Abstract
Central nervous system (CNS) infections continue to be an important cause of morbidity and mortality. Microbial invasion and traversal of the blood–brain barrier is a prerequisite for CNS infections. Pathogens can cross the blood–brain barrier transcellularly, paracellularly and/or in infected phagocytes (the so-called Trojan-horse mechanism). Consequently, pathogens can cause blood–brain barrier dysfunction, including increased permeability, pleocytosis and encephalopathy. A more complete understanding of the microbial–host interactions that are involved in microbial traversal of the blood–brain barrier and the associated barrier dysfunction should help to develop new strategies to prevent CNS infections.
Central nervous system (CNS) infections continue to be an important cause of morbidity and mortality throughout the world. For example, bacterial meningitis is one of the top ten causes of infection-related deaths worldwide1. Morbidity and mortality rates vary, however, depending on the age and location of the patient and the causative organism. Patient groups who are at risk of high rates of morbidity and mortality include newborns, the elderly and those living in developing countries, and the infections that have higher rates of morbidity and mortality are those caused by Gram-negative bacilli and Streptococcus pneumoniae2–5. A major contributing factor to the lack of preventive measures against CNS infections is our incomplete understanding of their pathogenesis6,7. Almost all microorganisms that are pathogenic to humans have the potential to penetrate the CNS, but it is still unclear why a comparatively small number of microbial pathogens account for most cases of CNS infection in humans.
This Review summarizes our current understanding of the mechanisms that are involved in traversal of the blood–brain barrier by selected meningitis-causing microorganisms and the associated blood–brain barrier dysfunction.
The blood–brain barrier
The blood–brain barrier (FIG. 1) is a structural and functional barrier that is formed by brain microvascular endothelial cells, astrocytes and pericytes. It maintains the neural microenvironment by regulating the passage of molecules into and out of the brain, and protects the brain from any microorganisms and toxins that are circulating in the blood. Brain microvascular endothelial cells have distinctive features, such as tight junctions and low rates of pinocytosis8–11. Astrocytes and pericytes help maintain the barrier property of brain microvascular endothelial cells, but their contributions to microbial traversal of the barrier remain incompletely understood. For example, the contributions of astrocytes and pericytes to Escherichia coli translocation of the barrier are minimal. By contrast, astrocytes, together with microglial cells, regulate the recruitment of infiltrating haematogenous cells12 and might affect the translocation of some microorganisms. In addition, the antimicrobial activities of astrocytes (such as indoleamine 2,3-dioxygenase activity) and the modulation of signal-transduction pathways in brain endothelial cells by pericytes might affect microbial traversal of the barrier13,14.
Figure 1. The blood–brain barrier.
The blood–brain barrier is formed by brain microvascular endothelial cells, astrocytes and pericytes. It maintains the neural microenvironment by regulating the passage of molecules into and out of the brain, and protects the brain from any microorganisms and toxins that are circulating in the blood.
Studies of microbial traversal of the blood–brain barrier and the associated barrier dysfunction have become feasible because of the availability of an in vitro human brain microvascular endothelial cell (HBMEC) model8–10,15,16 and in vivo models of experimental haematogenous meningitis in infant rats and mice6,7,17–19.
Microbial traversal of the blood–brain barrier
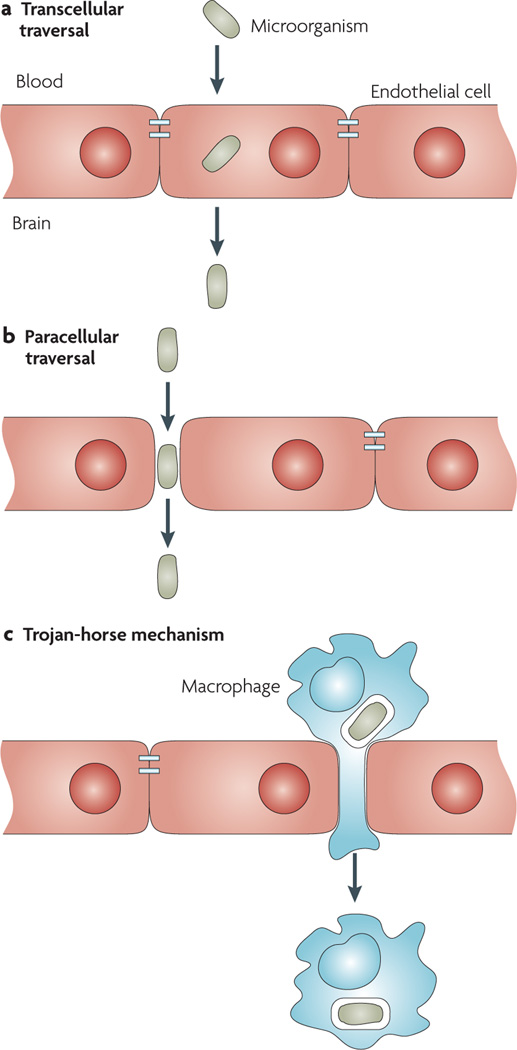
Pathogens can cross the blood–brain barrier transcellularly, paracellularly and/or by the Trojan-horse mechanism (FIG. 2). Transcellular traversal refers to microbial penetration through barrier cells without any evidence of microorganisms between the cells or of intercellular tight-junction disruption. Paracellular traversal is defined as microbial penetration between barrier cells with and/or without evidence of tight-junction disruption. The Trojan-horse mechanism involves microbial penetration of the barrier cells using transmigration within infected phagocytes.
Figure 2. Mechanisms involved in microbial traversal of the blood–brain barrier.
Pathogens can cross the blood–brain barrier transcellularly, paracellularly and/or in infected phagocytes (the Trojan-horse mechanism). a | In transcellular traversal, the pathogens cross the barrier without any evidence of intercellular tight-junction disruption or detection of microorganisms between cells. b | Paracellular traversal involves microbial penetration between barrier cells with and/or without evidence of tight-junction disruption. c | The Trojan-horse mechanism involves microbial penetration of the barrier cells using transmigration within infected phagocytes.
Transcellular traversal of the blood–brain barrier has been demonstrated for most meningitis-causing bacterial pathogens, including E. coli6,7,17, Streptococcus agalactiae6,7,20, S. pneumoniae6,7,21, Neisseria meningitidis6,7,22 and fungal pathogens, such as Candida albicans23 and Cryptococcus neoformans24 (BOX 1). Paracellular penetration of the blood–brain barrier has been suggested for the protozoan Trypanosoma spp.25,26 and Borrelia spp.27,28, although these microorganisms have also been shown to traverse the blood–brain barrier by transcellular penetration25,27,28. The Trojanhorse mechanism has been suggested for Listeria monocytogenes and Mycobacterium tuberculosis29, but transcellular penetration of the blood–brain barrier has also been demonstrated for these organisms6,7,30–32.
Box 1. Cryptococcal invasion and traversal of the blood–brain barrier.
Cryptococcus neoformans is a common cause of culture-proven meningitis in areas of the world where HIV-1 is endemic, and cryptococcal meningitis is known for its high morbidity and mortality rates118. C. neoformans is commonly acquired by inhalation. Extrapulmonary dissemination can lead to infection of the bloodstream and subsequent haematogenous dissemination to target organs, most commonly resulting in meningoencephalitis.
Results from experimental mouse models of cryptococcal meningitis following intravenous inoculation, as well as cases of human cryptococcal meningitis, have indicated that C. neoformans invasion into the brain follows fungaemia, and that cerebral capillaries, not the choroid plexus, are the portals of entry into the brain24,119,120. Cryptococcal invasion of the brain does not require recruitment of host inflammatory cells24,119,120, which eliminates the possibility that C. neoformans traverses the blood–brain barrier using the Trojan-horse mechanism.
A recent study showed that C. neoformans strains can enter and traverse human brain microvascular endothelial cells (HBMECs) without any obvious change in HBMEC integrity. Transmission and scanning electron microscopy has revealed that C. neoformans induces the formation of microvilli-like protrusions to initiate entry into HBMECs, that C. neoformans is found intracellularly in membrane-bound vacuoles and that no free C. neoformans cells are found in the HBMEC cytoplasm24. These findings indicate that C. neoformans uses a transcellular mechanism (FIG. 2a) to enter HBMECs that involves host cell actin cytoskeleton rearrangements.
