Abstract
Background
Disturbed sleep has been associated with increased insulin resistance and elevated inflammation. Although there is growing body of evidence that activation of inflammatory pathways plays a crucial role in the development of insulin resistance, the mediational model whereby sleep disturbances influence inflammation that drives insulin resistance has not been fully assessed in general population studies with objectively measured sleep. This study aimed to examine associations between objectively measured sleep, inflammatory markers, and insulin resistance simultaneously and in a mediational analysis, thereby offering insights into the possible causal model.
Methods
Cross-sectional data collected from 2004 to 2009 during the Midlife Development in the United States II biomarker project were used. The study population included 374 community-based participants (138 men and 236 women) who completed 7 nights of wrist actigraphy. Multiple regressions controlling for age and statistically significant variables in univariate regressions were performed to evaluate the associations between actigraphy-assessed sleep measures, inflammatory cytokines, and insulin resistance.
Results
The regression models showed that in women, higher sleep onset latency (SOL) was associated with higher insulin resistance after controlling for age, smoking, obesity, diabetes, depression, and inflammatory cytokines. Higher SOL was also associated with higher interleukin (IL)–6 and C-reactive protein (CRP) levels in women, but no association was found in men. Using mediation models in women, the association between SOL and insulin resistance was partially explained by the indirect effect of inflammatory cytokines.
Conclusion
A combination of inflammation and other unidentified pathways may contribute to the relationship between disturbed sleep and glucose homeostasis.
Keywords: Insulin resistance, Inflammation, Actigraphy, Women
1. Introduction
Sleep is thought to have an important role in glucose homeostasis [1–4]. Several cross-sectional and cohort studies conducted in various populations consistently demonstrate that disturbed sleep is associated with an increased prevalence of type 2 diabetes mellitus (DM) [5,6] and prediabetic conditions including impaired fasting glucose (IFG) [7] and impaired glucose tolerance (IGT) [6]. Furthermore, recent studies have noted that disturbed sleep [8] is also associated with increased insulin resistance.
However, there are mixed results and several debates regarding the association between disturbed sleep and insulin resistance. An epidemiological study of patients with type 2 DM reported a U-shaped relationship between self-reported sleep duration and insulin resistance [8]; that is, both short and long sleep are associated with insulin resistance. Another epidemiological investigation of adults without diabetes reported that self-reported long but not short sleep duration is associated with insulin resistance [9]. However, an actigraphic study of lean young adults [10] reported that only short sleep duration is associated with insulin resistance. Also, a study of healthy adolescents reported a U-shaped relationship between actigraphy determined sleep duration and insulin resistance [11], whereas another study of young adults found no association between actigraphy-assessed sleep duration and insulin resistance [12]. These mixed results are derived from investigations in heterogeneous populations that used self-reported sleep measures, mostly sleep duration, or likewise did not adjust for confounding factors such as depression. The self-reported sleep assessment adds considerable measurement error, with evidence that self-report corresponds poorly with more accurate objective sleep measures such as the actigraphy watch [13]. In addition, investigating the relationship between disturbed sleep and insulin resistance using sleep parameters besides sleep duration provides additional insight into the dimensions of sleep that might be most consequential for metabolic health. However, studies to date have not been able to examine associations of sleep parameters besides sleep duration and insulin resistance using an objective assessment of sleep. A recent actigraphic study showed a relationship between higher sleep fragmentation and higher insulin resistance among diabetic subjects [12]. However, this study had a relatively small sample size, used processed sleep measures, and did not make adjustments for depression symptoms.
Interestingly, existing literature also suggests that the relationship between sleep and health outcomes might vary by sex. Some have argued that women are more vulnerable to the effects of sleep disturbances, showing greater inflammatory activation [14–16]. For example, Suarez reported that poor sleep quality and problems falling asleep were associated with higher fasting insulin, fibrinogen, and inflammatory biomarkers, but only for women. Moreover, a number of studies reported associations of sleep disturbances with poor health outcomes including hypertension [17], cardiovascular disease [18], and type 2 diabetes [19] in women but not in men. Taken together, it is plausible that men and women may differ in the way in which sleep disturbances influence health, including insulin resistance.
The endocrine and molecular mechanisms underlying the association between disturbed sleep and insulin resistance are complex and poorly understood. Nevertheless, it has been suggested that inflammatory activation, sympathetic activation, hypothalamic–pituitary–adrenal (HPA) axis alterations, and changes in adipokine levels contribute to insulin resistance and the consequent development of type 2 DM [2]. Among the suggested mechanisms linking disturbed sleep and insulin resistance, there is a growing body of evidence that inflammation interacts with other factors to play a crucial role in the development of insulin resistance. Consistent with this, a systemic review and meta-analysis of cohort and experimental sleep deprivation studies reports that sleep disturbance is associated with increases in two markers of systemic inflammation: interleukin (IL)–6 and C-reactive protein (CRP) [20]. Increases in these inflammatory markers are also thought to predict increased insulin resistance and type 2 DM [21,22]. Therefore, we examined potential associations among actigraphy-assessed sleep measures, insulin resistance, and IL-6 and CRP levels in a community-based midlife sample of men and women after adjusting for multiple covariates including body mass and depression. Then with a mediation model, we investigated whether inflammation might explain the relationship between disturbed sleep and insulin resistance.
