Abstract
Human AlkB homolog 2 (ALKBH2) is a DNA repair enzyme that catalyzes the direct reversal of DNA methylation damage through oxidative demethylation. While ALKBH2 colocalizes with proliferating cell nuclear antigen (PCNA) in DNA replication foci, it remains unknown whether these two proteins alone form a complex or require additional components for interaction. Here, we demonstrate that ALKBH2 can directly interact with PCNA independent from other cellular factors, and we identify the hydrophobic pocket of PCNA as the key domain mediating this interaction. Moreover, we find that PCNA association with ALKBH2 increases significantly during DNA replication, suggesting that ALKBH2 forms a cell-cycle dependent complex with PCNA. Intriguingly, we show that an ALKBH2 germline variant, as well as a variant found in cancer, display altered interaction with PCNA. Our studies reveal the ALKBH2 binding interface of PCNA and indicate that both germline and somatic ALKBH2 variants could have cellular effects on ALKBH2 function in DNA repair.
Keywords: AlkB, ALKBH2, APIM, DNA repair, PCNA
1. Introduction
Escherichia coli AlkB is a DNA repair enzyme that represents the founding member of the AlkB family of non-heme iron-dependent dioxygenases (reviewed in [1]). The human genome encodes nine AlkB homologs termed AlkB homolog 1 through 8 (ALKBH1 through 8) plus a ninth member termed fat mass and obesity associated (FTO). Bacterial AlkB as well as human ALKBH2 and ALKBH3 catalyze the direct reversal of DNA methylation damage through a distinct oxidative demethylation reaction [2–5]. Importantly, ALKBH2 has been shown to play a role in DNA alkylation damage repair in human, mouse and plant cells [6–8].
Proliferating cellular nuclear antigen (PCNA) is the major eukaryotic DNA sliding clamp for both replicative and translesion DNA polymerases [9]. In addition to DNA polymerases, PCNA interacts with numerous proteins involved in DNA damage signaling and repair, cell cycle progression and the cellular stress response (reviewed in [10]). Many PCNA-interacting proteins such as p21, flap structure-specific endonuclease 1 and replication factor Ccontain a well-characterized PCNA-interacting protein (PIP) box motif that binds to a hydrophobic pocket in PCNA [9]. Importantly, the interaction between PCNA and the PIP box-containing proteins is regulated through post-translational modifications such as phosphorylation and ubiquitination [10].
In addition to the PIP box, the ALKBH2-PCNA interacting motif (APIM) represents anewly discovered PCNA-interacting domain found in numerous proteins that are required for coordination of DNA replication and repair [11]. The APIM sequence is found in the first seven amino acid residues of ALKBH2 and is required for close association between ALKBH2 and PCNA [11, 12]. In addition to ALKBH2, numerous other DNA repair proteins contain an APIM motif that is required for proper protein function in DNA repair pathways such as homologous recombination and nucleotide excision repair [13–15]. While an interaction between a subset of APIM-containing proteins and PCNA has been demonstrated, the specific contacts made between APIM-containing proteins with PCNA remains unknown.
Here, we demonstrate that human ALKBH2 directly interacts with PCNA through a hydrophobic pocket formed by the inter domain connector loop and internal region of PCNA. Moreover, we show that ALKBH2 interaction with PCNA is enhanced during S-phase, indicating a cell cycle dependent interaction. Finally, we find that naturally occurring variants of ALKBH2 in cancer cells or the human population can alter PCNA binding. Collectively, our results provide insight into the molecular determinants of ALKBH2-PCNA interaction and the potential effects caused by ALKBH2 isoforms.
2. Materials And Methods
All Materials and Methods such as recombinant protein expression, purification and analysis as well as cell culture and transfection can be found in Supplemental Information.
3. Results And Discussion
3.1 Mutation of the hydrophobic pocket in PCNA disrupts interaction with ALKBH2
Fluorescence resonance energy transfer (FRET)-based assays in human cells indicate a close association between ALKBH2 and PCNA [11]. Thus, we tested whether purified ALKBH2 and PCNA can interact in the absence of additional factors or modifications. As a control for binding specificity, we included ALKBH3, another AlkB homolog that has been shown to repair DNA in vitro and in vivo but lacks an APIM sequence [2, 4, 16]. For binding assays, purified ALKBH2 or ALKBH3 (Supplemental Figure 1) were pre-incubated with an equimolar amount of PCNA followed by binding of the SBP-tagged ALKBH2 or ALKBH3 to streptavidin resin. Using this assay, we found that PCNA interacts with ALKBH2 while no PCNA was detected with the buffer control (Figure 1A). However, we did not detect any interaction between PCNA and ALKBH3, consistent with the absence of a PCNA-interacting motif in ALKBH3. Our results demonstrate a direct and specific interaction between ALKBH2 and PCNA.
