Abstract
Despite the widespread prevalence of generalized anxiety disorder (GAD) in later life, almost nothing is known about the neural aspects of worry in adults over the age of 60. Given the ongoing rapid increase in the older adult population, the relatively poor response rates to current interventions for late life GAD, and the effects of age-related changes to the brain, additional research on worry neurobiology is needed. The study group comprised 15 older GAD patients and 15 matched controls who were compared on clinical measures and brain volumes. It was expected that prefrontal cortex (PFC) volumes [medial orbital cortex (mOFC), dorsolateral cortex (DLPFC)] would show positive relations to worry scores, and weaker relations to more general measures of anxiety and depression. Negative relations were expected between amygdala volumes and worry scores. As expected, mOFC volumes were positively related to worry scores; however, DLPFC and amygdala volumes were not. The mOFC is involved in emotional decision-making under uncertain conditions and has the ability to suppress the amygdala, both of which are hypothesized functions of worry. Results are partly consistent with GAD theory and suggest that worry may involve neural areas that are also involved in the successful control of anxiety.
Keywords: Aging, Worry, Geriatric anxiety, Prefrontal cortex, Anxiety neurobiology
1. Introduction
The world’s older adult population is increasing at an estimated rate of 800,000 people per month (Kinsella and Velkoff, 2001), which will have a dramatic impact on global health issues. For instance, along with the growing older adult population comes a corresponding expectation of increased rates of psychiatric conditions such as anxiety and depression (Jeste et al., 1999; Blazer, 2003). However, research in anxiety disorders has not kept pace with the aging trend. Mental health specialists now face a burgeoning public health problem, given the relative lack of empirical knowledge to inform the conceptualization and treatment of late life anxiety.
Generalized anxiety disorder (GAD), a condition characterized by excessive, uncontrollable worry, is the most common anxiety disorder among adults over the age of 60, with estimated prevalence rates ranging widely from 0.71 to 7.3% (Flint,1994; Beekman et al.,1998; Flint, 1999). Current diagnostic criteria according to the Diagnostic and Statistical Manual IV-TR (DSM-IV-TR; American Psychiatric Association, 2000) require at least 6 months of frequent worry about several real-life problems, occurring more days than not, accompanied by at least three associated symptoms such as tension, fatigue, irritability, trouble concentrating, or insomnia. GAD is a prevalent but underrecognized public health problem, associated with significant functional impairment (Mogotsi et al., 2000), serious disability (Kessler et al., 1999), and increased risk for acquisition of additional psychiatric disorders and medical conditions (Brown et al., 1994; Kennedy and Schwab, 1997; Noyes, 2001; Barger and Sydeman, 2005). Response rates in most studies of late life GAD pharmacological and psychosocial treatment are surprisingly low (Mohlman, 2004; Mitte, 2005), highlighting the need for additional data on the causal and maintenance factors of the disorder.
1.1. Neurobiology and theoretical models of GAD
Although the neurobiology of GAD remains largely obscure (Mathew et al., in press; Sinha et al., 2004), neural patterns characterizing two broad types of anxiety have been proposed (Heller et al.,1997; Nitschke et al., 2000). Anxious apprehension, a construct that encompasses cardinal symptoms of GAD such as worry and concern about imminent and future events (Borkovec et al., 1983; Barlow, 1991), is posited to involve increased activity in the left hemisphere, particularly in frontal areas (Heller et al., 1997). By contrast, those emotional states characterized by exaggerated somatic symptoms, sympathetic arousal, and a focus on imminent fear cues (such as panic disorder) are believed to be characterized by right posterior activity (Nitschke et al., 1999).
Models of GAD have posited a more specific relation between the PFC and the amygdala. In addition to a distinct left hemisphere profile, GAD may be associated with frontal overcontrol (e.g., excessive worry) and limbic hypoactivity (e.g., blunted sympathetic arousal; Hoehn-Saric et al., 1989; Thayer and Lane, 2000), which is in contrast to the common anxiety disorder profile of limbic overactivity coupled with poor frontal control of negative affect (e.g., Kent and Rauch, 2004; Lorberbaum et al., 2004). According to Borkovec (1994), GAD patients and high worriers use worry instrumentally as a strategy to dampen or avoid strong feelings of emotional arousal, which they may perceive to be more aversive than worry itself. Although it is likely that there are both excitatory and inhibitory processes at work during worry (Gray et al., 2003), these models and the few neurobiological studies that do exist implicate increased activity in the prefrontal cortex (PFC) both at rest and during worry in adults with GAD, perhaps due to the ability of areas of PFC to suppress or inhibit activation of the limbic system (Hoehn-Saric et al., 2005). Neuroimaging findings are mostly consistent with the notion that worry and related states of anxious apprehension recruit areas of the PFC (primarily DLPFC, mOFC), which may then suppress or inhibit activity in limbic regions including the amygdala (c.f. Sinha et al., 2004).
1.2. Additional considerations for older adults
All existing data on neurobiological correlates of worry have been collected from younger adults. However, factors related to aging are associated with changes in PFC structure, and could affect the neurobiological profile of worry. For instance, West’s (1996) frontal lobe hypothesis posits accelerated volume loss brought about by cell shrinkage (Hachinski et al., 1987), decreased dopamine (DA) concentration, and a reduction in the number of DA receptors in the frontal cortex (Goldman-Rakic and Brown, 1981) with advancing age. Some evidence exists for inordinate degradation of the PFC relative to other brain areas (Raz et al., 1998; Tisserand et al., 2002) and of the DLPFC relative to other frontal areas (MacPherson et al., 2002). Consistent with the age-related decline of PFC-governed cognitive operations (e.g., executive skills; Hasher and Zacks, 1988), these effects could lead to decreased PFC involvement in worry and states of anxious apprehension in older adults.
