Abstract
Our laboratory has recently demonstrated that low concentrations of ouabain increase blood pressure in rats associated with stimulation of Na-K ATPase activity and activation of the Src signaling cascade in NHE1-dependent manner. Proteomic analysis of human kidney proximal tubule cells (HKC11) suggested that the Angiotensin II type 1 receptor (AT1R) as an ouabain-associating protein. We hypothesize that ouabain-induced stimulation of Na-K ATPase activity is mediated through AT1R. To test this hypothesis, we examined the effect of ouabain on renal cell angiotensin II production, the effect of AT1R inhibition on ouabain-stimulated NKA activity, and the effect of ouabain on NKA-AT1R association. Ouabain increased plasma angiotensin II levels in rats treated with ouabain (1 μg/kg body wt./day) for 9 days and increased angiotensin II levels in cell culture media after 24 h treatment with ouabain in human (HKC11), mouse (MRPT), canine (MDCK) kidney cells, and human adrenal cells. Ouabain 10 pM stimulated NKA-mediated 86Rb uptake and phosphorylation of EGFR, Src, and ERK1/2. These effects were prevented by the AT1R receptor blocker candesartan. FRET and TIRF microscopy using Bodipy-labeled ouabain and mCherry-NKA or mCherry-AT1R demonstrated association of ouabain with AT1R and NKA. Further our FRET and TIRF studies demonstrated increased association between AT1R and NKA upon treatment with low dose ouabain. We conclude that ouabain stimulates NKA in renal proximal tubule cells through an angiotensin/AT1R-dependent mechanism and that this pathway contributes to cardiac glycoside associated hypertension.
Introduction
Sodium potassium ATPase (NKA) is a heterotrimeric enzyme consisting of an α, a β, and a γ subunit [1]. NKA is localized to the basolateral membrane in epithelial cells of renal tubules where it is responsible for the active transport of sodium and potassium. NKA maintains intracellular levels of sodium and energizes the secondary active transport of sodium into the cells from the apical membrane. NKA activity is tightly regulated in the renal proximal tubules. We and others have demonstrated that cardioglycosides like ouabain exert a biphasic effect on NKA activity, low concentrations stimulating and higher concentrations inhibiting the activity of NKA [2–5]. Our laboratory has demonstrated that ouabain-mediated stimulation of NKA involves an NHE1 dependent activation of a signaling complex that includes EGFR, Src kinase, ERK, and Akt [3,6]. We have also demonstrated that low concentrations of ouabain increase blood pressure in Sprague Dawley rats in an NHE1 dependent manner [3].
Cardiac glycosides have been identified predominantly as inhibitors of NKA, and their use in the treatment of cardiac disease has exploited this property. Ouabain and other cardioglycosides are endogenously synthesized in humans and other mammals and expressed at very low plasma (~ 100 pM) concentrations [7]. The physiologic relevance of endogenous ouabain production remains poorly understood. Recent studies have demonstrated that plasma levels of ouabain increase in individuals on a high salt diet [8]. Endogenous ouabain levels are also significantly increased in patients with several forms of hypertension [9], chronic heart disease [10], chronic kidney disease [11], and preeclampsia [12] to approximately 900 pM [13], a concentration that is well above normal but not sufficient to inhibit NKA activity. The synthesis of ouabain in the adrenal glands is regulated by adenocorticotropic hormone (ACTH), α adrenergic and dopaminergic stimulation, angiotensin II (Ang II) through stimulation of angiotensin receptor type 2 (AT2R), hypoxia, and physical exercise [14,15], suggesting a role in regulation of blood pressure. Ouabain increases circulating levels of angiotensin II through pathways involving the sympathetic nervous system [16,17]. Blaustein et al suggested that endogenous ouabain regulates blood pressure through a complex pathway involving aldosterone, the epithelial Na+ channel, NKA α2 subunit, and Ang II. This pathway modulates the activity of brain cardiovascular control centers that regulate the BP set point and induce sustained changes in sympathetic nerve activity [18].
A recent report suggests that ouabain can bind proteins other than NKA. Fujita-Sato et al demonstrated that cardioglycosides can bind to retinoic acid-related orphan nuclear receptor γt to suppress the differentiation of Th17 cells [19]. Based on these findings as well as our previous studies, we hypothesized that ouabain regulated blood pressure by increasing proximal tubule sodium reabsorption through mechanisms involving the renin angiotensin system. In the present study, using proteomic analysis of human kidney proximal tubule cell proteins immunoprecipitated with low dose ouabain, we identified the angiotensin receptor type I (AT1R) and type 2 (AT2R) receptors as potential binding partners (Tables 1–3). Using pharmacologic and genetic approaches, our results demonstrate that ouabain-stimulated signaling and NKA-mediated ion transport is AT1R dependent. Our results further demonstrate that NKA α1 subunit and AT1R can directly associate with each other and this association is enhanced by treatment with low dose ouabain.
Table 1.
Identification of ouabain-binding proteins in cells treated with 10 pM ouabain
| Proteins Pulled down from cells treated with 10 pM Ouabain |
|---|
| ADAM metallopeptidase domain 10 |
| adenosine kinase |
| adenosine A2a receptor |
| adenosine A2b receptor |
| angiotensin II receptor, type 1 |
| angiotensin II receptor, type 2 |
| alanine-glyoxylate aminotransferase |
| aryl hydrocarbon receptor |
| aryl-hydrocarbon receptor repressor |
| ankyrin 2, neuronal |
| ankyrin 3, node of Ranvier (ankyrin G) |
| apelin receptor |
| androgen receptor |
| ERBB4 Isoform JM-A of Receptor tyrosine-protein kinase erbB-4 |
| Rho GTPase activating protein 4 |
| Fas ligand (TNF superfamily, member 6) |
| ras homolog gene family, member A |
| ras homolog gene family, member C |
| ras homolog gene family, member C |
| RYR1 Isoform 2 of Ryanodine receptor 1 |
| shroom family member 2 |
| solute carrier family 16, member 2 (monocarboxylic acid transporter 8) |
Table 3.
Identification of ouabain-binding proteins common in cells treated with 10 pM and 100 nM ouabain
| Proteins Pulled common in cells treated with 10 pM and 100 nM Ouabain |
|---|
| actinin, alpha 2 |
| activin A receptor, type I |
| activin A receptor type II-like 1 |
| adenylate cyclase 9 |
| adenylate cyclase activating polypeptide 1 (pituitary) |
| adenylate cyclase activating polypeptide 1 (pituitary) receptor type I |
| adducin 3 (gamma) |
| adrenergic, alpha-1D-, receptor |
| adrenergic, alpha-1D-, receptor |
| adrenergic, alpha-2A-, receptor |
| adrenergic, beta-1-, receptor |
| adrenergic, beta-3-, receptor |
| adrenergic, beta, receptor kinase 1 |
| adaptor-related protein complex 2, alpha 1 subunit |
| adaptor-related protein complex 2, alpha 2 subunit |
| solute carrier family 39 (zinc transporter), member 4 |
| Cofilin |
| Na-K ATPase α1 subunit |
| NHE1 |
| Src |
| EGFR |
Materials and Experimental Methods
Materials
Ouabain and candesartan were purchased from Tocris (St. Louis, MO). Digibind, an FDA approved antibody against cardioglycosides was purchased from GlaxoSmithKline (Parma, Italy). The monoclonal antibody against NKA (α6F) developed by Dr. D.M. Fambrough was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of NIHCD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Phospho- and total EGFR, Src, and ERK1/2 antibodies were purchased from Cell Signaling. Antibodies against angiotensin converting enzyme (catalog number GTX100923, ACE) were purchased from GeneTex (Irvine, CA) and against angiotensinogen (catalog number ab198180, AGT) were purchased from Abcam (Cambridge, MA). HRP-linked secondary antibodies were purchased from Vector laboratories. Streptavidin agarose resin was purchased from Pierce Biotechnology (Rockford, IL). Phosphatase inhibitor cocktail -1 and protease inhibitor cocktail were purchased from Sigma (St. Louis, MO). All other chemicals were purchased from Sigma, unless otherwise specified.
Animal Model
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Louisville. Sprague Dawley rats weighing 200–250 gm were stabilized on the standard rat chow and water ad libitum for a week prior to experiments. Animals were divided into groups (6 animals in each group). The vehicle group received PBS intraperitoneally and the ouabain group received 1 μg/kg body weight/day ouabain for 9 days. Blood was collected from carotid artery and serum was separated after measuring blood pressure (blood pressure data was reported previously)[3,6].
Cell Culture
Human kidney proximal tubule cells HKC11 (a gift from Dr. Lorraine Racusen, Johns Hopkins University Baltimore MD), mouse renal proximal tubule cells (MRPT, a gift from Dr. Jeffrey R. Schelling, Case Western Reserve University, Cleveland), LLCPK1 cells stably transfected with vector or AT1R (a gift from Dr. Raymond Harris, Vanderbilt University), Madine Darby Canine Kidney (MDCK) cells (ATCC) and human adrenal cells (ATCC) were cultured as previously described [9]. Cells were maintained in DMEM-F12 (1:1, 5 mM glucose) supplemented with 10% FBS, and 1% penicillin/streptomycin, and cultured to 90–95% confluence. Cells were washed with serum-free medium 24 h before use.