FIGURE 1. Interaction of ALKBH2 with PCNA through the hydrophobic patch and the inter-domain connecting loop of PCNA.

(A) Immunoblot analysis of input and streptavidin resin from in vitro binding reactions containing recombinant ALKBH2 or ALKBH3 with PCNA. ALKBH2 and ALKBH3 contain a streptavidin binding peptide tag. Buffer represents a control purification containing PCNA and buffer but lacking ALKBH2 or ALKBH3. (B) Schematic of PCNA trimer with location of mutations found in SHV43, QLGI125 and VDK188 PCNA variants [17]. (C) Immunoblot analysis of input and streptavidin resin from binding reactions containing ALKBH2 and the indicated PCNA variants. Buffer represents a control purification containing wild-type (WT) PCNA and buffer but lacking ALKBH2. (D) Immunoblot analysis of input and purified samples from 293T cells expressing ALKBH2-FLAG and the indicated PCNA mutants. ALKBH2-FLAG was affinity purified using anti-FLAG resin and purifications were analyzed by immunoblotting.(−) represents a negative control purification from 293T cells transfected with empty vector.
Previous studies using FRET have suggested a common binding site on PCNA for both PIP-box containing proteins and APIM-containing proteins [12]. Our in vitro assay for measuring a direct interaction between ALKBH2 and PCNA allowed us to dissect the requirements for ALKBH2-PCNA interaction. We used a previously characterized panel of PCNA mutants to test the potential domains of PCNA that mediate interaction with ALKBH2 (Figure 1B) [17]. The PCNA mutants include: SHV43 (Ser43, His44 and Val45 to alanine), which disrupts a hydrophobic pocket on the front face of PCNA necessary for binding PIP-box containing proteins; QLGI125 (Gln125, Leu126, Gly127, and Ile128 to alanine), which disrupts the inter domain connector loop that composes a portion of the PCNA hydrophobic pocket; and VDK188 (Val188, Asp189 and Lys188 to alanine), which alters an unstructured loop on the backside of the PCNA trimer. While wild-type (WT) PCNA displayed robust interaction with ALKBH2, we found that PCNA mutants SHV43 and QLGI125 are severely deficient in ALKBH2 binding (Figure 1C). In contrast, the VDK188 PCNA mutant displayed interaction with ALKBH2 equivalent to that of WT PCNA (Figure 1C). Thus, mutation of the hydrophobic pocket of PNCA as well as the interdomain connecting loop that forms a portion of the hydrophobic pocket leads to greatly reduced interaction between PCNA and ALKBH2. On the other hand, mutations to the backside of PCNA appear to have minimal or no effect on ALKBH2 binding.
To confirm these in vitro results, we determined whether the same interaction requirements pertain to ALKBH2 and PCNA in human cells. We expressed ALKBH2 in 293T human embryonic kidney cells as a fusion protein with the FLAG epitope tag at either the amino- or carboxy-terminus. Following purification on anti-FLAG resin, we found that carboxy terminal-tagged ALKBH2 displayed interaction with endogenous PCNA while tagging ALKBH2 at the amino terminus disrupted interaction with PCNA (Supplemental Figure 2A). The lack of interaction between amino terminal-tagged ALKBH2 and PCNA is most likely due to steric hindrance by the 3xFLAG tag that blocks interaction between the amino-terminal APIM of ALKBH2 and PCNA. Furthermore, the interaction between ALKBH2 and PCNA was resistant to nuclease treatment, ruling out nucleic acids as bridging cofactors (Supplemental Figure 2B). The results are consistent with previous results using GFP-tagged ALKBH2 [11] and indicate that overexpressed ALKBH2 displays APIM-dependent interactions with PCNA without the need of DNA or RNA.