Indeed, GAD symptoms are typically less severe in older patients as compared with young patients. Self-report and clinician-rated measures of worry consistently reveal lower mean scores in older (e.g., Beck et al., 1996; Stanley et al., 1996a,b, 2003; Wetherell et al., 2003a,b; Mohlman and Gorman, 2005) than younger GAD samples (e.g., Molina and Borkovec, 1994; Behar et al., 2003; Fresco et al., 2002). Older adults may experience additional age-related physiological changes such as blunted reactivity to stress (Whitbourne, 1985; Kogan et al.,1999), decreased neurochemical reactivity (DeBeurs et al., 1999), long-term habituation effects, increased coping abilities, or less anxiety about future goals (Borkovec, 1988), all of which could produce less severe and frequent emotion states (Lawton et al., 1993; Kogan et al., 1999). Not surprisingly, it can be difficult to distinguish late life GAD from subclinical anxiety states, which are believed to be common among the elderly (Wetherell et al., 2003a,b), and many healthy older adults intermittently experience symptoms of GAD (Stanley and Novy, 2000). This is consistent with recent findings on the taxonomy and structure of worry implicating the appropriateness of a dimensional approach (Ruscio and Borkovec, 2001). Therefore, we expect differences between older GAD and control samples to be of generally lesser magnitude than those found in studies conducted with younger samples, even on measures of worry, the hallmark symptom of GAD.
1.3. The present study
The aim of this study was to test hypotheses derived from the avoidance model of GAD (Borkovec, 1994) regarding the relation of worry to PFC and amygdala volumes in patients and age- and sex-matched controls, a sample that was meant to represent the full range of scores on a measure of worry. Such data could be useful in identifying regions of interest (ROIs) and neurobiological targets for possible treatment optimization in older worriers, as well as providing support for popular and useful models of GAD and anxious apprehension.
Brain structure and function may be related due to use-dependent neuroplasticity as well as in cases where degraded structure constrains function. Thus, we used structural MRI to test the hypothesis that regional brain volumes would be related to symptom measures. Because worry involves the same neural areas and cognitive processes in nonclinical samples as in GAD patients (e.g., Hoehn-Saric et al., 2004, 2005), we did not expect substantial differences between the GAD and control groups on brain volumetric ROIs. Rather, consistent with a dimensional perspective, it was expected that scores on a continuous measure of worry would show a positive association with DLPFC and mOFC volumes, with stronger associations being found with left hemisphere ROIs due to the verbal component of worry. We also expected to find negative correlations between worry scores and amygdala volumes. Worry scores were expected to be significant predictors of PFC ROIs in linear regression models including variables related to PFC volume (age, hypertension [htn], whole brain volume [WBV]), and to outperform more general measures of anxiety and depression in these models, given the specific function of worry set forth in contemporary models (e.g., Borkovec, 1994).
2. Methods
2.1. Participants
Participants were 30 adults aged 60 and over (mean=67.87, S.D. =5.39, range=60–77), 50% female, mostly Caucasian (90%), recruited from an urban community through media ads and community outreach, and assessed in an outpatient hospital clinic setting. Fifteen met criteria for a primary diagnosis of GAD according to the Structured Clinical Interview Diagnostic for DSM-IV (SCID; First et al., 1995), and 15 did not meet criteria for any current psychiatric disorder.
2.2. Measures
The SCID (First et al., 1995) was administered by trained Master’s-or Ph.D.-level assessors who completed 6 months of training on administering the interview. Seventy-five percent of patient SCIDs (n=10) were observed by a supervisor to ensure reliability (J.M.). Additionally, a random sample of audiotaped SCID interviews (n=12) was then independently rated by a blind assessor to estimate interrater reliability. The kappa coefficient for the diagnosis of GAD was 0.92; however, all participants passed a preliminary phone screen that included a checklist of GAD symptoms, so this estimate might be somewhat inflated.
Rates of comorbidity were moderate; 57% of those in the GAD group also met criteria for at least one additional disorder, most often dysthymia (20%), specific phobia (20%), social phobia (20%), or panic disorder (14%). Because of the known differences in the neurobiology of GAD versus late life major depression (i.e., reduced PFC volumes in depression; Krishnan et al., 1992; Kumar et al., 2000; Taylor et al., 2004), we excluded any participant who reported major depressive episodes at the time of the interview (n=1). The mean age of GAD onset among patients was 43.50 (S.D. = 24.75; range =7–76), with 50% (n=7) reporting emergence of GAD at or prior to age 50.
The sample was generally in good health, as determined through self-reported medical history and a review of medical records. Most were retired, had at least a high school diploma, and were married or divorced. Participants were required to be right-handed, have no metal implanted in the body, have intact basic cognitive skills (Mini-Mental State Examination score >25; Folstein et al., 1975), read and write in English, have no history of suicidality in the previous year, have never experienced psychotic symptoms, have no history of stroke or heart disease, and be free of anxiolytic and antidepressant medications at the time of and at least 1 year before the interview.
Participants completed the Penn State Worry Questionnaire (PSWQ; Meyer et al., 1990), Beck Depression Inventory (BDI; Beck and Steer, 1990), and trait scale of the Spielberger State-Trait Anxiety Inventory-Form Y (STAI; Spielberger et al.,1983). All measures have been shown to have sound psychometric properties in older adult samples (e.g., Himmelfarb and Murrell, 1983; Beck et al., 1995; Stanley et al., 1996a, b). Internal consistency coefficients were 0.87, 0.79, and 0.90 for the PSWQ, STAI, and BDI, respectively.
2.3. Procedure
After completing the consent process, SCID, questionnaires, and cognitive tests, each participant underwent a 15-min MRI scan. Because participants were screened for the presence of claustrophobic symptoms, all were able to complete the scan successfully. Structural images were acquired on a Philips Medical full body MRI scanner, a 1.5 T Intera with Gyroscan version 8.1.3 software. The MR brain images were acquired using a quadrature radiofrequency receive-only head coil and inversion recovery prepped T1-weighted radiofrequency pulse sequence covering the brain in transaxial view with a total of 100 1.5-mm slice thickness images. The preparation time and weighting were optimized to give the best distinction for image post-processing reconstruction and brain structure segmentation. The MRI data were exported in DICOM format to a PC for subsequent post-processing analysis using MEASURE software (Barta et al., 1997).
Non-brain tissue was removed and frontal lobe tissue was isolated, using previously described protocols. The prefrontal cortex was divided into lateral and medial subregions of the dorsal and orbital prefrontal cortices using the measurement protocol described by Gur et al. (2000). The frontal lobes were rendered in order to delineate (1) the inferior border of the anterior commissure, (2) the anterior border of the genu of the corpus callosum and (3) the medial posterior border of the transverse orbital sulcus. The amygdala was delineated using the scheme described in Kates et al. (1997). Parcellation was performed by two raters trained to a gold standard (W.K.), and intraclass correlation coefficients ranged from 0.89 to 0.99.
