besnard M, Bochot A, Fattal E. Encapsulation of dexamethasone into biodegradable polymeric nanoparticles. Int J Pharm. 2007;331:153–9. doi: 10.1016/j.ijpharm.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 53.Verma A, Stellacci F. Effect of surface properties on nanoparticle-cell interactions. Small. 2009;6:12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 54.Jung T, Breitenbach A, Kissel T. Sulfobutylated poly(vinyl alcohol)-graft-poly(lactide-co-glycolide)s facilitate the preparation of small negatively charged biodegradable nanospheres. J Control Release. 2000;67:157–69. doi: 10.1016/s0168-3659(00)00201-7. [DOI] [PubMed] [Google Scholar]
- 55.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 56.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly (DL-lactide-co-glycolide) nanoparticles: Implications for drug and gene delivery. FASEB J. 2002;16:1217–26. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 57.Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63:170–83. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 58.Han FY, Thurecht KJ, Whittaker AK, Smith MT. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front Pharmacol. 2016;7:185. doi: 10.3389/fphar.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J, Wang BM, Schwendeman SP. Characterization of the initial burst release of a model peptide from poly(D,L-lactide-co-glycolide) microspheres. J Control Release. 2002;82:289–307. doi: 10.1016/s0168-3659(02)00137-2. [DOI] [PubMed] [Google Scholar]
- 60.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73:121–36. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 61.Ion RM. New trends in photodynamic therapy-review. In: Podbielska H, Sieron A, Strek W, editors. Aspects of photodynamic medicine. Biomedicine Engineering Acta. Vol. 3. Wroclaw, Poland: Indygo Zahir Media; 2008. pp. 123–59. [Google Scholar]
- 62.Ion RM. Derivative UV-Vis spectrophotometry for porphyrins interactions in photodynamic therapy. Anal Lett. 2010;43:1277–86. [Google Scholar]
- 63.Ricci-Júnior E, Marchetti JM. Zinc(II) phthalocyanine loaded PLGA nanoparticles for photodynamic therapy use. Int J Pharm. 2006;310:187–95. doi: 10.1016/j.ijpharm.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 64.da Silva CL, Del Ciampo JO, Rossetti FC, Bentley MV, Pierre MB. PLGA nanoparticles as delivery systems for protoporphyrin IX in topical PDT: Cutaneous penetration of photosensitizer observed by fluorescence microscopy. J Nanosci Nanotechnol. 2013;13:6533–40. doi: 10.1166/jnn.2013.7789. [DOI] [PubMed] [Google Scholar]
- 65.Ding B, Wu X, Fan W, Wu Z, Gao J, Zhang W, et al. Anti-DR5 monoclonal antibody-mediated DTIC-loaded nanoparticles combining chemotherapy and immunotherapy for malignant melanoma: target formulation development and in vitro anticancer activity. Int J Nanomedicine. 2011;6:1991–2005. doi: 10.2147/IJN.S24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jianbo G, Xue L, Hongdan Y, Zhaohui T, Xing T, Chenchen C, et al. The anti-melanoma efficiency of the intratumoral injection of cucurbitacin-loaded sustained-release carriers: A PLGA particle system. J Pharm Sci. 2013;102:2550–63. doi: 10.1002/jps.23604. [DOI] [PubMed] [Google Scholar]
- 67.Basu S, Harfouche R, Soni S, Chimote G, Mashelkar RA, Sengupta S. Nanoparticle-mediated targeting of MAPK signaling predisposes tumor to chemotherapy. Proc Natl Acad Sci USA. 2009;106:7957–61. doi: 10.1073/pnas.0902857106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhattacharyya SS, Paul S, De A, Das D, Samadder A, Boujedaini N, et al. Poly (lactide-co-glycolide) acid nanoencapsulation of a synthetic coumarin: Cytotoxicity and bio-distribution in mice, in cancer cell line and interaction with calf thymus DNA as target. Toxicol Appl Pharmacol. 2011;253:270–81. doi: 10.1016/j.taap.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Misak H, Zacharias N, Song Z, Hwang S, Man KP, Asmatulu R, et al. Skin cancer treatment by albumin/5-Fu loaded magnetic nanocomposite spheres in a mouse model. J Biotechnol. 2013;164:130–6. doi: 10.1016/j.jbiotec.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 70.Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, et al. Drug delivery systems: An updated review. Int J Pharm Investig. 2012;2:2–11. doi: 10.4103/2230-973X.96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomes AJ, Espreafico EM, Tfouni E. Trans-[Ru(NO)Cl(cyclam)](PF6)2 and [Ru(NO)(Hedta)] incorporated in PLGA nanoparticles for the delivery of nitric oxide to B16-F10 cells: Cytotoxicity and phototoxicity. Mol Pharm. 2013;10:3544–54. doi: 10.1021/mp3005534. [DOI] [PubMed] [Google Scholar]