Next, we tested whether PCNA variants display differential binding to ALKBH2 in cells by coexpression of PCNA variants with FLAG-tagged ALKBH2 [18]. The over-expressed PCNA contains a His-Myc tag at the C-terminus that allows differentiation from endogenous PCNA. Using this strategy, we found that the amount of SHV43 and QLGI125 PCNA variants associated with ALKBH2 was greatly reduced relative to WT PCNA (Figure 1D). Unlike the pure populations of PCNA variants used for in vitro studies, the PCNA trimer associated with AKLBH2 purified from human cells represents a mixture of endogenous and transiently expressed PCNA. Thus, the remaining amount of SHV43 and QLGI125 PCNA variant associated with ALKBH2 is likely due to incorporation of endogenous PCNA into a trimer containing both endogenous and variant PCNA that can associate with ALKBH2.
Overall, these data reveal that the hydrophobic pocket of PCNA as a critical determinant of ALKBH2-PCNA interaction both in vitro and in vivo. The identification of a common binding site on PCNA for APIM and PIP box-containing proteins suggests that two classes of PCNA-interacting proteins can compete for the same site. Future studies will be devoted to understanding the interplay between APIM versus PIP box-containing proteins for binding to PCNA as well as the exact molecular contacts made between the APIM motif and PCNA.
3.2 ALKBH2 displays enhanced interaction with PCNA during S-phase
To gain insight into the dynamics of the ALKBH2-PCNA interaction, we investigated whether the two proteins display any changes in association after DNA damage and during various phases of the cell cycle. For DNA damage, we chose alkylation damage caused by MMS since this agent is known to induce replication-blocking lesions repaired by ALKBH2 [19, 20]. However, we were unable to detect any differences in PCNA-ALKBH2 interaction between untreated or MMS-treated cells (Supplemental Figure 2C). Since DNA replication stalling due to alkylation damage is known to trigger PCNA modification, our results suggest that PCNA-ALKBH2 interaction is not affected by either alkylation DNA damage or damage-induced PCNA modifications.
Previous studies using fluorescent tagged proteins and FRET have shown that ALKBH2 colocalizes with replication foci containing PCNA [11]. Thus, we examined the ALKBH2-PCNA interaction throughout the cell cycle to determine if there are any cell cycle-dependent changes in physical interaction between ALKBH2 and PCNA. For this purpose, we purified ALKBH2-FLAG from cells synchronized at different phases of the cell cycle using a double thymidine block and quantified the amount of PCNA associated relative to purified ALKBH2. Notably, we detected a considerable increase in association between ALKBH2 and PCNA at four hours after cell synchronization, which represents the time at which the highest number of cells are in S-phase (Figures 2A–C). The amount of PCNA associated with ALKBH2 decreases significantly at the eight-hour time point, which correlates with cellular exit from S-phase and the beginning of G2/mitosis (Figures 2A–C).
FIGURE 2. Enhanced interaction of PCNA with ALKBH2 during S-phase.
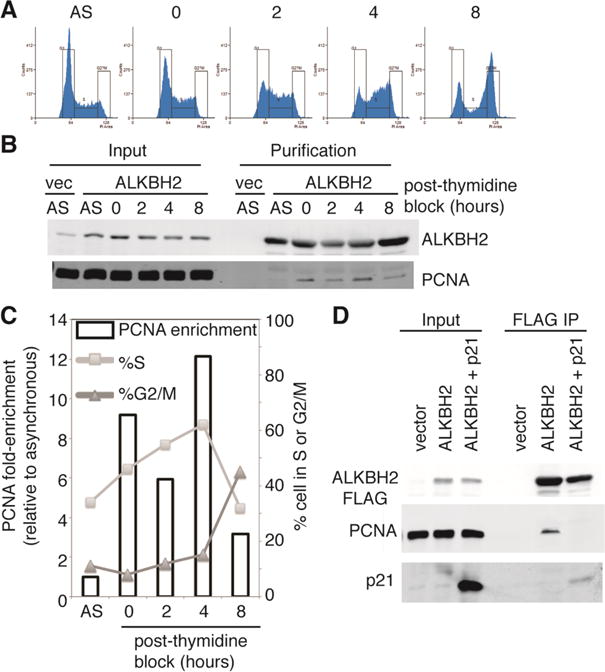
(A) Cell cycle profiles of 293T cells transfected with ALKBH2-FLAG. “AS” represents an asynchronous population and time after the double thymidine block is represented in hours. (B) Immunoblot analysis of input and purified samples from 293T cells transfected with ALKBH2 at the indicated time points post double thymidine block. Vector represents a control purification from 293T cells transfected with empty vector. PCNA fold-enrichment was relative to asynchronous and normalized to purified ALKBH2. (C) Immunoblot analysis of input and purified samples from 293T cells expressing ALKBH2 with or without p21 overexpression. The experiment was repeated twice with identical results.
