Abstract
Significant hemodynamic changes ensue with aging, leading to an ever-growing epidemic of hypertension. Alterations in central arterial properties play a major role in these hemodynamic changes. These alterations are characterized by an initial decline in aortic distensibility and a rise of diastolic blood pressure, followed by a sharp increase in pulse wave velocity (PWV), and a rise in pulse pressure (PP) beyond the sixth decade. However, the trajectories of PWV and PP diverge with advancing age, more profoundly in men than women, likely reflecting the more pronounced aortic dilatation in men. There is an increased prevalence of salt-sensitive hypertension with advancing age, that is, in part, mediated by marinobufagenin, an endogenous sodium pump ligand, which is also linked to central arterial stiffness. Within the arterial wall, biomechanical and humoral changes are accompanied by significant biomolecular alterations producing a proinflammatory state, in which activation of angiotensin II signaling plays a pivotal role. This proinflammatory state is in origin a reparatory response to a damaged arterial wall under a pulsatile injury. However the same reparatory process results in fibrosis, which in turn worsens arterial stiffness and produce more pulsatile hemodynamics; this relationship between the pulsatile damage and proinflammatory state is best described as a feed-forward loop. Effective efforts to counter the surging epidemic of hypertension with the aging of our population should be aimed at revealing early, pre-clinical hemodynamic and arterial wall alterations, and develop interventions that halt these processes before they reach the stage the medical community defined as “disease”.
Keywords: Hemodynamics, aging, arterial wall remodeling, age-dependent salt-sensitive hypertension, pulse wave velocity, arterial fibrosis, angiotensin II, marinobufagenin, an endogenous steroidal Na pump inhibitor
Introduction
Major hemodynamic alterations ensue with aging and are primarily attributable to central arterial stiffening;1 these alterations become manifest in the ever expanding epidemic of hypertension affecting 1 of every 3 Americans in general, and a staggering rate of 7 of every 10 of those aged 65 and older.2 The burden of this epidemic is projected to increase with the aging of our population as the percentage of people aged 65 and older increasing from 15% in 2014 to 22% in 2030.3 This shift in demographics makes predominantly systolic hypertension, a challenging form hypertension that becomes more prevalent with advancing age and that dominates the hypertension field.4 All of these factors make hypertension a growing health burden with a predicted hypertension-related health care costs reaching $389 billion in 2030.5 Because aging is the major risk factor for predominantly systolic hypertension the pivotal question to effectively address this hypertension epidemic is: What underlies arterial aging?
Evolutionary biologists proclaim that most of us are wired to be very healthy until around the end of child-bearing age, because the main reason for our reality, they would say, is to perpetually insure the next generation of our species; after that, from an evolutionary perspective, there is no essential reason for us to be alive. However, we do remain alive longer well beyond our evolutionary life expectancy prescription because our environment has been enhanced by improved hygiene, better nutrition, better health care, etc. But, in outliving our paleolithic gene set, disorder among molecules within our body progressively increases and functional declines accumulate with advancing age and beyond 40 years we become vulnerable to what are referred to as “degenerative chronic diseases of aging”. Arterial aging and the associated alteration in hemodynamics are no exception.
The hypertension field has been struggling to better understand the complex relationship between arterial wall mechanical changes, i.e. arterial wall stiffness, and hemodynamics, i.e. arterial pressure alterations, and has been fixated on defining: “which factor is the culprit?” The failure to reach an unequivocal answer is not surprising and is probably a reflection of the naivety of the question is. In health “homeostasis”, a functional crosstalk between central and peripheral segments of the circulation is required for optimal operation. Once this homeostasis is broken, for any reason, a vicious cycle of minute alterations in central arterial mechanical and hemodynamics ensues and propagates, leading to the dramatic changes in arterial properties observed with aging. Thus, in this paradigm, it is close to impossible to detect the “initial” minute alteration and point to it as the “culprit”.
Given this extreme complexity, any efforts directed to treating or preventing the increase in blood pressure would be infertile without major efforts are committed to further explore the underpinning aging of the arterial wall. These efforts should be aimed at revealing early alterations, starting in young adulthood, before reaching the clinical threshold, and to develop stage/process-specific interventions rather than the” one-size fits all” approach that dominate the field of hypertension. In the meantime, targeting elements of this vicious cycle, “the master perpetuators” of arterial aging appears to be the most promising strategy to reduce the health burden of hypertension.
Age-Associated Dysfunction of Central Arteries and Hemodynamic Alterations
Epidemiological studies have pursued description of changes in arterial stiffness with aging and to answer the question of whether central arterial stiffness is a cause or an effect of elevated systolic and pulse blood pressure. One of the difficulties in addressing this issue relates to a degree of ambiguity and restrictions of the terms “blood pressure” and “arterial stiffness”. This question might be better articulated if we expand these terms and rename “arterial stiffness” as “arterial mechanical alterations”, and “elevated blood pressure” as “hemodynamic alterations”; then, it becomes apparent that arterial mechanical properties and hemodynamics are inseparable and the question on what starts first, mechanical or hemodynamic alterations appears to be somewhat naïve.
In the beginning… Early 3rd decade arterial mechanical alterations and the rise in diastolic blood pressure
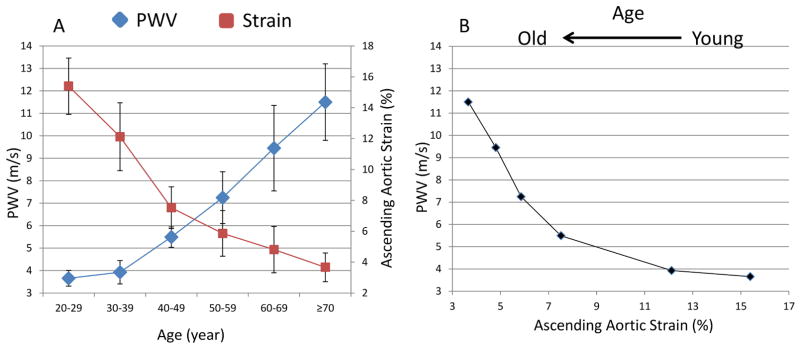
The initial evidence of age-associated arterial mechanical alterations is observed by the third decade of age with sharp declines in aortic strain, the difference between aortic systolic and diastolic diameter relative to the diastolic diameter, and in aortic distensibility, i.e. aortic strain divided by pulse pressure; analysis of cardiac magnetic resonance (CMR) imaging of 111 healthy participants have shown that nearly 80% of the total decline of aortic strain occurs before the 5th decade of age after which the decline in strain is less dramatic 6 (Figure 1A), however the small decline in aortic strain beyond the age of 50 is was associated with an exponential increase in PWV with aging (Figure 1B).
Figure 1.
Aortic strain (red) decreases sharply between the 3rd and 5th decade of life after which there is a sharp rise in aortic pulse wave velocity (blue) (A). Aortic strain and PWV plotted against each other showing an exponential increase in PWV with declining aortic strain with aging (B)
Adapted from Redheuil A, Yu WC, Wu CO, et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension 2010;55(2):319–26; with permission.
During the same stages of life, as central arterial strain is becoming reduced, there is an increase in diastolic blood pressure,7,8 which could be associated with progression of increased endothelial dysfunction with aging, leading to increased peripheral vascular resistance. While such changes are not very well studied in normal human subjects, those with essential hypertension demonstrate eutrophic, inward remodeling of small arteries,9,10 It is not clear whether the increase in diastolic blood pressure alters the optimal conformation of the arterial wall, making it prone to an increase in hemodynamic stress, and to additional mechanical alterations beyond those resulting from simply stretching elastin fibers at higher pressures and shifting the load to the stiffer collagen fibers.
Beyond the 6th Decade of Age: Central Arterial Mechanics and the Pulsatile Hemodynamic
Dramatic hemodynamic alterations ensue beyond the 6th decade of life, as increases in SBP and PP become the hallmarks of arterial aging.8 PWV and blood pressure parameters have been shown to be strongly associated in cross-sectional and prospective studies.11,12 The Framingham Heart Study, using data from Cycle 7 to predict SBP and PWV in Cycle 8, has shown that higher PWV in Cycle 7 is associated with higher SBP in Cycle 8;13 however, the opposite was not true: SBP at Cycle 7 was not associated with higher PWV in Cycle 8. It is noteworthy, however, that one of the shortcomings of The Framingham Study design with a relatively short follow-up time of 7 years is that it does not address that the magnitude and the direction of association between SBP and PWV are not addressed and could change with aging and differ by gender; merely adjusting for these variables does not inform whether these associations differed between the different categories of these variables. Hence, the findings reflect that the average associations over the age spectrum studied for both genders.