Immunoprecipitation (IP)
HKC11 cells were treated with 10 pM or 10 nM ouabain for 15 min at 37°C in a humidified incubator. Excess ouabain was washed and bound ouabain was cross-linked to the proteins using DSS reagent. Crude membranes were prepared as described previously [20]. IP was performed as described previously with modifications [6]. Briefly, crude membranes from cells were solubilized in IP lysis buffer (20 mM Tris-HCl pH 7.4, 150 mM NaCl, 20 mM NaF, 1 mM EDTA, 1 mM EGTA, 100 μl/ml phosphatase inhibitor cocktail, 100 μl/ml protease inhibitor cocktail, 1% N-octyl-β-D-glucopyranoside). 2 mg protein from the solubilized membranes was pre-cleared with Protein A Sepharose beads for 2 h at 4°C. The beads were separated by centrifugation at 14,000 rpm for 1 min in a tabletop centrifuge (Spectrafuge, National Labnet Co., NJ). The supernatant was incubated overnight at 4°C with 1 μg/100 μg protein rabbit polyclonal anti-ouabain (Digibind) or monoclonal anti-NKA antibodies (α6F). Protein A sepharose beads were added and incubated for 4 h at 4°C. The beads were washed three times with IP buffer by centrifugation at 14,000 rpm for 1 min in a tabletop centrifuge. Proteins were eluted in 100 mM glycine pH 2 by incubation for 1 h on a rotator at room temperature. The samples were centrifuged at 14,000 rpm for 1 min in a tabletop centrifuge and the supernatant was collected in 1/10th volume 1M Triethyl ammonium bicarbonate, pH 8.5. Half of the eluted sample was used for LC/MS identification of proteins immunoprecipitated with ouabain. The other half was mixed with an equal volume of 2× Laemmli sample buffer and heated at 65°C for 10 min. The proteins were separated by 10% SDS-PAGE transferred to nitrocellulose membranes and probed with the indicated antibodies.
Proteomic sample handling
The immunoprecipitated complexes and beads were washed using cold PBS, eluted using 0.2M glycine pH2.7, immediately neutralized with 1M triethylammonium bicarbonate pH8.5, and prior to digestion using 50ng of sequencing grade modified trypsin (Promega, Madison, WI, USA) the samples were reduced and alkylated; all as previously described [21]. Each digest was desalted and concentrated using a Michrom Peptide MicroTrap (Bruker-Michrom Inc., Auburn, CA, USA) prior to dissolution in chromatography buffer A (5% acetonitrile/0.05% formic acid).
LCMS data acquisition
Two-dimensional liquid chromatography (2DLC)-mass spectrometry (MS) data were collected as previously described [21] using a Dionex Ultimate 3000 nanoflow system (Thermo Fisher Scientific, Waltham, MA, USA) and a 10cm, 100μm inner diameter fused silica column packed with 3cm of Luna 5μ SCX 400Å material (Phenomenex, Torrance, CA, USA), and then 3cm of Jupiter 5μm C18 300Å material (Phenomenex). Peptides were eluted in a stepped salt bump fashion using seven ammonium acetate salt steps (50, 75, 100, 150, 250, 350, and 500mM) onto an 14cm, 100μm inner diameter analytical column packed with 10cm of Jupiter 5μm C18 300Å material. Peptides were eluted from the C18 resin using a 180 minute linear gradient elution from 5% to 60% acetonitrile in 0.05% formic. Peptides were introduced into a LTQ mass spectrometer (Thermo) using a custom nanospray source (Thermo) with the transfer capillary temperature set at 180°C, and the spray voltage set at 1.4kV. A Top 6 DDA method was created in Xcalibur with scan event one for the ITMS MS1 scan (normal mass range, normal scan rate, full scan type, positive polarity, centroid data type) for the range 300–2000m/z. Scan events two through seven obtained ITMS MS2 scans (normal mass range, normal scan rate, centroid data type) on the top six peaks that had a minimum signal threshold of 1,000 (1E3) counts from scan event one.
Analysis of LCMS datasets
The LCMS data for the eight fractions of each sample was analyzed together in Proteome Discoverer (v1.4.1.14) by enabling the MudPIT option. These data were searched Mascot (v2.5) and SequestHT searches against the human IPI protein database (v3.74). The data were searched with a using a Target Decoy PSM Validator and separately to address low abundant protein identification with a Fixed Value PSM Validator. The Proteome Discover analysis workflows have been included in the supplemental information.
For comparative analysis of high abundant, higher probability searches, the .msf files from the Proteome Discoverer Target Decoy search were loaded into Scaffold Q+S (v4.4.1). Scaffold was used to calculate the false discovery rate using the Peptide and Protein Prophet algorithms. Proteins were accepted if they had greater than 95% confidence and at least two peptides match with greater than 95% confidence (Supplemental Tables 1–3).
For comparative analyses of lower abundant, lower probability searches, the .msf files from the Fixed Value search were loaded together in Proteome Discoverer (Supplemental Table 4). For each MudPIT experiment, peptides were grouped by mass and sequence and only medium confidence peptides were retained. Next, proteins were grouped considering PSMs with at least low confidence and a delta Cn better than 1. To address the lack of a decoy database search strategy and the increased false discovery probability we included strict inclusion filters for proteins including having multiple unique peptides, at least five peptide spectral matches, counting only peptides from top scored proteins, and requiring that a minimum of 10% protein sequence coverage. These data were then filtered using Gene Ontology classification for the following: molecular activity- receptor function; cellular component- membrane proteins; and Biological Process- response to stimulus.
Western blot analysis
Western blot was performed exactly as described previously[6].
Determination of Angiotensin II levels
Angiotensin II levels in the media and plasma were measured using Angiotensin II EIA kit from Enzo Life Sciences (Plymouth Meeting, PA) according to the manufacturer’s protocol. Briefly, angiotensin II was first extracted using C18 Sep-Pak columns from Waters (Milford, MA). The samples (500 μl) were diluted with 1:1 (500 μl) with 1% trifluoroacetic acid (TFA), mixed vigorously and centrifuged at 14,000×g at 4°C in a cooling table top centrifuge. The supernatant was passed through methanol and 1% TFA pre-washed C18 Sep-Pak column. The sample was allowed to flow through the column slowly. The column was washed three times with 1% TFA. Bound angiotensin II was eluted using in 1 ml solution containing 60% acetonitrile and 1% TFA by centrifugation at 300 rpm for 5 min. The eluted sample was lyophilized and reconstituted in 100 μl assay buffer. Angiotensin II levels were measured by EIA following the manufacturer’s protocol using angiotensin II standards provided in the kit.
Ouabain-sensitive 86Rb uptake
Ouabain-sensitive 86Rb uptake in cultured cells was measured at 37°C exactly as described previously [22,23].
Biotinylation
Surface biotinylation was performed as described previously [3]. Briefly, cells were treated with ouabain for 15 min, washed with cold PBS, and incubated with N-hydroxysulfosuccinimidobiotin (10 μl /ml) in borate buffer, pH 9.0 (20 mM Tris, 150 mM NaCl, 10 mM boric acid, 7.2 mM KCl, 1.8 mM CaCl2) for 2 h at 4°C. Cells were then washed 3 times with cold PBS, excess biotin was quenched with 100mM glycine in PBS for 15 min at 4°C, and then washed 3 times more. Next, crude membranes were isolated and incubated with streptavidin agarose resins overnight on a rotator at 4°C. Proteins bound to the resins were eluted and then resolved on 10% SDS-PAGE followed by immunoblotting using monoclonal antibodies against Na-K ATPase α1 subunit (α6F).
Plasmid Constructs
GFP labeled human NKA was purchased from Origene, mCherry labeled rat NKA was provided by Dr. Thomas A Pressley (Texas Tech University) and mCherry labeled AT1R was provided by Dr. Marcela Herrera (Duke University).
Transfection
HKC11 cells were transfected with the indicated plasmids by electroporation using Neon Transfection System (Invitrogen) in OPTI-MEM following manufacturer’s instruction for rat proximal tubular cells and as described previously [24].
Sensitized FRET
FRET imaging experiments were performed in living cells exactly as described previously [3,24]. Briefly, HKC11 cells were transfected with either mCherry-NKA or mCherry-AT1R (acceptor) for 24 h followed by 15 min treatment with Bodipy-ouabain (donor). Cells were washed five times with PBS to wash excess Bodipy-ouabain. To determine association of NKA with AT1R, cells were transfected with GFP labeled human NKA and mCherry labeled AT1R for 24 h followed by treatment with 100 pM ouabain. Cells were viewed and analyzed with an Olympus FRET/TIRF microscope (Center Valley, PA). FRET image acquisition and analysis was performed using SlideBook software version 4.2 as described previously [3,24]. The software is based on the three-filter “micro-FRET” image subtraction method described by Jiang and Sorkin [25]. Briefly, three images (100-ms or 250-ms exposure sets, 2 × 2 binning) were obtained: a mCherry excitation/mCherry emission image; a GFP or Bodipy excitation/GFP or Bodipy emission image; and a GFP or Bodipy excitation/mCherry emission image (raw, uncorrected FRET). After imaging, background images were taken. Background-subtracted mCherry and GFP or Bodipy images were fractionally subtracted from raw FRET images based on measurements for GFP or Bodipy bleed-through (0.50–0.56) and mCherry cross-excitation (0.015–0.02). This fractional subtraction generates corrected FRETc images. The corrected images were represented in pseudocolor (gated to mCherry acceptor levels), showing sensitized FRET within cells. The subtraction Pearson’s coefficients were rounded up from average cross-bleed values determined in cells expressing Bodipy/GFP- or mCherry-tagged constructs alone. Thus, these coefficients result in the underestimation of FRETc signals for true FRET partners but prevent false positive detection of FRET.