3. Results
3.1. Data analysis plan
Data were checked for deviations from normality and outliers using appropriate diagnostic tests. When adjusted for whole brain volume (WBV, which is known to differ between males and females, and show positive relations to PFC volumes; Goldstein et al., 2001), data from one male in the GAD group on left mOFC (2.89 cm3) met criteria for an outlying value as compared with the GAD group mean (12.63 cm3, S.D.=2.69) and thus was removed for the analysis of this ROI, leaving 14 participants in the GAD group. Due to scheduling problems, one male in the control group completed the SCID interview and MRI but not the questionnaire packet; therefore, the control group was adjusted to n=14 whenever pertinent. We tested for between-group differences based on GAD status, then investigated the relation of worry as a continuous variable to ROIs.
3.2. Participant characteristics
Participants were compared on age, number of medical problems, daily medications, and alcoholic beverages consumed per month with analyses of variance (ANOVA) with alpha set at 0.013 to control for four comparisons. There were no significant differences on any variables. Certain health problems (e.g., hypertension, diabetes, coronary heart disease) are associated with reduced PFC volumes (Kumar et al., 2007; Salerno et al., 1992; Strassburger et al., 1997). Participants with a history of heart disease or stroke were excluded from the current study; however, those with diabetes and hypertension were allowed; thus, these two conditions were examined in the sample. According to Fisher’s exact test, rates of diabetes were equally distributed, reported by a total of four participants; two in the GAD group and two in the control group. Rates of hypertension (htn), which is common in older adults with anxiety disorders (Kim et al., 2000), particularly those who report frequent worry (Kubzansky et al., 1997), were also tested using a chi-square test. The GAD group had a higher proportion (57%) of hypertensive individuals than the control group (20%), χ2 (1, N=28)=2.77, P<0.10, a difference that was marginally significant, indicating the use of htn as a covariate in subsequent analyses. Additional chi-square and Fisher’s exact tests were used to compare the GAD and control groups on sex, marital, educational, ethnic, and occupational status. There were no other differences between the patient and control groups.
3.3. Psychiatric measures, brain volumes
Bonferroni-adjusted univariate ANOVAs with alpha set to 0.017 were conducted on PSWQ, STAI, and BDI scores. The GAD group scored significantly higher than controls on the PSWQ, F(1,26)=17.64, P<0.001, η2=0.40, STAI, F(1,26)=41.97, P<0.001, η2=0.62, and BDI, F(1,26)=5.91, P<.01, η2=0.30. These results did not appreciably change when htn was included as a covariate. Consistent with prior findings, the mean PSWQ score from the GAD group was lower than published means from younger GAD samples (Antony et al., 2001), but similar to those published from other older samples (e.g., Beck et al., 1996; Stanley et al., 2003). Scores on the MMSE did not differ between patients and controls. Descriptive data are shown in Table 1.
Table 1.
Participant characteristics, scores on self-report measures, brain volumes.
| Control (n=15) | GAD (n=15) | ||
|---|---|---|---|
| Age | 67.50 (4.94) | 67.39 (5.42) | |
| Female | 60% | 40% | |
| Hypertension | 20% | 57%† | |
| Alcoholic drinks/month | 13.67 (19.17) | 9.88 (16.22) | |
| MMSEa | 28.93 (1.19) | 27.69 (1.89) | |
| PSWQa | 39.31 (11.55) | 59.38 (7.47)* | |
| STAI-traita | 29.07 (6.34) | 54.61 (14.28)* | |
| BDIa | 5.92 (4.18) | 14.47 (10.72)* | |
| WBV | 1176.12 (85.65) | 1164.33 (102.93) | |
| Amygdala | 3.30 (0.62) | 3.52 (0.74) | |
| Right | 1.76 (0.44) | 1.88 (0.48) | |
| Left | 1.54 (0.31) | 1.64 (0.41) | |
| DLPFC | 41.03 (4.23) | 41.06 (7.82) | |
| Right | 21.20 (2.09) | 21.09 (4.34) | |
| Left | 19.63 (3.45) | 20.04 (3.94) | |
| mOFCb | 22.26 (4.58) | 23.34 (5.50) | |
| Right | 11.07 (2.30) | 11.64 (2.85) | |
| Left | 11.25 (2.40) | 12.03 (2.54) | |
Note.
P<.017;
P<.10.
=control n=14;
=GAD n=14.
MMSE=Mini-Mental State Exam; PSWQ=Penn State Worry Questionnaire; STAI-trait=trait scale of State Trait Anxiety Inventory; BDI=Beck Depression Inventory. WBV=whole brain volume; DLPFC=dorsolateral cortex; mOFC=medial orbital cortex. All brain volumes were measured in cubic centimeters, no cerebrospinal fluid.
Three brain ROIs (DLPFC, mOFC, amygdala) were examined across groups with ANOVAs. Because age, htn, and WBV were significantly or marginally related to PFC ROIs, analyses of covariance were also run (ANCOVAs), with alpha adjusted to 0.017 for three comparisons. The ANOVAs failed to reach significance (all Ps>0.25), indicating that there were no differences in ROIs between the GAD and control groups regardless of inclusion of the covariates. Brain volumes are displayed in Table 1.
Comorbidity in GAD is the rule rather than the exception; however, it is possible that findings could have been influenced by co-occurring psychiatric disorders characterized by limbic overactivity and PFC hypoactivity, which would have obscured between-group effects. To test this possibility in the current small sample, we compared the GAD group according to those with (n=8) and without (n=7) comorbid conditions, and found no differences on any self-report measure or brain ROI (all Ps>0.29). Although we allowed comorbidity, we excluded cases of recent major depression, which is one of the most frequently occurring conditions with GAD (Bruce et al., 2001; Noyes, 2001; Gorwood, 2004). However, inclusion of these patients would have potentially obscured the effects of GAD per se, due to the chances of reduction in PFC volume in depressed older adults (Krishnan et al., 1992; Kumar et al., 2000; Taylor et al., 2004).
3.4. Correlation and regression analyses
Partial correlations controlling for age, htn, and WBV were run between psychiatric measures and brain volumes in the full sample, as shown in Table 2. Significant relations were found between PSWQ and right and left mOFC. These relations were then tested within the control and GAD groups. As shown in Tables 3 (GAD group) and 4 (control group), after alpha was adjusted to 0.005 to account for 10 correlations and after controlling for age, htn, and WBV, scores on the PSWQ were positively related to left mOFC, partial r=0.872, P<0.005 in the GAD group, but not in controls. No other correlations between ROIs and clinical measures reached significance.