Longitudinal perspective on the conundrum of arterial wall stiffness, blood pressure, and aging: A vicious cycle between teammates that eventually diverge
Indications of a vicious cycle between arterial stiffness and systolic blood pressure
The Baltimore Longitudinal Study of Aging (BLSA) is a cohort study of community-swelling populations with extended follow-up time and multiple repeated measures of PWV and blood pressure (1988–2013). Earlier analyses from the BLSA have shown that greater PWV was associated with larger increase in SBP with aging, and predicted the incidence of hypertension.14 Recent analysis from the BLSA, however, using linear mixed effects models, shed light on the vicious cycle, showing that higher SBP, in a dose-dependent fashion, is also associated with a greater rate of increase in PWV; this association was more pronounced in men with accelerating rates of increase in PWV at higher SBP with advancing age.15
Dissociation between PWV and SBP trajectories
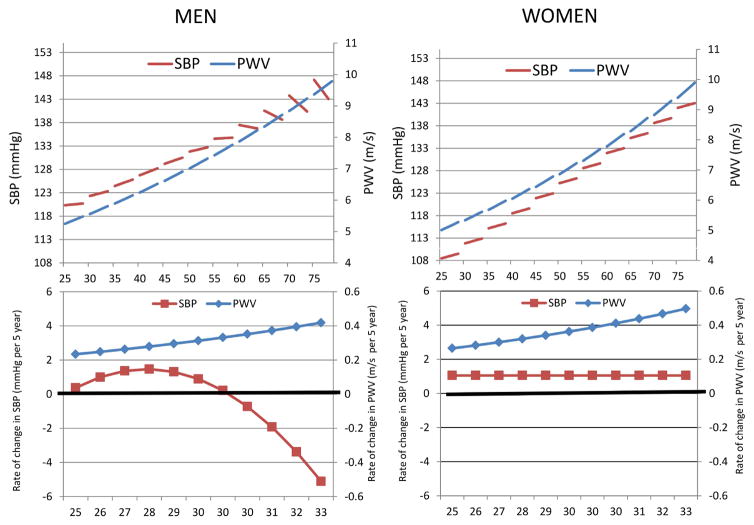
More recent insights on the longitudinal changes in PWV and SBP parameters came from the SardiNIA Study of concurrent trajectories of repeated measures of PWV and SBP; using linear mixed effects models allows the examination of whether the longitudinal changes of these parameters over time vary by starting age.7 This analysis demonstrated a striking dissociation in the trajectories of these parameters with advancing age; a dissociation more pronounced in men than women.7 Figures 2A and B illustrate the cross-sectional differences “beginning of the splines” and the longitudinal changes (slopes of the splines) with aging (Rates of changes are illustrated in the lower panels) of PWV and SBP in both men and women. In men (Figure 2) PWV increased with age at rates that increase linearly with advancing age; however, although cross-sectional SBP continues to increase, the longitudinal rates of change, while initially increasing begin to decline with time, thus the rates of change in SBP diverge from those of PWV by the fifth decade. A similar, but a less dramatic, divergence is observed in women (Figure 2); while, PWV showed the same pattern of longitudinal changes in men with linearly increasing rates of change with advancing age, SBP increased longitudinally at a steady, rather than increasing, rates throughout the age range studied. Preliminary analyses from the BLSA using the same approach of examining the concurrent trajectories showed a similar pattern of dissociations between PWV trajectories and those of SBP and PP, which were more pronounced in men than women.16
Figure 2.
Linear mixed-effects models predicted PWV and SBP values illustrating gender-specific cross-sectional differences “beginning of the splines” and the longitudinal changes (slopes of the splines) with aging (Rates of changes are illustrated in the lower panels) in men and women from the SardiNIA project.
Adapted from Scuteri A, Morrell CH, Orru M, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension 2014;64(6):1219–27; with permission.
Physiological explanations for the dissociation between PWV and the decline in SBP with aging in men
Dissociations between the rates at which PWV and SBP change over time bring our attention again to terminology; “Arterial stiffness” usually implies an increase in arterial opposition to flow i.e. characteristics (Zc) impedance. However, Zc is a function of both PWV and aortic diameter squared.17 Hence, an explanation for the dissociation between SBP and PWV longitudinal trajectories in men is an increase in aortic diameter. Preliminary analysis from the BLSA show a greater increase in aortic root dilatation with increasing age in men than in women.16 The net effect of increasing PWV and aortic diameter approximated by applying the water hammer equation is, in fact, a less pronounced increase in calculated Zc in men despite their more pronounced increase in PWV which was offset by the greater increase in diameter.16 These results are in agreement with cross-sectional data from the Asklepios study showing that with advancing age, men have lower Zc than women.18 While aortic diameter and Zc might explain how PWV and SBP/PP trajectories would diverge, the role of wave reflection in this dissociation is not well clear and it is worth further examination.
Salt-sensitive hypertension and aging
The incidence of hypertension and salt-sensitivity increases with advancing age.19–23 High NaCl intake in addition to its effect on blood pressure, 24 increases arterial stiffness by altering vascular structure, vascular smooth muscle cell (VSMC) and endothelial cell function, and producing arterial wall fibrosis. 25, 26, 27 Both clinical and experimental evidence indicate that NaCl induces hypertrophy of the arterial wall in the absence of changes in arterial pressure26 and induces hypertrophy of cultured VSMC.27 Excessive NaCl intake reduces the bioavailability of nitric oxide by interfering with the induction of nitric oxide synthase,28 and by elevating levels of peroxinitrite due to an increase in NADPH oxidase activity, marker of oxidative stress, and production of reactive oxygen species.29 These NaCl effects lead to hypertension by reducing arterial compliance and increasing peripheral vascular resistance (PVR),30 and to oxidative damage to the arterial wall.31 An age-associated decline in nitric oxide mediated dilation becomes particularly apparent during the 6th decade, a time when pulse pressure, a barometer of large artery stiffness, begins to appreciably elevate.32
The endogenous steroids, sodium pump ligands
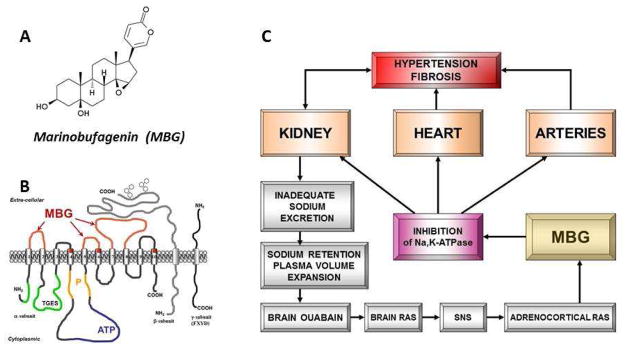
Evidence is mounting that a NaCl-induced signaling cascade involving tissue renin angiotensin aldosterone signaling (RAAS), initiates the production of an endogenous ouabain-like substance in the brain, which then acts as a neurohormone to activate brain AT1R (Figure 3).33,34 Sympathetic signaling to the adrenal cortex leads to production of marinobufagenin (MBG), another recently discovered Na pump ligand that is an endogenous inhibitor of the alpha-1 isoform of the Na/K-ATPase (NKA),35 which is the exclusive NKA isoform in renal tubules, and a main isoform in VSMC (Figure 3). Inhibition of NKA i n renal tubular cells leads to decreased reabsorption of Na and promotes natriuresis. However, Na-pump ligands are not selective for renal NKA, but also inhibit N K A in the vasculature, leading to arterial constriction, and an increase in PVR and arterial blood pressure (Figure 3).35,36,37,38,39 Interestingly, compared to normotensive control, older patients with resistant hypertension demonstrate greater blood pressure and PWV, higher plasma MBG (an NKA inhibitor), and decreased erythrocyte NKA activity.40 In this regard, it is of note that the increase in arterial pressure induced by a chronic high NaCl intake (i.e. salt-sensitive hypertension) in rodents is substantially reduced by an anti-MBG antibody.35,41
Figure 3.
Structures of marinobufagenin (MBG) (A) and Na/K-ATPase with binding sites for MBG (B). Interaction between RAAS and MBG in the pathogenesis of salt-sensitive hypertension (C).
Adapted from Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacological reviews 2009;61(1):9–38; with permission.