2. Methods
2.1. Study population and design
The data for this study are from the Midlife Development in the U.S II (MIDUS II) biomarker project, a longitudinal follow-up of the original MIDUS I study (N = 7,108). MIDUS I was designed to investigate the roles of behavioral, psychological, and social factors in physical and mental health. The MIDUS II biomarker project was conducted to obtain comprehensive biologic assessments in a subsample of MIDUS I respondents and was specifically designed to identify biopsychosocial pathways that contribute to diverse health outcomes. The MIDUS II biomarker project included 1255 participants aged 34 to 84 years who were required to visit one of three general clinical research centers (GCRCs): the University of California at Los Angeles, Georgetown University, or the University of Wisconsin–Madison. With the exception of sleep assessments, all project data were obtained by a standardized protocol during the overnight stay at a GCRC between July 2004 and May 2009. The protocol included fasting blood samples, a 12-hour urine sample, medical history, physical examination, and self-administered questionnaires. Of the 1255 participants, 441 participants who attended the MIDUS Research Center at the University of Wisconsin– Madison were asked to wear an Actiwatch wrist activity monitor (Philips, Amsterdam, the Netherlands) for 1 week. All participants gave their written informed consent to participate in this study at the clinic prior to beginning the study procedures, and each MIDUS research center obtained institutional review board approval.
2.2. Measures
2.2.1. Sleep
Subjective sleep measures were obtained from the Pittsburgh Sleep Quality Index (PSQI), which is a self-administered questionnaire about usual sleep habits during the past month [23].
Participants were also invited to participate in a 7-day sleep study. The protocol required that participants continuously wear the Mini Mitter Actiwatch-64 activity monitor for 7 consecutive days and also complete a daily sleep diary for the same time period. The Actiwatch data collection period began on the morning of the Tuesday after the day the respondent returned home following the GCRC visit and ended when they woke up on the following Tuesday. The Actiwatch was configured to detect the number of movements in every 30-second epochs, and all epochs were scored as either sleep or wake by comparing the calculated total activity counts to a wake threshold value. If the total activity counts was less than or equal to the wake threshold value, the epoch was scored as sleep. Bed and rise times in the sleep diary were used as start and end times for the actigraphic records. If participants provided incomplete information (eg, forgot to put the watch on, took it off too early, etc), or had unexpected experiences (eg, traveled to a different time zone, worked an extra shift, etc) during the data collection period, they were flagged for review, and intervals were marked or deleted as appropriate according to standard data cleaning guidelines. Objective sleep measures including total sleep time (TST), sleep onset latency (SOL), and wake after sleep onset (WASO) were derived from the actigraphy data and averaged across the valid nights of the study for use in analyses. Finally, we manually reviewed the daily sleep measures and identified data errors in 10 entries and recorded as missing. Specifically, their time in bed per day were calculated to be longer than 24 hours (eg, SE = 18%, SOL = 10 minutes, TST = 955 minutes, WASO = 85 minutes) or SE were higher than 100% (eg, SE = 300%, SOL = 257 minutes, TST = 145 minutes, WASO = 10 minutes).
2.2.2. Blood samples and insulin resistance
Fasting blood samples were collected from each participant before breakfast on day 2 of their overnight hospital stay. To ensure consistency, all samples were collected and processed at the GCRC using standardized procedures. Hemoglobin A1c (HbA1c) assays were performed at Meriter Labs (GML, Madison, WI) using a Cobas Integra analyzer (Roche Diagnostics, Indianapolis, IN). Fasting glucose was measured at ARUP laboratories (Salt Lake City, UT) using an automated analyzer (Roche Modular Analytics P). Fasting insulin was measured at ARUP laboratories using a Siemens Adivia Centaur analyzer (Munich, Germany). CRP assays were performed at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT) using a BNII nephelometer from Dade Behring (Deerfield, IL). IL-6 is assayed in the MIDUS Biocore laboratory (University of Wisconsin, Madison, WI) using a high-sensitivity enzyme-linked immunosorbent assay kit (R & D Systems, Minneapolis, MN). A homeostasis model assessment of insulin resistance (HOMA-IR), an indicator of insulin resistance, was calculated using an established formula: the product of fasting glucose and fasting insulin, divided by a constant [24]. HOMA-IR, IL-6, and CRP values were natural log-transformed to achieve normal distributions.
2.2.3. Covariates
Age, sex, and current smoking status were obtained from self-reported data. Current smoking status was dichotomously coded. Because obesity is an established risk factor in type 2 DM pathogenesis and is associated with insulin resistance, body mass index (BMI) was included as a covariate. We created dichotomous variables to identify the presence of diabetes and significant depressive symptoms. A participant was considered to have diabetes if one of three criteria was met: a participant reported having been diagnosed with diabetes; a participant reported taking any medications for diabetes; or both HbA1c and fasting glucose level were above 6.5% and 125 mg/d, respectively. However, a participant was considered not to have diabetes if two criteria were met: they reported not taking any diabetes medications; and both HbA1c and fasting glucose level were within normal limits. Depressive symptoms were assessed with the Center for Epidemiological Studies Depression (CESD) Scale, and participants with a total score ≥16 were considered to have current depression. In addition, participants were instructed to bring all their medications to the GCRC, and the use of medication for depression was dichotomously coded. The use of medication for sleep for more than 3 days per week was assessed with a PSQI item and dichotomously coded. Because we hypothesized that inflammation plays a crucial role in mediating the relationship between sleep and insulin resistance, natural log-transformed IL-6 and CRP values were continuously coded and used for analyses.
2.3. Statistical analyses
SPSS for Windows version 18.0 (SPSS Inc, Chicago, IL) was used for statistical analyses. Among 441 participants who provided actigraphy-assessed sleep measures, we excluded 15 men and 10 women to minimize the effect of hypoxic stress due to obstructive sleep apnea on insulin resistance development using two PSQI items as a proxy for sleep-disordered breathing. Participants were thought to have sleep-disordered breathing if they had trouble sleeping because they “could not breathe comfortably” and “coughed and snored” more than once per week during the past month of the study. Furthermore, we excluded 20 participants who had been taking insulin or whose glucose levels on fasting blood samples were greater than 200 mg/d because HOMA-IR cannot be reliably calculated using the established formula in these conditions [25].