Several studies have shown that various cryptococcal virulence factors contribute to extrapulmonary dissemination, including the capsular polysaccharide, mannitol, the mating type, melanin, phenotypic switching, phospholipase, prostaglandins and urease118 (TABLE 1). For example, mutants of C. neoformans that lacked laccase were defective in dissemination to extrapulmonary sites in mice following intratracheal inoculation compared with the parental strain, but seemed to be as effective as the parental strain in dissemination to the brain following intravenous inoculation121. Similarly, mutants of C. neoformans that lacked phospholipase B were defective in extrapulmonary dissemination following intratracheal inoculation compared with the parental strain; the ability of these mutants to disseminate to the brain following intravenous inoculation is unknown122,123. A recent report described how cryptococcal urease contributes to dissemination to the central nervous system (CNS) following intratracheal and intravenous inoculation120, but it is unclear how urease contributes to C. neoformans traversal of the blood–brain barrier. The cryptococcal inositolphosphosphingolipid phospholipase C1 gene (isc1) has been shown to be important for controlling the dissemination of C. neoformans to the brain in mice in which macrophages are depleted124. This suggests that macrophage activation is important for preventing fungal dissemination of the Δisc1 mutant to the CNS and the development of C. neoformans meningoencephalitis. Additional studies are therefore needed to elucidate the microbial–host interactions and associated signal-transduction pathways that are involved in C. neoformans traversal of HBMECs and penetration into the CNS.
The availability of in vivo models has enabled us to elucidate some of the mechanisms that are involved in the traversal of the blood–brain barrier by microorganisms. For example, studies have indicated that the primary site of entry into the CNS for circulating E. coli, S. agalactiae and C. neoformans is the cerebral vasculature, not the choroid plexus17,24,33,34. The interaction between E. coli and HBMECs is currently the best-characterized system to study how meningitis-causing pathogens cross the blood–brain barrier6,7. In the following sections, the mechanisms that are involved in traversal of the blood–brain barrier by microorganisms that commonly cause CNS infections in humans are summarized, with an emphasis on the similarities and differences between different organisms.
Bacterial invasion and traversal
Several studies in humans and experimental animals point to a relationship between the magnitude of bacteraemia and the development of meningitis following E. coli, S. agalactiae and S. pneumoniae infections17,33,35–39. Other routes of bacterial entry into the CNS include spread from a contiguous source of infection, such as sinusitis or mastoiditis. For example, S. pneumoniae has been shown to enter the CNS through a non-haematogenous route in experimental animals after intranasal inoculation and otitis media39,40. E. coli penetration into the CNS is similar in infant and adult animals with similar levels of bacteraemia17, which suggests that a threshold level of bacteraemia must be reached before the blood–brain barrier can be breached.
The prevention of bacterial multiplication in the blood could therefore be a potential approach for the prevention of bacterial meningitis. E. coli K1 capsular polysaccharides and O-chain lipopolysaccharides have been recognized as crucial surface structures that contribute to resistance to serum- and polymorphonuclear leukocyte-mediated killing17,41. Such resistance is a factor in the induction of a high degree of bacteraemia, and studies are underway to identify additional microbial structures that contribute to a high level of bacteraemia and that could be used as protective epitopes in vaccine design42. This concept has been successfully applied to Haemophilus influenzae serotype b and S. pneumoniae, and protein-conjugated capsular polysaccharide vaccines for these organisms have almost completely eliminated the meningitis that is caused by the vaccine serotypes. For example, introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) led to a substantial reduction in the incidence of pneumococcal meningitis that was caused by PCV7 serotypes in the target population of children aged less than 5 years43,44. Use of the vaccine also reduced pneumococcal meningitis among unvaccinated populations through reductions in nasopharyngeal colonization and transmission of vaccine-type pneumococci from vaccinated children43,44. One caveat is the apparent increase in the incidence of invasive pneumococcal disease that is caused by non-PCV7 serotypes, such as serotype 19A45. By contrast, it is unclear whether the development of meningococcal (N. meningitidis) meningitis is associated with a threshold level of bacteraemia, although prevention of invasive meningococcal disease has been achieved by the use of meningococcal group C conjugate vaccines46.
Recent studies have shown that a high degree of bacteraemia is necessary, but not sufficient, for the development of meningitis and that microbial binding to, and invasion of, HBMECs is a prerequisite for penetration of the blood–brain barrier in vivo. This was demonstrated using infant rats with experimental haematogenous E. coli meningitis that had been infected with several isogenic mutants of E. coli K1 strain RS218 in which proteins that contribute to HBMEC binding (such as outer-membrane protein A (OmpA)) and invasion (such as IbeA, IbeB, IbeC and cytotoxic necrotizing factor 1 (CNF1)) had been deleted. The deleted strains were significantly less able to traverse the blood–brain barrier than the parental strain despite causing similar levels of bacteraemia (FIG. 3), which indicates that those E. coli proteins that contribute to HBMEC binding and invasion are necessary for crossing the blood–brain barrier in vivo6,7,47–51.
Figure 3. The effect of microbial determinants on Escherichia coli meningitis.
In infant rats with experimental haematogenous E. coli meningitis, isogenic mutants of E. coli K1 strain RS218 in which outer-membrane protein A (OmpA), IbeA, IbeB, IbeC, arylsulfatase-like protein (AslA) and cytotoxic necrotizing factor 1 (CNF1) had been deleted were less able to traverse the blood–brain barrier and cause meningitis than the parental strain, despite causing similar levels of bacteraemia. CFU, colony-forming unit.
Transmission electron microscopy studies have revealed that E. coli K1 and S. agalactiae invade HBMECs. Internalized bacteria are found within membrane-bound vacuoles and transmigrate HBMECs in an enclosed vacuole without intracellular multiplication and without any change in the integrity of HBMEC monolayers20,52. No free bacteria are found in the cytoplasm of HBMECs or between adjacent HBMECs, which confirms that E. coli K1 and S. agalactiae cross the blood–brain barrier by transcellular penetration.
Pathogenic microorganisms use various strategies to invade host cells, such as endothelial cells. Two of the major mechanisms involve rearrangements of the host cell actin cytoskeleton: the zipper mechanism, which involves the formation of cell protrusions that contact the pathogen, and the trigger mechanism, which involves the formation of membrane ruffles around the pathogen53,54. Invasion of HBMECs by meningitis-causing microorganisms also requires rearrangement of the actin cytoskeleton6,7,20,30,52. However, the mechanisms that are involved differ between microorganisms6,7,16,55–57. Once internalized into membrane-bound vacuoles6,20,52,57,58, some bacteria can modulate intracellular trafficking to avoid lysosomal fusion.
In vivo, HBMECs are exposed to shear stress through blood flow, which enhances the barrier properties of a HBMEC monolayer59. N. meningitidis adhesion to brain endothelial cells is inversely affected by shear stress60, which suggests that cerebral microcirculation is an important attribute in N. meningitidis adhesion to the blood–brain barrier.
It is important to note that E. coli K1 binding to, and invasion of, endothelial cells has been demonstrated in cells that are derived from the brain, such as HBMECs, but not in cells of non-brain origin, such as human umbilical vein endothelial cells (HUV ECs), human aortic arterial endothelial cells and human iliac vein endothelial cells61,62, which suggests that the interaction between meningitis-causing E. coli K1 and HBMECs is unique. Similarly, Streptococcus suis (a causative agent of meningitis and septicaemia) has been shown to interact with HBMECs but not HUV ECs63. It is therefore unclear whether the interactions between meningitis-causing microorganisms and non-brain endothelial cells, such as bone-marrow-derived endothelial cells64, can be extrapolated to interactions with HBMECs.
Bacterial ligand–receptor interactions
E. coli
As discussed above, microbial binding to and invasion of HBMECs is a prerequisite for successful penetration into the CNS6,7. Type 1 fimbriae and OmpA are the two major determinants that contribute to E. coli K1 binding to HBMECs65,66. FimH (a type 1 fimbrial adhesin) interacts with CD48 on HBMECs, and the addition of exogenous FimH- or CD48-specific antibodies inhibits E. coli K1 binding to HBMECs67. It has also been shown that the amino-terminal region and surface-exposed loops of OmpA contribute to binding to HBMECs68 and that OmpA interacts with HBMECs through N-acetylglucosamine (GlcNAc) residues in HBMEC glycoproteins, including gp96 (Refs 66,69). Exogenous OmpA and gp96 (also known as GRP94) and anti-gp96 antibodies inhibit E. coli K1 binding to HBMECs, but do not inhibit binding of an OmpA mutant66,68. The ability of the OmpA mutant to penetrate into the CNS was significantly reduced compared with the parental E. coli K1 strain, and GlcNAc-β1,4-GlcNAc oligomers blocked E. coli K1 traversal of the blood–brain barrier in the infant-rat model of experimental haematogenous meningitis51,62. These findings indicate that proteins such as FimH and OmpA contribute to the binding of E. coli to HBMECs through an interaction with their respective HBMEC receptors (TABLE 1). gp96 is an endoplasmic reticulum paralogue of heat shock protein 90 that is not restricted to the endoplasmic reticulum and has been detected at the surface of HBMECs66. gp96 is also a cellular receptor for L. monocytogenes Vip, which is involved in infection of the spleen, liver and brain of mice70; however, gp96 interactions with OmpA and Vip involve different signal-transduction pathways70.