The age-associated increase in the secretion of Na pump ligands, including MBG, linked to reduced r e n a l NaCl excretion, could be an explanation for the moderate increases in PVR in older persons with predominantly systolic hypertension. The effects of MBG to increase PVR in salt-sensitive hypertension may be substantially enhanced via their interaction with other vasoactive substances that are implicated in the pathogenesis of NaCl- dependent effects to increase arterial pressure. A NaCl induced up regulation of the tissue activity of RAAS, via PKC dependent phosphorylation of the Na/K-pump, may sensitize this pump to both vasoconstrictive and NaCl-dependent growth promoting MBG effects.42 Additionally, NaCl-dependent angiotensin II (Ang II) signaling induced deficit in the bioavailability of endothelium-derived vasorelaxants, e.g., nitric oxide and C-type natriuretic peptide, which oppose the vasopressor action of MBG, but enhance its adaptive natriuretic action, may further reinforce the deleterious effects of the Na pump ligand.
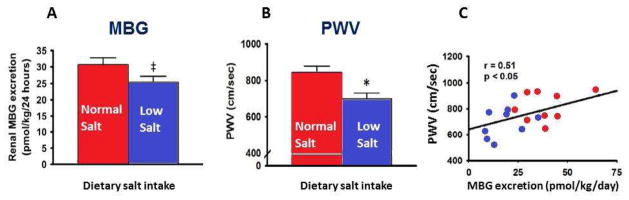
Thus, Na pump ligands, including MBG, link high dietary salt intake to the increase in arterial stiffness and hypertension.20,21,34,36,43 High salt intake is associated with an increase in MBG and is accompanied by marked salt-sensitivity of blood pressure.44,45 Dietary sodium restriction is an effective lifestyle approach for reducing both blood pressure and arterial stiffness in middle-age and older individuals.46,47 In older humans after 5 weeks of a low salt diet vs. 5 weeks of a normal salt diet a reduction in urinary MBG excretion was positively related to reductions in urinary Na excretion and arterial stiffness (Figure 4).48 Furthermore, the expression of NADPH oxidase was correlated with MBG levels, indicating that low-salt-dependent reduction in MBG may contribute to the reductions in large elastic artery stiffness and SBP through decreased oxidative stress.48 Thus, MBG is a molecular link of increased salt-sensitivity and arterial stiffness in aging.
Figure 4.
Effect of low and normal dietary salt intake on urinary MBG excretion (A), aortic pulse-wave velocity (PWV) (B), and correlation between urinary MBG and PWV (C) in older patients
Adapted from Jablonski KL, Fedorova OV, Racine ML, et al. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clin J Am Soc Nephrol 2013;8(11):1952–9; with permission.
Interaction of MBG and ANP in age-dependent salt-sensitivity
In addition to MBG, high salt intake stimulates atrial natriuretic peptide (ANP) (Figure 5).49 Inhibition of renal NKA by MBG is enhanced via ANP-dependent phosphorylation of NKA, whereas, in the aorta, ANP exerts the opposite effect.50 Vasorelaxant ANP and vasoconstrictor MBG potentiate each other's natriuretic effects, but ANP may offset the deleterious vasoconstrictor effect of MBG.50 An imbalance of ANP-MBG signaling increases with advancing age and is a factor that underlies the phenomenon of salt-sensitivity of blood pressure with aging.50
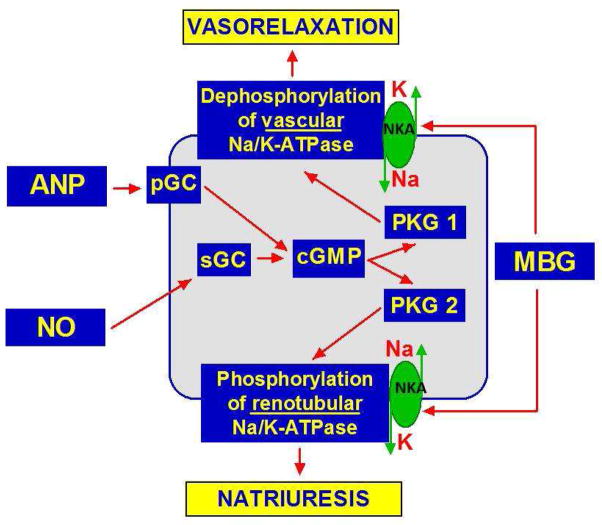
Figure 5.
Factors implicated in the modulation of cGMP-dependent phosphorylation / dephosphorylation of renal and vascular Na/K-ATPase (NKA).
ANP sensitizes renal NKA to MBG inhibitory activity and reduces MBG-induced inhibition of vascular NKA via cyclic guanosine monophosphate/protein kinase G-dependent mechanism (cGMP/PKG). Because downregulation of cGMP/PKG signaling is associated with aging, ANP does not potentiate renal effects of MBG and does not oppose vasoconstrictive effects of MBG in older rats (Figure 6).49,50
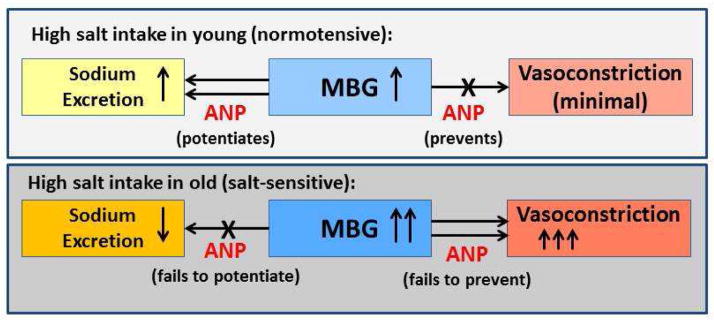
Figure 6.
Scheme of age-associated shift of the modulation of renal and vascular effects of MBG by ANP.
The NaCl- dependence of blood pressure in older persons with predominantly systolic hypertension can be attributed to multiple mechanisms that underlie arterial compliance and vascular resistance. Excessive dietary NaCl may also alter vascular structure and function via Ang II or Na pump ligands driven mechanisms (Figure 7) in the setting of age-associated reductions in renal blood flow and in the ability to excrete Na. NaCl activates tissue Ang II and MBG, affects endothelial and vascular cell functions and affects arterial structural remodeling, which results in arterial stiffening (Figure 7). NaCl also activates ANP, via a cGMP/PKG-dependent mechanism that attenuates pro-fibrotic effect of MBG on vascular NKA (see below).50 Remarkably, both phenomena, MBG-dependent activation of pro-fibrotic signaling and down-regulation of cGMP/PKG-dependent signaling, which accelerates the pro-fibrotic and pro-hypertensive MBG effects, are the hallmarks of arterial aging (Figure 6).50 Pro-fibrotic MBG activity, involving MBG/NKA association, is considered to be an important therapeutic target for immunoneutralization and modulation of MBG/NKA interactions in salt-sensitivity and in aging.
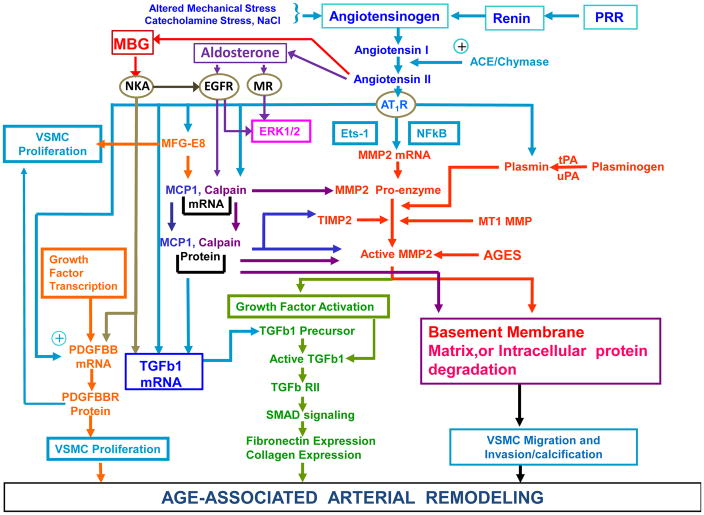
Figure 7.
Pathways that participate in age-associated remodeling of large arteries. Ang II initiates both inflammatory and repair processes From Lakatta EG. The reality of aging viewed from the arterial wall. Artery research 2013;7(2):73–80; with permission.
Excessive dietary NaCl, in fact, may be an etiologic factor in the increased central arterial stiffening that accompanies advancing age, and the attendant age-associated increase in pulse pressure, as this does not occur in populations that do not consume excess NaCl.24 The combined effects of NaCl to increase arterial stiffness and resistance progresses to the point of raising systolic pressure, in nearly half of the individuals in our society, to the current epidemiologically defined hypertensive threshold (140 mm Hg). It is imperative, therefore, that we not lose sight of the reality that the NaCl baseline diet consumed in the US population at large, is 60% higher than the DASH recommendations47,51 and appears to accelerate aging, and to increase the likelihood of salt-sensitive hypertension.