For the remaining 396 participants, preliminary regression analyses showed no significant association between insulin resistance and any of the sleep measures (Supplementary Table 1). However, a general linear model showed a significant adjusted interaction effect between sex and actigraphy-assessed SOL on insulin resistance (p = 0.001). Therefore, we classified the participants into two groups by sex and performed separate analyses. Because 13 men outliers and 9 women outliers were excluded to obtain normal distributions of variables within each group, our analyses were based on 374 participants.
Independent sample t tests and χ2 tests were used to compare continuous and categorical variables between sexes, respectively. To identify factors associated with insulin resistance, age and statistically significant (p < 0.05) variables from the univariate regression analyses were entered to multivariate regression analyses (dependent variable: log-transformed HOMA-IR). We investigated both regression analysis models without (model 1) and with (models 2 and 3) inflammatory cytokines’ effects. Because some studies have reported a U-shaped relationship between sleep duration and insulin resistance [8,11], we performed additional regression analyses in which the continuous variable TST was categorized as shorter (<6 hours), intermediate (6–8 hours), or longer (>8 hours). Furthermore, univariate and subsequent multivariate regression analyses were performed to identify factors associated with inflammation (dependent variable: log-transformed IL-6 or CRP). We used enter-method multiple regression analyses because statistically significant variables from the results of enter- and stepwise-method analyses were identical. Finally, mediation analyses with bootstrapping method were performed, in which variables were statistically significant from the regression analyses, to investigate the relationship between actigraphy-assessed sleep measures and insulin resistance by considering inflammation as a mediator [26].
3. Results
3.1. Subject characteristics
Table 1 presents the participants’ characteristics by sex. Their age ranged from 34 to 83 years. Women showed greater use of antidepressant medication and longer actigraphy-assessed TST than men. Correlations between self-reported sleep parameters and actigraphy-assessed data were significant (r = 0.190, p < 0.001 for SOL and r = 0.376, p < 0.001 for TST).
Table 1.
Characteristics of study participants.
| Women (n = 236) |
Men (n = 138) |
p Valuea | |
|---|---|---|---|
| Age (y) | 53.46 (11.64) | 55.78 (11.58) | 0.063 |
| BMI (kg/m2) | 30.29 (7.28) | 29.37 (4.95) | 0.151 |
| Smoking | 29 (12.3%) | 28 (20.3%) | 0.038 |
| Diabetes | 26 (11.0%) | 15 (10.9%) | 0.965 |
| CESD total score ≥ 16 | 33 (14.2%) | 26 (18.8%) | 0.234 |
| Medication for depression | 38 (16.1%) | 11 (8%) | 0.025 |
| Medication for sleep ≥ 3 days/week |
30 (12.7%) | 12 (8.7%) | 0.235 |
| Fasting glucose (mg/dL) | 98.53 (14.36) | 100.78 (16.29) | 0.165 |
| Fasting insulin (µIU/mL) | 12.62 (9.81) | 13.19 (9.10) | 0.581 |
| HbA1C (%) | 5.97 (0.63) | 5.89 (0.67) | 0.235 |
| HOMA-IR | 3.22 (2.85) | 3.40 (2.63) | 0.562 |
| IL-6 (pg/mL) | 3.27 (3.26) | 3.12 (2.47) | 0.626 |
| CRP (µg/mL) | 3.39 (4.42) | 2.65 (6.01) | 0.177 |
| Actigraphy SOL (h) |
0.60 (0.31) | 0.65 (0.29) | 0.184 |
| TST (h) | 6.41 (1.03) | 6.12 (0.97) | 0.007 |
| WASO (h) | 0.78 (0.31) | 0.82 (0.36) | 0.221 |
| PSQI | |||
| Global score | 6.22 (3.66) | 5.65 (3.33) | 0.150 |
| SOL (h) | 0.32 (0.25) | 0.29 (0.24) | 0.205 |
| TST (h) | 6.91 (1.27) | 6.79 (1.17) | 0.363 |
Abbreviations: BMI, body mass index; CESD, Center for Epidemiological Studies Depression scale; CRP, C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance; PSQI, Pittsburgh Sleep Quality Index; SOL, sleep onset latency; TST, total sleep time; WASO, wake after sleep onset.
Values are expressed as mean (SD) for continuous variables and as n (%) for categorical variables.
p Values were calculated with independent-sample t tests for continuous variables and χ2 tests for categorical variables. Bold indicates p < 0.05.
3.2. Sleep, inflammation, and insulin resistance in the entire cohort
Univariate regression analyses with all 396 participants showed that higher BMI, presence of diabetes, higher SOL, shorter TST, and inflammatory cytokines were associated with higher insulin resistance, which was estimated by log-transformed HOMA-IR. The subsequent multivariate regression analyses showed that in the combined cohort of both males and females, higher BMI and presence of diabetes, CRP, but none of the sleep measures, were associated with higher insulin resistance (Supplementary Table 1).