Table 2.
Partial correlations with age, htn, and wbv controlled below the diagonal (n=28).
| Lamyg | Ramyg | Ldlpfc | Rdlpfc | LmOFC | RmOFC | PSWQ | BDI | STAI | |
|---|---|---|---|---|---|---|---|---|---|
| Lamyg | – | 0.405 | 0.014 | −0.304 | −0.074 | 0.202 | 0.093 | −0.146 | −0.087 |
| Ramyg | – | 0.071 | 0.098 | 0.069 | 0.186 | 0.186 | 0.037 | 0.016 | |
| Ldlpfc | – | 0.071 | −0.327 | −0.200 | −0.056 | 0.044 | −0.050 | ||
| Rdlpfc | – | 0.186 | −0.040 | 0.135 | 0.235 | 0.052 | |||
| LmOFCa | – | −0.872** | 0.582** | 0.428 | 0.488 | ||||
| RmOFCa | – | 0.546** | 0.488 | 0.403 | |||||
| PSWQ | – | 0.547** | 0.551** | ||||||
| BDI | – | 0.598** |
Note.
P<0.005;
P<0.001.
=n=28 due to one outlying value on mOFC volumes; amyg = amygdala; dlpfc = dorsolateral cortex; mOFC = medial orbital cortex; wbv=whole brain volume; PSWQ=Penn State Worry Questionnaire; BDI=Beck Depression Inventory; STAI=Trait Scale, State Trait Anxiety Inventory. All brain volumes were measured in cubic centimeters, without cerebrospinal fluid.
Table 3.
Within group partial correlations between brain ROIs (left, right) and clinical measures, controlling for wbv, age, and htn; GAD group.
| Lamyg | Ramyg | Ldlpfc | Rdlpfc | LmOFC | RmOFC | PSWQ | BDI | STAI | Onset | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lamyg | – | 0.388 | 0.199 | −0.476 | −0.099 | 0.234 | −0.471 | −0.371 | −0.261 | −0.179 |
| Ramyg | – | 0.120 | −0.143 | 0.119 | 0.271 | −0.040 | −0.073 | −0.159 | 0.309 | |
| Ldlpfc | – | 0.592 | −0.264 | −0.204 | −0.190 | 0.084 | −0.007 | 0.371 | ||
| Rdlpfc | – | 0.206 | 0.058 | 0.467 | 0.409 | 0.122 | 0.348 | |||
| LmOFC | – | 0.879** | 0.872** | 0.459 | 0.513 | −0.022 | ||||
| RmOFC | – | 0.661 | 0.195 | 0.373 | 0.168 | |||||
| PSWQ | – | 0.579 | 0.538 | 0.199 | ||||||
| BDI | – | 0.427 | 0.090 | |||||||
| STAI | – | 0.159 |
Note.
P<0.001.
amyg=amygdala; dlpfc=dorsolateral cortex; mOFC=medial orbital cortex; wbv=whole brain volume; PSWQ=Penn State Worry Questionnaire; BDI=Beck Depression Inventory; STAI=Trait Scale, State Trait Anxiety Inventory; Onset=age of GAD onset. All brain volumes were measured in cubic centimeters, without cerebrospinal fluid.
Table 4.
Within group partial correlations between brain ROIs (left, right) and clinical measures, controlling for wbv, age, and htn; control group.
| Lamyg | Ramyg | Ldlpfc | Rdlpfc | LmOFC | RmOFC | PSWQ | BDI | STAI | |
|---|---|---|---|---|---|---|---|---|---|
| Lamyg | – | 0.431 | 0.176 | 0.073 | −0.295 | −0.092 | 0.248 | −0.109 | −0.187 |
| Ramyg | – | 0.319 | 0.400 | −0.396 | −0.295 | 0.174 | −0.011 | −0.302 | |
| Ldlpfc | – | 0.462 | −0.241 | −0.008 | 0.532 | −0.001 | 0.224 | ||
| Rdlpfc | – | −0.072 | −0.214 | −0.184 | 0.021 | −0.016 | |||
| LmOFC | – | 0.839** | 0.269 | 0.463 | 0.592 | ||||
| RmOFC | – | 0.488 | 0.337 | 0.681 | |||||
| PSWQ | – | 0.461 | 0.497 | ||||||
| BDI | – | 0.550 |
Note.
P<0.001.
amyg=amygdala; dlpfc=dorsolateral cortex; mOFC=medial orbital cortex; wbv=whole brain volume; PSWQ=Penn State Worry Questionnaire; BDI=Beck Depression Inventory; STAI=Trait Scale, State Trait Anxiety Inventory; All brain volumes were measured in cubic centimeters, without cerebrospinal fluid.
Hierarchical linear regression models were tested to further investigate the relationship between scores on PSWQ, other psychiatric measures, and PFC and amygdala volumes. PFC ROIs (DLPFC, mOFC) were modeled with age, htn, and WBV entered on the first step, GAD status (coded ‘0’ for the absence and ‘1’ for the presence of GAD) on the second step, PSWQ scores on the third step, and the interaction term (PSWQ×GAD status) on the fourth step. Due to the relatively small sample and consequent threats to model reliability, ROIs were kept unified rather than lateralized in an attempt to minimize the number of predictors tested in each model.
The model of DLPFC (F(5,24)=4.259, P<0.008) was significant, adjusted r2=0.40, however WBV was the only significant predictor, t (28)=4.03, P=0.001. The model of mOFC was significant (F(5,23)=6.643, P=0.001), adjusted r2=0.54. WBV (t(27)=5.09, P=0.001), htn (t(27)=−2.51, P=0.05), and PSWQ (t(27)=3.60, P=0.002) were significant predictors. The model of amygdala (F(5,24)=1.906, P<0.40) was not significant. Entering the psychiatric measures on the first step of each model did not change the set of predictors identified for each ROI as described above.
Alternative models of DLPFC and mOFC including the BDI and STAI rather than the PSWQ were also tested. Regardless of order of entry of the set of variables (BDI or STAI, htn, WBV, GAD status), neither measure emerged a significant predictor of any ROI, with full model r2’s ranging from 0.26 to 0.41. When the model of mOFC was run with WBV and htn entered on the first step, BDI or STAI on the second, GAD status on the third, and PSWQ on the fourth, PSWQ was the only psychiatric measure to emerge as a significant predictor. Additional details of regression models appear in Table 5 and Fig. 1.
Table 5.