Table 1.
Ligand–receptor interactions in microbial traversal of the blood–brain barrier
| Ligand | Receptor and mechanism | Refs |
|---|---|---|
| Escherichia coli | ||
| FimH | CD48; binding to HBMECs | 65,67 |
| OmpA | gp96; binding to HBMECs | 6,68,69 |
| CNF1 | 37 LRP–67 LR; invasion of HBMECs | 49,78,80 |
| IbeA | 45 kDa protein; invasion of HBMECs | 47,74 |
| Listeria monocytogenes | ||
| InlB | gClq or Met; invasion of HBMECs | 30,89,90 |
| Vip | gp96; penetration into the brain | 70 |
| Neisseria meningitidis | ||
| Opc | Fibronectin; invasion of HBMECs through α5β1 integrin | 22,97,108 |
| Pili | Possibly CD46; penetration into the brain | 97,98 |
| LOS | Unknown; penetration into the brain owing to a high level of bacteraemia | 100 |
| Streptococcus pneumoniae | ||
| Phosphorylcholine | PAF receptor; invasion of HBMECs | 21,93 |
| Haemophilus influenzae serotype b | ||
| Phosphorylcholine | PAF receptor; unknown | 95 |
| Streptococcus agalactiae | ||
| Lmb | Laminin; invasion of HBMECs | 85 |
| FbsA | Fibrinogen; binding to HBMECs | 86 |
| Pili (PilA and PilB) | Unknown; binding to HBMECs | 88 |
| iagA | Unknown; binding to HBMECs through LTA anchoring | 87 |
| Cryptococcus neoformans | ||
| Capsule, laccase, phospholipase B, urease and Isc1 |
Unknown; penetration into the brain | 24, 118–124 |
| Plasmodium falciparum | ||
| Pf-IRBCs or PfEMP1 | Thrombospondin, CD36, ICAM1, gClqR, PECAM or CD31, VCAM1, ELAM1 and chondroitin sulphate A; HBMEC dysfunction | 92,113–116 |
37 LRP–67 LR, 37-kDa laminin receptor precursor–67-kDa laminin receptor; CNF1, cytotoxic necrotizing factor 1; ELAM1, endothelial-leukocyte adhesion molecule 1; gp96, glycoprotein 96; HBMEC, human brain microvascular endothelial cell; iagA, invasion-associated gene A; ICAM1, intercellular adhesion molecule 1; InlB, internalin B; Isc1, inositolphosphosphingolipid phospholipase C1; Lmb, laminin-binding protein; LOS, lipooligosaccharide; LTA, lipoteichoic acid; OmpA, outer-membrane protein A; Pf-IRBC, P. falciparum-infected red blood cell; PfEMP1, P. falciparum erythrocyte membrane protein 1; PAF, platelet-activating factor; PECAM, platelet–endothelial-cell adhesion molecule.
A recent study used an E. coli DNA microarray to compare an OmpA mutant with its parental E. coli K1 strain and revealed that the OmpA mutant expressed significantly fewer fim cluster genes71. This suggested that the decreased binding of the OmpA mutant might be related to lower expression of type 1 fimbriae. Additional studies are needed to determine whether the in vitro and in vivo defects of the OmpA mutant are related to the decreased expression of type 1 fimbriae and to understand how the deletion of ompA affects type 1 fimbriae expression.
As mentioned earlier, several other E. coli determinants that contribute to invasion of HBMECs have been identified47–50,72. Recombinant Ibe proteins inhibit E. coli K1 invasion of HBMECs47,73, which suggests that these proteins also contribute to HBMEC invasion through ligand–receptor interactions. This was supported by the identification of a HBMEC receptor protein for IbeA and the fact that a polyclonal antibody raised against this receptor inhibited E. coli K1 invasion of HBMECs74. In addition, enrichment of IbeA-receptor-expressing HBMECs by fluorescence-activated cell sorting resulted in significantly enhanced invasion by the IbeA-positive E. coli K1 strain74. CNF1 is a virulence factor that is associated with pathogenic E. coli strains that cause extraintestinal infections, including meningitis. CNF1 is an AB-type toxin, is composed of an amino-terminal cell-binding domain and a carboxy-terminal catalytic domain that has deaminase activity, and activates Rho GTPases, such as Rho, Rac and Cdc42 (Refs 75,76). CNF1 contributes to E. coli K1 invasion of HBMECs in vitro and traversal of the blood–brain barrier in vivo, and these in vitro and in vivo effects are dependent on RhoA activation49. However, it is unclear how CNF1 enters HBMECs and activates Rho GTPases. It has been suggested that CNF1 is internalized by receptor-mediated endocytosis upon binding to a cell-surface receptor77. The HBMEC receptor for CNF1 has been identified as a 37-kDa laminin receptor precursor (37 LRP)78. The 37 LRP is a ribosome-associated cytoplasmic protein and is a precursor of the 67-kDa laminin receptor (67 LR). It is unclear how the mature 67 LR is synthesized from the 37 LRP, but mature 67 LR is present on the cell surface and functions as a membrane receptor for the adhesive basement-membrane protein laminin79. It has been shown that the expression levels of 37 LRP and 67 LR are directly correlated with CNF1-mediated E. coli K1 invasion of HBMECs78. Recent studies have shown that CNF1-expressing E. coli K1 upregulates the expression of 67 LR in HBMECs and recruits 67 LR along with focal adhesion kinase (FAK) and paxillin to the site of invading E. coli K1 in a CNF1-dependent manner80. The 37 LRP–67 LR has been shown to be a cellular target for a range of CNS-infecting microorganisms, including dengue virus, adeno-associated virus and Venezuelan equine encephalitis virus, as well as the prion protein PrP81–84. It remains to be determined how these different organisms interact with the same receptor.
S. agalactiae and L
monocytogenes. Recent studies have indicated that other meningeal pathogens invade HBMECs through ligand–receptor interactions (TABLE 1). The neonatal meningitis pathogens S. agalactiae and L. monocytogenes possess several proteins that allow binding to, and invasion of, HBMECs. S. agalactiae binding to HBMECs occurs through laminin-binding protein (Lmb), the fibrinogen-binding protein FbsA, pili and invasion-associated gene A (IagA) (through lipoteichoic acid anchoring)85–88, but it is unclear whether these proteins are unique to cerebrospinal fluid isolates of S. agalactiae and whether they contribute to S. agalactiae penetration into the CNS.
L. monocytogenes invasion of HBMECs is mediated by internalin B (InlB)30. Several HBMEC receptors for InlB have been identified, including gC1q-R (the receptor for the globular head of the complement component C1q) and Met tyrosine kinase89,90, but their contributions to L. monocytogenes invasion of HBMECs are not completely understood. For example, InlB does not compete for the same interaction site on Met as the natural ligand hepatocyte growth factor91. L. monocytogenes penetration into the CNS has also been attributed to transmigration of L. monocytogenes-infected monocytes and myeloid cells across the blood–brain barrier29, and further studies are needed to determine the major route of L. monocytogenes penetration into the CNS. gC1q-R has also been shown to be the HBMEC receptor for Plasmodium falciparum-infected red blood cells (Pf-IRBCs)92.
S. pneumoniae
S. pneumoniae crosses the blood–brain barrier partly through interactions between cell-wall phosphorylcholine and the platelet-activating-factor (PAF) receptor. This was shown by its partial inhibition of pneumococcal invasion of HBMECs by a PAF-receptor antagonist21,93 and delayed translocation of pneumococci from the lung to the blood (as well as from the blood to the cerebrospinal fluid) in PAF-receptor-knockout mice94. The PAF receptor has also been shown to interact with H. influenzae serotype b95, but the role of the PAF receptor in H. influenzae penetration into the CNS remains unclear. Several studies suggested that the role of the PAF receptor differs in S. pneumoniae and H. influenzae infections. For example, a PAF-receptor antagonist attenuated the pleocytosis that was elicited by intracisternal inoculation of S. pneumoniae but had no effect on the pleocytosis that was induced by H. influenzae96.