3.3. Sex differences in sleep, inflammation, and insulin resistance
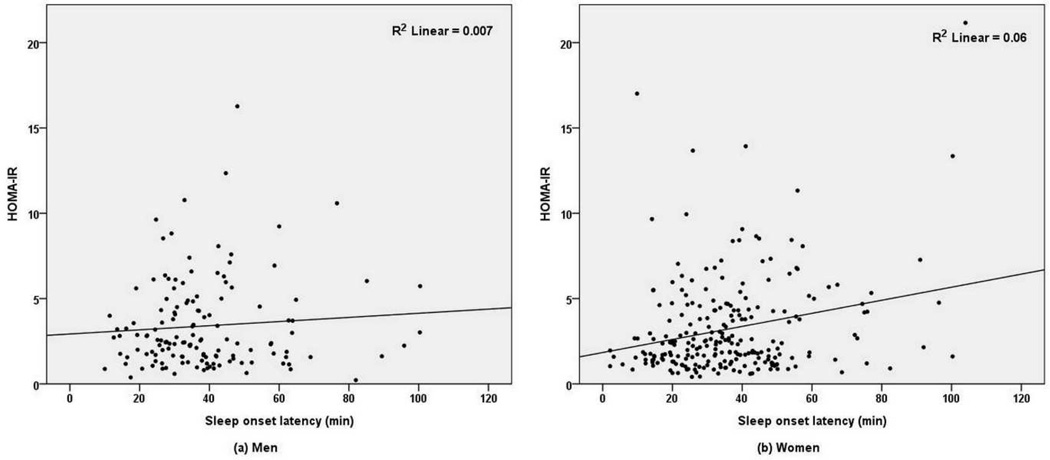
Examination of these relationships separately within women revealed unique effects. In women only, univariate regression analyses showed that higher BMI, presence of diabetes, presence of current depression (CESD total score ≥ 16), longer SOL and WASO, shorter TST, and higher IL-6 and CRP were associated with higher insulin resistance (Fig 1, Supplementary Table 2). The subsequent multivariate regression analysis of women only, in which IL-6 and CRP were not entered (model 1), showed that SOL, BMI, and diabetes were associated with insulin resistance (Table 2). IL-6 (model 2) and CRP (model 3) were next entered as independent variables; however, longer SOL was still associated with higher insulin resistance among women. Higher IL-6 and CRP levels were also associated with greater insulin resistance in women, but TST and WASO were not. In additional multivariate regression analyses with categorized TST (short, intermediate, or long sleep duration), TST was still not associated with insulin resistance in women.
Fig 1.
Relationship between sleep onset latency and homeostasis model assessment of insulin resistance (HOMA-IR), by sex. SOL, sleep onset latency.
Table 2.
Multivariate linear regression analyses of factors associated with insulin resistance.
| Women B (SE) |
tr | p Value | Men B (SE) |
β | p Value | |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| Age | −0.006(0.004) | −0.097 | 0.090 | 0.001(0.005) | 0.019 | 0.803 |
| BMI | 0.041 (0.006) | 0.397 | <0.001 | 0.075(0.011) | 0.495 | <0.001 |
| Diabetes | 0.622 (0.145) | 0.251 | <0.001 | 0.596(0.178) | 0.250 | 0.001 |
| Depressiona | 0.127(0.123) | 0.059 | 0.301 | 0.096(0.139) | 0.050 | 0.493 |
| SOLb | 0.427 (0.151) | 0.167 | 0.005 | −0.034(0.192) | −0.014 | 0.859 |
| TSTb | −0.029(0.043) | −0.039 | 0.500 | 0.041(0.057) | 0.053 | 0.477 |
| WASOb | 0.043(0.141) | 0.018 | 0.760 | 0.050(0.154) | 0.024 | 0.745 |
| Model 2 | ||||||
| Age | −0.007(0.004) | −0.109 | 0.055 | −0.002(0.005) | −0.024 | 0.760 |
| BMI | 0.035 (0.007) | 0.342 | <0.001 | 0.071(0.011) | 0.471 | <0.001 |
| Diabetes | 0.597 (0.144) | 0.241 | <0.001 | 0.555(0.179) | 0.232 | 0.002 |
| Depressiona | 0.146(0.122) | 0.068 | 0.233 | 0.082(0.138) | 0.043 | 0.556 |
| IL-6c | 0.131 (0.061) | 0.136 | 0.034 | 0.135(0.086) | 0.128 | 0.118 |
| SOLb | 0.372 (0.152) | 0.146 | 0.015 | −0.052(0.192) | −0.020 | 0.787 |
| TSTb | −0.027(0.042) | −0.037 | 0.520 | 0.056(0.058) | 0.073 | 0.335 |
| WASOb | 0.007(0.141) | 0.003 | 0.963 | 0.031(0.153) | 0.015 | 0.839 |
| Model 3 | ||||||
| Age | −0.006(0.004) | −0.094 | 0.086 | <0.001(0.005) | −0.002 | 0.977 |
| BMI | 0.036 (0.006) | 0.345 | <0.001 | 0.066(0.012) | 0.437 | <0.001 |
| Diabetes | 0.569 (0.137) | 0.233 | <0.001 | 0.570(0.177) | 0.239 | 0.002 |
| Depressiona | 0.116(0.118) | 0.054 | 0.327 | 0.067(0.139) | 0.035 | 0.632 |
| CRPc | 0.149 (0.040) | 0.228 | <0.001 | 0.093(0.054) | 0.141 | 0.085 |
| SOLb | 0.290 (0.147) | 0.115 | 0.050d | −0.036(0.191) | −0.014 | 0.850 |
| TSTb | −0.017(0.041) | −0.023 | 0.674 | 0.039(0.057) | 0.051 | 0.495 |
| WASOb | 0.012(0.134) | 0.005 | 0.931 | 0.026(0.153) | 0.012 | 0.867 |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; SOL, sleep onset latency; TST, total sleep time; WASO, wake after sleep onset.
Dependent variable: log-transformed (natural log) homeostasis model assessment of insulin resistance (HOMA-IR). Bold indicates p < 0.05. In model 1, independent variables include age, body mass index, presence of diabetes, presence of depression (Center for Epidemiological Studies Depression scale [CESD] total score ≥ 16), sleep onset latency, total sleep time and wake after sleep onset. Model 2 included model 1 variables plus IL-6. Model 3 included model 1 variables plus CRP. Independent variables: age and statistically significant variables from univariate regression analyses in women.
Presence of significant depressive symptoms (CESD total score ≥ 16).
Actigraphy-assessed.
Log-transformed for analyses.
t = 1.971.
Examination of the relationships in men only showed that in univariate regression analyses higher BMI, presence of diabetes, and higher IL-6 and CRP levels were associated with greater insulin resistance (Fig 1, Supplementary Table 2). In subsequent multivariate regression analyses of men, BMI and diabetes were associated with insulin resistance (Supplementary Table 3). However, none of the sleep variables including SOL, WASO, and TST were predictive of insulin resistance. This lack of association was not changed in additional multivariate regressions analyses, which adjusted for potential confounds same as in women (Table 2).