Summary of linear regression models predicting brain volumes (n=28).
| Predictor | B | SE B | β | Δ adj. r2 | F Δ adj. r2 | |
|---|---|---|---|---|---|---|
| DLPFC | ||||||
| Step 1 | WBV* | 0.035 | 0.007 | 0.709 | 0.490 | 11.529*** |
| age | −0.097 | 0.218 | −0.087 | |||
| htn | 1.359 | 2.158 | 0.109 | |||
| Step 2 | GAD | 0.998 | 2.451 | 0.085 | 0.002 | 0.085 |
| Step 3 | PSWQ | −0.032 | 0.108 | −0.065 | 0.004 | 0.090 |
| Step 4 | GAD×PSWQ | −0.106 | 0.247 | −0.491 | 0.005 | 0.183 |
| mOFC | ||||||
| Step 1 | WBV* | 0.033 | 0.008 | 0.628 | 0.293 | 6.328** |
| age | 0.197 | 0.155 | 0.221 | |||
| htn | −2.296 | 1.647 | −0.245 | |||
| Step 2 | GAD | −0.899 | 1.871 | −0.097 | 0.043 | 0.954 |
| Step 3 | PSWQ* | 0.205 | 0.082 | 0.546 | 0.145 | 12.932*** |
| Step 4 | GAD×PSWQ | 0.094 | 0.156 | 0.607 | 0.007 | 0.362 |
Note.
P<0.05;
P<0.01;
P<0.005;
WBV=whole brain volume; htn=hypertension; DLPFC=dorsolateral cortex; mOFC=medial orbital cortex. Volumes measured in cubic centimeters, no cerebrospinal fluid.
Fig. 1.
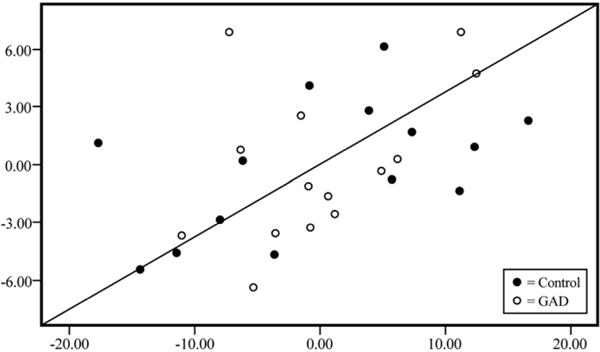
Standardized partial regression plot of medial orbital cortex volumes (y-axis) and PSWQ scores (x-axis) in control and GAD groups, controlling for age, htn, and WBV.
4. Discussion
Adding to limited research on the neurobiology of worry among older adults, this investigation provides support for the hypothesized link between PFC volume and worry, but not between amygdala volume and worry. As predicted on the basis of contemporary theory (Borkovec, 1994), the mOFC, a PFC region of interest, was significantly and positively related to worry scores in the GAD group, but not to more general measures of anxiety, depression, or illness chronicity (as measured by age of GAD onset). Consistent with earlier studies of anxious apprehension showing left hemisphere asymmetry at rest (e.g., Heller et al., 1997) and during worry (Carter et al., 1986; Hofmann et al., 2005), we also found a stronger relation of left than right mOFC to worry scores in the GAD group.
Amygdala volume was not associated with any PFC regions or worry, standing in contrast to the assertion by Hoehn-Saric et al. (2005) that the neural substrate of worry is likely to include the mOFC-amygdalar connection. The circuit connecting DLPFC to amygdala is indirect, and both regions may exert influence on each other via mutual projections to the orbital PFC and via thalamic and striatal circuits (Hariri et al., 2003). Alternatively, age-related changes in symptom presentation, including decreased severity of worry in older versus younger adults (Babcock et al., 2000; Skarborn and Nicki, 2000), could potentially obscure associations between regions that might have been present earlier in life. Unlike areas of the PFC, which are known to show structural changes with age (West, 1996), the amygdala is largely preserved, even in older individuals (Good et al., 2001; Grieve et al., 2005).
Interestingly, no relation was found between the diagnosis of GAD and any of the variables of interest in regression analyses, indicating that it was worry specifically, rather than the more general GAD syndrome, that drove the current results. When participants were dichotomously categorized on the basis of the full GAD symptom profile (insomnia, feeling restless or ‘keyed up,’ difficulty concentrating, mind going blank, muscle tension, irritability, and fatigue in addition to frequent and uncontrollable worry), the prediction of mOFC volume did not improve. The present findings are then consistent with the increasingly popular notion that worry in GAD is likely to be more quantitatively than qualitatively different from normal worry (Ruscio, 2002; Borkovec et al., 2004; Ruscio et al., 2005), and indicate a continued focus on the specific process of worry as a phenomenon of clinical interest.
The mOFC is part of the orbitofrontal cortex (OFC), an area that is known to play a role in inhibiting or terminating inappropriate, and initiating appropriate, responses according to changing context (Beer et al., 2006; Evans et al., 2004). Elliot et al. (2000) posit that the role of the OFC is to inform choices made under uncertain or unpredictable circumstances. Indeed, in the clinical setting the subjective experience of worry is often described as a ‘holding pattern.’ It interferes with task initiation and subsequently leads to procrastination and mounting anxiety, which is perhaps an example of an adaptive behavioral modification system gone awry. The OFC also contributes to perseverative and obsessional thoughts and behaviors. Lesions to the OFC in rats lead to impaired reversal learning (i.e., impairment in learning to return to a strategy that was previously unrewarded) despite intact set switching abilities; McAlonan and Brown, 2003; Schoenbaum et al., 2003), suggesting that high worriers might be particularly prone to engage in repetitive nonreinforced thoughts and behaviors when attempting to solve problems. In other words, they might be likely to return to a strategy that was previously ineffective or unsuccessful, when contingencies have remained the same. This maladaptive pattern (which would not be expected in low worriers) in some ways parallels the unproductive nature of pathological worry and related behaviors, such as repeated checking or reassurance-seeking under unchanging conditions.
The finding of a link between mOFC and worry suggests support for a limited resource model; interventions for worry and GAD should include worry reduction techniques imparted early in treatment (e.g., stimulus control, relaxation, acceptance and mindfulness), which would ostensibly increase the availability of frontal resources such as the mOFC, followed by training in alternative decision making and emotion regulation strategies (e.g., ruling out nonreinforced behaviors, conducting cost–benefit analyses, resolution of perceived uncertainty or unpredictability, accepting unknown aspects of situations). These are adaptive skills that could supplant worry as a strategy for managing anxiety and other negative emotions, but are also likely to involve (and perhaps compete for) activity in areas such as the mOFC.