N. meningitidis
N. meningitidis is a human-specific pathogen that interacts with human endothelial and epithelial cells. This interaction involves several microbial structures and proteins, including type IV pili, PilC, N. meningitidis adhesin A (NadA) and the Opa and Opc proteins64,97–99. However, these observations were derived from N. meningitidis interactions with non-brain endothelial cells, such as human bone-marrow-derived endothelial cells, and their relevance to HBMECs remains unclear. Interestingly, the invasion of HBMECs by unencapsulated N. meningitidis is mediated by Opc binding to fibronectin, which anchors the bacteria to the integrin α5β1 receptor on the HBMEC surface22. However, in the bloodstream, N. meningitidis is encapsulated, and the in vivo relevance of Opc– fibronectin-mediated binding to integrin is therefore unclear. N. meningitidis pili bind to CD46 on HBMECs98, and their lipooligosaccharides have been shown to contribute to a high level of bacteraemia and subsequent penetration into the CNS100; CD46 has also been shown to be the receptor for measles virus, adenovirus and human herpesvirus 6 (Refs 101–103).
M. tuberculosis
Tuberculosis of the CNS is a serious and often fatal disease that affects young children disproportionally. M. tuberculosis can cross the blood–brain barrier as a free organism or in infected phagocytes104 (FIG. 2). One recent study showed that strains of M. tuberculosis can invade and traverse HBMECs; invasion was significantly increased for the M. tuberculosis strains H37RV and CDC 1551 than for the non-pathogenic Mycobacterium smegmatis, and traversal occurred with M. tuberculosis strains but not M. smegmatis31.
DNA microarray analysis identified at least 33 genes that were upregulated by eightfold or more and 147 genes that were downregulated by eightfold or more in M. tuberculosis that was associated with HBMECs compared with M. tuberculosis that was not associated with HBMECs31. Mutants of five M. tuberculosis genes were attenuated in their ability to invade and/or survive in the brain32. The identification and characterization of M. tuberculosis genes that are involved in traversal of the blood–brain barrier should help elucidate the pathogenesis of CNS tuberculosis.
Spirochaetes
Neurosyphilis and neuroborreliosis are prototypic spirochaete infections of the CNS, but the mechanisms that are involved in their traversal of the blood–brain barrier remain unclear. Treponema pallidum can invade through the intercellular junction of aortic endothelial cells105, which suggests that a mechanism of paracellular penetration of the vascular endothelium occurs, but it is unclear whether a similar mechanism is involved in T. pallidum penetration of the blood–brain barrier. A previous study showed that http://www.ncbi.nlm.nih.gov/sites/entrez?db=genomeprj&cmd=Retrieve&dopt=Overview&list_uids=19847 strains traverse HBMECs without any obvious change in the integrity of HBMECs and that this traversal is facilitated by proteases27. These findings suggest that the fibrinolytic system — linked by an activation cascade — might lead to focal and transient degradation of tight-junction proteins, which would allow B. burgdorferi to traverse the blood–brain barrier. Other recent studies revealed that Borrelia turicatae serotype 1, which is defined by the presence of Vsp1 (variable small protein 1), infects the CNS significantly more than the isogenic serotype, which is defined by the presence of Vsp2, and that Vsp1 binds to HBMECs28. B. burgdorferi expressing recombinant Vsp1 exhibited an increased interaction with HBMECs. These findings suggest that Vsp1 binding to HBMECs might play a part in borrelial penetration into the CNS.
Receptors and cytokines
Cytokines have been shown to regulate expression of the HBMEC receptors and/or signalling molecules that are involved in the interaction between HBMECs and microorganisms. For example, tumour necrosis factor (TNF)-α treatment of HBMECs resulted in increased invasion of S. pneumoniae owing to upregulation of the PAF receptor21, which is the receptor for S. pneumoniae phosphorylcholine. Transforming growth factor (TGF)-β1 treatment of HBMECs significantly increased E. coli K1 invasion and traversal of HBMECs106, whereas treatment with TNF-α and interferon (IFN)-γ had no effect. TGF-β1 has been shown to affect the host cell signal-transduction pathways that are involved in microbial invasion and traversal of HBMECs, such as Rho GTPases and cytosolic phospholipase A2α (cPLA2α), and more work is needed to understand the mechanisms that are associated with cytokine-mediated modulation of microbial–HBMEC interactions.
Further studies are needed to elucidate the ligand–receptor interactions that are involved in microbial traversal of the blood–brain barrier, particularly as the same receptors have been shown to be involved in the pathogenesis of CNS infection by different microorganisms (TABLE 1). It remains speculative whether the expression levels of these receptors dictate the tropism for CNS infection by meningitis-causing microorganisms.
Signal-transduction mechanisms
Recent advances in our understanding of eukaryotic signal-transduction pathways have expedited our understanding of microbial invasion and traversal of the blood–brain barrier, including identification of the signalling molecules that are involved in rearrangements of the host cell actin cytoskeleton. The signal-transduction mechanisms that are involved in actin cytoskeleton rearrangements and HBMEC invasion differ between meningitis-causing microorganisms. For example, actin cytoskeleton rearrangements are a prerequisite for HBMEC invasion by E. coli K1, S. agalactiae and L. monocytogenes. E. coli K1 invasion and traversal of the blood–brain barrier involves FAK, paxillin, phosphatidylinositol 3-kinase, Src kinase, Rho GTPases, cPLA2α and 5-lipoxygenase6,7,49,55,56,66,76,80 (FIG. 4). By contrast, S. agalactiae invasion of HBMECs is independent of Src activation, and L. monocytogenes invasion of HBMECs is independent of FAK and cPLA2 activation. The microbial–host interactions that contribute to invasion of HBMECs and the relevant signalling mechanisms that are involved have not yet been fully elucidated.
Figure 4. Signalling mechanisms involved in Escherichia coli K1-mediated actin cytoskeleton rearrangements and traversal of the blood–brain barrier.
E. coli K1 invasion and traversal of the blood–brain barrier occurs as the result of specific bacterial interactions with receptors (CD48, gp96, R and LR) on brain microvascular endothelial cells (BMECs) and involves FAK, paxillin, phosphatidylinositol 3-kinase, Rho GTPases, 5-LO and cPLA2α. 5-LO, 5-lipoxygenase; CNF1, cytotoxic necrotizing factor 1; cPLA2α, cytosolic phospholipase A2α; FAK, focal adhesion kinase; gp96, glycoprotein 96; OmpA, outer-membrane protein A; PH, pleckstrin homology; PIP, phosphatidylinositol phosphate.
It is important to note that the mechanisms which are involved in entry of HBMECs by meningitis-causing microorganisms differ from those that are involved in the release of cytokines and chemokines in response to these microorganisms. For example, E. coli proteins that are involved in binding to and invasion of HBMECs (OmpA and CNF1, respectively) did not affect the release of interleukin-8 (IL-8) from HBMECs107. Similar findings were observed for an S. agalactiae Lmb mutant, which was defective for the invasion of HBMECs but induced the same amount of IL-8 as the parental strain85. Additionally, N. meningitidis invasion of HBMECs involves c-Jun kinases 1 and 2 (JNK1 and JNK2), but the release of IL-6 and IL-8 from HBMECs involves the p38 mitogen-activated protein kinase (MAPK) pathway108.
Another crucial factor for the development of meningitis is the ability of pathogens to cross the blood–brain barrier as live organisms. As discussed earlier, transmission electron microscopy studies with E. coli and S. agalactiae (as well as C. neoformans, C. albicans and M. tuberculosis) revealed that these microorganisms are found within membrane-bound vacuoles in HBMECs and transmigrate HBMECs in an enclosed vacuole. HBMECs possess the complete trafficking machinery that is necessary to deliver microorganism-containing vacuoles to lysosomes57. Vacuoles that contained a capsule-deletion mutant of E. coli K1 interacted sequentially with early endosomal marker proteins (such as early endosomal auto-antigen 1 and the transferrin receptor) and late endosome and late endosome–lysosomal markers (such as Rab7 and lysosome-associated membrane proteins, respectively), and allowed lysosomal fusion with subsequent degradation inside vacuoles. By contrast, capsule-positive E. coli K1-containing vacuoles reached early and late endosomes without fusion with lysosomes, thereby allowing E. coli K1 to cross the blood–brain barrier as live bacteria57. These findings indicate that the capsule of E. coli K1 modulates the maturation of E. coli K1-containing vacuoles and prevents fusion with lysosomes, which is necessary for traversal of the blood–brain barrier as live bacteria. Additional studies are needed to understand how the capsule can modulate intracellular vacuolar trafficking and whether similar events occur with other meningitis-causing microorganisms.