An age-associated increase in renin angiotensin aldosterone signaling (RAAS) in VSMC promotes central arterial wall remodeling
Age-associated changes in vascular structure/function, per se, set the stage for the pathogenesis of vascular diseases such as hypertension in older persons.52–54 The fact that the age-associated changes in arterial wall remodeling, including proinflammation, proliferation, migration/invasion of VSMCs, elastin fragmentation and calcification of Ang II signaling that are observed in rats also occur across a wide range of other species such as rabbits, nonhuman primates, and humans (Table 1; Figure 7), provides insights into the pathogenesis of hypertension with aging and age-associated hypertension.52–54 The Table 1 and Figure 7 illustrate the proinflammatory profile of arterial wall remodeling. Components of the Ang II signaling cascade are “master perpetrators” of age-associated stress signaling that is linked to arterial wall remodeling.52–54
Table 1.
Age-associated proinflammatory arterial remodeling
| Aging
|
Hyper-tension | Ang II Signaling | |||||
|---|---|---|---|---|---|---|---|
| Humans | Monkeys | Rats | Rabbits | ||||
| >56 vs. <20 yrs | 15–20 vs. <10 yrs | 24–30 vs. 3–8 mo | 2–6 yrs vs. <10 mo | ||||
| Inflammation-association Molecules | Local Ang II/AT1 | ↑ | ↑ | ↑ | ? | ↑ | ↑ |
| ET-1 | ↑ | ? | ↑ | ↑ | ↑ | ↑ | |
| MMPs | ↑ | ↑ | ↑ | ? | ↑ | ↑ | |
| Calpain-1 | ↑ | ↑ | ↑ | ? | ↑ | ↑ | |
| MCP-1/CCR2 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| TGF-β1/TβIIR | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| NADPH Oxidase | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| NO Bioavailability | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| TNF-α1 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| ICAM-1 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| MFG-E8 | ↑ | ↑ | ↑ | ↑ | ? | ↑ | |
| PDGF/PDGF-R | ↑ | ? | ↑ | ? | ↑ | ↑ | |
| tPA/uPA | ? | ? | ↑ | ? | ↑ | ↑ | |
| AGEs/RAGE | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| IL-1/-6/-8 | ↑ | ? | ↑ | ↑ | ↑ | ↑ | |
| MR | ? | ? | ↑ | ? | ? | ↑ | |
| NF-κB | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Ets-1 | ? | ? | ↑ | ? | ↑ | ↑ | |
| SirT1 | ↓ | ? | ↓ | ? | ↓ | ↓ | |
|
| |||||||
| Cellular-Matrix structure and function | EC dysfunction | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Diffuse IMT | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Stiffness | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Matrix | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Calcification | ↑ | ↑ | ↑ | ? | ↑ | ↑ | |
| FN/Collagen | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| VSMC migration | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| VSMC proliferation | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
Adapted from Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends in endocrinology and metabolism 2014;25(2):72–9; with permission.
Proinflammation
In a hybrid FXBN strain old rat (30 mo) aortae exhibit a dramatic increase in intimal-medial thickness compared as young adults (8 mo), due to increased VSMCs infiltration into the intime, collagen deposition, calcification, elastin fracture, known as arterial wall remodeling.55–57 Arterial Ang II signaling components (Figure 7) are increased in age-associated aortic remodeling in FXBN rats, including an increased abundance of Ang II, the AT-1 receptor, angiotensin converting enzyme (ACE), and their downstream molecules aldosterone, endothelin-1 (ET-1), matrix metalloproteinase type II (MMP-2), calpain-1, transformation growth factor beta 1 (TGF-β1), monocyte chemoattractant protein-1 (MCP-1), milk fat globule EGF-8 (MFG-E8) in FXBN rat with aging (Figure 7).52,55–64 In addition, aldosterone mineralocorticoid receptor (MR) activation is increased, promoting arterial wall remodeling.65 Interestingly, aortic prorenin receptor (PPR) ACE, Ang II and AT1 receptor proteins are also upregulated in C57/BL6 mice with aging (Figure 7).66
Chronic (30 days) Ang II infusion into young FXBN rats increases the intimal-medial thickness, enhances calpain-1, MMP-2, MCP-1, TGF-β1, MFG-E8 expression or activities, collagen deposition, and elastin network breakdown in the arterial wall, mimicking arterial wall remodeling that accompanies an advancing age.58,62,67 In other terms in response to chronic administration of Ang II to young rats, the arterial VSM and matrix take on the appearance of their counterparts in old rats.
Chronic ACE inhibition or AT1 receptor blockade, beginning at an early age, markedly inhibits the expression of proinflammatory molecules and delays the progression of age-associated aortic remodeling and senescence.68,69 Interestingly, long-term AT1 blockade improves endothelial function and decreases blood pressure, doubles lifespan of hypertensive rats rendering it similar to normotensives.70 Disruption of the AT1 receptor, retards arterial inflammation, promotes longevity and improves survival after MI in mice.71 These findings provide strong support to the hypothesis that increased Ang II proinflammatory signaling is involved in arterial wall remodeling (Figure 7).
Proliferation
VSMCs within the aged aortic wall have an enhanced proliferation capacity compared to young cells linked to proinflammation.60 The proliferation rate in cultured VSMCs is increased in old vs. young adult rats.59 An increase in CDK4 and PCNA, an increase in the acceleration of cell cycle S and G2 phases, a decrease in the G1 / G0 phase, and an increase in PDGF and its receptors drive the elevated proliferative capacity in early passage old VSMC vs. young VSMC.60
Ang II signaling increases MFG-E8 expression in both arterial walls and VSMCs (Figure 7).67,72 An increase in MFG-E8, a cell adhesion protein, is a signature of aging arterial walls.60,72–74 Aortic MFG-E8 mRNA and protein levels and its integrin receptor, avβ 3/5, increase with aging.60,72 MFG-E8 signaling via integrins activates proliferation of VSMC, PCNA and Ki67, markers of cell cycle activation.60 In young VSMC in vitro, MFG-E8 treatment triggers p-ERK-1/2, augments levels of PCNA and CDK4, increases BrdU incorporation, and promotes proliferation via αvβ5 integrins.60 MFG-E8 silencing, or its receptor inhibition, or the blockade of p-ERK1 / 2 in these cells reduces PCNA and CDK4 levels, and decelerates the cell cycle S phase, conferring a reduction in proliferative capacity.60 Collectively, MFG-E8 coordinates the expression of cell cycle molecules and facilitates VSMC proliferation via integrin / ERK1 / 2 signaling (Figure 7).
Migration/Invasion
The migration/invasion of VSMCs, which is a key cellular event in age-associated diffuse intimal thickening, is driven by proinflammatory molecular signaling.52 VSMCs from old vs. young adult rats also have a 50% increase in migration potential.59 Ang II triggers the activation of MMP-2, calpain 1, MCP-1, and MFG-E8, which play a causal role in the migration/ invasion of VSMC due to its cleavage of the basement membrane and cytoskeletal remodeling (Figure 7). 58,61,62,72 In cultured young VSMCs, Ang II exposure increases VSMC migration to the level of isolated old cells.58,62 The Ang II-mediated, age-associated increases in VSMC migration capacity is blocked by inhibition of MMP-2.58 Calpain-1 is an intracellular Ca2+-activated cysteine protease and downstream molecule of Ang II signaling cascade. Its transcription, translation, and activation are significantly up-regulated in rat aortae and VSMCs in culture with aging.55,58 Calpain-1 and MMP-2 are colocalized within old VSMC.55 Over-expression of calpain-1 in young VSMC results in cleavage of intact vimentin (as an index of calpain-1 activity) and an increased migratory capacity, mimicking old VSMC. These actions are blocked by the MMP inhibitor, GM6001 and its inhibitor calpastatin.58 Thus, calpain-1 and MMP-2 activation are pivotal molecular events in the age-associated arterial Ang II signaling/migration cascade of VSMC migration.