TIRF Microscopy
Membrane TIRF was performed as described previously [3,24]. Briefly, HKC11 cells were grown to 60% confluence in a dish with a collagen coated coverslip bottom (no. 1.5, MatTek, Ashland, MA). Cells were transfected with the indicated plasmids as described in FRET method. Samples were observed using an Olympus TIRF microscope equipped with a 60× 1.45 numerical aperture (NA) objective under the control of SlideBook software (version 4.2, Olympus, Center Valley, PA). Laser excitation was derived from a multiline argon ion laser run at the same current setting for all experiments. The power at the sample was controlled by a neutral density filter wheel. Excitation and emission wavelengths were selected using filter set for mCherry and GFP. The laser was aligned per the manufacturer’s instructions to achieve TIRF illumination. Images were taken using a Hamamatsu camera operating with 2 by 2 binning. Oxygen was provided by the ambient air which was supplemented by 5% CO2 and warmed to 37°C in an environmental chamber surrounding the specimen.
Protein determination
Protein concentration was determined using a bicinchoninic acid protein assay kit (Sigma) using BSA standard.
Statistics
Data are shown as mean ± SE. The n values represent the number of independent experiments. Each experiment was performed in triplicate. P values were calculated using GraphPad Prism software utilizing Student’s t-test or by ANOVA, followed by Bonferroni analysis. A p value <0.05 was a priori considered statistically significant.
Results
Identification of ouabain-binding proteins
To identify proteins bound to ouabain, HKC11 cells were treated with either 10 pM or 10 nM ouabain for 15 min. Ouabain-bound proteins were immunoprecipitated and subjected to LTQ mass spec analysis. Since our hypothesis was guided by the theory that ouabain would bind to the plasma membrane of the HKC11 cells and likely to a low abundant membrane protein, in addition to NKA α1 subunit, we used a two-dimensional LC workflow with SCX- and RP-HPLC to increase the amount of sample that could be loaded onto the LCMS system and to correspondingly increase the sensitivity to low abundant proteins. As shown in Table 1, in cells treated with 10 pM ouabain, our data suggest the presence of membrane receptors that are involved in regulation of salt homeostasis including Angiotensin II type 1 (AT1R) and 2 (AT2R) receptors, adenosine A2a and A2b receptors, several aquaporins, Akt1, and receptor kinases. In cells treated with 10 nM ouabain (Table 2), we identified adducin 3γ, arachidonate 12 and 15 lipoxygenase, arachidonate-5 lipoxygenase activating protein, aquaporins 5, 7, and 9, receptor tyrosine kinase erbB2, PDZ containing protein 3, non-colonic H+, K+ ATPase and Akt2. The proteins common to samples treated with both 10 pM and 10 nM treated cells (Table 3) were NKA α1subunit, NHE1, Src, EGFR, actinin, activin A receptors type I and II, adenylate cyclase 9, adrenergic receptors alpha 1D, 2A, β1, and β3, and β adrenergic receptor kinase 1 (Table 3). A complete list of identified proteins is presented in supplementary tables (Supplementary tables 1–5). Association of NKA α1subunit, NHE1, Src, and EGFR with ouabain was confirmed by western blot. As shown in Figure 1A, western blot analysis of ouabain immunoprecipitated proteins showed NKA α1 subunit, NHE1, Src, EGFR, and caveolin-1 were immunoprecipitated with ouabain in cells treated with both 10 pM and 10 nM ouabain (Figure 1A).
Table 2.
Identification of ouabain-binding proteins in cells treated with 100 nM ouabain
| Proteins Pulled down from cells treated with 100 nM Ouabain |
|---|
| adenylate cyclase 3 |
| adducin 3 (gamma) |
| AE binding protein 1 |
| advanced glycosylation end product-specific receptor |
| anaplastic lymphoma receptor tyrosine kinase |
| arachidonate 12-lipoxygenase, 12R type |
| arachidonate 15-lipoxygenase, type B |
| arachidonate 5-lipoxygenase-activating protein |
| ALX homeobox 3 |
| alpha-1-microglobulin/bikunin precursor |
| annexin A8-like 2 |
| ERBB2 Isoform 1 of Receptor tyrosine-protein kinase erbB-2 |
| Rho GTPase activating protein 5 |
| FRMPD3 FERM and PDZ domain-containing protein 3 |
| H+-K+ ATPase non colonic |
| ras homolog gene family, member B |
| SLC4A4 Isoform 2 of Electrogenic sodium bicarbonate cotransporter 1 |
Figure 1. Identification of ouabain-binding proteins.
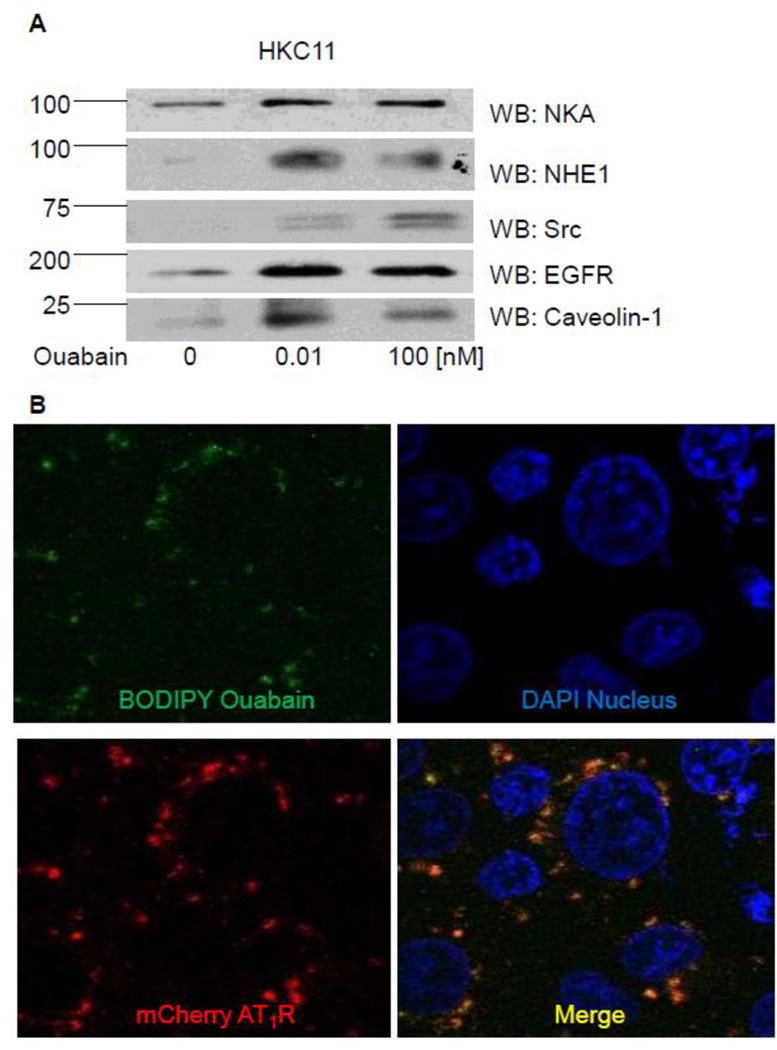
HKC11 cells were treated with 10 pM or 100 nM ouabain for 15 min at 37°C in humidified incubator. Cells were washed with ice-cold PBS, homogenized, and crude membranes were prepared as described in Methods. Cell membranes were solubilized in immunoprecipitation buffer and ouabain-binding proteins were immunoprecipitated with 1 μg Digibind (an antibody against cardioglycosides) per 100 μg protein as described in Methods. Proteins were eluted with 100 mM glycine pH 2 and collected in 1/10 volume 1 mM Tris-base pH 10 to neutralize the pH. Half of the proteins were subjected to LC/MS for identification of the proteins (Table 1–3 and Supplementary Tables 1–4) and the rest were used for western blot. A, Representative western blot from two individual experiments for NKA, NHE1, Src, EGFR, and caveolin-1 is shown. B, Cells transiently transfected with mCherry-labeled AT1R were treated with 10 nM Bodipy-ouabain for 15 min. Cells were washed with PBS to remove excess Bodipy-ouabain and live cells were imaged by confocal microscopy using Olympus FV1000 confocal microscope. A representative image from three independent experiments is shown.