Consequences of CNS infection
As discussed above, CNS-infecting microorganisms can induce blood–brain barrier dysfunction by affecting the release and/or expression of cytokines, chemokines and cell-adhesion molecules and/or inducing cytotoxicity and apoptosis in HBMECs, which results in increased blood–brain barrier permeability, pleocytosis and encephalopathy6. For example, blockade of pleocytosis by intravenous administration of an anti-CD18 monoclonal antibody that was directed against the β-chain of β2-integrin reduced blood–brain barrier permeability in experimental meningitis that involved intracisternal injection of S. pneumoniae, N. meningitidis and H. influenzae serotype b109. However, intracisternal injection of S. pneumoniae and H. influenzae serotype b resulted in increased blood–brain barrier permeability in both normal animals and leukopenic animals110,111. In addition, pleocytosis was not affected in intercellular adhesion molecule 1-knockout and E- and P-selectin knockout mice with haematogenous meningitis caused by S. pneumoniae and H. influenzae serotype b18,19. These findings indicate that increased blood–brain barrier permeability and pleocytosis might develop independently of each other in bacterial meningitis.
As noted above, meningitis-causing microorganisms such as E. coli, S. agalactiae, N. meningitidis and S. suis induce the release of cytokines and chemokines from HBMECs, but the mechanisms that are involved differ from those which are involved in their binding to, and invasion of, HBMECs. For example, the release of IL-8 in response to E. coli and S. agalactiae infection was independent of the ability of these organisms to bind to and invade HBMECs85,107. In addition, the signalling pathways that are involved in the release of IL-8 differed from the pathways that are involved in the invasion of HBMECs by N. meningitidis (p38 MAPK and JNK, respectively)108. Notably, the release of IL-8 in response to meningitis-causing microorganisms did not occur in non-brain endothelial cells such as HUV ECs107,112,113, which illustrates how the IL-8 response to meningitis-causing microorganisms is specific to HBMECs.
Another consequence of the microbial interaction with HBMECs is encephalopathy, which has been observed in patients with cerebral malaria and B. pertussis infection. The mechanisms that are involved in infection-related encephalopathy remain incompletely understood. For example, Pf-IRBCs have been shown to bind to several molecules on HBMECs92,114–116 (TABLE 1) and the binding of Pf-IRBCs to HBMECs and/or the sequestration of Pf-IRBCs in the cerebral microvasculature has been shown to cause blood–brain barrier dysfunction, including the release of cytokines and chemokines, increased expression of cell-adhesion molecules, alterations in intercellular proteins and the induction of apoptosis114–116. Encephalopathy is a serious complication of B. pertussis infection, and a recent study has shown that pertussis toxin induces a transient increase in permeability and transendothelial migration of monocytes in HBMEC monolayers117, which suggests that pertussis-toxin-mediated blood–brain dysfunction could contribute to encephalopathy.
Conclusions
A major limitation to advances in the prevention and treatment of CNS infection is our incomplete understanding of these diseases and the associated blood–brain barrier dysfunction. Studies of the in vitro model of the blood–brain barrier and the in vivo animal model of experimental haematogenous meningitis have shed some light on the mechanisms of microbial traversal of the blood–brain barrier, the key step for the development of CNS infections. This traversal is the result of specific microorganism–host interactions and involves host cell signal-transduction pathways that affect host cell actin cytoskeleton rearrangements. It is unclear, however, whether the mechanisms that are involved in the traversal of the blood–brain barrier are similar and/or different in the various pathogens that cause CNS infection, such as bacteria, fungi and parasites (BOXes 1,2; TABLE 1). A more complete knowledge of microbial interactions with the blood–brain barrier might facilitate the development of novel strategies for the prevention and therapy of CNS infections.
Box 2. Parasite invasion and traversal of the blood–brain barrier.
The neurological manifestations of sleeping sickness in humans that are caused by Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense are attributed to the penetration of the central nervous system by these organisms25,26. Bloodstream forms of T. brucei gambiense efficiently cross human brain microvascular endothelial cells (HBMECs) by a paracellular route26. In experimental rodent models, the parasite can pass through the blood–brain barrier across or between endothelial cells and the vessel basement membranes25. The laminin composition of the basement membranes determines whether they are permissive to parasite penetration, and interferon (IFN)-γ has been shown to have an important role in regulating trypanosome trafficking into the brain25.
Toxoplasma encephalitis is a serious complication of infection with the obligate intracellular parasite Toxoplasma gondii. Stimulation of HBMECs with IFN-γ resulted in the restricted growth of T. gondii, which was enhanced in the presence of tumour necrosis factor (TNF)-α. This anti-parasitic activity was due to the induction of indoleamine 2,3-dioxygenase (IDO) in HBMECs by IFN-γ and TNF-α13. Repletion of the essential amino acid tryptophan abrogated this inhibition, suggesting that IDO activation and the subsequent degradation of tryptophan is the main mechanism for IFN-γ-mediated and TNF-α-mediated toxoplasmosis. IDO-positive HBMECs can cleave tryptophan to kynurenine and therefore reduce the transport of tryptophan to astrocytes. As IDO is the main effector mechanism in astrocytes against T. gondii, reduced tryptophan influx enhances the antimicrobial effect of IDO-positive astrocytes. Additional studies are needed to elucidate the mechanisms that are involved in IDO-mediated toxoplasmosis.
Acknowledgments
The information contained in this Review is derived from studies carried out by members of the author’s laboratory and his collaborators’ laboratories. Work in the laboratory of K.S.K. was supported by National Institutes of Health grants.
Glossary
- Astrocyte
A star-shaped glial cell that supports the tissue of the central nervous system
- Pericyte
A cell that is found around capillaries and is related to smooth muscle cells. Pericytes surround the endothelium as single cells. Association with pericytes reduces endothelial apoptosis and stabilizes the vasculature
- Pinocytosis
The cellular uptake of extracellular fluid. Involves the formation of caveolae by the cell membrane that pinch off to form vesicles in the cytoplasm
- Microglial cell
An immune cell of the central nervous system that is derived from mesodermal precursor cells and could be of haematopoietic lineage
- Choroid plexus
A site of production of cerebrospinal fluid in the adult brain that is formed by the invagination of ependymal cells into the ventricles, which then become vascularized
- AB-type toxin
A bacterial toxin that modifies target proteins within the cytosol of host cells and is composed of two domains: one that is responsible for the enzymatic activity (A) and one that is responsible for cell-receptor binding (B)
- Pleocytosis
The presence of a higher than normal number of cells in the cerebrospinal fluid
- Fibrinolytic system
A broad spectrum of proteolytic enzymes that includes the plasminogen activator system and plasmin Plasmin and plasmin activators proteolytically degrade the extracellular matrix
- Encephalopathy
Brain dysfunction that is associated with alterations of the neural microenvironment and results from metabolic, toxic, vascular, infectious and/or inflammatory insults
Footnotes
DATABASES
Entrez Genome Project: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=genomeprj
Borrelia burgdorferi | Borrelia turicatae | Candida albicans | Cryptococcus neoformans | Escherichia coli | Haemophilus influenzae | Listeria monocytogenes | Mycobacterium smegmatis | Mycobacterium tuberculosis | Neisseria meningitidis | Plasmodium falciparum | Streptococcus agalactiae | Streptococcus pneumoniae | Streptococcus suis | Toxoplasma gondii | Treponema pallidum
Entrez Protein: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=protein
CNF1 | FbsA | IbeA | IbeB | IL-8 | InlB | Lmb | NadA | OmpA
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.World Health Organization. Geneva: WHO; 2004. World health report: changing history. [Google Scholar]
- 2.Klinger G, Chin C-N, Beyene J, Perlman M. Predicting the outcome of neonatal bacterial meningitis. Pediatrics. 2000;106:477–482. doi: 10.1542/peds.106.3.477. [DOI] [PubMed] [Google Scholar]
- 3.Stevens JP, et al. Long-term outcome of neonatal meningitis. Arch. Dis. Child. Fetal Neonatal Ed. 2003;88:F179–F184. doi: 10.1136/fn.88.3.F179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang C-J, et al. Bacterial meningitis in infants: the epidemiology, clinical features, and prognostic factors. Brain Dev. 2004;26:168–175. doi: 10.1016/S0387-7604(03)00122-0. [DOI] [PubMed] [Google Scholar]
- 5.van de Beek D, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N. Engl. J. Med. 2004;351:1849–1859. doi: 10.1056/NEJMoa040845. [DOI] [PubMed] [Google Scholar]
- 6. Kim KS. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nature Rev. Neurosci. 2003;4:376–385. doi: 10.1038/nrn1103. A comprehensive review on the pathogenesis of meningitis and the associated neuronal injury.