In addition, MFG-E8 plays an important role as a relay element within the AngII/MCP-1 signaling cascade that modulates VSMC invasion with aging.72 MCP-1 and its receptor, CCR2 (Figure 7), are upregulated in age-associated aortic remodeling.59,61 Exposure of young VSMCs to Ang II markedly increases MFG-E8 and enhances their invasive capacity to old cell levels72. Treatment of VSMCs with MFG-E8 increases MCP-1 expression and VSMC invasion, and both are inhibited by the MCP-1 receptor blocker vCCI.72 Exposure of young VSMC to MCP-1 also increases their migration, up to levels of old cells.59 Silencing MFG-E8 substantially reduces MFG-E8 expression and VSMC invasion capacity.72 Thus MFG-E8, a protein secreted by VSMC, significantly increases with aging and is a pivotal relay element within the Ang II/MCP-1/VSMC invasion signaling cascade (Figure 7).
Elastin fragmentation
Rat aortic wall elastin fraction is significantly decreased (by 60%) with advancing age and the network of elastin is diminished.56 The age-dependent, Ang II-mediated increase in arterial MMP-2 activity is involved in the cleavage of the elastin fibrillin-1 leading to the degradation of the elastin arterial network (Figure 8).55–57,62 These age-associated changes within elastin lamina are associated with an imbalance of synthesis and degradation of tropoelastin. The level of tropoelastin production by old aortic VSMCs in vitro is markedly reduced and tropoelastin is also degraded more rapidly in tertiary culture with increased passage number than in primary culture.75,76 Importantly, elastin fibers are cleaved by age-associated activation of the gelatinases MMP-2/9 and elastase.76,77 Chronic administration of a broad-spectrum MMP inhibitor, PD166793, via a daily gavage, to 16-month–old rats for 8 months markedly blunted the expected age-associated increases in aortic MMP activity and a release of fibrillin-1 and preserved the elastic fiber network integrity.63 Importantly, degraded fibrillin-1 initiates fibrosis, and ends of fractured fiber become an impetus to calcification (Figure 8).78
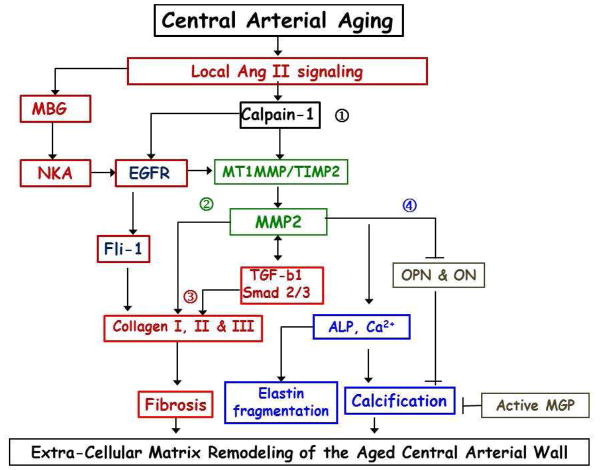
Figure 8.
Age-Associated Matrix Remodeling of Central Arteries. MBG – marinobufagenin; NKA – Na/K-ATPase (MBG receptor); EGFR – epidermal growth factor receptor; MT1 – metallothionein 1; MMP – matrix metalloprotease; TIMP – metalloprotease inhibitor; TGF-b1 – transforming growth factor beta 1; OPN – osteoponin; ON – osteonectine; ALP – alkaline phosphatase; MGP – matrix Gla protein. Numerous other molecules, not shown on that diagram, have important role in fibrosis, calcification and elastin fragmentation.
Arterial calcification
Arterial calcification is also the calcium build-up consequence of a reparative or reactive process to chronic proinflammation. Arterial calcification, the deposition of calcium phosphate mineral, most often hydroxyapatite within or outside of arterial cells is a salient feature of age-associated arterial remodeling (Figure 8). Old cultured VSMCs, like osteoblasts, are able to produce large amounts of bone-like substrates, including collagen II, which become bio-mineralized and calcified. 63 Over-expression of calpain-1 reduces the calcification inhibitors, osteonectin, and osteopontin (OPN), and induces alkaline phosphatase activity in young VSMCs, mimicking that of old cells.63 In addition, the activity of tissue transglutaminase (TG2), a protein crosslinking enzyme, increases in the old arterial wall, and is closely associated with reduction of NO bioavailability.79–82 Activated TG2 up-regulates calcification promoter genes, i.e. Runx2 and down-regulates the expression of calcification inhibitor genes, i.e. OPN within VSMCs and increases arterial stiffness (Figure 8). 80–82
Arterial fibrosis
Arterial wall fibrosis is a hallmark of aging and vascular diseases.83,84 Arterial fibrosis is the formation of excessive extracellular fibrous tissue in a reparative or reactive process in response to chronic proinflammation (Figures 7 and 8). VSMCs are stretched via longitudinal or circumferential strain causing an age-associated increase in Ang II and TGF-β1 signaling in the arterial wall over time.85,86 Inflammation induces tissue repair,87 which is important in the scenario of tissue damage or blood pressure increase, and TGF-beta is an important player in this reparative process.87,88 Increased aortic calpain-1 associated MMP-2 activity mediates age-associated arterial Ang II profibrotic signaling effect (Figures 7 and 8).58 Overexpression of calpain-1 induces MMP-2 transcription, following by an increase in protein levels and activity, in part, by increasing the ratio of MT1-MMPs to TIMP2.55 The increased MMP-2 activity of the old rat aorta colocalizes with TGF-β1.57 The latent TGF-β1 precursor linked to fibrillin 1, and its intermediate degradation form, latent associated protein (LAP), as well as active TGF-β1 within VSMC increase with aging via a stepwise of cleavage by MMP-2.57 The expression of TGF-β1 receptor TβRII also increases with aging. Downstream receptor signaling molecules (Figure 7) of p-SMAD2, 3 and 4 increases while the medial expression of the suppressor SMAD7 is decreased with aging.57 The effect of calpain-1-induced MMP-2 activation (Figure 8) results in increased collagen I, II and III production in VSMCs.55 Chronic inhibition of MMP markedly reduces arterial interstitial collagenase activity, TGF-β1 activation, the profibrogenic signaling molecule SMAD-2/3 phosphorylation, and collagen deposition63. Collectively, in vitro and in vivo results indicate that MMP inhibition retards age-associated arterial profibrotic signaling (Figures 7 and 8).
The role of the endogenous NKA ligand, MBG, in pro-fibrotic signaling
It is known that aging is a predominant factor for most diseases.89 Chronic diseases even in younger ages also resemble aging by alteration of expression of genes in pro-inflammatory and pro-fibrotic pathways, creating an aging profile at the genetic, molecular, cellular, physiological levels especially in cardiovascular system.83,90,91,92 Fibrosis of arterial wall accompanies aging and vascular diseases. 83,90,91,92 Levels of the pro-fibrotic factor, MBG, are implicated in development of vascular fibrosis in preeclampsia, chronic renal failure, and salt-sensitive hypertension at any age.39,93–99 Production of this steroid, MBG, is regulated by Ang II (Figure 7).34 MBG links salt-sensitive arterial stiffness and hypertension. MBG initiates TGFβ- and Fli-1- dependent pro-fibrotic signaling via binding to NKA (Figure 8).100,101 Activation of TGFβ pro-fibrotic signaling by MBG was demonstrated in the Dahl-S model of salt-sensitive hypertension and aging. In vivo, immunoneutralization of high MBG levels in old Dahl-S rats by an anti-MBG monoclonal antibody reverses arterial fibrosis, and down-regulates genes, implicated in TGFβ signaling.