3.4. Sex-specific relationship of sleep with inflammation
Univariate regression analyses of data from women showed that older age, higher BMI, diabetes, longer SOL, longer WASO, and shorter TST were associated with higher IL-6 level. The factors associated with higher CRP were higher BMI, diabetes, use of sleep medications more than 3 days per week, use of antidepressant medication, longer SOL, WASO, and shorter TST (Supplementary Tables 4 and 5). The subsequent multivariate regression analyses of women (Table 3) revealed that longer SOL and higher BMI were associated with higher IL-6 (model 1). In addition, longer SOL, higher BMI, and antidepressant medication use were associated with higher CRP (model 2).
Table 3.
Multivariate linear regression analyses of factors associated with IL-6 and CRP levels.
| Women B (SE) |
β |
p Value |
Men B (SE) |
β |
p Value |
|
|---|---|---|---|---|---|---|
| Model 1 (IL-6) | ||||||
| Age | 0.007 (0.004) | 0.103 | 0.080 | 0.020 (0.005) | 0.338 | <0.001 |
| BMI | 0.042 (0.007) | 0.387 | <0.001 | 0.027 (0.011) | 0.187 | 0.018 |
| Diabetes | 0.250 (0.152) | 0.099 | 0.102 | 0.304 (0.180) | 0.135 | 0.094 |
| SOLa | 0.387 (0.156) | 0.150 | 0.014 | 0.137 (0.194) | 0.057 | 0.481 |
| TSTa | –0.023 (0.045) |
– 0.030 |
0.614 | −0.114 (0.058) |
– 0.158 |
0.050 |
| WASOa | 0.228 (0.153) | 0.089 | 0.136 | 0.140 (0.155) | 0.072 | 0.368 |
| Model 2 (CRP) | ||||||
| Age | –0.008 (0.006) |
– 0.083 |
0.164 | 0.016 (0.008) | 0.160 | 0.048 |
| BMI | 0.063 (0.010) | 0.386 | <0.001 | 0.096 (0.018) | 0.420 | <0.001 |
| Diabetes | 0.290 (0.224) | 0.080 | 0.196 | 0.307 (0.294) | 0.085 | 0.298 |
| Medication for sleepb | 0.321 (0.210) | 0.092 | 0.126 | 0.645 (0.337) | 0.162 | 0.058 |
| Medication for depression |
0.396 (0.188) | 0.126 | 0.036 | −0.294 (0.354) |
– 0.071 |
0.409 |
| SOLa | 0.677 (0.232) | 0.181 | 0.004 | −0.027 (0.333) |
– 0.007 |
0.936 |
| TSTa | –0.090 (0.068) |
– 0.081 |
0.184 | −0.029 (0.096) |
– 0.025 |
0.763 |
| WASOa | –0.068 (0.228) |
– 0.018 |
0.766 | 0.255 (0.251) | 0.082 | 0.310 |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; IL, interleukin; SOL, sleep onset latency; TST, total sleep time; WASO, wake after sleep onset.
Bold indicates p < 0.05. Dependent variable: log-transformed IL-6 in Model 1 and logtransformed CRP in Model 2, respectively. Independent variables: age and statistically significant variables from univariate regression analyses in women.
Actigraphy-assessed.
Use of medication for sleep more than 3 days a week.
Conversely, in men, the univariate regression analyses of sleep-related variables and inflammatory cytokine showed no significant association (Supplementary Tables 4–6). Model 1 multivariate regressions analyses adjusting for age, BMI, and diabetes found that shorter TST was associated with higher IL-6 levels (p = 0.05) (Table 3). Further adjustments for medications for sleep and depression in model 2 eliminated this effect.
3.5. Mediation of the effect of sleep onset latency on insulin resistance by inflammation in women
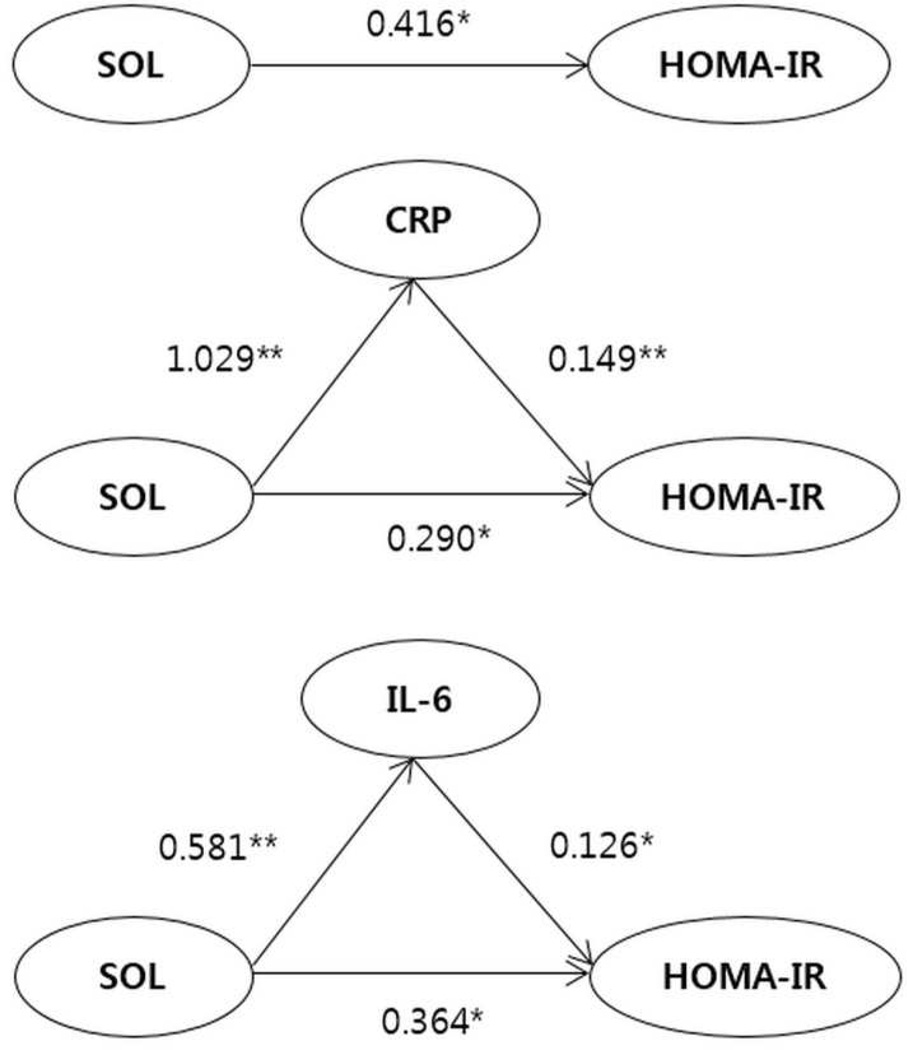
Because associations between SOL, inflammatory markers and insulin resistance were all significant in women, mediation analyses were performed by partitioning the total effect of SOL on insulin resistance into its indirect effect through inflammatory markers and its direct effect. In women, mediation analyses among SOL, insulin resistance, and CRP showed both significant direct effect of SOL on insulin resistance (β = 0.290, 95% confidence interval [CI] = 0.080–0.218) and indirect mediating effect by CRP (β = 0.154, 95% CI = 0.070–0.294). Also, mediation analyses of SOL, insulin resistance, and IL-6 showed that direct effect of SOL on insulin resistance (β = 0.364, 95% CI = 0.097–0.631) and indirect mediating effect by IL-6 (β = 0.073, 95% CI = 0.010–0.169) were statistically significant (Fig 2, Supplementary Table 7).
Fig 2.
Structural equation model of the relationship among SOL, inflammatory markers, and insulin resistance in women.
Abbreviations: CRP, C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance; IL-6, interleukin 6; SOL, sleep onset latency.
Values are path coefficients. *p < 0.05, **p < 0.001.
4. Discussion