- 7.Kim KS. Microbial translocation of the blood–brain barrier. Int. J. Parasitol. 2006;36:607–614. doi: 10.1016/j.ijpara.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Kim YV, Di Cello F, Hillaire CS, Kim KS. Differential Ca2+ signaling by thrombin and protease-activated receptor-1-activating peptide in human brain microvascular endothelial cells. Am. J. Physiol. Cell Physiol. 2004;286:C31–C42. doi: 10.1152/ajpcell.00157.2003. [DOI] [PubMed] [Google Scholar]
- 9.Ruffer C, Strey A, Janning A, Kim KS, Gerke V. Cell–cell junctions of dermal microvascular endothelial cells contain tight and adherens junction proteins in spatial proximity. Biochemistry. 2004;43:5360–5369. doi: 10.1021/bi035517c. [DOI] [PubMed] [Google Scholar]
- 10.Stins MF, Badger JL, Kim KS. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb. Pathog. 2001;30:19–28. doi: 10.1006/mpat.2000.0406. [DOI] [PubMed] [Google Scholar]
- 11.Rubin LL, Staddon JM. The cell biology of the blood–brain barrier. Annu. Rev. Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 12.Mucke L, Eddleston M. Astrocytes in infectious and immune-mediated diseases of the central nervous system. FASEB J. 1993;7:1226–1232. doi: 10.1096/fasebj.7.13.8405808. [DOI] [PubMed] [Google Scholar]
- 13.Daubener W, et al. Restriction of Toxoplasma gondii growth in human brain microvascular endothelial cells by activation of indoleamine 2,3-dioxygenase. Infect. Immun. 2001;69:6527–6531. doi: 10.1128/IAI.69.10.6527-6531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anfuso CD, et al. Endothelial cell–pericyte cocultures induce PLA2 protein expression through activation of PKCα and MAPK/ERK cascade. J. Lipid Res. 2007;48:782–793. doi: 10.1194/jlr.M600489-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Stins MF, Gilles F, Kim KS. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 16. Das A, et al. Differential role of cytosolic phospholipase A2 in the invasion of brain microvascular endothelial cells by Escherichia coli and Listeria monocytogenes. J. Infect. Dis. 2001;184:732–737. doi: 10.1086/322986. Described the contribution of cPLA2 to E. coli invasion of brain microvascular endothelial cells.
- 17.Kim KS, et al. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J. Clin. Invest. 1992;90:897–905. doi: 10.1172/JCI115965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan TQ, Smith CW, Hawkins EP, Mason EO, Jr, Kaplan SL. Hematogenous bacterial meningitis in an intercellular adhesion molecule-1-deficient infant mouse model. J. Infect. Dis. 1995;171:342. doi: 10.1093/infdis/171.2.342. [DOI] [PubMed] [Google Scholar]
- 19.Munoz FM, Hawkins EP, Bullard DC, Beauch AL, Kaplan SL. Host defense against systemic infection with Streptococcus pneumoniae is impaired in E-, P-, and E-/P-selectin-deficient mice. J. Clin. Invest. 1997;100:2099–2106. doi: 10.1172/JCI119744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nizet V, et al. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. The first study to show transcellular penetration of HBMECs by S. agalactiae.
- 21.Ring A, Weiser JN, Tuomanen EI. Pneumococcal trafficking across the blood–brain barrier. Molecular analysis of a novel bi-directional pathway. J. Clin. Invest. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unkmeir A, et al. Fibronectin mediates Opc-dependent internalization of Neisseria meningitidis in human brain microvascular endothelial cells. Mol. Microbiol. 2002;46:933–946. doi: 10.1046/j.1365-2958.2002.03222.x. [DOI] [PubMed] [Google Scholar]
- 23.Jong AY, Stins MF, Huang SH, Kim KS. Traversal of Candida albicans across human blood–brain barrier in vitro. Infect. Immun. 2001;69:4536–4544. doi: 10.1128/IAI.69.7.4536-4544.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang YC, et al. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood–brain barrier. Infect. Immun. 2004;72:4985–4995. doi: 10.1128/IAI.72.9.4985-4995.2004. The first study to show transcellular penetration of the blood–brain barrier by C. neoformans.
- 25.Masocha W, Rottenberg ME, Kristensson K. Migration of African trypanosomes across the blood–brain barrier. Physiol. Behav. 2007;92:110–114. doi: 10.1016/j.physbeh.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 26.Grab DJ, et al. African trypanosome interactions with an in vitro model of the human blood–brain barrier. J. Parasitol. 2004;90:970–979. doi: 10.1645/GE-287R. [DOI] [PubMed] [Google Scholar]
- 27.Grab D, et al. Borrelia burgdorferi, host-derived proteases, and the blood–brain barrier. Infect. Immun. 2005;73:1014–1022. doi: 10.1128/IAI.73.2.1014-1022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sethi N, Sondey M, Bai Y, Kim KS, Cadavid D. Interaction of a neurotropic strain of Borrelia turicatae with the cerebral microcirculation system. Infect. Immun. 2006;74:6408–6418. doi: 10.1128/IAI.00538-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drevets DA, Leenen PJ, Greenfield RA. Invasion of the central nervous system by intracellular bacteria. Clin. Microbiol. Rev. 2004;17:323–347. doi: 10.1128/CMR.17.2.323-347.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greiffenberg L, Goebel W, Kim KS, Kuhn M. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect. Immun. 1998;66:5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jain SK, Paul-Satyaseela M, Lamichhane G, Kim KS, Bishai WR. Mycobacterium tuberculosis invasion and traversal across an in vitro human blood–brain barrier as a pathogenic mechanism for central nervous system tuberculosis. J. Infect. Dis. 2006;193:1287–1295. doi: 10.1086/502631. The first paper to show transcellular penetration of HBMECs by M. tuberculosis.
- 32.Be NA, et al. Bacterial genetic determinants for Mycobacterium tuberculosis invasion and survival in the central nervous system. J. Infect. Dis. (in the press) [Google Scholar]
- 33.Ferrieri P, Burke B, Nelson J. Production of bacteremia and meningitis in infant rats with group B streptococcal serotypes. Infect. Immun. 1980;27:1023–1032. doi: 10.1128/iai.27.3.1023-1032.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berman PH, Banker BQ. Neonatal meningitis. A clinical and pathological study of 29 cases. Pediatrics. 1966;38:6–24. [PubMed] [Google Scholar]
- 35.Kim KS. Effect of antimicrobial therapy for experimental infections due to group B streptococcus on mortality and clearance of bacteria. J. Infect. Dis. 1987;155:1233–1241. doi: 10.1093/infdis/155.6.1233. [DOI] [PubMed] [Google Scholar]
- 36.Dietzman DE, Fischer GW, Schoenknecht FD. Neonatal Escherichia coli septicemia — bacterial counts in blood. J. Pediatr. 1974;85:128–130. doi: 10.1016/s0022-3476(74)80308-2. [DOI] [PubMed] [Google Scholar]
- 37.Bell LM, Alpert G, Campos JM, Plotkin SA. Routine quantitative blood cultures in children with Haemophilus influenzae or Streptococcus pneumoniae bacteremia. Pediatrics. 1985;76:901–904. [PubMed] [Google Scholar]
- 38.Sullivan TD, LaScolea LJ, Neter E. Relationship between the magnitude of bacteremia in children and the clinical disease. Pediatrics. 1982;69:699–702. [PubMed] [Google Scholar]
- 39.Marra A, Brigham MD. Streptococcus pneumoniae causes experimental meningitis following intranasal and otitis media infections via a nonhematogenous route. Infect. Immun. 2001;69:7318–7325. doi: 10.1128/IAI.69.12.7318-7325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Ginkle FW, et al. Pneumococcal carriage results in ganglioside-mediated olfactory tissue infection. Proc. Natl Acad. Sci. USA. 2003;24:14363–14367. doi: 10.1073/pnas.2235844100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cross AS, Kim KS, Wright DC, Sadoff JC, Gemski P. Role of lipopolysaccharide and capsule in the serum resistance of bacteremic strains of Escherichia coli. J. Infect. Dis. 1986;154:497–503. doi: 10.1093/infdis/154.3.497. [DOI] [PubMed] [Google Scholar]
- 42. Xie Y, et al. Identification and characterization of Escherichia coli RS218-derived islands in the pathogenesis of E. coli meningitis. J. Infect. Dis. 2006;194:358–364. doi: 10.1086/505429. The first comparative genomics study to demonstrate that E. coli K1-derived genomic islands contribute to meningitis.
- 43.Whitney CG, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 44.Tsai CJ, Griffin MR, Pekka Nuorti J, Grijalva CG. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin. Infect. Dis. 2008;46:1664–1672. doi: 10.1086/587897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore MR, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 2008;197:1016–1027. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- 46.Borrow R, Miller E. Long-term protection in children with meningococcal C conjugate vaccination: lessons learned. Expert Rev. Vaccines. 2006;5:851–857. doi: 10.1586/14760584.5.6.851. [DOI] [PubMed] [Google Scholar]
- 47. Huang SH, et al. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect. Immun. 1995;63:4470–4475. doi: 10.1128/iai.63.11.4470-4475.1995. First identification of the E. coli gene that contributes to the invasion of HBMECs and penetration into the brain.