Association of AT1R and NKA with ouabain
To determine whether AT1R can bind to ouabain we performed confocal microscopy in live HKC11 cells transiently transfected with mCherry-labeled AT1R. Transfected cells were treated with 10 pM Bodipy-ouabain for 15 min. The cells were washed with PBS at room temperature to remove excess Bodipy-ouabain. Cells were imaged using Olympus FV1000 microscope as described previously [26]. As shown in Fig. 1B, Bodipy-ouabain colocalized with mCherry-labeled AT1R. We further examined the nature of the association between AT1R and ouabain, by using FRET and TIRF microscopy. HKC11 cells were transiently transfected with either mCherry-AT1R or rat mCherry-NKA. After 24 h following transfection, cells were treated with 10 pM (AT1R transfected) or 100 nM (rat NKA transfected) Bodipy-ouabain. Cells were extensively washed with PBS to remove excess Bodipy-ouabain and subjected to FRET and TIRF microscopy as described in Methods. As shown in Fig. 2A, epifluorescence showed expression of AT1R and NKA. As shown in Fig. 2B, FRET analysis of the cells showed that low dose ouabain binds more to NKA than AT1R. However, there was no statistically significant difference between cells transfected with AT1R and NKA to ouabain binding (Fig. 2C). As shown in Fig. 2D, TIRF microscopy, similar to FRET data, showed binding of ouabain to both NKA and AT1R but more prominently to NKA than to AT1R particles in the plasma membrane.
Figure 2. Association between ouabain and NKA or AT1R in kidney proximal tubule cells.
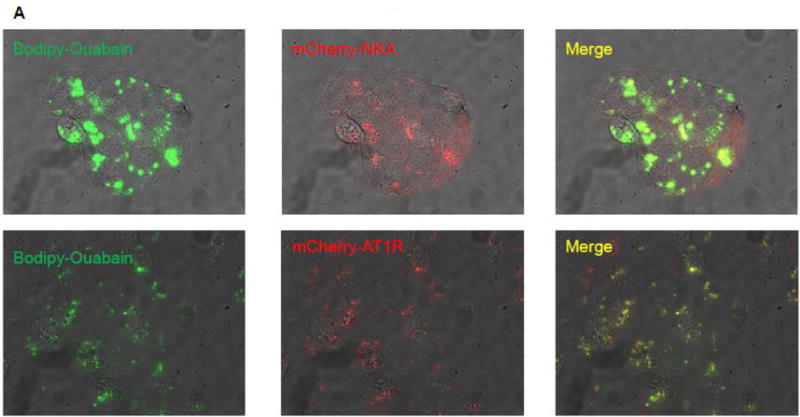
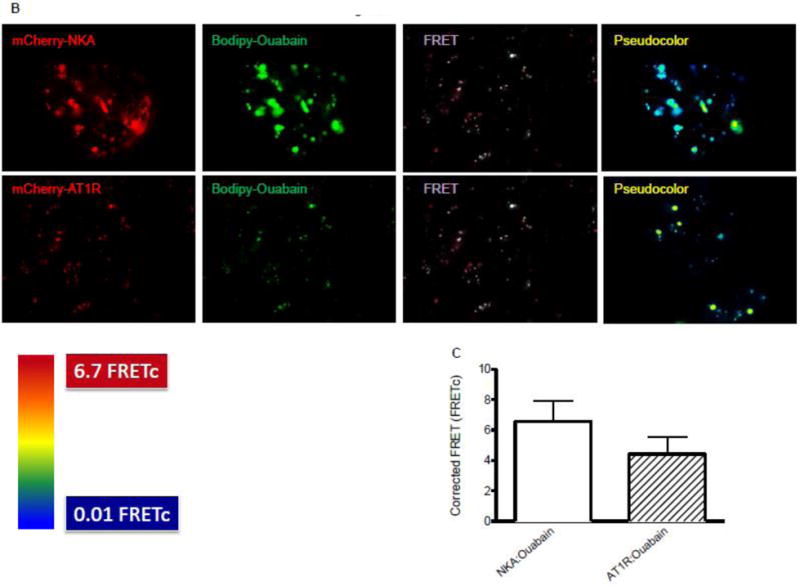
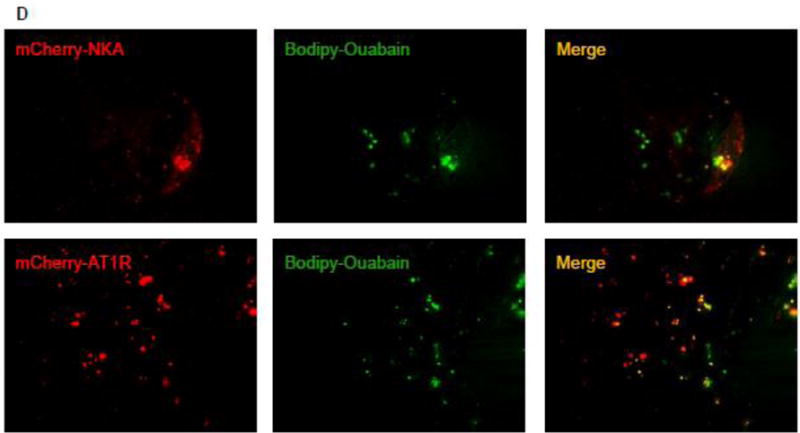
Immortalized human proximal tubule cells (HKC11) were transfected with either mCherry-tagged rat NKA or AT1R (red fluorescence). Cells were treated with bodipy-ouabain (10 pM (AT1R transfected cells) or 100 nM (rat NKA transfected cells), green fluorescence) for 15 min. Excess ouabain was washed with PBS and the cells were imaged for epifluorescence (A), sensitized FRET (B), or TIRF (D) microscopy as described in Methods. Representative images from 8 individual experiments are shown (n=8). C, Sensitized FRET after photo bleaching was calculated using SlideBook4 software as described in Methods. Each bar represents corrected FRET (FRETc) as mean±se from 8 individual experiments (n=8). In each experiment 20–30 cells were imaged and data from all the cells were pooled and considered as one data point.
Effect of low dose ouabain on Angiotensin II levels
The above proteomic, confocal, and FRET data suggested that low dose ouabain may bind to AT1R. However, recent reports suggest that AT1R can associate with NKA [27]. Data from several laboratories also suggest that ouabain can increase synthesis and release of angiotensin II [14]. We therefore, measured angiotensin II levels in the media of cells treated with a low (NKA stimulatory) and a high (NKA inhibitory) dose of ouabain in different cell culture models viz., human adrenal cortical cells (Fig. 3A), human kidney proximal tubule cells (HKC11, Fig. 3B), and mouse renal proximal tubule cells (MRPT, Fig. 3C). The low and high concentrations of ouabain were chosen based upon the sensitivity of NKA α1 subunit to ouabain and our published dose response curve data [6]. Cells were treated with the indicated ouabain concentration for 24 h, media was collected, and angiotensin II concentrations were measured. As shown in Fig. 3, ouabain at low dose significantly increased angiotensin II concentration in all cells studied. However, at higher concentrations ouabain had no significant effect on angiotensin II levels. We also measured plasma concentrations of angiotensin II from animals treated with ouabain (1μg/kg body wt. /day) for 9 days [3,6]. As shown in Fig. 3D, plasma concentrations of angiotensin II were significantly higher in ouabain treated animals as compared to vehicle treated animals.
Figure 3. Effect of ouabain on Angiotensin II release.
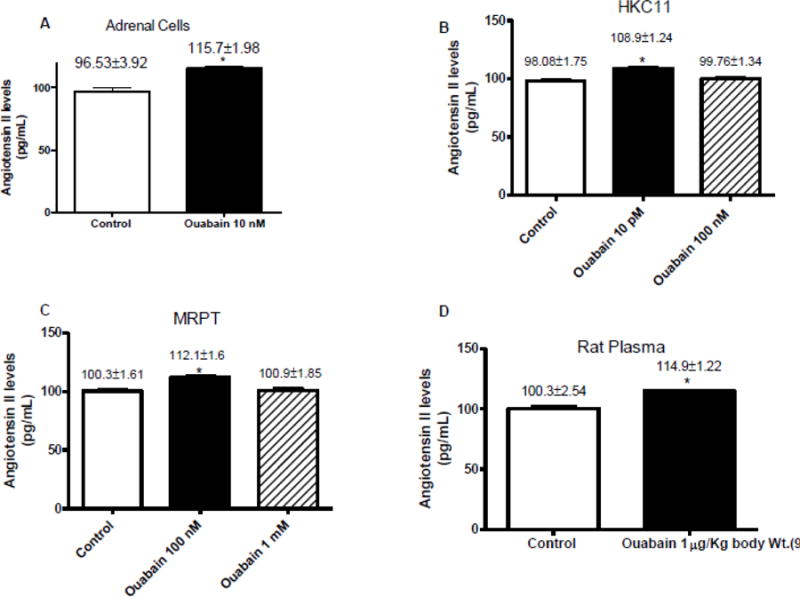
Cells (A: adrenal cells, B: MDCK, C: HKC11, and D: MRPT) were treated with indicated ouabain concentrations for 24 h. Cell media (A–D) or plasma (E) from ouabain treated rats was collected and proteins were purified using C18 HPLC columns followed by determination of angiotensin concentration EIA as described in Methods. Each bar represents data as pg/mL (mean±se) from 4 independent experiments (n=4 for cell culture) or plasma from 6 animals (n=6 for plasma). * indicates P < 0.05 as calculated by two-way ANOVA followed by Bonferroni analysis.