Fli1-dependent signaling, another pro-fibrotic pathway, is also activated by MBG. MBG-NKA-Src complex activates of epidermal growth factor receptor (EGFR) signaling resulting in a degradation of Fli1 (a negative nuclear regulator of the procollagen-1 gene) and induction of collagen-1 synthesis (Figure 8).101 This mechanism could be relevant to the pathogenesis of several conditions, including preeclampsia, in which elevated plasma MBG levels are associated with development of fibrosis in umbilical arteries accompanied by the reduction of Fli-1.102 Cardiovascular fibrosis activated by high endogenous MBG levels in rat model of chronic renal disease (CRD), was reversed by immunization of these rats with monoclonal anti-MBG antibody.103 Of note, the pro-fibrotic effect of MBG and anti-fibrotic effect of anti-MBG antibody are pressure-independent.104 High NaCl intake in normotensive rats increases MBG levels and induces aortic fibrosis in the absence of a hypertensive response.104 In this study, immunoneutralization of MBG reduces aortic fibrosis and restores the aortic relaxation. 104
Anti-fibrotic effects of the MR antagonists, spironolactone, and its common metabolite, canrenone,105 are also associated with Na pump ligands.106,107 The MR antagonist, canrenone, blockes the pro-fibrotic activity of an endogenous MBG in animal models of CRD, and also suppresses cardiac fibrosis in rats chronically treated by MBG in the absence of changes in aldosterone levels.108 In addition, canrenone significantly attenuates pressure-independent pro-fibrotic activity of MBG in cultures aortic VSMCs in vitro. Incubation of rat aortic rings with MBG reduces aortic Fli1, increases collagen-1, and attenuates relaxation of aorta. Canrenone restores aortic rexation, restores Fli1 levels and reduces collagen abundance in aortic wall.40
Older patients with resistant hypertension exhibit an increase in PWV, higher plasma MBG, and inhibited erythrocyte NKA vs. normotensive age-matched control.40 Administration of spironolactone to these patients with resistant hypertension for 6 months in addition to the conventional triple anti-hypertensive therapy restores of NKA activity, decreased SBP and DBP, and significantly reduced PWV. These data further demonstrate the intimate relationship of MBG and arterial stiffness. Ang II-driven MBG is a novel target for MR antagonists in MBG-induced arterial remodeling and arterial stiffness. In addition, the immunoneutralization of pro-fibrotic steroid MBG and also blocking its inhibiting effect on NKA by MR antagonists are additional approaches in treatment of salt-sensitive hypertension and conditions when heightened MBG level cause a devastating pro-fibrotic and pro-hypertensive effect, which is accelerated by aging.
Summary
The aforementioned evidence indicates that accelerated arterial wall remodeling via Ang II signaling within the arteries, per se, ought to be considered a type of hypertensive effect, because the molecular disorder and the inflammatory milieu it creates within the arteries with advancing age are the roots of the pathophysiology of hypertension (Table 1). Thus, therapies to prevent or reduce signaling that drive arterial wall remodeling may ultimately reduce the epidemic of hypertension in the older population by reducing the undisputed major risk factor for hypertension i.e., arterial aging, per se. Targeting “the master perpetuators” of arterial aging, i.e. therapies to prevent or delay Ang II signaling related vascular changes that accompany aging, appears to be the most promising strategy to reduce the health burden of hypertension and ultimately reduce the prevalence of hypertension and arterial fibrosis.
Key Points.
While pre-clinical hemodynamic alterations observed early in life are not directly harmful, they form the basis for the deleterious hemodynamic effects observed with aging.
The increase of salt-sensitivity with advancing age appears to be linked to aortic biomechanics.
A proinflammatory state in the arterial a wall, with a pivotal role for angiotensin II, is a key component of arterial aging
An endogenous sodium pump ligand, marinobufagenin is a novel marker that links aging, salt-sensitivity, and arterial stiffness.
Pulsatile damage to arterial wall and the proinflammatory state within the arterial wall interact in a vicious cycle, resulting in increasing arterial wall fibrosis.
Interventions should be aimed at breaking the vicious cycle at its early stages.
Therapies to prevent or delay Ang II signaling related vascular changes that accompany aging may ultimately reduce the prevalence of hypertension.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.AlGhatrif M, Lakatta EG. The conundrum of arterial stiffness, elevated blood pressure, and aging. Current hypertension reports. 2015 Feb;17(2):12. doi: 10.1007/s11906-014-0523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.High Blood Pressure Fact Sheet|Data & Statistics|DHDSP|CDC [Internet] 2016 [cited 2016 May 11]. Available from: http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_bloodpressure.htm. http://www.ncbi.nlm.nih.gov/pubmed/16193230.
- 3.Colby SL, Ortman JM. Current Population Report. 2015 [Google Scholar]
- 4.Franklin SS, Jacobs MJ, Wong ND, L'Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001 Mar;37(3):869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011 Mar 1;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 6.Redheuil A, Yu WC, Wu CO, et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010 Feb;55(2):319–326. doi: 10.1161/HYPERTENSIONAHA.109.141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scuteri A, Morrell CH, Orru M, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension. 2014 Dec;64(6):1219–1227. doi: 10.1161/HYPERTENSIONAHA.114.04127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklin SS, Gustin Wt, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997 Jul 1;96(1):308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 9.Rizzoni D, Muiesan ML, Porteri E, et al. Interrelationships between macro and microvascular structure and function. Artery research. 2010 Apr 4;4(4):114–117. [Google Scholar]
- 10.Mulvany MJ. Small artery remodelling in hypertension. Basic & clinical pharmacology & toxicology. 2012 Jan;110(1):49–55. doi: 10.1111/j.1742-7843.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- 11.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009 Dec;54(6):1328–1336. doi: 10.1161/HYPERTENSIONAHA.109.137653. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension. 2014 Aug;64(2):210–214. doi: 10.1161/HYPERTENSIONAHA.114.03449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. Jama. 2012 Sep 5;308(9):875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. Journal of the American College of Cardiology. 2008 Apr 8;51(14):1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AlGhatrif M, Strait JB, Morrell CH, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013 Nov;62(5):934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AlGhatrif M, Strait JB, Morrell C, et al. Attenuated Aortic Dilatation, Not Increased Wall Stiffness Best Explains the Rise in Pulse Pressure in Women With Aging: Results From the Baltimore Longitudinal Study of Aging. Circulation. 2013 Nov 26;128(22) [Google Scholar]
- 17.Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility-Reykjavik Study. Hypertension. 2008 Apr;51(4):1123–1128. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segers P, Rietzschel ER, De Buyzere ML, et al. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. 2007 Jun;49(6):1248–1255. doi: 10.1161/HYPERTENSIONAHA.106.085480. [DOI] [PubMed] [Google Scholar]
- 19.Folkow B. Physiological aspects of primary hypertension. Physiological reviews. 1982 Apr;62(2):347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 20.Wasserstrom JA, Aistrup GL. Digitalis: new actions for an old drug. American journal of physiology. Heart and circulatory physiology. 2005 Nov;289(5):H1781–1793. doi: 10.1152/ajpheart.00707.2004. [DOI] [PubMed] [Google Scholar]
- 21.de Wardener HE, Clarkson EM. Concept of natriuretic hormone. Physiological reviews. 1985 Jul;65(3):658–759. doi: 10.1152/physrev.1985.65.3.658. [DOI] [PubMed] [Google Scholar]
- 22.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. The American journal of physiology. 1998 Nov;275(5 Pt 2):F633–650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986 Jun;8(6 Pt 2):II127–134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 24.De Wardener HE, MacGregor GA. Sodium and blood pressure. Current opinion in cardiology. 2002 Jul;17(4):360–367. doi: 10.1097/00001573-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 25.de Wardener HE, MacGregor GA. Harmful effects of dietary salt in addition to hypertension. Journal of human hypertension. 2002 Apr;16(4):213–223. doi: 10.1038/sj.jhh.1001374. [DOI] [PubMed] [Google Scholar]
- 26.Tobian L, Hanlon S. High sodium chloride diets injure arteries and raise mortality without changing blood pressure. Hypertension. 1990 Jun;15(6 Pt 2):900–903. doi: 10.1161/01.hyp.15.6.900. [DOI] [PubMed] [Google Scholar]
- 27.Gu JW, Anand V, Shek EW, et al. Sodium induces hypertrophy of cultured myocardial myoblasts and vascular smooth muscle cells. Hypertension. 1998 May;31(5):1083–1087. doi: 10.1161/01.hyp.31.5.1083. [DOI] [PubMed] [Google Scholar]