- 48.Huang SH, et al. Identification and characterization of an Escherichia coli invasion gene locus ibeB, required for penetration of brain microvascular endothelial cells. Infect. Immun. 1999;67:2103–2109. doi: 10.1128/iai.67.5.2103-2109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan NA, et al. Cytotoxic necrotizing factor-1 contributes to Escherichia coli K1 invasion of the central nervous system. J. Biol. Chem. 2002;277:15607–15612. doi: 10.1074/jbc.M112224200. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Huang SH, Wass C, Kim KS. The gene locus yijP contributes to Escherichia coli K1 invasion of brain microvascular endothelial cells. Infect. Immun. 1999;67:4751–4756. doi: 10.1128/iai.67.9.4751-4756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Kim KS. Role of OmpA and IbeB in Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo. Pediatr. Res. 2002;51:559–563. doi: 10.1203/00006450-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Prasadarao NV, Wass CA, Stins M, Shimada H, Kim KS. Outer membrane protein A-promoted actin condensation of brain microvascular endothelial cells is required for Escherichia coli invasion. Infect. Immun. 1999;67:5775–5783. doi: 10.1128/iai.67.11.5775-5783.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knodler LA, Celli J, Finlay BB. Pathogenic trickery: deception of host cell processes. Nature Rev. Mol. Cell Biol. 2001;2:578–588. doi: 10.1038/35085062. [DOI] [PubMed] [Google Scholar]
- 54.Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 55.Reddy MA, Wass CA, Kim KS, Schlaepfer DD, Prasadarao NV. Involvement of focal adhesion kinase in Escherichia coli invasion of human brain microvascular endothelial cells. Infect. Immun. 2000;68:6423–6430. doi: 10.1128/iai.68.11.6423-6430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reddy MA, Nemani PV, Wass CA, Kim KS. Phosphatidylinositol 3-kinase activation and interaction with focal adhesion kinase in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J. Biol. Chem. 2000;275:36769–36774. doi: 10.1074/jbc.M007382200. Showed for the first time that activation of FAK and phosphatidylinositol 3-kinase is required for E. coli K1 invasion of HBMECs.
- 57. Kim KJ, Elliott SA, DiCello F, Stins MF, Kim KS. The K1 capsule modulates trafficking of E. coli-containing vacuoles and enhances intracellular bacterial survival in human brain microvascular endothelial cells. Cell. Microbiol. 2003;5:245–252. doi: 10.1046/j.1462-5822.2003.t01-1-00271.x. First demonstration of the role of the K1 capsule in the modulation of E. coli movement in HBMECs.
- 58.Nikulin J, Panzner U, Frosch M, Schubert-Unkmeir A. Intracellular survival and replication of Neisseria meningitidis in human brain microvascular endothelial cells. Int. J. Med. Microbiol. 2006;296:553–558. doi: 10.1016/j.ijmm.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mairey E, et al. Cerebral microcirculation shear stress levels determine Neisseria meningitidis attachment sites along the blood–brain barrier. J. Exp. Med. 2006;203:1939–1950. doi: 10.1084/jem.20060482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stins MF, et al. Binding characteristics of S fimbriated Escherichia coli to isolated brain microvascular endothelial cells. Am. J. Pathol. 1994;145:1228–1236. [PMC free article] [PubMed] [Google Scholar]
- 62.Prasadarao NV, Wass CA, Kim KS. Endothelial cell GlcNAcβ1–4 GlcNAc epitopes for outer membrane protein A enhance traversal of Escherichia coli across the blood–brain barrier. Infect. Immun. 1996;64:154–160. doi: 10.1128/iai.64.1.154-160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charland N, et al. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect. Immun. 2000;68:637–643. doi: 10.1128/iai.68.2.637-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffmann I, Eugene E, Nassif X, Couraud P, Bourdoulous S. Activation of ErbB2 receptor tyrosine kinase supports invasion of endothelial cells by Neisseria meningitidis. J. Cell Biol. 2001;155:133–145. doi: 10.1083/jcb.200106148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Teng CH, et al. Escherichia coli K1 RS218 interacts with human brain microvascular endothelial cells via type 1 fimbria bacteria in the fimbriated state. Infect. Immun. 2005;73:2923–2931. doi: 10.1128/IAI.73.5.2923-2931.2005. Used DNA microarrays to identify type 1 fimbriae in E. coli that bind to HBMECs.
- 66.Khan NA, et al. Outer membrane protein A and cytotoxic necrotizing factor-1 use diverse signaling mechanisms for Escherichia coli K1 invasion of human brain microvascular endothelial cells. Microb. Pathog. 2003;35:35–42. doi: 10.1016/s0882-4010(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 67.Khan NA, Kim Y, Shin S, Kim KS. FimH-mediated Escherichia coli K1 invasion of human brain microvascular endothelial cells. Cell. Microbiol. 2007;9:169–178. doi: 10.1111/j.1462-5822.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- 68.Shin S, Lu G, Cai M, Kim KS. Escherichia coli outer membrane protein A adheres to human brain microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2005;330:1199–1204. doi: 10.1016/j.bbrc.2005.03.097. [DOI] [PubMed] [Google Scholar]
- 69.Prasadarao NV, et al. Cloning and expression of the Escherichia coli K1 outer membrane protein A receptor, a gp96 homologue. Infect. Immun. 2003;71:1680–1688. doi: 10.1128/IAI.71.4.1680-1688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cabanes D, et al. Gp96 is a receptor for a novel Listeria monocytogenes virulence factor, Vip, a surface protein. EMBO J. 2005;24:2827–2838. doi: 10.1038/sj.emboj.7600750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teng C-H, et al. Effects of ompA deletion on expression of type 1 fimbriae in Escherichia coli K1 strain RS218 and on the association of E. coli with human brain microvascular endothelial cells. Infect. Immun. 2006;74:5609–5616. doi: 10.1128/IAI.00321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badger J, Wass C, Weissman S, Kim KS. Application of signature-tagged mutagenesis for identification of Escherichia coli K1 genes that contribute to invasion of human brain microvascular endothelial cells. Infect. Immun. 2000;68:5056–5061. doi: 10.1128/iai.68.9.5056-5061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang S-H, Wan Z-S, Chen Y-H, Jong AY, Kim KS. Further characterization of Escherichia coli brain microvascular endothelial cell invasion gene ibeA by deletion, complementation, and protein expression. J. Infect. Dis. 2001;183:1071–1078. doi: 10.1086/319290. [DOI] [PubMed] [Google Scholar]
- 74.Nemani PV, Wass CA, Huang S-H, Kim KS. Identification and characterization of a novel Ibe10 binding protein that contributes to Escherichia coli invasion of brain microvascular endothelial cells. Infect. Immun. 1999;67:1131–1138. doi: 10.1128/iai.67.3.1131-1138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flatau G, et al. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt G, et al. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-I. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 77.Contamin S, et al. The p21 Rho-activating toxin cytotoxic necrotizing factor 1 is endocytosed by a clathrin-independent mechanism and enters the cytosol by an acidic-dependent membrane translocation step. Mol. Biol. Cell. 2000;11:1775–1787. doi: 10.1091/mbc.11.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chung JW, et al. 37-kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1 -mediated RhoA activation and bacterial uptake. J. Biol. Chem. 2003;278:16857–16862. doi: 10.1074/jbc.M301028200. The first study to identify 37 LRP as the receptor for CNF1.