The study had a range of limitations. The small sample might have limited statistical power to detect all but large effects. All GAD patients were participating in a clinical trial of cognitive behavior therapy and might have had a different clinical profile than those who do not seek treatment. Additionally, the high demand on participants (MRI, neuropsychological testing) might have biased our sample toward those older adults who are high functioning and mobile. It is unclear what role age-related neural atrophy could have played in the older sample. Although there is support for the notion that orbitofrontal regions contribute to anxiety even in pediatric samples (Vasa et al., 2004), the inclusion of a younger GAD group would have eased interpretation regarding age-specific effects. The correlational design of this study precludes interpretation of directionality. It is possible that interindividual differences in regional PFC volumes in older adults could impact the availability of prefrontal resources for symptomatic behaviors such as worry. Thus, individuals with larger PFC volumes may be more vulnerable to developing chronic worry habits; conversely, habitual behaviors such as worry may gradually alter brain structure as a result of use-dependent neuroplasticity. Or, an unidentified third variable could contribute to or cause both worry and PFC volume changes, such as a temperamental disposition for cognitive overcontrol. Additionally, the lack of functional brain measures limits inference about brain activity, a more sensitive measure. Clearly, more research on anxiety, worry, and brain integrity in the OFC in participants of all ages is needed.
This is the first study to examine brain volumes and worry in older adults with and without GAD. Findings were somewhat consistent with those from studies of younger analog and patient samples, and suggest a potentially unique neurobiology of worry that diverges from other types of anxiety states (i.e., those characterized by high somatic arousal and PFC hypoactivity; Kent and Rauch, 2004; Lorberbaum et al., 2004). GAD is considered the most refractory anxiety disorder, poses risk for the acquisition of additional psychiatric and medical conditions, and is prevalent among older adults. Additional studies are needed to increase knowledge of late life anxiety and worry, with the overarching goal of reducing the mental health care burden for upcoming cohorts of older adults.
Acknowledgments
This research was supported in part by a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Grant (YI2002) awarded to the first author. We gratefully acknowledge Gwen Tillapaugh-Fay for assistance with MRI scans, and Nuria Abdul-Sabur and Jena Conchelos for MRI data processing.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders—Text Revision. 4th. APA; Washington, DC: 2000. [Google Scholar]
- Antony MM, Orsillo SM, Roemer L. Practitioner’s Guide to Empirically Based Measures of Anxiety. Kluwer Academic/Plenum Publishers; New York: 2001. [Google Scholar]
- Babcock RL, Laguna LB, Laguna KD, Urusky DA. Age differences in the experience of worry. Journal of Mental Health and Aging. 2000;6:227–234. [Google Scholar]
- Barger SD, Sydeman SJ. Does generalized anxiety disorder predict coronary heart disease risk factors independently of major depressive disorder? Behaviour Research & Therapy. 2005;88:87–91. doi: 10.1016/j.jad.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Disorders of emotion. Psychological Inquiry. 1991;2:58–71. [Google Scholar]
- Barta PE, Dhingra L, Royall R, Schwartz E. Improving stereological estimates for the volume of structures identified in three-dimensional arrays of spatial data. Journal of Neuroscience Methods. 1997;75:111–118. doi: 10.1016/s0165-0270(97)00049-6. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. the Psychological Corporation; San Antonio, TX: 1990. [Google Scholar]
- Beck JG, Stanley MA, Zebb BJ. Psychometric properties of the Penn State Worry Questionnaire in older adults. Journal of Clinical Geropsychology. 1995;1:33–42. [Google Scholar]
- Beck JG, Stanley MA, Zebb BJ. Characteristics of generalized anxiety disorder in older adults: a descriptive study. Behaviour Research & Therapy. 1996;34:225–234. doi: 10.1016/0005-7967(95)00064-x. [DOI] [PubMed] [Google Scholar]
- Beekman ATF, Bremmer MA, Deeg DJH, VanBalkom AJLM, Smit JH, DeBeurs E. Anxiety disorders in later life: a report from the longitudinal aging study Amsterdam. International Journal of Geriatric Psychiatry. 1998;13:717–726. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Beer JS, Knight RT, D’Esposito M. Controlling the integration of emotion and cognition: the role of frontal cortex in distinguishing helpful from hurtful emotional information. Psychological Science. 2006;17:448–453. doi: 10.1111/j.1467-9280.2006.01726.x. [DOI] [PubMed] [Google Scholar]
- Behar E, Alcaine O, Zuellig AR, Borkovec TD. Screening for generalized anxiety disorder using the Penn State Worry Questionnaire: a receiver operating characteristics analysis. Journal of Behavior Therapy and Experimental Psychiatry. 2003;34:25–43. doi: 10.1016/s0005-7916(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Blazer DG. Depression in late life: review and commentary. Journal of Gerontology: Medical Sciences. 2003;56A:249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- Borkovec TD. Comments on “Worry as a phenomenon related to the elderly”. Behavior Therapy. 1988;19:381–383. [Google Scholar]
- Borkovec TD. The nature, functions, and origins of worry. In: Davey G, Tallis F, editors. Worrying: Perspectives on Theory, Assessment, and Treatment. Wiley & Sons; Sussex, England: 1994. pp. 5–33. [Google Scholar]
- Borkovec TD, Robinson E, Pruzinsky T, DePree JA. Preliminary exploration of worry: some characteristics and processes. Behaviour Research and Therapy. 1983;21:9–16. doi: 10.1016/0005-7967(83)90121-3. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Alcaine OM, Behar E. Avoidance theory of worry and generalized anxiety disorder. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized Anxiety Disorder. The Guilford Press; New York: 2004. pp. 77–108. [Google Scholar]
- Brown TA, Barlow DH, Liebowitz MR. The empirical basis of generalized anxiety disorder. American Journal of Psychiatry. 1994;151:1272–1280. doi: 10.1176/ajp.151.9.1272. [DOI] [PubMed] [Google Scholar]
- Bruce SE, Machan JT, Dyck I, Keller MB. Infrequency of “pure” GAD: impact of psychiatric comorbidity on clinical course. Depression and Anxiety. 2001;14:219–225. doi: 10.1002/da.1070. [DOI] [PubMed] [Google Scholar]