Effect of low dose ouabain on ACE and AGT expression
Angiotensin II is synthesized and modified by proteolysis of angiotensinogen (AGT) by angiotensin converting enzyme (ACE). To determine if low dose ouabain has any effect on the expression of ACE and AGT, we performed western blot in HKC11 cells treated with a low and a high dose of ouabain. As shown in Fig. 4, ouabain at both low and high dose increased the expression of ACE and AGT. These results are consistent with the studies by Wu et al [28] that demonstrated increased AGT expression at the mRNA level with the increase in ACE expression.
Figure 4. Effect of ouabain on ACE and AGT expression.
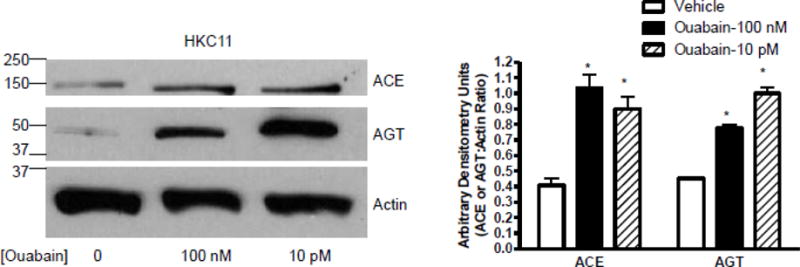
Human kidney proximal tubule cells (HKC11) were treated with 100 nM or 10 pM ouabain for 24 h at 37°C in humidified incubator. Cells were washed with ice-cold PBS and expression of ACE and AGT was determined by western blot in nuclear-free homogenates. A representative blot from 4 individual experiments is shown. Each bar represents data as ratio of arbitrary densitometry units between ACE or AGT and actin (mean±se) from 4 independent experiments (n=4). * indicates P < 0.05 as calculated by two-way ANOVA followed by Bonferroni analysis.
Effect of candesartan on NKA-dependent 86Rb uptake
We have previously demonstrated that low dose ouabain stimulates NKA-dependent ion transport [6]. The above data suggested that low dose ouabain may increase angiotensin II levels. Together, the data suggest that ouabain at low dose may increase NKA-dependent ion transport through activation of AT1R. To test this hypothesis, cells were treated with 10 pM (HKC11) or 100 nM (MRPT) ouabain for 15 min in the presence or absence of 100 nM candesartan, an AT1R blocker. As shown in Fig. 5A, ouabain increased NKA-dependent 86Rb uptake in both HKC11 and MRPT cells. Pre-treatment with candesartan prevented ouabain-mediated NKA-dependent 86Rb uptake in both HKC11 and MRPT cells. Candesartan by itself had no effect on NKA-dependent 86Rb uptake. To confirm that AT1R is required for ouabain-mediated increase in NKA-dependent 86Rb uptake, we treated LLC-PK1 cells stably transfected with either vector or AT1R with low dose ouabain. As shown in Fig. 5B, ouabain increased NKA-dependent 86Rb uptake in cells stably expressing AT1R but not in vector transfected cells.
Figure 5. Effect of candesartan on ouabain-stimulated NKA activity in renal proximal tubule cells.
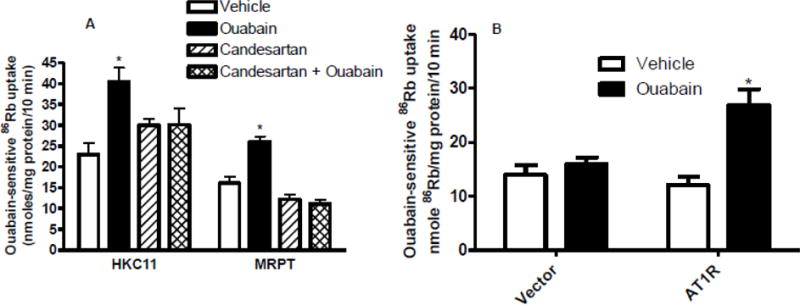
Human (HKC11) or mouse (MRPT) kidney proximal tubule cell lines were treated for 15 min with ouabain (10 pM, HKC11; 100 nM, MRPT) in the presence or absence of AT1R blocker, candesartan (100 nM). Ouabain (1 mM)-sensitive 86Rb uptake was determined as a measure of NKA-mediated ion transport. Each bar represents data as nmoles 86Rb/mg protein/10 min (mean±se) from 6 individual experiments (n=6) performed in triplicate. * indicates P < 0.05 as calculated by one-way ANOVA followed by Bonferroni analysis.
Effect of candesartan on NKA phosphorylation and expression
We have previously demonstrated that ouabain-mediated stimulation of NKA activity and ion transport is dependent upon increased NKA expression and tyrosine phosphorylation [3]. To determine if candesartan prevents ouabain-mediated increase in expression and phosphorylation of NKA, we performed biotinylation and immunoprecipitation followed by western blot analysis of crude membranes from HKC11 cells treated with 10 pM ouabain (15 min) in the presence and absence of candesartan (100 nM). As shown in Fig. 6A, surface biotinylation showed that ouabain significantly increased surface expression of NKA which was prevented by pretreatment with candesartan. Similarly, as shown in Fig. 6B, ouabain significantly increased tyrosine phosphorylation of NKA which was prevented by candesartan. To confirm that AT1R is required for ouabain-mediated increase in tyrosine phosphorylation, we treated LLC-PK1 cells stably transfected with either vector or AT1R with low dose ouabain. As shown in Fig. 6C, ouabain increased tyrosine phosphorylation in cells stably expressing AT1R but not in vector transfected cells.
Figure 6. Effect of candesartan on ouabain-stimulated NKA phosphorylation and expression in human kidney proximal tubule cells.

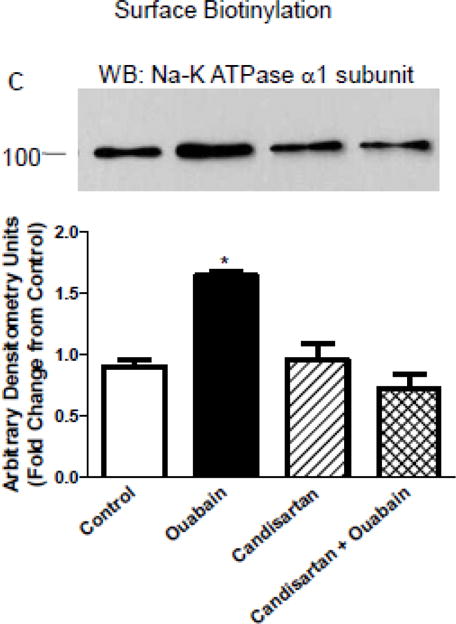
HKC11 (A and C) or LLCPK1 cells stably expressing AT1R (B) were treated for 15 min with 10 pM or 1 nM ouabain in the presence or absence of AT1R blocker, candesartan (100 nM). A and B, cell membranes were immunoprecipitated with NKA antibodies (α6F) followed by western blot using phospho-tyrosine antibodies. Nitrocellulose membranes were stripped and reprobed with NKA antibodies to show equal loading (bottom panel). A representative blot from 4 (HKC11, n=4) or 2 (LLCPK1, n=2) independent experiments. Each bar represents data as ratio of band intensity of phospho to total NKA (mean±se) from 4 independent experiments (n=4) as Arbitrary Units (AU). C, cell surface was biotinylated as described in Methods. Crude membrane proteins were separated by 10% SDS-PAGE and analyzed by immunoblot using NKA (upper panel) or caveolin-1 (lower panel) antibodies. Each bar represents data (mean±se) densitometry data (Arbitrary Units (AU)) from three independent experiments as fold difference from the vehicle-treated group as. * indicates P < 0.05 as calculated by two-way ANOVA followed by Bonferroni analysis.
Effect of candesartan on ouabain-mediated signaling
We and others have demonstrated that ouabain at low dose increases phosphorylation of several signaling molecules including EGFR, Src kinase, p42/p44 ERK kinase, and Akt [2,29,30]. To determine if AT1R plays a role in ouabain-stimulated signaling, we treated cells with low dose ouabain in the presence and absence of AT1R blocker candesartan (100 nM). As shown in Supplementary Figure 1, treatment with 10 pM ouabain increased phosphorylation of EGFR (A), Src kinase (B), and ERK (C). Pretreatment with candesartan prevented ouabain-stimulated phosphorylation of EGFR, Src kinase, and ERK. Candesartan by itself has no effect on phosphorylation of EGFR, Src kinase, and ERK. To confirm the role of AT1R, we treated LLC-PK1 cells stably transfected with vector or AT1R with low dose ouabain. As shown in D, ouabain increased EGFR and Src phosphorylation in cells stably expressing AT1R but not in vector transfected cells.