This cross-sectional community-based cohort study evaluated the relationship between actigraphy-assessed sleep measures and insulin resistance considering levels of IL-6 and CRP, inflammatory biomarker, among men and women in midlife after adjusting for covariates including age, body mass, diabetes status, and depression. Actigraphy-assessed SOL in women, but not men, was positively associated with insulin resistance and inflammatory cytokines. Mediation analysis revealed that the association between SOL and insulin resistance was partially explained by CRP and the inflammatory cytokine IL-6. This finding suggests that inflammatory pathways partially explain the association of SOL with insulin resistance in women. The unexplained variance also points to other potential unidentified mechanisms being involved. Meta-analytic analysis of the literature has linked sleep disturbances with elevated inflammation [20], whereas another body of literature has demonstrated a link between sleep disturbances and insulin resistance [5]. Our results merge this literature and demonstrate that the association of SOL with insulin resistance is statistically partially mediated through elevated inflammation. To the best of our knowledge, these study is the first to show a mediational analysis with objectively assessed sleep linking SOL with insulin resistance via inflammatory pathways.
Our results are largely in line with those of previous studies regarding the impact of disturbed sleep on insulin resistance, but differ in a number of ways. To the best of our knowledge, our study is the first to show associations between objectively measured SOL and insulin resistance in a midlife community population, and to demonstrate differences by sex. Previous studies investigating the association between insulin resistance and sleep (mostly sleep duration) reported mixed results, which are limited due to a combination of self-reported sleep measures, heterogeneous populations, and a lack of adjustment for confounding factors.
We also examined other sleep parameters. Here, initial models examining both men and women combined suggested shorter TST to be related to insulin resistance; however, this was no longer statistically significant in the fully adjusted multivariate regression analyses controlling for age BMI, diabetes, and medications for sleep and depression. Additional analyses with categorized TST did not reveal a relationship with insulin resistance. Moreover, we found no marked associations of TST with inflammation. Although most previous studies have reported associations of TST rather than SOL with adverse health outcomes, the significant association of SOL with insulin resistance in our study is not contrary to the existing literature. In the context of hyperarousal theory [27], constant sympathetic hyperactivation, which is prevalent in patients with primary insomnia [28] and particularly pronounced in those with difficulty in initiating sleep [29], may drive the mobilization of glucose stores, raising blood sugar levels and leading to insulin resistance. Moreover, sympathetic hyperactivation has been shown to be associated with adverse health outcomes including hypertension [30], cardiovascular events [31] and all-cause mortality [32]. Thus, although not directly tested, it remains plausible that SOL may reflect a hyperarousal state that drives both insulin resistance and inflammatory markers.
Overall, our findings linking SOL with insulin resistance and inflammation are consistent with a growing body of evidence that disturbed sleep activates inflammatory pathways that interacts with other mechanisms to play a crucial role in insulin resistance development [20]. For example, disturbed sleep is thought to affect the sympathetic nervous system and HPA axis to influence the development of adverse health outcome by promoting inflammation [33]. Especially, β-adrenergic signaling induces inflammatory gene expression, proinflammatory cytokine production, and increased systemic inflammation [20]. In addition, a study in healthy adults without a history of sleep disorders showed that PSQI-reported poor sleep quality, more frequent difficulty falling asleep, and longer SOL, but not sleep duration, were associated with higher inflammatory marker and insulin levels in women [14].