To provide further evidence that ALKBH2 is associated with PCNA predominantly during S-phase, we selectively blocked cells in G1 or G2 by p21 overexpression, which is known to arrest cells mainly in G1 along with a fraction in G2 [21]. Indeed, the interaction between PCNA and ALKBH2 could be abrogated by p21 overexpression and a fraction of p21 copurified with ALKBH2 after p21 overexpression (Figure 2D). Thus, p21 overexpression could be disrupting the ALKBH2-PCNA interaction by cell cycle arrest in G1/G2 and/or direct competition with ALKBH2. Our results demonstrate that the physical association between ALKBH2 and PCNA is modulated throughout the cell cycle, potentially through modifications on either ALKBH2 or PCNA that are independent of DNA damage [11].
3.3 ALKBH2 variants in cancer cells and the human population perturb PCNA binding
Since PCNA and ALKBH2 exhibit specific and dynamic association in cells, we investigated whether there are naturally occurring variants that could affect the PCNA-ALKBH2 interaction. Based upon the Catalogue of Somatic Mutations In Cancer (COSMIC) and Ensembl genome database [22, 23], we identified two ALKBH2 variants containing amino acid residue substitutions near the APIM motif (Figure 3A). The first variant was identified as a somatic missense mutation in an endometrial tumor resulting in an alanine to valine mutation (A9V) (Mutation ID: COSM934680). The second variant is a heterozygous allele pair found through genome wide exon sequencing which leads to a glutamine to lysine substitution (Q10K) (Reference SNP: rs138073204). The Q10K variant was identified as a heterozygous allele pair present at low frequency (0.023%) among 13,006 individuals of European and African-American ancestry (Exome Variant Server, NHLBI GO Exome Sequencing Project) (Supplemental Table 1). Due to their proximity to the APIM motif, we tested whether these variants affect binding to PCNA.
FIGURE 3. Alteration of PCNA interaction in ALKBH2 variants found in cancer or the human population.

(A) Schematic representation of the amino terminus of ALKBH2 (amino acid residues 1–10). The APIM motif is shaded. ALKBH2 variants F4A, A9V and Q10K are indicated by arrows. (B) Immunoblot analysis of input and purified samples from 293T cells transfected with the indicated ALKBH2 variants. “vec” represents a control purification from 293T cells transfected with empty vector. PCNA enrichment was normalized to purified ALKBH2. (C) Denaturing gels of DNA products from double-stranded DNA substrates reacted with the indicated samples. (D) Quantification of ALKBH2-catalyzed repair. The percent repaired substrate represents the amount of repaired product to unrepaired substrate normalized against the signal in (−) negative control reactions. Purifications and activity assays were performed at least three times with comparable results.
Wild-type ALKBH2 or either of the two ALKBH2 variants, A9V or Q10K, was expressed in human cells followed by affinity purification and analysis of copurifying PCNA. As a control, we included a previously characterized ALKBH2 variant that contains a directed mutation of phenylalanine to alanine in the APIM motif (F4A) and lacks interaction with PCNA [11]. As expected, the F4A mutation completely abolishes the interaction between ALKBH2 and PCNA (Figure 3B). Moreover, the amount of PCNA copurifying with the A9V tumor-associated variant of ALKBH2 is slightly but reproducibly decreased relative to WT ALKBH2. In striking contrast, we find that the Q10K polymorphic variant of ALKBH2 leads to significantly increased association with PCNA compared to WT ALKBH2 (Figure 3B, Q10K). The amount of PCNA copurified with ALKBH2 Q10K is approximately 5-fold higher than that with WT ALKBH2. We also note a slower migrating PCNA band that copurifies with the ALKBH2 Q10K variant. While the exact nature of the higher molecular weight PCNA band is currently unknown, the additional PCNA species could represent an acetylated form of PCNA based upon its migration [24]. By using a restriction enzyme-mediated AlkB DNA repair assay, the ALKBH2 variants display comparable enzymatic activity to WT ALKBH2 (Figure 3C and D). Thus, the amino acid residue changes in the ALKBH2 variants affect PCNA binding but do not alter in vitro DNA repair activity of the purified enzymes. Thus, it appears that naturally occurring ALKBH2 variants alter APIM-dependent interactions with PCNA, leading to either decreased or increased binding.
