Abstract
Escherichia coli K1 invasion of microvascular endothelial cells of human brain (HBMEC) is required for E. coli penetration into the central nervous system, but the microbial-host interactions that are involved in this invasion of HBMEC remain incompletely understood. We have previously shown that FimH, one of the E. coli determinants contributing to the binding to and invasion of HBMEC, induces Ca2+ changes in HBMEC. In the present study, we have investigated in detail the role of cellular calcium signaling in the E. coli K1 invasion of HBMEC, the main constituents of the blood-brain barrier. Addition of the meningitis-causing E. coli K1 strain RS218 (O18:K1) to HBMEC results in transient increases of intracellular free Ca2+. Inhibition of phospholipase C with U-73122 and the chelating of intracellular Ca2+ by BAPTA/AM reduces bacterial invasion of HBMEC by approximately 50%. Blocking of transmembrane Ca2+ fluxes by extracellular lanthanum ions also inhibits the E. coli invasion of HBMEC by approximately 50%. In addition, E. coli K1 invasion is significantly inhibited when HBMEC are pretreated by the calmodulin antagonists, trifluoperazine or calmidazolium, or by ML-7, a specific inhibitor of Ca2+/calmodulin-dependent myosin light-chain kinase. These findings indicate that host intracellular Ca2+ signaling contributes in part to E. coli K1 invasion of HBMEC.
Keywords: Brain, Endothelial cells, Invasion, Calcium signaling, Calmodulin, Myosin light-chain kinase, Human, Escherichia coli
Introduction
Neonatal gram-negative bacillary meningitis is associated with high mortality and morbidity. The key aspect of neonatal meningitis is related to the ability of pathogens to invade the blood-brain barrier and to penetrate the central nervous system (CNS). However, the ways in which pathogens invade the blood-brain barrier remain incompletely understood. For example, Escherichia coli K1 is the most common gram-negative organism causing neonatal meningitis, with most cases of neonatal E. coli meningitis developing via hematogenous spread (Kim 2003), but the mechanisms that circulating E. coli uses to traverse the blood-brain barrier are unclear.
We have shown that E. coli binding to brain microvascular endothelial cells and its invasion of these cells is required for its traversal of the blood-brain barrier (Huang et al. 1995; Kim 2001, 2003). E. coli binds to and invades microvascular endothelial cells of human brain (HBMEC) via specific bacteria-host cell interactions, involving specific signal transduction pathways such as focal adhesion kinase (FAK), phosphatidylinositol 3-kinase (PI3K), Rho GTPases, and cytosolic phospholiase A2 (cPLA2) (Kim 2003), but details of the involvement of all the above-mentioned signaling molecules in the interaction of E. coli K1 with HBMEC are lacking.
We have also shown that the E. coli K1 invasion of HBMEC requires the rearrangement of the actin cytoskeleton of the host cell, as shown by blockade of E. coli invasion with microfilament-disrupting agents, such as cytochalasin D and latrunculin A (Nemani et al. 1999b). Transmission- and scanning electron-microscopic studies have revealed that invading E. coli K1 is associated with microvilli-like membrane protrusions at the entry site of the HBMEC surface, and internalized E. coli is located within membrane-bound vacuoles of HBMEC and transmigrates through an enclosed vacuole (Nemani et al. 1999b; Kim 2003). We have also shown that HBMEC have all the trafficking machinery required to deliver the microbe-containing vacuoles to late endosomes and lysosomes (Kim et al. 2003).
While investigating the microbial-host interactions involved in E. coli K1 invasion of HBMEC, we have noted that purified E. coli FimH protein, one of the E. coli determinants contributing to the binding to and invasion of HBMEC, induces transient increases of intracellular free Ca2+ ([Ca2+]i) in HBMEC (Khan et al. 2007). We have also observed that E. coli K1 induces transient increases of [Ca2+]i in HBMEC. The present study has therefore examined the role of [Ca2+]i and calcium-dependent mechanisms in the E. coli K1 invasion of HBMEC. We show that [Ca2+]i changes and Ca2+/calmodulin (CaM)-dependent myosin light-chain kinase (MLCK) contribute in part to the E. coli K1 invasion of HBMEC.