In our results, women showed longer actigraphy-assessed TST than men, but there was no sex difference in PSQI-assessed TST. This discrepancy between subjectively and objectively measured TST is consistent with previous studies reporting that women exhibit objectively defined longer sleep and less sleep fragmentation than men, but subjectively report shorter sleep and poorer sleep quality [34,35]. It is also noteworthy that we observed significant associations only between objectively assessed SOL with insulin resistance and inflammatory marker levels in women, whereas self-reported SOL was not significantly associated with these biomarkers. Although the underlying mechanisms are not clear, the observed sex difference is not contrary to the existing literature. For example, it has been suggested that women are more vulnerable to the effects of sleep disturbance and thus show greater inflammation [14–16] and more adverse health outcomes including insulin resistance. Likewise, a number of studies reported that disturbed sleep was associated with several medical conditions including prevalence and incidence of hypertension [17], cardiovascular disease mortality [18], and incidence of type 2 DM [19] in women only. Sex differences in β-adrenergic signaling and reproductive hormone levels have been suggested as reasons why women are more vulnerable to inflammatory processes. Suarez reported that sleep disturbances were associated with inflammatory marker and insulin levels only in women [14], and there is substantial evidence that disturbed sleep has a greater influence on inflammation in women compared to men [15,16]. During undisturbed sleep, women exhibit greater monocyte IL-6 expression due to sex differences in tonic sympathovagal activity [36]. In addition, both estradiol and testosterone are thought to inhibit inflammatory pathways [37,38]. The women in this study were mostly postmenopausal, suggesting that lower reproductive hormone levels might contribute to inflammatory pathway activation associated with disturbed sleep.
The strengths of this study include the use of objective and continuous sleep variables, our population-based design, adjustments for a wide range of possible confounding factors, and relatively consistent results with previous studies. The simultaneous examination of the associations among objective sleep measures, inflammatory markers, and insulin resistance allowed us to statistically test a mediational model. Although this approach offers insight into the mechanisms through which SOL influences insulin resistance, this does not demonstrate causation. Additional limitations also exist. First, due to its cross-sectional design, the determination of causal direction was inherently limited. However, growing evidence from prospective epidemiological studies indicates that sleep influences glucose homeostasis [39,40]. Future prospective studies that include objective sleep measures are necessary to confirm and refine our results. Second, we did not perform polysomnography and therefore did not objectively assess sleep apnea. Instead, we used two PSQI items as a proxy for sleep-disordered breathing and then excluded 25 participants. The effect of sleep apnea was further minimized in the multiple regression analyses because we adjusted for BMI known to be associated with sleep apnea. Third, instead of the gold-standard euglycemic–hyperinsulinemic clamp test, we estimated insulin resistance with the HOMA-IR. However, we excluded 20 participants because it may be a poor indicator of insulin resistance in insulin-treated subjects or those with poor glycemic control. In subjects with mild diabetes, HOMA-IR exhibits good correlation with euglycemic clamp data [25,41].
5. Conclusions
Our community-based cohort study demonstrated linear associations between actigraphy-assessed SOL, inflammatory marker levels, and insulin resistance in midlife women, but not in men. Difficulty initiating sleep may contribute to insulin resistance and the consequent development of type 2 DM in women. Our mediation model suggests that inflammatory pathway activation may be one of the key underlying mechanisms through which disturbed sleep affects glucose homeostasis. Interventions for women who experience difficulty initiating sleep may reduce the risk of developing type 2 DM.
Supplementary Material
Highlights.
Higher sleep onset latency was associated with higher insulin resistance in women.
Women may be more vulnerable than men to the effects of sleep disturbance.
The association in women is partially mediated by inflammatory pathways.
Disturbed sleep influences glucose homeostasis partially through inflammation
Acknowledgments
The authors gratefully and sincerely thank Dr. Michael Irwin and the Cousins Center for Psychoneuroimmunology at UCLA. This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2014S1A3A2044496) and a grant of the Korean Mental Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HM15C0995).
Abbreviations
Sleep onset latency
- BMI
body mass index
- CESD
center for epidemiological studies depression scale
- CI
confidence interval
- CRP
C-reactive protein
- HbA1c
hemoglobin A1c
- HOMA-IR
homeostasis model assessment of insulin resistance
- IL-6
interleukin 6
- PSQI
Pittsburgh sleep quality index
- SOL
sleep onset latency
- TST
total sleep time
- WASO
wake after sleep onset.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The MIDUS I study (Midlife Development in the U.S.) was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. The MIDUS II research was supported by a grant from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the MIDUS I investigation. The research was further supported by the following grants: M01-RR023942 (Georgetown), M01-RR00865 (UCLA) from the General Clinical Research Centers Program, and UL1TR000427 (UW) from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health.