- Carter WR, Johnson MC, Borkovec TD. Worry: an electrocortical analysis. Advances in Behaviour Research and Therapy. 1986;8:193–204. [Google Scholar]
- DeBeurs E, Beekman ATF, van Balkom AJLM, Deeg DJH, van Dyck R, van Tilburg W. Consequences of anxiety in older persons: its effect on disability, well being, and use of health services. Psychological Medicine. 1999;29:583–593. doi: 10.1017/s0033291799008351. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Evans DW, Lewis MD, Iobst E. The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and obsessive–compulsive disorder. Brain and Cognition. 2004;55:220–234. doi: 10.1016/S0278-2626(03)00274-4. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders. (SCID-I, version 2.0, October 1995, Final Version) Biometrics Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Flint A. Epidemiology and comorbidity of anxiety disorders in the elderly. American Journal of Psychiatry. 1994;150:640–649. doi: 10.1176/ajp.151.5.640. [DOI] [PubMed] [Google Scholar]
- Flint A. Anxiety disorders in late life. Canadian Family Physician. 1999;45:2672–2679. [PMC free article] [PubMed] [Google Scholar]
- Folstein SE, Folstein MF, McHugh PR. “Mini-mental state”: a practical method for grading the mental state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fresco DM, Mennin DS, Heimberg RG, Turk CL. Confirmatory factor analysis of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 2002;28:487–495. doi: 10.1016/s0005-7967(00)00113-3. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience. 1981;6:177–187. doi: 10.1016/0306-4522(81)90053-1. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Cainess VS. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11I:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Fristen KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gorwood P. Generalized anxiety disorder and major depressive disorder comorbidity: an example of genetic pleiotropy? European Psychiatry. 2004;19:27–33. doi: 10.1016/j.eurpsy.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Gray M, Kemp AH, Silberstein RB, Nathan PJ. Cortical neurophysiology of anticipatory anxiety: an investigation utilizing steady state probe topography (SSPT) NeuroImage. 2003;20:975–986. doi: 10.1016/S1053-8119(03)00401-4. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Human Brain Mapping. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporolimbic and frontal brain volumes of healthy adults. Cerebral Cortex. 2000;12:998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Archives of Neurology. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay A, Tessitore F, Fera D, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: a review and a new view. In: Bower G, editor. The Psychology of Learning and Motivation: Advances in Research and Theory. Vol. 22. Academic Press; San Diego: 1988. pp. 193–225. [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106:376–385. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- Himmelfarb S, Murrell SA. Reliability and validity of five mental health scales in older persons. Journal of Gerontology. 1983;38:333–339. doi: 10.1093/geronj/38.3.333. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod DR, Zimmerli W. Somatic manifestations in women with generalized anxiety disorder: psychophysiological responses to psychological stress. Archives of General Psychiatry. 1989;46:1113–1119. doi: 10.1001/archpsyc.1989.01810120055009. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, Schlund MW, Wong SHY. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Research: Neuroimaging. 2004;131:11–21. doi: 10.1016/j.pscychresns.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, Lee JS, McLeod DR, Wong DF. Effect of worry on regional cerebral blood flow in nonanxious subjects. Psychiatry Research: Neuroimaging. 2005;140:259–269. doi: 10.1016/j.pscychresns.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Moscovitch DA, Litz BT, Kim HJ, Davis LL, Pizzagalli D. The worried mind: autonomic and prefrontal activation during worrying. Emotion. 2005;5:464–475. doi: 10.1037/1528-3542.5.4.464. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Alexopoulos GS, Bartels SJ, Cummings JL, Gallo JJ, Gottlieb GL, Halpain MC, Palmer BW, Patterson TL, Reynolds CF, Lebowitz BD. Consensus statement on the upcoming crisis in geriatric mental health. Archives of General Psychiatry. 1999;56:848–853. doi: 10.1001/archpsyc.56.9.848. [DOI] [PubMed] [Google Scholar]
- Kates W, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Research: Neuroimaging. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Kennedy BL, Schwab JJ. Utilization of medical specialists by anxiety disorder patients. Psychosomatics. 1997;38:109–112. doi: 10.1016/S0033-3182(97)71478-6. [DOI] [PubMed] [Google Scholar]
- Kent JM, Rauch SL. Neuroimaging studies of anxiety disorders. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. 2nd. Oxford University Press, Inc; New York: 2004. pp. 639–660. [Google Scholar]
- Kessler RC, DuPont RL, Berglund P, Wittchen H. Impairment in pure and comorbid generalized anxiety disorder and major depression at 12 months in two national surveys. American Journal of Psychiatry. 1999;156:1915–1923. doi: 10.1176/ajp.156.12.1915. [DOI] [PubMed] [Google Scholar]
- Kim HF, Kunik ME, Molinari V, Hillman S, Lalani S, Orengo CA, et al. Functional impairment in COPD patients: the impact of anxiety and depression. Psychosomatics. 2000;41:465–471. doi: 10.1176/appi.psy.41.6.465. [DOI] [PubMed] [Google Scholar]
- Kinsella K, Velkoff VA. US Census Bureau, Series 95/0-1, An Aging World. US Government Printing Office; Washington DC: 2001. [Google Scholar]
- Kogan JN, Edelstein BA, McKee DR. Assessment of anxiety in older adults: current status. Journal of Anxiety Disorders. 1999;14:109–132. doi: 10.1016/s0887-6185(99)00044-4. [DOI] [PubMed] [Google Scholar]
- Krishnan KRR, McDonald WM, Doraiswamy PM, Tupler LA, Husain M, Boyko OB. Neuroanatomical substrates of depression in the elderly. European Archives of Psychiatry and Clinical Neuroscience. 1992;243:41–46. doi: 10.1007/BF02191522. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Kawachi I, Spiro A, Weiss ST, Vokonas PS, Sparrow D. Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the normative aging study. Circulation. 1997;95:818–824. doi: 10.1161/01.cir.95.4.818. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bilker W, Zhisong J, Udupa J. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late life major depression. Neuropsychopharmacology. 2000;22:264–274. doi: 10.1016/S0893-133X(99)00124-4. [DOI] [PubMed] [Google Scholar]