- 79.Massia SP, Rao SS, Hubbell JA. Covalently immobilized laminin peptide Tyr-Ile-Gly-Ser-Arg (YIGSR) supports cell spreading and co-localization of the 67-kilodalton laminin receptor with α-actinin and vinculin. J. Biol. Chem. 1993;268:8053–8059. [PubMed] [Google Scholar]
- 80.Kim KJ, Chung JW, Kim KS. 67-kDa laminin receptor promotes internalization of cytotoxic necrotizing factor 1-expressing Escherichia coli K1 into human brain microvascular endothelial cells. J. Biol. Chem. 2005;280:1360–1368. doi: 10.1074/jbc.M410176200. [DOI] [PubMed] [Google Scholar]
- 81.Ludwig GV, Kondig JP, Smith JF. A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. J. Virol. 1996;70:5592–5599. doi: 10.1128/jvi.70.8.5592-5599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gauczynski S, et al. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J. 2001;20:5863–5875. doi: 10.1093/emboj/20.21.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thepparit C, Smith DR. Serotype-specific entry of dengue virus into liver cells: identification of the 37-kilodalton/67-kilodalton high-affinity laminin receptor as a dengue virus serotype 1 receptor. J. Virol. 2004;78:12647–12656. doi: 10.1128/JVI.78.22.12647-12656.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akache B, et al. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tenenbaum T, et al. Streptococcus agalactiae invasion of human brain microvascular endothelial cells is promoted by the laminin-binding protein Lmb. Microbes Infect. 2007;9:714–720. doi: 10.1016/j.micinf.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 86.Tenenbaum T, Bloier C, Adam R, Reinscheid DJ, Schroten H. Adherence to and invasion of human brain microvascular endothelial cells are promoted by fibrinogen-binding protein FbsA of Streptococcus agalactiae. Infect. Immun. 2005;73:4404–4409. doi: 10.1128/IAI.73.7.4404-4409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doran KS, et al. Blood–brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J. Clin. Invest. 2005;115:2499–2507. doi: 10.1172/JCI23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maisey HC, Hensler M, Nizet V, Doran KS. Group B streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. J. Bacteriol. 2007;189:1464–1467. doi: 10.1128/JB.01153-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Braun L, Ghebrehiwet B, Cossart P. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 2000;19:1458–1466. doi: 10.1093/emboj/19.7.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shen Y, Naujokas M, Park M, Ireton K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell. 2000;103:501–510. doi: 10.1016/s0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 91.Niemann HH, et al. Structure of the human receptor tyrosine kinase Met in complex with the Listeria invasion protein InIB. Cell. 2007;130:235–246. doi: 10.1016/j.cell.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 92.Biswas AK, et al. Plasmodium falciparum uses gC1qR/HABP1/p32 as a receptor to bind to vascular endothelium and for platelet-mediated clumping. PloS Pathog. 2007;3:1271–1280. doi: 10.1371/journal.ppat.0030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 94.Radin JN, et al. β-arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect. Immun. 2005;73:7827–7835. doi: 10.1128/IAI.73.12.7827-7835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weiser JN, et al. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J. Exp. Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cabellos C, et al. Differing roles of plateletactivating factor during inflammation of the lung and subarachnoid space. The special case of Streptococcus pneumoniae. J. Clin. Invest. 1992;90:612–618. doi: 10.1172/JCI115900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nassif X, Pujol C, Morand P, Eugene E. Interactions of pathogenic Neisseria with host cells. Is it possible to assemble the puzzle? Mol. Microbiol. 1999;32:1124–1132. doi: 10.1046/j.1365-2958.1999.01416.x. [DOI] [PubMed] [Google Scholar]
- 98.Johansson L, et al. CD46 in meningococcal disease. Science. 2003;301:373–375. doi: 10.1126/science.1086476. [DOI] [PubMed] [Google Scholar]
- 99.Capecchi B, et al. Neisseria meningitis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol. Microbiol. 2005;55:687–698. doi: 10.1111/j.1365-2958.2004.04423.x. [DOI] [PubMed] [Google Scholar]
- 100.Plant L, et al. Lipooligosaccharide structure contributes to multiple steps in the virulence of Neisseria meningitidis. Infect. Immun. 2006;74:1360–1367. doi: 10.1128/IAI.74.2.1360-1367.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Manchester M, et al. Clinical isolates of measles virus use CD46 as a cellular receptor. J. Virol. 2000;74:3967–3974. doi: 10.1128/jvi.74.9.3967-3974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nature Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 103.Santoro F, et al. Interaction of glycoprotein H of human herpesvirus 6 with the cellular receptor CD46. J. Biol. Chem. 2003;278:25964–25969. doi: 10.1074/jbc.M302373200. [DOI] [PubMed] [Google Scholar]
- 104.Rich AR, McCordock HA. The pathogenesis of tuberculous meningitis. Bull. Johns Hopkins Hosp. 1933;52:5–37. [Google Scholar]
- 105.Thomas DD, et al. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc. Natl Acad. Sci. USA. 1988;85:3608–3612. doi: 10.1073/pnas.85.10.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang GW, Khan NA, Kim KJ, Stins M, Kim KS. Transforming growth factor-β increases Escherichia coli K1 adherence, invasion and transcytosis in human brain microvascular endothelial cells. Cell Tissue Res. 2002;309:281–286. doi: 10.1007/s00441-002-0549-4. [DOI] [PubMed] [Google Scholar]
- 107.Galanakis E, Di Cello F, Paul-Satyaseela M, Kim KS. Escherichia coli K1 induces IL-8 expression in human brain microvascular endothelial cells. Eur. Cytokine Netw. 2006;17:260–265. [PubMed] [Google Scholar]
- 108.Sokolova O, et al. Interaction of Neisseria meningitidis with human brain microvascular endothelial cells: role of MAP- and tyrosine kinases in invasion and inflammatory cytokine release. Cell. Microbiol. 2004;6:1153–1166. doi: 10.1111/j.1462-5822.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 109.Tuomanen E, Saukkonen K, Sande S, Cioffe C, Wright SD. Reduction of inflammation, tissue damage and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J. Exp. Med. 1989;170:959–968. doi: 10.1084/jem.170.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tauber MG, Borschberg U, Sande MA. Influence of granulocytes on brain edema, intracranial pressure, and cerebrospinal fluid concentrations of lactate and protein in experimental meningitis. J. Infect. Dis. 1988;157:456–464. doi: 10.1093/infdis/157.3.456. [DOI] [PubMed] [Google Scholar]
- 111.Lesse AJ, Moxon ER, Zwahten A, Scheid WM. Role of cerebrospinal fluid pleocytosis and Haemophilus influenzae type b capsule on blood brain barrier permeability during experimental meningitis in the rat. J. Clin. Invest. 1988;82:102–109. doi: 10.1172/JCI113556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vadeboncoeur N, Segura M, Al-Numani D, Vanier G, Gottschalk M. Pro-inflammatory cytokine and chemokine release by human brain microvascular endothelial cells stimulated by Streptococcus suis serotype 2. FEMS Immunol. Med. Microbiol. 2003;35:49–58. doi: 10.1111/j.1574-695X.2003.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 113.Frigerio S, et al. Immunocompetence of human microvascular brain endothelial cells: cytokine regulation of IL-1β, MCP-1, IL-10, sICAM-1 and sVCAM-1. J. Neurol. 1998;245:727–730. doi: 10.1007/s004150050275. [DOI] [PubMed] [Google Scholar]
- 114.Medana IM, Turner GD. Human cerebral malaria and the blood–brain barrier. Int. J. Parasitol. 2006;36:555–568. doi: 10.1016/j.ijpara.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 115.van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 2006;22:503–508. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 116.Tripathi AK, Sullivan DJ, Stins MP. Plasmodium falciparum-infected erythrocytes decrease the integrity of human blood–brain barrier endothelial cell monolayers. J. Infect. Dis. 2007;195:942–950. doi: 10.1086/512083. [DOI] [PubMed] [Google Scholar]
- 117.Kugler S, et al. Pertussis toxin transiently affects barrier integrity, organelle organization and transmigration of monocytes in a human brain microvascular endothelial cell barrier model. Cell. Microbiol. 2007;9:619–632. doi: 10.1111/j.1462-5822.2006.00813.x. [DOI] [PubMed] [Google Scholar]
- 118.Perfect JR, Casadevall A, et al. Cryptococcus. Infect. Dis. Clin. North Am. 2002;4:837–874. doi: 10.1016/s0891-5520(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 119.Chretien F, et al. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J. Infect. Dis. 2002;4:522–530. doi: 10.1086/341564. [DOI] [PubMed] [Google Scholar]
- 120.Olszewski MA, et al. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am. J. Pathol. 2004;164:1761–1771. doi: 10.1016/S0002-9440(10)63734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Noverr MC, Williamson PR, Fajardo RS, Huffnagle GB. CNLAC1 is required for extrapulmonary dissemination of Cryptococcus neoformans but not pulmonary persistence. Infect. Immun. 2004;72:1693–1699. doi: 10.1128/IAI.72.3.1693-1699.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Noveer MC, Cox GM, Perfect JR, Huffnagle GB. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect. Immun. 2003;71:1538–1547. doi: 10.1128/IAI.71.3.1538-1547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Santangelo R, et al. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect. Immun. 2004;72:2229–2239. doi: 10.1128/IAI.72.4.2229-2239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shea JM, Kechichian TB, Luberto C, Del Poeta M. The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect. Immun. 2006;74:5977–5988. doi: 10.1128/IAI.00768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]