References
- 1.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 2.Briancon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: Endocrine and molecular mechanisms. Diabetol Metab Syndr. 2015;7:25. doi: 10.1186/s13098-015-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2015;3:52–62. doi: 10.1016/S2213-8587(14)70012-9. [DOI] [PubMed] [Google Scholar]
- 4.Depner CM, Stothard ER, Wright KP., Jr Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14:507. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katano S, Nakamura Y, Nakamura A, Murakami Y, Tanaka T, Takebayashi T, et al. Association of short sleep duration with impaired glucose tolerance or diabetes mellitus. J Diabetes Investig. 2011;2:366–372. doi: 10.1111/j.2040-1124.2011.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung HC, Yang YC, Ou HY, Wu JS, Lu FH, Chang CJ. The relationship between impaired fasting glucose and self-reported sleep quality in a Chinese population. Clin Endocrinol (Oxf) 2013;78:518–524. doi: 10.1111/j.1365-2265.2012.04423.x. [DOI] [PubMed] [Google Scholar]
- 8.Ohkuma T, Fujii H, Iwase M, Ogata-Kaizu S, Ide H, Kikuchi Y, et al. U-shaped association of sleep duration with metabolic syndrome and insulin resistance in patients with type 2 diabetes: The Fukuoka Diabetes Registry. Metabolism. 2014;63:484–491. doi: 10.1016/j.metabol.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Pyykkonen AJ, Isomaa B, Pesonen AK, Eriksson JG, Groop L, Tuomi T, et al. Sleep duration and insulin resistance in individuals without type 2 diabetes: The PPP-Botnia study. Ann Med. 2014;46:324–329. doi: 10.3109/07853890.2014.902226. [DOI] [PubMed] [Google Scholar]
- 10.Darukhanavala A, Booth JN, 3rd, Bromley L, Whitmore H, Imperial J, Penev PD. Changes in insulin secretion and action in adults with familial risk for type 2 diabetes who curtail their sleep. Diabetes Care. 2011;34:2259–2264. doi: 10.2337/dc11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Association of short and long sleep durations with insulin sensitivity in adolescents. J Pediatr. 2011;158:617–623. doi: 10.1016/j.jpeds.2010.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34:1171–1176. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girschik J, Fritschi L, Heyworth J, Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22:462–468. doi: 10.2188/jea.JE20120012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: Evidence for gender disparity. Brain Behav Immun. 2008;22:960–968. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: Sex differences. Brain Behav Immun. 2010;24:54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prather AA, Epel ES, Cohen BE, Neylan TC, Whooley MA. Gender differences in the prospective associations of self-reported sleep quality with biomarkers of systemic inflammation and coagulation: Findings from the Heart and Soul Study. J Psychiatr Res. 2013;47:1228–1235. doi: 10.1016/j.jpsychires.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: The Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rod NH, Kumari M, Lange T, Kivimaki M, Shipley M, Ferrie J. The joint effect of sleep duration and disturbed sleep on cause-specific mortality: Results from the Whitehall II cohort study. PLoS One. 2014;9:e91965. doi: 10.1371/journal.pone.0091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuomilehto H, Peltonen M, Partinen M, Seppa J, Saaristo T, Korpi-Hyovalti E, et al. Sleep duration is associated with an increased risk for the prevalence of type 2 diabetes in middle-aged women—The FIN-D2D survey. Sleep Med. 2008;9:221–227. doi: 10.1016/j.sleep.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunner EJ, Kivimaki M, Witte DR, Lawlor DA, Davey Smith G, Cooper JA, et al. Inflammation, insulin resistance, and diabetes—Mendelian randomization using CRP haplotypes points upstream. PLoS Med. 2008;5:e155. doi: 10.1371/journal.pmed.0050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 26.Preacher KJ, Kelley K. Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychol Methods. 2011;16:93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- 27.Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 28.de Zambotti M, Covassin N, De Min Tona G, Sarlo M, Stegagno L. Sleep onset and cardiovascular activity in primary insomnia. J Sleep Res. 2011;20:318–325. doi: 10.1111/j.1365-2869.2010.00871.x. [DOI] [PubMed] [Google Scholar]
- 29.Tsai HJ, Kuo TB, Lin YC, Yang CC. The association between prolonged sleep onset latency and heart rate dynamics among young sleep-onset insomniacs and good sleepers. Psychiatry Res. 2015;230:892–898. doi: 10.1016/j.psychres.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Vgontzas AN, Fernandez-Mendoza J, Bixler EO, Sun Y, Zhou J, et al. Insomnia with physiological hyperarousal is associated with hypertension. Hypertension. 2015;65:644–650. doi: 10.1161/HYPERTENSIONAHA.114.04604. [DOI] [PubMed] [Google Scholar]
- 31.Palatini P, Reboldi G, Beilin LJ, Eguchi K, Imai Y, Kario K, et al. Predictive value of night-time heart rate for cardiovascular events in hypertension. The ABP-International study. Int J Cardiol. 2013;168:1490–1495. doi: 10.1016/j.ijcard.2012.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Blunted heart rate dip during sleep and all-cause mortality. Arch Intern Med. 2007;167:2116–2121. doi: 10.1001/archinte.167.19.2116. [DOI] [PubMed] [Google Scholar]
- 33.Hall MH, Smagula SF, Boudreau RM, Ayonayon HN, Goldman SE, Harris TB, et al. Association between sleep duration and mortality is mediated by markers of inflammation and health in older adults: The Health, Aging and Body Composition Study. Sleep. 2015;38:189–195. doi: 10.5665/sleep.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, et al. Objectively measured sleep characteristics among early-middle-aged adults: The CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 35.van den Berg JF, Miedema HM, Tulen JH, Hofman A, Neven AK, Tiemeier H. Sex differences in subjective and actigraphic sleep measures: A population-based study of elderly persons. Sleep. 2009;32:1367–1375. doi: 10.1093/sleep/32.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Connor MF, Motivala SJ, Valladares EM, Olmstead R, Irwin MR. Sex differences in monocyte expression of IL-6: Role of autonomic mechanisms. Am J Physiol Regul Integr Comp Physiol. 2007;293:R145–R151. doi: 10.1152/ajpregu.00752.2006. [DOI] [PubMed] [Google Scholar]
- 37.Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156:595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- 38.Ray P, Ghosh SK, Zhang DH, Ray A. Repression of interleukin-6 gene expression by 17 beta-estradiol: Inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-kappa B by the estrogen receptor. FEBS Lett. 1997;409:79–85. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- 39.Hayashino Y, Fukuhara S, Suzukamo Y, Okamura T, Tanaka T, Ueshima H, et al. Relation between sleep quality and quantity, quality of life, and risk of developing diabetes in healthy workers in Japan: the High-risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) Study. BMC Public Health. 2007;7:129. doi: 10.1186/1471-2458-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meisinger C, Heier M, Loewel H Study MKAC. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48:235–241. doi: 10.1007/s00125-004-1634-x. [DOI] [PubMed] [Google Scholar]
- 41.Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, Araki-Sasaki R, et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24:362–365. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.