- 28.Scuteri A, Stuehlinger MC, Cooke JP, et al. Nitric oxide inhibition as a mechanism for blood pressure increase during salt loading in normotensive postmenopausal women. Journal of hypertension. 2003 Jul;21(7):1339–1346. doi: 10.1097/00004872-200307000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Manning RD, Jr, Hu L, Tan DY, Meng S. Role of abnormal nitric oxide systems in salt-sensitive hypertension. American journal of hypertension. 2001 Jun;14(6 Pt 2):68S–73S. doi: 10.1016/s0895-7061(01)02072-6. [DOI] [PubMed] [Google Scholar]
- 30.Kinlay S, Creager MA, Fukumoto M, et al. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001 Nov;38(5):1049–1053. doi: 10.1161/hy1101.095329. [DOI] [PubMed] [Google Scholar]
- 31.Aviv A. Salt consumption, reactive oxygen species and cardiovascular ageing: a hypothetical link. Journal of hypertension. 2002 Apr;20(4):555–559. doi: 10.1097/00004872-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. Journal of the American College of Cardiology. 1994 Aug;24(2):471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 33.Leenen FH, Ruzicka M, Huang BS. The brain and salt-sensitive hypertension. Current hypertension reports. 2002 Apr;4(2):129–135. doi: 10.1007/s11906-002-0037-y. [DOI] [PubMed] [Google Scholar]
- 34.Fedorova OV, Agalakova NI, Talan MI, Lakatta EG, Bagrov AY. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signalling in NaCl-loaded Dahl-S rats. Journal of hypertension. 2005 Aug;23(8):1515–1523. doi: 10.1097/01.hjh.0000174969.79836.8b. [DOI] [PubMed] [Google Scholar]
- 35.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Endogenous ligand of alpha(1) sodium pump, marinobufagenin, is a novel mediator of sodium chloride--dependent hypertension. Circulation. 2002 Mar 5;105(9):1122–1127. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- 36.Hamlyn JM, Hamilton BP, Manunta P. Endogenous ouabain, sodium balance and blood pressure: a review and a hypothesis. Journal of hypertension. 1996 Feb;14(2):151–167. doi: 10.1097/00004872-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Fedorova OV, Shapiro JI, Bagrov AY. Endogenous cardiotonic steroids and salt-sensitive hypertension. Biochimica et biophysica acta. 2010 Dec;1802(12):1230–1236. doi: 10.1016/j.bbadis.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaustein MP. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. The American journal of physiology. 1977 May;232(5):C165–173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- 39.Periyasamy SM, Liu J, Tanta F, et al. Salt loading induces redistribution of the plasmalemmal Na/K-ATPase in proximal tubule cells. Kidney international. 2005 May;67(5):1868–1877. doi: 10.1111/j.1523-1755.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 40.Fedorova OV, Emelianov IV, Bagrov KA, et al. Marinobufagenin-induced vascular fibrosis is a likely target for mineralocorticoid antagonists. Journal of hypertension. 2015 Aug;33(8):1602–1610. doi: 10.1097/HJH.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fedorova OV, Kolodkin NI, Agalakova NI, et al. Antibody to marinobufagenin lowers blood pressure in pregnant rats on a high NaCl intake. Journal of hypertension. 2005 Apr;23(4):835–842. doi: 10.1097/01.hjh.0000163153.27954.33. [DOI] [PubMed] [Google Scholar]
- 42.Fedorova OV, Dorofeeva NA, Lopatin DA, Lakatta EG, Bagrov AY. Phorbol diacetate potentiates na(+)-k(+) ATPase inhibition by a putative endogenous ligand, marinobufagenin. Hypertension. 2002 Feb;39(2):298–302. doi: 10.1161/hy0202.104344. [DOI] [PubMed] [Google Scholar]
- 43.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacological reviews. 2009 Mar;61(1):9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson DE, Fedorova OV, Morrell CH, et al. Endogenous sodium pump inhibitors and age-associated increases in salt sensitivity of blood pressure in normotensives. American journal of physiology. Regulatory, integrative and comparative physiology. 2008 Apr;294(4):R1248–1254. doi: 10.1152/ajpregu.00782.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fedorova OV, Lakatta EG, Bagrov AY, Melander O. Plasma level of the endogenous sodium pump ligand marinobufagenin is related to the salt-sensitivity in men. Journal of hypertension. 2015 Mar;33(3):534–541. doi: 10.1097/HJH.0000000000000437. discussion 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cappuccio FP, Markandu ND, Carney C, Sagnella GA, MacGregor GA. Double-blind randomised trial of modest salt restriction in older people. Lancet. 1997 Sep 20;350(9081):850–854. doi: 10.1016/S0140-6736(97)02264-2. [DOI] [PubMed] [Google Scholar]
- 47.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004 Jul;44(1):35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- 48.Jablonski KL, Fedorova OV, Racine ML, et al. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clinical journal of the American Society of Nephrology : CJASN. 2013 Nov;8(11):1952–1959. doi: 10.2215/CJN.00900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fedorova OV, Kashkin VA, Zakharova IO, Lakatta EG, Bagrov AY. Age-associated increase in salt sensitivity is accompanied by a shift in the atrial natriuretic peptide modulation of the effect of marinobufagenin on renal and vascular sodium pump. Journal of hypertension. 2012 Sep;30(9):1817–1826. doi: 10.1097/HJH.0b013e328356399b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fedorova OV, Agalakova NI, Morrell CH, Lakatta EG, Bagrov AY. ANP differentially modulates marinobufagenin-induced sodium pump inhibition in kidney and aorta. Hypertension. 2006 Dec;48(6):1160–1168. doi: 10.1161/01.HYP.0000248129.20524.d0. [DOI] [PubMed] [Google Scholar]
- 51.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. The New England journal of medicine. 2001 Jan 4;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 52.Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends in endocrinology and metabolism: TEM. 2014 Feb;25(2):72–79. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang M, Kim SH, Monticone RE, Lakatta EG. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension. 2015 Apr;65(4):698–703. doi: 10.1161/HYPERTENSIONAHA.114.03618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang M, Shah AM. Age-associated pro-inflammatory remodeling and functional phenotype in the heart and large arteries. Journal of molecular and cellular cardiology. 2015 Jun;83:101–111. doi: 10.1016/j.yjmcc.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang L, Zhang J, Monticone RE, et al. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension. 2012 Nov;60(5):1192–1199. doi: 10.1161/HYPERTENSIONAHA.112.196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension. 2002 Apr;39(4):865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- 57.Wang M, Zhao D, Spinetti G, et al. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arteriosclerosis, thrombosis, and vascular biology. 2006 Jul;26(7):1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 58.Jiang L, Wang M, Zhang J, et al. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PloS one. 2008;3(5):e2231. doi: 10.1371/journal.pone.0002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arteriosclerosis, thrombosis, and vascular biology. 2004 Aug;24(8):1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 60.Wang M, Fu Z, Wu J, et al. MFG-E8 activates proliferation of vascular smooth muscle cells via integrin signaling. Aging cell. 2012 Jun;11(3):500–508. doi: 10.1111/j.1474-9726.2012.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang M, Spinetti G, Monticone RE, et al. A local proinflammatory signalling loop facilitates adverse age-associated arterial remodeling. PloS one. 2011;6(2):e16653. doi: 10.1371/journal.pone.0016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang M, Zhang J, Spinetti G, et al. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. The American journal of pathology. 2005 Nov;167(5):1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang M, Zhang J, Telljohann R, et al. Chronic matrix metalloproteinase inhibition retards ageassociated arterial proinflammation and increase in blood pressure. Hypertension. 2012 Aug;60(2):459–466. doi: 10.1161/HYPERTENSIONAHA.112.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. Journal of molecular and cellular cardiology. 2010 Apr;48(4):765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krug AW, Allenhofer L, Monticone R, et al. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension. 2010 Jun;55(6):1476–1483. doi: 10.1161/HYPERTENSIONAHA.109.148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoon HE, Kim EN, Kim MY, et al. Age-Associated Changes in the Vascular Renin-Angiotensin System in Mice. Oxidative medicine and cellular longevity. 2016;2016:6731093. doi: 10.1155/2016/6731093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang M, Fu Z, Lakatta EG, Van Eyk J. Google Patents. 2012. Method for the diagnosis of age-associated vascular disorders. [Google Scholar]
- 68.Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. American journal of physiology. Heart and circulatory physiology. 2007 Sep;293(3):H1351–1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- 69.Michel JB, Heudes D, Michel O, et al. Effect of chronic ANG I-converting enzyme inhibition on aging processes. II. Large arteries. The American journal of physiology. 1994 Jul;267(1 Pt 2):R124–135. doi: 10.1152/ajpregu.1994.267.1.R124. [DOI] [PubMed] [Google Scholar]
- 70.Linz W, Heitsch H, Scholkens BA, Wiemer G. Long-term angiotensin II type 1 receptor blockade with fonsartan doubles lifespan of hypertensive rats. Hypertension. 2000 Apr;35(4):908–913. doi: 10.1161/01.hyp.35.4.908. [DOI] [PubMed] [Google Scholar]