In conclusion, we identify the regions of PCNA and ALKBH2 that are required for interaction with each other and uncover a dynamic physical interaction that is modulated by the cell cycle. Notably, a germline variant of ALKBH2 (Q10K) leads to an increased PCNA binding while a cancer-associated mutation (A9V) leads to decreased PCNA binding. Future studies will be devoted to understanding the exact cellular consequences arising from altered binding of ALKBH2 variants to PCNA. Moreover, we are investigating additional cancer-association mutations or naturally-occurring SNPs in ALKBH2 that might affect PCNA binding or other aspects of ALKBH2 repair function. Intriguingly, overexpression of GFP fusion proteins with the APIM motif can sensitize human cells to chemotherapeutic DNA damaging agents [11, 12]. Thus, our studies could provide a novel insight into potential anti-cancer therapies based upon APIM motifs [25].
Supplementary Material
HIGHLIGHTS.
ALKBH2 directly interacts with PCNA through a hydrophobic pocket.
PCNA and ALKBH2 associate in cell-cycle dependent manner.
Naturally-occurring variants of ALKBH2 display altered binding to PCNA.
Acknowledgments
We thank Antonia Furrer, Elena Ferrari and Zophonías O. Jónssonfor reagents and assistance used in this study. D.F and U.H were funded by Swiss National Science Foundation (31003A_133100/1). L.D.S. is an American Cancer Society Research Professor. This work was supported by NIH grants CA055042 and ES002109. B.v.L. was funded by the University of Zurich.
Abreviations
- ALKBH2
AlkB homolog 2
- APIM
ALKBH2-PCNA interacting motif
- PCNA
proliferating cell nuclear antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict Of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Yi C, He C. DNA repair by reversal of DNA damage, Cold Spring Harbor perspectives in biology. 2012;5 doi: 10.1101/cshperspect.a012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aas PA, Otterlei M, Falnes PO, Vågbø CB, Skorpen F, Akbari M, Sundheim O, Bjørås M, Slupphaug G, Seeberg E, Krokan HE. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature. 2003;421:859–863. doi: 10.1038/nature01363. [DOI] [PubMed] [Google Scholar]
- 3.Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature. 2002;419:174–178. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- 4.Duncan T, Trewick SC, Koivisto P, Bates PA, Lindahl T, Sedgwick B. Reversal of DNA alkylation damage by two human dioxygenases. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16660–16665. doi: 10.1073/pnas.262589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falnes PO, Johansen RF, Seeberg E. AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature. 2002;419:178–182. doi: 10.1038/nature01048. [DOI] [PubMed] [Google Scholar]
- 6.Ringvoll J, Nordstrand LM, Vågbø CB, Talstad V, Reite K, Aas PA, Lauritzen KH, Liabakk NB, Bjørk A, Doughty RW, Falnes PØ, Krokan HE, Klungland A. Repair deficient mice reveal mABH2 as the primary oxidative demethylase for repairing 1meA and 3meC lesions in DNA. The EMBO journal. 2006;25:2189–2198. doi: 10.1038/sj.emboj.7601109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nay SL, Lee D-HH, Bates SE, O’Connor TR. Alkbh2 protects against lethality and mutation in primary mouse embryonic fibroblasts. DNA repair. 2012;11:502–510. doi: 10.1016/j.dnarep.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meza TJ, Moen MN, Vågbø CB, Krokan HE, Klungland A, Grini PE, Falnes PØ. The DNA dioxygenase ALKBH2 protects Arabidopsis thaliana against methylation damage. Nucleic acids research. 2012;40:6620–6631. doi: 10.1093/nar/gks327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Mailand N, Gibbs-Seymour I, Bekker-Jensen S. Regulation of PCNA-protein interactions for genome stability. Nature reviews Molecular cell biology. 2013;14:269–282. doi: 10.1038/nrm3562. [DOI] [PubMed] [Google Scholar]