Role of ouabain in NKA α1 subunit and AT1R association
Khan et al demonstrated that NKA and AT1R can colocalize in renal proximal tubular cells [27]. To determine if treatment with ouabain increases association between NKA α1 subunit and AT1R we performed FRET and TIRF microscopy in cells transiently transfected with human GFP-tagged NKA α1 subunit and mCherry-tagged AT1R before and after treatment with ouabain. As shown in Fig. 7A, epifluorescence showed that both NKA α1 and AT1R are expressed in the same cells. As shown in Fig. 7B, FRET microscopy showed that under basal conditions NKA α1 subunit and AT1R can colocalize. Treatment of cells with 10 pM ouabain significantly increased the association between NKA α1 subunit and AT1R. Fig. 7C shows the calculated FRETc from 8 different experiments was significantly higher in cells treated with ouabain suggesting an increase in direct association between NKA α1 subunit and AT1R. TIRF microscopy was performed to determine if the association was occurring in the plasma membrane. As shown in Fig. 7D and Supplemental data (video recording), ouabain increased membrane expression of both NKA α1 subunit and AT1R and increased the association between NKA α1 subunit and AT1R in plasma membrane.
Figure 7. Effect of ouabain on association between NKA and AT1R in kidney proximal tubule cells.
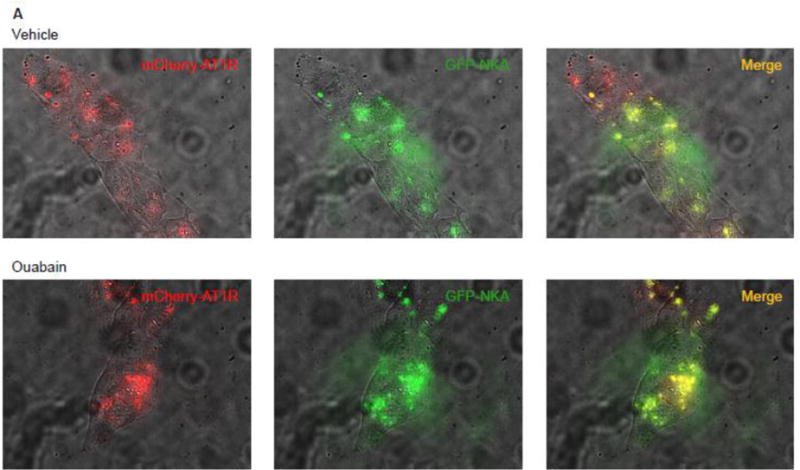
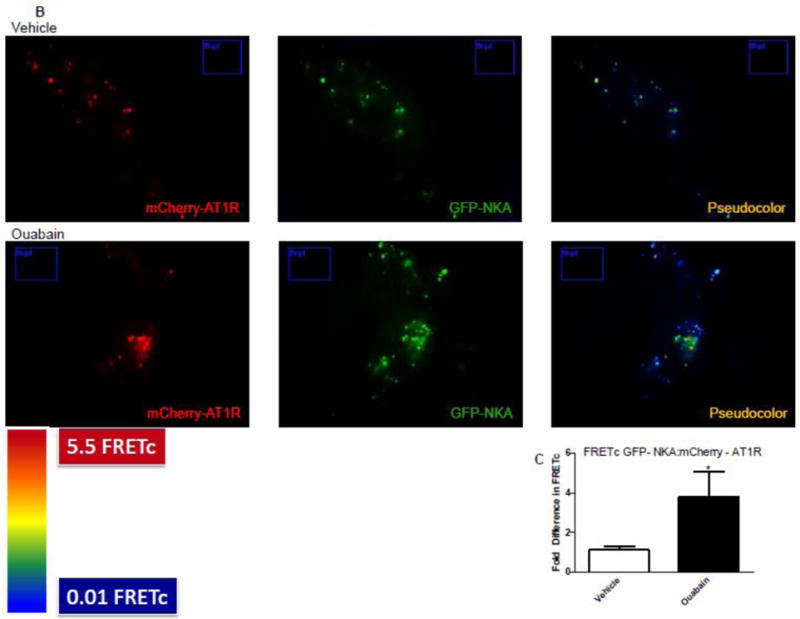

Immortalized human proximal tubule cells (HKC11) were transfected with mCherry-tagged AT1R (red fluorescence) and/or GFP-tagged human NKA (green fluorescence). Cells were treated with ouabain (1 nM) for 15 min. Excess ouabain was washed with PBS and the cells were imaged for epifluorescence (A), sensitized FRET (B), or TIRF (D) microscopy before and after treatment with ouabain (1 nM) as described in Methods. Representative images are shown from 8 individual experiments are shown (n=8). C, Sensitized FRET after photo bleaching was calculated using SlideBook4 software as described in Methods. Each bar represents corrected FRET (FRETc) as mean±se from 8 individual experiments (n=8). In each experiment 20–30 cells were imaged and data from all the cells were pooled and considered as one data point.
Discussion
The present study for the first time provides evidence that ouabain at low picomolar concentrations activates the angiotensin pathway in the regulation of proximal tubular sodium reabsorption. Using cell lines from human and mouse kidney proximal tubules, we demonstrated that low dose ouabain increases angiotensin II synthesis and release into the media. Our results further demonstrate that expression of AT1R is critical for ouabain-stimulated signaling through EGFR/Src kinase/ERK pathway and stimulation of NKA-mediated 86Rb uptake. These results have significant implications in the understanding of the molecular mechanisms by which ouabain at low picomolar concentrations, concentrations as seen in circulation, regulate sodium transport and activation of signaling molecules in kidney proximal tubules.
The major function of NKA has been established to maintain intracellular sodium and potassium concentrations. In the renal proximal tubules, NKA is heavily regulated by several hormones including aldosterone [31], angiotensin II [32], insulin [33], and PTH [34], neurotransmitters [35], and cardioglycosides [6]. Cardioglycosides were identified as molecules that inhibit NKA. However, data over the past decade suggest that NKA can function as a receptor for cardioglycosides to stimulate a signaling pathway involved in cell proliferation and survival [36]. We have previously demonstrated that at these low concentrations, ouabain increases blood pressure in an NHE1 dependent manner [3,6]. The involvement of angiotensin II in this process, although not studied in detail in renal tubules, is not surprising as angiotensin II is well known for the regulation of sodium transport in the renal tubules and blood pressure. Interestingly, the synthesis and release of angiotensin II by the renal tubules reported here were observed at low picomolar concentrations of ouabain, concentrations that stimulate the activity of NKA. The mechanisms by which low dose ouabain activates the synthesis and release of angiotensin II are not understood. Furthermore, whether all cardioglycosides have similar effects is not known.
Proteomic data following immunoprecipitation with Digibind, an antibody that binds to ouabain, from cells treated with low dose ouabain identified AT1R as one of the co-immunoprecipitating protein suggesting that perhaps ouabain can bind to AT1R. Confocal microscopy studies in live cells expressing mCherry-AT1R treated with Bodipy-ouabain suggest the possibility that ouabain may associate with AT1R. FRET and TIRF analysis are consistent with a direct interaction of ouabain with AT1R. However, Khan et al demonstrated that AT1R can directly associate with NKA α1 subunit [27]. Thus, we cannot exclude the possibility that the interaction between ouabain and AT1R may be indirect through ouabain-binding directly to NKA α1 subunit but not to AT1R and the observed association by FRET and TIRF analysis may be due to proximity of NKA α1 subunit and AT1R.
Synthesis of cardioglycosides in adrenal glands has been shown to be regulated by angiotensin II through activation of AT2R [14,37]. In turn, cardioglycosides have been demonstrated to regulate angiotensin II synthesis and release into the circulation [17,38]. Blaustein et al proposed the existence of a nexus between renin-angiotensin-aldosterone-system and cardioglycosides to regulate blood pressure through activation of sympathetic nervous system [38,39]. In fact, angiotensin II synthesis and release were demonstrated in animals that were injected with low dose cardioglycosides in the brain [16,40,41]. In a reciprocal manner treatment with angiotensin II resulted in increase in circulating endogenous ouabain levels and that this effect was absent in animals treated with eplerenone, a mineralocorticoid receptor (MR) blocker or FAD286, an inhibitor of aldosterone synthase [40,42]. These data suggest that ouabain synthesis in response to angiotensin II may be dependent upon aldosterone synthesis or activation of MR. It is not known whether ouabain at low doses can stimulate aldosterone synthesis. Further studies are needed to understand these complex mechanisms. It is also not known whether ouabain has a similar effect in the renal proximal tubules, another important site of angiotensin II synthesis, which participates in regulation of renal sodium transport and blood pressure regulation. It has demonstrated that ouabain is synthesized in adrenal cortex [43]. Cow [44] has demonstrated a direct microcirculation between adrenal glands and kidneys. Taken together, we speculate that ouabain synthesized in the adrenal glands may enter the kidneys directly at higher than circulating concentrations through the microcirculation to increase angiotensin II synthesis and release in the tubular lumen. Our results from cells treated with ouabain for 24 h suggest an increase in expression of angiotensinogen, angiotensin converting enzyme, and the release of angiotensin II in the media suggest the possibility that ouabain can increase synthesis of angiotensin II in the renal tubules that may have direct effect on renal sodium transport and blood pressure regulation.
