Abstract
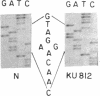
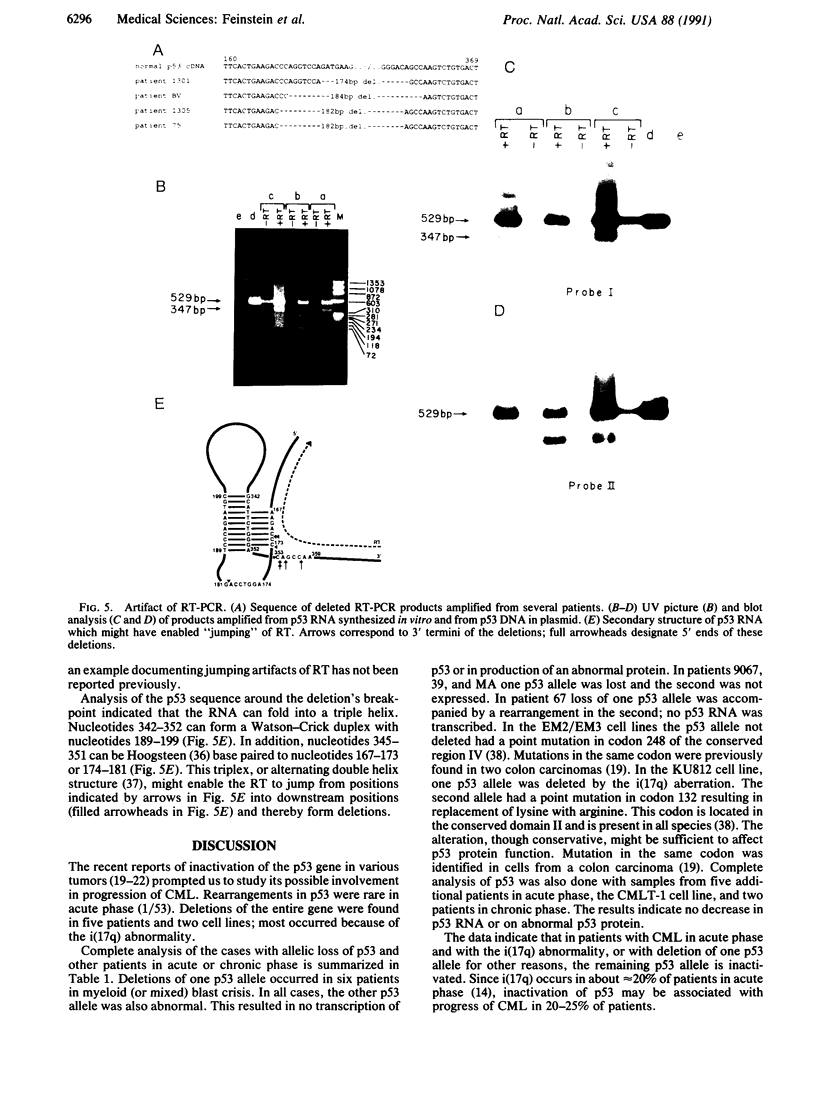
All patients with chronic myelogenous leukemia (CML) undergo clinical transition from chronic to acute phase. This transition is often associated with deletion of the short arm of chromosome 17 in the form of the i(17q) aberration. Since the p53 gene is a suppressor gene and is located on 17p13, we examined the possibility that it is inactivated during progression of CML. Therefore, we studied the structure and expression of p53 in the leukemic cells of a large number of CML patients in acute phase. We found that although the gene is rarely rearranged, one p53 allele is completely deleted in patients with the i(17q) aberration as well as in some patients who do not show karyotypic changes. In all of these patients the remaining allele is inactivated through loss of expression, rearrangement, or point mutation. Detailed analysis of some patients who carry both p53 alleles indicated neither loss of expression nor structural alterations. It appears that p53 loss of function is associated with progression of around 25% of CML patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahuja H., Bar-Eli M., Advani S. H., Benchimol S., Cline M. J. Alterations in the p53 gene and the clonal evolution of the blast crisis of chronic myelocytic leukemia. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6783–6787. doi: 10.1073/pnas.86.17.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. F., 3rd, Collins S. J. Heterogeneity in expression of the bcr-abl fusion transcript in CML blast crisis. Leukemia. 1987 Oct;1(10):718–724. [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Champlin R. E., Golde D. W. Chronic myelogenous leukemia: recent advances. Blood. 1985 May;65(5):1039–1047. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainstein E., Einat M., Gokkel E., Marcelle C., Croce C. M., Gale R. P., Canaani E. Nucleotide sequence analysis of human abl and bcr-abl cDNAs. Oncogene. 1989 Dec;4(12):1477–1481. [PubMed] [Google Scholar]
- Fainstein E., Marcelle C., Rosner A., Canaani E., Gale R. P., Dreazen O., Smith S. D., Croce C. M. A new fused transcript in Philadelphia chromosome positive acute lymphocytic leukaemia. 1987 Nov 26-Dec 2Nature. 330(6146):386–388. doi: 10.1038/330386a0. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Canaani E. An 8-kilobase abl RNA transcript in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5648–5652. doi: 10.1073/pnas.81.18.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Isobe M., Emanuel B. S., Givol D., Oren M., Croce C. M. Localization of gene for human p53 tumour antigen to band 17p13. Nature. 1986 Mar 6;320(6057):84–85. doi: 10.1038/320084a0. [DOI] [PubMed] [Google Scholar]
- Kelman Z., Prokocimer M., Peller S., Kahn Y., Rechavi G., Manor Y., Cohen A., Rotter V. Rearrangements in the p53 gene in Philadelphia chromosome positive chronic myelogenous leukemia. Blood. 1989 Nov 15;74(7):2318–2324. [PubMed] [Google Scholar]
- Kishi K. A new leukemia cell line with Philadelphia chromosome characterized as basophil precursors. Leuk Res. 1985;9(3):381–390. doi: 10.1016/0145-2126(85)90060-8. [DOI] [PubMed] [Google Scholar]