- 71.Benigni A, Corna D, Zoja C, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. The Journal of clinical investigation. 2009 Mar;119(3):524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu Z, Wang M, Gucek M, et al. Milk fat globule protein epidermal growth factor-8: a pivotal relay element within the angiotensin II and monocyte chemoattractant protein-1 signaling cascade mediating vascular smooth muscle cells invasion. Circulation research. 2009 Jun 19;104(12):1337–1346. doi: 10.1161/CIRCRESAHA.108.187088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu Z, Wang M, Everett A, Lakatta E, Van Eyk J. Can proteomics yield insight into aging aorta? Proteomics. Clinical applications. 2013 Aug;7(7–8):477–489. doi: 10.1002/prca.201200138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang M, Wang HH, Lakatta EG. Milk fat globule epidermal growth factor VIII signaling in arterial wall remodeling. Current vascular pharmacology. 2013 Sep;11(5):768–776. doi: 10.2174/1570161111311050014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruckman JL, Luvalle PA, Hill KE, Giro MG, Davidson JM. Phenotypic stability and variation in cells of the porcine aorta: collagen and elastin production. Matrix biology : journal of the International Society for Matrix Biology. 1994 Mar;14(2):135–145. doi: 10.1016/0945-053x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 76.Antonicelli F, Bellon G, Debelle L, Hornebeck W. Elastin-elastases and inflamm-aging. Current topics in developmental biology. 2007;79:99–155. doi: 10.1016/S0070-2153(06)79005-6. [DOI] [PubMed] [Google Scholar]
- 77.Cauchard JH, Berton A, Godeau G, Hornebeck W, Bellon G. Activation of latent transforming growth factor beta 1 and inhibition of matrix metalloprotease activity by a thrombospondin-like tripeptide linked to elaidic acid. Biochemical pharmacology. 2004 Jun 1;67(11):2013–2022. doi: 10.1016/j.bcp.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 78.Kumata C, Mizobuchi M, Ogata H, et al. Involvement of matrix metalloproteinase-2 in the development of medial layer vascular calcification in uremic rats. Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. 2011 Jun;15( Suppl 1):18–22. doi: 10.1111/j.1744-9987.2011.00921.x. [DOI] [PubMed] [Google Scholar]
- 79.Chabot N, Moreau S, Mulani A, Moreau P, Keillor JW. Fluorescent probes of tissue transglutaminase reveal its association with arterial stiffening. Chemistry & biology. 2010 Oct 29;17(10):1143–1150. doi: 10.1016/j.chembiol.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 80.Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circulation research. 2008 Mar 14;102(5):529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santhanam L, Tuday EC, Webb AK, et al. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circulation research. 2010 Jul 9;107(1):117–125. doi: 10.1161/CIRCRESAHA.109.215228. [DOI] [PubMed] [Google Scholar]
- 82.Steppan J, Sikka G, Jandu S, et al. Exercise, vascular stiffness, and tissue transglutaminase. Journal of the American Heart Association. 2014;3(2):e000599. doi: 10.1161/JAHA.113.000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lakatta EG. So! What's aging? Is cardiovascular aging a disease? Journal of molecular and cellular cardiology. 2015 Jun;83:1–13. doi: 10.1016/j.yjmcc.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003 Jan 7;107(1):139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 85.Shyu KG, Chao YM, Wang BW, Kuan P. Regulation of discoidin domain receptor 2 by cyclic mechanical stretch in cultured rat vascular smooth muscle cells. Hypertension. 2005 Sep;46(3):614–621. doi: 10.1161/01.HYP.0000175811.79863.e2. [DOI] [PubMed] [Google Scholar]
- 86.Li Q, Muragaki Y, Hatamura I, Ueno H, Ooshima A. Stretch-induced collagen synthesis in cultured smooth muscle cells from rabbit aortic media and a possible involvement of angiotensin II and transforming growth factor-beta. Journal of vascular research. 1998 Mar-Apr;35(2):93–103. doi: 10.1159/000025570. [DOI] [PubMed] [Google Scholar]
- 87.Hold GL, Untiveros P, Saunders KA, El-Omar EM. Role of host genetics in fibrosis. Fibrogenesis & tissue repair. 2009;2(1):6. doi: 10.1186/1755-1536-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leask A. Potential Therapeutic Targets for Cardiac Fibrosis TGF beta, Angiotensin, Endothelin, CCN2, and PDGF, Partners in Fibroblast Activation. Circulation research. 2010 Jun 11;106(11):1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 89.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014 Nov 6;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kovacic JC, Moreno P, Hachinski V, Nabel EG, Fuster V. Cellular Senescence, Vascular Disease, and Aging Part 1 of a 2-Part Review. Circulation. 2011 Apr 19;123(15):1650–1660. doi: 10.1161/CIRCULATIONAHA.110.007021. [DOI] [PubMed] [Google Scholar]
- 91.Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation. 2011 May 3;123(17):1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- 92.Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. The Canadian journal of cardiology. 2016 May;32(5):659–668. doi: 10.1016/j.cjca.2016.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fedorova OV, Doris PA, Bagrov AY. Endogenous marinobufagenin-like factor in acute plasma volume expansion. Clinical and experimental hypertension. 1998 Jul-Aug;20(5–6):581–591. doi: 10.3109/10641969809053236. [DOI] [PubMed] [Google Scholar]
- 94.Fedorova OV, Anderson DE, Lakatta EG, Bagrov AY. Interaction of NaCl and behavioral stress on endogenous sodium pump ligands in rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2001 Jul;281(1):R352–358. doi: 10.1152/ajpregu.2001.281.1.R352. [DOI] [PubMed] [Google Scholar]
- 95.Kennedy DJ, Vetteth S, Periyasamy SM, et al. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006 Mar;47(3):488–495. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 96.Tian J, Haller S, Periyasamy S, et al. Renal ischemia regulates marinobufagenin release in humans. Hypertension. 2010 Nov;56(5):914–919. doi: 10.1161/HYPERTENSIONAHA.110.155564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kolmakova EV, Haller ST, Kennedy DJ, et al. Endogenous cardiotonic steroids in chronic renal failure. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011 Sep;26(9):2912–2919. doi: 10.1093/ndt/gfq772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lopatin DA, Ailamazian EK, Dmitrieva RI, et al. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. Journal of hypertension. 1999 Aug;17(8):1179–1187. doi: 10.1097/00004872-199917080-00018. [DOI] [PubMed] [Google Scholar]
- 99.Gonick HC, Ding Y, Vaziri ND, Bagrov AY, Fedorova OV. Simultaneous measurement of marinobufagenin, ouabain, and hypertension-associated protein in various disease states. Clinical and experimental hypertension. 1998 Jul-Aug;20(5–6):617–627. doi: 10.3109/10641969809053240. [DOI] [PubMed] [Google Scholar]
- 100.Xie Z, Askari A. Na(+)/K(+)-ATPase as a signal transducer. European journal of biochemistry / FEBS. 2002 May;269(10):2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 101.Elkareh J, Periyasamy SM, Shidyak A, et al. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: implications for uremic cardiomyopathy. American journal of physiology. Renal physiology. 2009 May;296(5):F1219–1226. doi: 10.1152/ajprenal.90710.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nikitina ER, Mikhailov AV, Nikandrova ES, et al. In preeclampsia endogenous cardiotonic steroids induce vascular fibrosis and impair relaxation of umbilical arteries. Journal of hypertension. 2011 Apr;29(4):769–776. doi: 10.1097/HJH.0b013e32834436a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haller ST, Kennedy DJ, Shidyak A, et al. Monoclonal antibody against marinobufagenin reverses cardiac fibrosis in rats with chronic renal failure. American journal of hypertension. 2012 Jun;25(6):690–696. doi: 10.1038/ajh.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grigorova YN, Juhasz O, Zernetkina V, et al. Aortic Fibrosis, Induced by High Salt Intake in the Absence of Hypertensive Response, Is Reduced by a Monoclonal Antibody to Marinobufagenin. American journal of hypertension. 2016 May;29(5):641–646. doi: 10.1093/ajh/hpv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Mendonca M, Grichois ML, Pernollet MG, et al. Antihypertensive effect of canrenone in a model where endogenous ouabain-like factors are present. Journal of cardiovascular pharmacology. 1988 Jan;11(1):75–83. doi: 10.1097/00005344-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 106.Safar ME, Blacher J, Jankowski P. Arterial stiffness, pulse pressure, and cardiovascular disease-is it possible to break the vicious circle? Atherosclerosis. 2011 Oct;218(2):263–271. doi: 10.1016/j.atherosclerosis.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 107.Semplicini A, Serena L, Valle R, et al. Ouabain-inhibiting activity of aldosterone antagonists. Steroids. 1995 Jan;60(1):110–113. doi: 10.1016/0039-128x(94)00005-w. [DOI] [PubMed] [Google Scholar]
- 108.Tian J, Shidyak A, Periyasamy SM, et al. Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension. 2009 Dec;54(6):1313–1320. doi: 10.1161/HYPERTENSIONAHA.109.140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lakatta EG. The reality of aging viewed from the arterial wall. Artery research. 2013 Jun 1;7(2):73–80. doi: 10.1016/j.artres.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]