- Kumar A, Haroon E, Darwin C, Pham D, Ajilore O, Rodriguez G, et al. Gray matter prefrontal changes in type 2 diabetes detected using MRI. Journal of Magnetic Resonance Imaging. 2007;27:14–19. doi: 10.1002/jmri.21224. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Kleban MH, Dean J. Affect and age: cross sectional comparisons of structure and prevalence. Psychology & Aging. 1993;8:165–175. doi: 10.1037//0882-7974.8.2.165. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Mamner MB. Neural correlates of speech anticipatory anxiety in generalized social phobia. NeuroReport. 2004;15:2702–2705. [PubMed] [Google Scholar]
- MacPherson SE, Phillips LH, Della Sala S. Age, executive function, and social decision making: a dorsolateral prefrontal theory of cognitive aging. Psychology & Aging. 2002;17:598–609. [PubMed] [Google Scholar]
- Mathew SJ, Steinbugler M, Smith ELP. Neurochemistry of generalized anxiety disorder. In: Kinrys G, Renshaw PF, editors. Understanding Anxiety: Its Neurobiological Basis and Treatment. Taylor & Francis; New York: in press. [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Meyer T, Miller ML, Metzger RL, Borkovec TD. Development and validity of the Penn State Worry Scale. Behaviour Research & Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mitte K. Meta-analysis of cognitive behavioral treatments for generalized anxiety disorder: a comparison with pharmacotherapy. Psychological Bulletin. 2005;131:785–795. doi: 10.1037/0033-2909.131.5.785. [DOI] [PubMed] [Google Scholar]
- Mogotsi M, Kaminer D, Stein DJ. Quality of life in the anxiety disorders. Harvard Review of Psychiatry. 2000;8:273–282. [PubMed] [Google Scholar]
- Mohlman J. Psychosocial treatment of late life generalized anxiety disorder: current status and future directions. Clinical Psychology Review. 2004;24:149–169. doi: 10.1016/j.cpr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Mohlman J, Gorman JM. The role of executive functioning in CBT: a pilot study with anxious older adults. Behaviour Research and Therapy. 2005;43:447–465. doi: 10.1016/j.brat.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Molina S, Borkovec TD. The Penn State Worry Questionnaire: psychometric properties and associated characteristics. In: Davey GCL, Tallis F, editors. Worrying: Perspectives on Theory, Assessment and Treatment. Wiley; New York: 1994. pp. 265–283. [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–637. [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Miller GA. Anxiety, stress, and cortical brain function. In: Borod JC, editor. The Neuropsychology of Emotion. Oxford University Press; New York: 2000. pp. 298–319. [Google Scholar]
- Noyes R. Comorbidity in generalized anxiety disorder. Psychiatric Clinics of North America. 2001;24:41–55. doi: 10.1016/s0193-953x(05)70205-7. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Ruscio AM. Delimiting the boundaries of generalized anxiety disorder. Journal of Anxiety Disorders. 2002;16:377–400. doi: 10.1016/s0887-6185(02)00130-5. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Borkovec TD. A taxometric investigation of the latent structure of worry. Journal of Abnormal Psychology. 2001;110:413–422. doi: 10.1037//0021-843x.110.3.413. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Lane M, Roy-Byrne P, Stang PE, Stein DJ, Wittchen HU. Should excessive worry be required for a diagnosis of generalized anxiety disorder? Results from the US National Comorbidity Study. Psychological Medicine. 2005;35:1–12. doi: 10.1017/S0033291705005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno JA, Murphy DG, Horwitz B, DeCarli C, Haxby JV, Rapoport SI. Brain atrophy in hypertension. A volumetric magnetic resonance imaging study Hypertension. 1992;20:340–348. doi: 10.1161/01.hyp.20.3.340. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discrimination and reversals. Learning and Memory. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Mohlman J, Gorman JM. Neurobiology of generalized anxiety disorder. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized Anxiety Disorder: Advances in Research and Practice. Guilford; New York: 2004. pp. 187–216. [Google Scholar]
- Skarborn M, Nicki R. Worry in pre- and post-retirement persons. International Journal of Aging and Human Development. 2000;50:61–71. doi: 10.2190/CFQ1-7HG3-9APX-1NMA. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, Vagg PR, Jacobs GA. State-Trait Anxiety Inventory: Test Manual. Consulting Psychologists; Palo Alto, CA: 1983. [Google Scholar]
- Stanley MA, Novy DM. Cognitive behavior therapy for generalized anxiety disorder in late life. Journal of Anxiety Disorders. 2000;14:191–207. doi: 10.1016/s0887-6185(99)00048-1. [DOI] [PubMed] [Google Scholar]
- Stanley MA, Beck JG, Glassco JD. Treatment of generalized anxiety in older adults: a preliminary comparison of cognitive-behavioral and supportive approaches. Behavior Therapy. 1996a;27:565–581. [Google Scholar]
- Stanley MA, Beck JG, Zebb BJ. Characteristics of generalized anxiety disorder in older adults: a descriptive study. Behaviour Research & Therapy. 1996b;34:225–234. doi: 10.1016/0005-7967(95)00064-x. [DOI] [PubMed] [Google Scholar]
- Stanley MA, Beck JG, Novy DM, Averill PM, Swann AC, Diefenbach GJ, Hopko D. Cognitive behavioral treatment of late-life generalized anxiety disorder. Journal of Consulting & Clinical Psychology. 2003;71:309–319. doi: 10.1037/0022-006x.71.2.309. [DOI] [PubMed] [Google Scholar]
- Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ. Interactive effects of age and hypertension on volumes of brain structures. Stroke. 1997;28:1410–1417. doi: 10.1161/01.str.28.7.1410. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. American Journal of Psychiatry. 2004;617:1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanzarigita E, Van Boxtel MPJ, Evans AC, Jolles J. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel based morphometry. Neuro-Image. 2002;17:657–669. [PubMed] [Google Scholar]
- Vasa RA, Gradod M, Slomine B, Herskovits EH, Thompson RE, Salorio C, et al. Neuroimaging correlates of anxiety after pediatric traumatic brain injury. Biological Psychiatry. 2004;55:208–216. doi: 10.1016/s0006-3223(03)00708-x. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Gatz M, Craske MG. Treatment of generalized anxiety disorder in older adults. Journal of Consulting & Clinical Psychology. 2003a;71:31–40. doi: 10.1037//0022-006x.71.1.31. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, LeRoux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychology & Aging. 2003b;18:622–627. doi: 10.1037/0882-7974.18.3.622. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Whitbourne S. The Aging Body: Physiological Changes and Psychological Consequences. Springer Verlag; New York: 1985. [Google Scholar]