- 11.Gilljam KM, Feyzi E, Aas PA, Sousa MML, Müller R, Vågbø CB, Catterall TC, Liabakk NB, Slupphaug G, Drabløs F. Identification of a novel, widespread, and functionally important PCNA-binding motif. The Journal of cell biology. 2009;186:645–654. doi: 10.1083/jcb.200903138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller R, Misund K, Holien T, Bachke S, Gilljam KM, Vatsveen TK, Ro TB, Bellacchio E, Sundan A, Otterlei M. Targeting proliferating cell nuclear antigen and its protein interactions induces apoptosis in multiple myeloma cells. PloS one. 2013;8:e70430. doi: 10.1371/journal.pone.0070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciccia A, Nimonkar AV, Hu Y, Hajdu I, Achar Y, Izhar L, Petit SA, Adamson B, Yoon JC, Kowalczykowski SC. Polyubiquitinated PCNA recruits the ZRANB3 translocase to maintain genomic integrity after replication stress. Molecular cell. 2012;47:396–409. doi: 10.1016/j.molcel.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilljam KM, Muller R, Liabakk NB, Otterlei M. Nucleotide excision repair is associated with the replisome and its efficiency depends on a direct interaction between XPA and PCNA. PloS one. 2012;7:e49199. doi: 10.1371/journal.pone.0049199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacquin A, Pouvelle C, Siaud N, Perderiset M, Salome-Desnoulez S, Tellier-Lebegue C, Lopez B, Charbonnier JB, Kannouche PL. The helicase FBH1 is tightly regulated by PCNA via CRL4 (Cdt2)-mediated proteolysis in human cells. Nucleic Acids Res. 2013;41:6501–6513. doi: 10.1093/nar/gkt397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dango S, Mosammaparast N, Sowa ME, Xiong LJ, Wu F, Park K, Rubin M, Gygi S, Harper JW, Shi Y. DNA unwinding by ASCC3 helicase is coupled to ALKBH3-dependent DNA alkylation repair and cancer cell proliferation. Mol Cell. 2011;44:373–384. doi: 10.1016/j.molcel.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonsson ZO, Hindges R, Hubscher U. Regulation of DNA replication and repair proteins through interaction with the front side of proliferating cell nuclear antigen. EMBO J. 1998;17:2412–2425. doi: 10.1093/emboj/17.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu D, Samson LD. Direct repair of 3,N (4)-ethenocytosine by the human ALKBH2 dioxygenase is blocked by the AAG/MPG glycosylase. DNA Repair (Amst) 2012;11:46–52. doi: 10.1016/j.dnarep.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furrer A, van Loon B. Handling the 3-methylcytosine lesion by six human DNA polymerases members of the B-, X- and Y-families. Nucleic Acids Res. 2014;42:553–566. doi: 10.1093/nar/gkt889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrivastav N, Li D, Essigmann JM. Chemical biology of mutagenesis and DNA repair: cellular responses to DNA alkylation. Carcinogenesis. 2010;31:59–70. doi: 10.1093/carcin/bgp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niculescu AB, Chen X, Smeets M, Hengst L, Prives C, Reed SI. Effects of p21 (Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Molecular and cellular biology. 1997;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, Kok CY, Jia M, De T, Teague JW, Stratton MR, McDermott U, Campbell PJ. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805–811. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham F, Amode MR, Barrell D, Beal K, Billis K, Brent S, Carvalho-Silva D, Clapham P, Coates G, Fitzgerald S, Gil L, Giron CG, Gordon L, Hourlier T, Hunt SE, Janacek SH, Johnson N, Juettemann T, Kahari AK, Keenan S, Martin FJ, Maurel T, McLaren W, Murphy DN, Nag R, Overduin B, Parker A, Patricio M, Perry E, Pignatelli M, Riat HS, Sheppard D, Taylor K, Thormann A, Vullo A, Wilder SP, Zadissa A, Aken BL, Birney E, Harrow J, Kinsella R, Muffato M, Ruffier M, Searle SM, Spudich G, Trevanion SJ, Yates A, Zerbino DR, Flicek P. Ensembl 2015. Nucleic Acids Res. 2015;43:D662–669. doi: 10.1093/nar/gku1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cazzalini O, Sommatis S, Tillhon M, Dutto I, Bachi A, Rapp A, Nardo T, Scovassi AI, Necchi D, Cardoso MC, Stivala LA, Prosperi E. CBP and p300 acetylate PCNA to link its degradation with nucleotide excision repair synthesis. Nucleic Acids Res. 2014;42:8433–8448. doi: 10.1093/nar/gku533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang SCC. PCNA: a silent housekeeper or a potential therapeutic target? Trends in pharmacological sciences. 2014;35:178–186. doi: 10.1016/j.tips.2014.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