- 72.Srivastava AK, Bhatnagar P, Singh M, Mishra S, Kumar P, Shukla Y, et al. Synthesis of PLGA nanoparticles of tea polyphenols and their strong in vivo protective effect against chemically induced DNA damage. Int J Nanomedicine. 2013;8:1451–62. doi: 10.2147/IJN.S26364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alshamsan A, Haddadi A, Hamdy S, Samuel J, El-Kadi AO, Uludag H, et al. STAT3 silencing in dendritic cells by siRNA polyplexes encapsulated in PLGA nanoparticles for the modulation of anticancer immune response. Mol Pharm. 2010;7:1643–54. doi: 10.1021/mp100067u. [DOI] [PubMed] [Google Scholar]
- 74.Wallet MA, Sen P, Tisch R. Immunoregulation of dendritic cells. Clin Med Res. 2005;3:166–75. doi: 10.3121/cmr.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Das J, Das S, Paul A, Samadder A, Bhattacharyya SS, Khuda-Bukhsh AR. Assessment of drug delivery and anticancer potentials of nanoparticles-loaded siRNA targeting STAT3 in lung cancer, in vitro and in vivo. Toxicol Lett. 2014;225:454–66. doi: 10.1016/j.toxlet.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 76.Liu CW, Lin WJ. Using doxorubicin and siRNA-loaded heptapeptide-conjugated nanoparticles to enhance chemosensitization in epidermal growth factor receptor high-expressed breast cancer cells. J Drug Target. 2013;21:776–86. doi: 10.3109/1061186X.2013.811511. [DOI] [PubMed] [Google Scholar]
- 77.He XX, Wang K, Tan W, Liu B, Lin X, Huang S, et al. Bioconjugated nanoparticles for DNA protection from cleavage. J Am Chem Soc. 2003;125:7168–9. doi: 10.1021/ja034450d. [DOI] [PubMed] [Google Scholar]
- 78.Mittal G, Sahana DK, Bhardwaj V, Ravi Kumar MN. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release. 2007;119:77–85. doi: 10.1016/j.jconrel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 79.Tomoda K, Watanabe A, Suzuki K, Inagi T, Terada H, Makino K. Enhanced transdermal permeability of estradiol using combination of PLGA nanoparticles system and iontophoresis. Colloids Surf B Biointerfaces. 2012;97:84–9. doi: 10.1016/j.colsurfb.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 80.Donnelly RF, Morrow DI, Fay F, Scott CJ, Abdelghany S, Singh RRT, et al. Microneedle-mediated intradermal nanoparticle delivery: Potential for enhanced local administration of hydrophobic pre-formed photosensitisers. Photodiagnosis Photodyn Ther. 2010;7:222–31. doi: 10.1016/j.pdpdt.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 81.Gomaa YA, Garland MJ, McInnes FJ, Donnelly RF, El-Khordagui LK, Wilsona CG. Microneedle/nanoencapsulation-mediated transdermal delivery: Mechanistic insights. Eur J Pharm Biopharm. 2014;86:145–55. doi: 10.1016/j.ejpb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giudice EL, Campbell JD. Needle-free vaccine delivery. Adv Drug Deliv Rev. 2006;58:68–89. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 83.Donatus U, Bruce G, Bull RT. Model-based estimates of risks of disease transmission and economic costs of seven injection devices in sub-Saharan Africa. Bull World Health Organ. 2002;80:859–70. [PMC free article] [PubMed] [Google Scholar]
- 84.Pruss-Ustun A, Mathers C, Corvalán CF, Woodward A. Introduction and methods: Assessing the environmental burden of disease at national and local levels. (WHO Environmental Burden of Disease Series, No. 1) Geneva: World Health Organization; 2003. [Google Scholar]
- 85.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Glenn GM, Kenney RT, Ellingsworth LR, Frech SA, Hammond SA, Zoeteweij JP. Transcutaneous immunization and immunostimulant strategies: Capitalizing on the immunocompetence of the skin. Expert Rev Vaccines. 2003;2:253–67. doi: 10.1586/14760584.2.2.253. [DOI] [PubMed] [Google Scholar]
- 87.Zhang W, Gao J, Zhu Q, Zhang M, Ding X, Wang X, et al. Penetration and distribution of PLGA nanoparticles in the human skin treated with microneedles. Int J Pharm. 2010;402:205–12. doi: 10.1016/j.ijpharm.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 88.Naguib YW, Kumar A, Cui Z. The effect of microneedles on the skin permeability and antitumor activity of topical 5-fluorouracil. Acta Pharm Sin B. 2014;4:94–9. doi: 10.1016/j.apsb.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma D, Chelvi TP, Kaur J, Ralhan R. Thermosensitive liposomal taxol formulation: Heat-mediated targeted drug delivery in murine melanoma. Melanoma Res. 1998;8:240–4. doi: 10.1097/00008390-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Vader P, Fens MH, Sachini N, van Oirschot BA, Andringa G, Egberts AC, et al. Taxol(®)-induced phosphatidylserine exposure and microvesicle formation in red blood cells is mediated by its vehicle Cremophor(®) EL. Nanomedicine (Lond) 2013;8:1127–35. doi: 10.2217/nnm.12.163. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X, Chibli H, Kong D, Nadeau J. Comparative cytotoxicity of gold-doxorubicin and InP-doxorubicin conjugates. Nanotechnology. 2012;23:275103. doi: 10.1088/0957-4484/23/27/275103. [DOI] [PubMed] [Google Scholar]