- Kloetzer W., Kurzrock R., Smith L., Talpaz M., Spiller M., Gutterman J., Arlinghaus R. The human cellular abl gene product in the chronic myelogenous leukemia cell line K562 has an associated tyrosine protein kinase activity. Virology. 1985 Jan 30;140(2):230–238. doi: 10.1016/0042-6822(85)90361-7. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Lamb P., Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986 May;6(5):1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo T. G., Witte O. N. The BCR-ABL oncogene transforms Rat-1 cells and cooperates with v-myc. Mol Cell Biol. 1989 Mar;9(3):1263–1270. doi: 10.1128/mcb.9.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbert M., Miller C. W., Crawford L., Koeffler H. P. p53 in chronic myelogenous leukemia. Study of mechanisms of differential expression. J Exp Med. 1988 Mar 1;167(3):873–886. doi: 10.1084/jem.167.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelle C., Gale R. P., Prokocimer M., Berrebi A., Merle-Beral H., Canaani E. Analysis of BCR-ABL mRNA in chronic myelogenous leukemia patients and identification of a new BCR-related sequence in human DNA. Genes Chromosomes Cancer. 1989 Nov;1(2):172–179. doi: 10.1002/gcc.2870010211. [DOI] [PubMed] [Google Scholar]
- Mashal R., Shtalrid M., Talpaz M., Kantarjian H., Smith L., Beran M., Cork A., Trujillo J., Gutterman J., Deisseroth A. Rearrangement and expression of p53 in the chronic phase and blast crisis of chronic myelogenous leukemia. Blood. 1990 Jan 1;75(1):180–189. [PubMed] [Google Scholar]
- Masuda H., Miller C., Koeffler H. P., Battifora H., Cline M. J. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7716–7719. doi: 10.1073/pnas.84.21.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat M., Cheng A., Kimura N., Bernstein A., Benchimol S. Rearrangements of the cellular p53 gene in erythroleukaemic cells transformed by Friend virus. Nature. 1985 Apr 18;314(6012):633–636. doi: 10.1038/314633a0. [DOI] [PubMed] [Google Scholar]
- Mulligan L. M., Matlashewski G. J., Scrable H. J., Cavenee W. K. Mechanisms of p53 loss in human sarcomas. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5863–5867. doi: 10.1073/pnas.87.15.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOWELL P. C., HUNGERFORD D. A. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960 Jul;25:85–109. [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D., Testa J. R. Chromosome abnormalities in malignant hematologic diseases. Adv Cancer Res. 1982;36:103–148. doi: 10.1016/s0065-230x(08)60423-6. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Gale R. P., Dreazen O., Berrebi A., Zaizov R., Kubonishi I., Miyoshi I., Canaani E. bcr-abl RNA in patients with chronic myelogenous leukemia. Blood. 1987 Mar;69(3):971–973. [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Sklenár V., Feigon J. Formation of a stable triplex from a single DNA strand. Nature. 1990 Jun 28;345(6278):836–838. doi: 10.1038/345836a0. [DOI] [PubMed] [Google Scholar]
- Soussi T., Caron de Fromentel C., May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene. 1990 Jul;5(7):945–952. [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Valiron O., Clemancey-Marcille G., Troesch A., Schweitzer A., Prenant M., Hollard D., Berthier R. Immunophenotype of blast cells in chronic myeloid leukemia. Leuk Res. 1988;12(10):861–872. doi: 10.1016/0145-2126(88)90040-9. [DOI] [PubMed] [Google Scholar]