The results from this study suggest that ouabain-mediated activation of its signaling pathway requires the presence of AT1R. Our results in cell culture models suggest that blocking AT1R with candesartan prevents ouabain-stimulated EGFR/Src/ERK pathway. Our results also demonstrate that ouabain stimulated the EGFR/Src/ERK pathway in LLC-PK1 cells stably expressing AT1R but not in cells transfected with vector although ouabain stimulated similar levels of angiotensin II in both cells (data not shown). Taken together, these data suggest the importance of AT1R expression in the regulation of signaling mechanisms by low dose ouabain. It is suggested that NKA serves as a receptor for ouabain in the activation of the signaling pathway. The current results point to a more complex pathway that involves AT1R. We speculate that binding of ouabain to high affinity NKA in the caveolin pockets may trigger activation of angiotensin II synthesis and release into the lumen. Angiotensin II then binds to its canonical receptor, AT1R, to stimulate the signaling pathway. Activation of the EGFR/Src cascade may phosphorylate NKA α1 subunit at tyrosine 10 to increase its membrane translocation and activity (Figure 8). However, this hypothesis needs to be tested.
Figure 8. Proposed model of ouabain action in the proximal tubule.
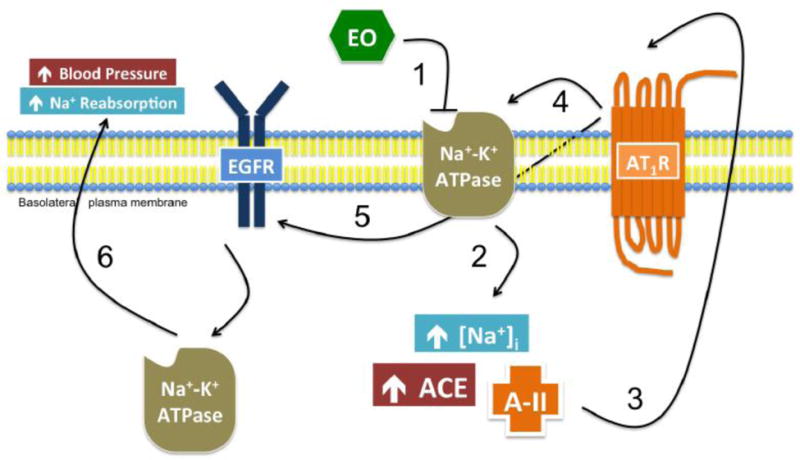
(1) Endogenous ouabain binds to and inhibits high-affinity NKA in the basolateral membrane of the proximal tubule, (2) resulting in an increase in intracellular sodium, ACE, and Ang II. (3) Ang II binds AT1 receptors in the basolateral membrane in an autocrine manner. (4) AT1R activation stimulates its association with NKA and (5) the activation of EGFR. (6) EGFR signaling stimulates the translocation of intracellular NKA to the plasma membrane, ultimately resulting in increased sodium reabsorption in the kidney and higher systemic blood pressure.
Our results demonstrate that the increase in 86Rb uptake by low dose ouabain is accompanied by an increase in plasma membrane expression and phosphorylation of NKA α1 subunit as demonstrated by biotinylation experiments. The increase in expression and phosphorylation was prevented by treatment with candesartan and in cells lacking expression of AT1R. Consistent with this data, TIRF and FRET analysis showed increased membrane expression of both NKA α1 subunit and AT1R in the plasma membrane and increased association of NKA α1 subunit and AT1R in human kidney proximal tubule cells. These data suggest that ouabain stimulates translocation of both NKA α1 subunit and AT1R to the plasma membrane. However, whether synthesis and release of angiotensin II by ouabain increases this association or that the increased association triggers a signal to increase synthesis of angiotensin II remains to be studied.
In summary, we have demonstrated an ouabain-stimulated increase in synthesis and release of angiotensin II and a direct interaction between NKA α1 subunit and AT1R which is critical for ouabain-stimulated NKA activity and signal transduction. The regulatory role for AT1R in these processes suggests potential therapeutic targets for regulation of both proximal tubule sodium transport, cell survival and proliferation.
Supplementary Material
Highlights.
We demonstrate ouabain may bind to and Na-K ATPase and AT1R in renal proximal tubules. Association with AT1R may not be direct.
We demonstrate low dose ouabain increases association between Na-K ATPase and AT1R.
We demonstrate that ouabain may mediate stimulation of Na-K ATPase expression and activity through AT1R dependent mechanism.
Acknowledgments
“The opinions expressed in this manuscript do not reflect the opinions of the Department of Veteran Affairs”. We acknowledge the technical assistance of Nina Lesousky and Caryl Conklin. We thank Dr. Marcela Herrera, Duke University for providing mCherry-AT1R construct and Dr. Thomas Pressley, Texas Tech University, Lubbock, Texas, USA for providing rat mCherry-NKA α1 construct. We thank Dr. Lorraine Racusen, Johns Hopkins University, Baltimore, MD for providing HKC11 cells Dr. Jeffrey R. Schelling, Case Western Reserve University, Cleveland, OH for mouse renal proximal tubule cells, and Dr. Raymond Harris, Vanderbilt University, Nashville, TN for AT1R expressing LLC-PK1 cells.
Sources of Funding: The work was supported by Grant-in-Aid, Great River Affiliate from American Heart Association, and NIH-5R21AG047474-02 (SJK) and Veteran Affairs Merit Review grant (EDL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Disclosure: No Conflicts/Disclosure to report
References
- 1.Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol. 2005;25:292–303. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Khundmiri SJ, Metzler MA, Ameen M, Amin V, Rane MJ, Delamere NA. Ouabain induces cell proliferation through calcium-dependent phosphorylation of Akt (protein kinase B) in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol. 2006;291:C1247–C1257. doi: 10.1152/ajpcell.00593.2005. [DOI] [PubMed] [Google Scholar]
- 3.Khundmiri SJ, Salyer SA, Farmer B, Qipshidze-Kelm N, Murray RD, Clark BJ, et al. Structural determinants for the ouabain-stimulated increase in Na-K ATPase activity. Biochim Biophys Acta - Mol Cell Res. 2014;1843:1089–1102. doi: 10.1016/j.bbamcr.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Meira EF, Siman FDM, Faria T de O, Júnior RFR, de Batista PR, Stefanon I, et al. Low-dose ouabain administration increases Na+,K+-ATPase activity and reduces cardiac force development in rats. Pharmacol Rep. 2015;67:253–9. doi: 10.1016/j.pharep.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Gao J, Wymore RS, Wang Y, Gaudette GR, Krukenkamp IB, Cohen IS, et al. Isoform-specific stimulation of cardiac Na/K pumps by nanomolar concentrations of glycosides. J Gen Physiol. 2002;119:297–312. doi: 10.1085/jgp.20028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holthouser KA, Mandal A, Merchant ML, Schelling JR, Delamere NA, Valdes RR, Jr, et al. Ouabain stimulates Na-K-ATPase through a sodium/hydrogen exchanger-1 (NHE-1)-dependent mechanism in human kidney proximal tubule cells. Am J Physiol Ren Physiol. 2010;299:F77–90. doi: 10.1152/ajprenal.00581.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manunta P, Hamilton BP, Hamlyn JM. Salt intake and depletion increase circulating levels of endogenous ouabain in normal men. Am J Physiol Regul Integr Comp Physiol. 2006;290:R553–9. doi: 10.1152/ajpregu.00648.2005. [DOI] [PubMed] [Google Scholar]
- 8.Hamlyn JM, Manunta P. Endogenous ouabain: a link between sodium intake and hypertension. Curr Hypertens Rep. 2011;13:14–20. doi: 10.1007/s11906-010-0161-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manunta P, Iacoviello M, Forleo C, Messaggio E, Hamlyn JM, Lucarelli K, et al. High circulating levels of endogenous ouabain in the offspring of hypertensive and normotensive individuals. J Hypertens. 2005;23:1677–1681. doi: 10.1097/01.hjh.0000177049.38417.67. [DOI] [PubMed] [Google Scholar]
- 10.Manunta P, Stella P, Rivera R, Ciurlino D, Cusi D, Ferrandi M, et al. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension. 1999;34:450–456. doi: 10.1161/01.hyp.34.3.450. [DOI] [PubMed] [Google Scholar]
- 11.Pamnani MB, Swindall BT, Schooley JF, Ghai R, Haddy FJ. Sodium-potassium pump inhibitor in the mechanism of one-kidney, one wrap hypertension in dogs. Cell Mol Biol (Noisy-Le-Grand) 1999;45:115–21. [PubMed] [Google Scholar]
- 12.Amler E, Cester N, Magnanelli R, Mazzanti L, Kotyk A, Romanini C. Na,K-ATPase from placenta of women with pregnancy-induced hypertension exhibits an increased affinity for cardiac glycosides. Physiol Res. 1994;43:33–6. [PubMed] [Google Scholar]
- 13.Manunta P, Ferrandi M, Bianchi G, Hamlyn JM. Endogenous ouabain in cardiovascular function and disease. J Hypertens. 2009;27:9–18. doi: 10.1097/HJH.0b013e32831cf2c6. [DOI] [PubMed] [Google Scholar]
- 14.Laredo J, Shah JR, Lu ZR, Hamilton BP, Hamlyn JM. Angiotensin II stimulates secretion of endogenous ouabain from bovine adrenocortical cells via angiotensin type 2 receptors. Hypertension. 1997;29:401–407. doi: 10.1161/01.hyp.29.1.401. [DOI] [PubMed] [Google Scholar]
- 15.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293:C509–36. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 16.Budzikowski AS, Huang BS, Leenen FH. Brain “ouabain”, a neurosteroid, mediates sympathetic hyperactivity in salt-sensitive hypertension. Clin Exp Hypertens. 1998;20:119–40. doi: 10.3109/10641969809053211. [DOI] [PubMed] [Google Scholar]
- 17.Huang BS, Leenen FH. Brain “ouabain” mediates the sympathoexcitatory and hypertensive effects of high sodium intake in Dahl salt-sensitive rats. Circ Res. 1994;74:586–95. doi: 10.1161/01.res.74.4.586. [DOI] [PubMed] [Google Scholar]
- 18.Hamlyn JM, Blaustein MP. Salt sensitivity, endogenous ouabain and hypertension. Changes. 2013;29:997–1003. doi: 10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita-Sato S, Ito S, Isobe T, Ohyama T, Wakabayashi K, Morishita K, et al. Structural basis of digoxin that antagonizes RORgamma t receptor activity and suppresses Th17 cell differentiation and interleukin (IL)-17 production. J Biol Chem. 2011;286:31409–17. doi: 10.1074/jbc.M111.254003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khundmiri SJ, Lederer E. PTH and DA regulate Na-K ATPase through divergent pathways. Am J Physiol Ren Physiol. 2002;282:F512–F522. doi: 10.1152/ajprenal.00111.2000. [DOI] [PubMed] [Google Scholar]
- 21.Merchant ML, Powell DW, Wilkey DW, Cummins TD, Deegens JK, Rood IM, et al. Microfiltration isolation of human urinary exosomes for characterization by MS. Proteomics Clin Appl. 2010;4:84–96. doi: 10.1002/prca.200800093. [DOI] [PubMed] [Google Scholar]
- 22.Khundmiri SJ, Bertorello AM, Delamere NA, Lederer ED. Clathrin-mediated endocytosis of Na+,K+-ATPase in response to parathyroid hormone requires ERK-dependent phosphorylation of Ser-11 within the alpha1-subunit. J Biol Chem. 2004;279:17418–17427. doi: 10.1074/jbc.M311715200. [DOI] [PubMed] [Google Scholar]
- 23.Khundmiri SJ, Amin V, Henson J, Lewis J, Ameen M, Rane MJ, et al. Ouabain stimulates protein kinase B (Akt) phosphorylation in opossum kidney proximal tubule cells through an ERK-dependent pathway. Am J Physiol Cell Physiol. 2007;293:C1171–C1180. doi: 10.1152/ajpcell.00535.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ketchem CJ, Khundmiri SJ, Gaweda AE, Murray R, Clark BJ, Weinman EJ, et al. Role of the sodium hydrogen exchanger regulatory factor 1 (NHERF1) in forward trafficking of the type IIa sodium phosphate cotransporter (NpT2a) Am J Physiol Renal Physiol. 2015;309 doi: 10.1152/ajprenal.00133.2015. ajprenal.00133.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang X, Sorkin A. Coordinated traffic of Grb2 and Ras during epidermal growth factor receptor endocytosis visualized in living cells. Mol Biol Cell. 2002;13:1522–35. doi: 10.1091/mbc.01-11-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khundmiri SJ, Weinman EJ, Steplock D, Cole J, Ahmad A, Baumann PD, et al. Parathyroid hormone regulation of NA+,K+-ATPase requires the PDZ 1 domain of sodium hydrogen exchanger regulatory factor-1 in opossum kidney cells. J Am Soc Nephrol. 2005;16:2598–2607. doi: 10.1681/ASN.2004121049. [DOI] [PubMed] [Google Scholar]
- 27.Khan F, Spicarova Z, Zelenin S, Holtback U, Scott L, Aperia A. Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am J Physiol Ren Physiol. 2008;295:F1110–6. doi: 10.1152/ajprenal.90336.2008. [DOI] [PubMed] [Google Scholar]
- 28.Wu SJ, Soulez M, Yang YH, Chu CS, Shih SC, Hébert MJ, et al. Local Augmented Angiotensinogen Secreted from Apoptotic Vascular Endothelial Cells Is a Vital Mediator of Vascular Remodelling. PLoS One. 2015;10:e0132583. doi: 10.1371/journal.pone.0132583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammadi K, Liu L, Tian J, Kometiani P, Xie Z, Askari A. Positive inotropic effect of ouabain on isolated heart is accompanied by activation of signal pathways that link Na+/K+-ATPase to ERK1/2. J Cardiovasc Pharmacol. 2003;41:609–614. doi: 10.1097/00005344-200304000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Xie Z, Askari A. Na(+)/K(+)-ATPase as a signal transducer. Eur J Biochem. 2002;269:2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 31.Salyer SA, Parks J, Barati MT, Lederer ED, Clark BJ, Klein JD, et al. Aldosterone regulates Na(+), K(+) ATPase activity in human renal proximal tubule cells through mineralocorticoid receptor. Biochim Biophys Acta. 2013;1833:2143–52. doi: 10.1016/j.bbamcr.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Bharatula M, Hussain T, Lokhandwala MF. Angiotensin II AT1 receptor/signaling mechanisms in the biphasic effect of the peptide on proximal tubular Na+,K+-ATPase. Clin Exp Hypertens. 1998;20:465–80. doi: 10.3109/10641969809053225. [DOI] [PubMed] [Google Scholar]
- 33.Sweeney G, Niu W, Canfield VA, Levenson R, Klip A. Insulin increases plasma membrane content and reduces phosphorylation of Na(+)-K(+) pump alpha(1)-subunit in HEK-293 cells. Am J Physiol Cell Physiol. 2001;281:C1797–803. doi: 10.1152/ajpcell.2001.281.6.C1797. [DOI] [PubMed] [Google Scholar]
- 34.Khundmiri SJ, Ameen M, Delamere NA, Lederer ED. PTH-mediated regulation of Na+-K+-ATPase requires Src kinase-dependent ERK phosphorylation. Am J Physiol Ren Physiol. 2008;295:F426–37. doi: 10.1152/ajprenal.00516.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cinelli AR, Efendiev R, Pedemonte CH. Trafficking of Na-K-ATPase and dopamine receptor molecules induced by changes in intracellular sodium concentration of renal epithelial cells. Am J Physiol Renal Physiol. 2008;295:F1117–25. doi: 10.1152/ajprenal.90317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aizman O, Aperia A. Na,K-ATPase as a signal transducer. Ann N Y Acad Sci. 2003;986:489–496. doi: 10.1111/j.1749-6632.2003.tb07233.x. [DOI] [PubMed] [Google Scholar]
- 37.Shah JR, Laredo J, Hamilton BP, Hamlyn JM. Different signaling pathways mediate stimulated secretions of endogenous ouabain and aldosterone from bovine adrenocortical cells. Hypertension. 1998;31:463–468. doi: 10.1161/01.hyp.31.1.463. [DOI] [PubMed] [Google Scholar]
- 38.Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, et al. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Hear Circ Physiol. 2012;302:H1031–49. doi: 10.1152/ajpheart.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamlyn JM, Blaustein MP. Salt sensitivity, endogenous ouabain and hypertension. Curr Opin Nephrol Hypertens. 2013;22:51–58. doi: 10.1097/MNH.0b013e32835b36ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamlyn JM, Linde CI, Gao J, Huang BS, Golovina VA, Blaustein MP, et al. Neuroendocrine humoral and vascular components in the pressor pathway for brain angiotensin II: a new axis in long term blood pressure control. PLoS One. 2014;9:e108916. doi: 10.1371/journal.pone.0108916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padilha AS, Rossoni LV, Xavier FE, Vassallo DV. Ouabain at nanomolar concentration promotes synthesis and release of angiotensin II from the endothelium of the tail vascular bed of spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2004;44:372–80. doi: 10.1097/01.fjc.0000138165.96364.51. [DOI] [PubMed] [Google Scholar]
- 42.Leenen FHH. Actions of circulating angiotensin II and aldosterone in the brain contributing to hypertension. Am J Hypertens. 2014;27:1024–32. doi: 10.1093/ajh/hpu066. [DOI] [PubMed] [Google Scholar]
- 43.Doris PA, Hayward-Lester A, Bourne D, Stocco DM. Ouabain production by cultured adrenal cells. Endocrinology. 1996;137:533–539. doi: 10.1210/endo.137.2.8593799. [DOI] [PubMed] [Google Scholar]
- 44.Cow D. The suprarenal bodies and diuresis. J Physiol. 1914;48:443–452. doi: 10.1113/jphysiol.1914.sp001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