Materials and methods
Materials
Fura-2/AM and BAPTA/AM were purchased from Molecular Probes (La Jolla, Calif.). CaM antagonists, trifluoperazine and calmidazolium, were obtained from Sigma (St. Louis, Mo.). U-73122 and ML-7 were from CalBiochem (San Diego, Calif.).
Bacterial strains and culture conditions
E. coli K1 strain RS218 (O18:K1:H7) is the cerebrospinal fluid isolate from a neonate with meningitis (Huang et al. 1995). E. coli K-12 strain HB101 was used as a negative control for invasion assays of HBMEC. E. coli strains were routinely grown at 37°C in Luria-Bertani broth.
Endothelial cell cultures and bacterial invasion assay
HBMEC were isolated and cultured as previously described (Stins et al. 1997). The HBMEC cultures thus obtained were routinely grown in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (FBS), 10% Nu-Serum, 2 mM glutamine, 1 mM pyruvate, penicillin (100 U/ml), streptomycin (100 µg/ml), essential amino acids, and vitamins.
Invasion was analyzed by using gentamicin-protection assays as previously described (Huang et al. 1995). Briefly, confluent cultures of HBMEC grown in 24-well plates were incubated with 107 colony-forming units (CFU) of E. coli (multiplicity of infection of 100) in experimental medium (M199-HamF12 [1:1] containing 5% heat-inactivated FBS, 2 mM glutamine, and 1 mM pyruvate). Plates were incubated at 37°C in a 5% CO2 incubator for 90 min. The monolayers were washed with RPMI 1640 and incubated with experimental medium containing gentamicin (100 µg/ml) for 1 h to kill extracellular bacteria. After being washed, the monolayers were lysed in 0.5% Triton X-100. The released intracellular bacteria were enumerated by plating on sheep-blood/Agar plates. Results are presented as percent invasion, calculated as (number of bacteria recovered/number of bacteria inoculated)×100, or as relative invasion, defined as the percentage invasion compared with invasion of the parent strain. Assays were performed in triplicates and repeated at least two or three times.
To assess the effects on E. coli invasion of phospholipase C (PLC) inhibitor U-73122, the intracellular Ca2+ chelator BAPTA/AM, the CaM antagonists trifluoperazine and calmidazolium, and the inhibitor of MLCK ML-7, the cell monolayers were pretreated for 30 min with the corresponding agent, and then the bacterial suspension was added. In experiments with LaCl3, this agent was added 5 min before the addition of E. coli. Subsequently, invasion assays were carried out as described above.
Measurements of [Ca2+]i
To demonstrate the effect of E. coli on [Ca2+]i levels, assays were performed by using our previously published protocols (Kim et al. 2004). Briefly, HBMECs were grown in M199 medium supplemented with 10% FBS and 10% Nu-serum on collagen-coated glass-bottomed 35-mm culture dishes (World Precision Instruments, Fl.) until at least 80% confluence. Cells were washed with serum-free medium and cultured overnight in low-serum (0.5% FBS) M199 medium. Before starting the experiments, the monolayers were washed with phenol-red-free HEPES-buffered Hanks’ balanced salt solution (HBSS; 137 mM NaCl, 4.2 mM NaHCO3, 0.4 mM Na2HPO4, 5.4 mM KCl, 0.4 mM KH2PO4, 1.3 mM CaCl2, 0.5 mM MgCl2, 0.4 mM MgSO4, 5.6 mM D-glucose, 2 mM Na pyruvate, 15 mM HEPES, pH 7.4) and incubated for 40 min with 3 µM Fura-2/AM and 0.04% Pluronic-123 in the dark at room temperature. After loading, the cells were washed to remove extracellular Fura-2/AM and incubated in HBSS for an additional 20 min. Fura-2-loaded cells were mounted into the recording chamber (Warner Instruments) on the microscope stage, and fluorescence images were captured by using an Olympus fluorescence microscopy system equipped with an inverted Olympus microscope IX70, a cooled OlymPix charge-coupled device camera (model TE3/A/S), and a 40×, 1.3-numerical aperture oil-immersion objective, and a computer-controlled Sutter Filter Wheel (Sutter Instrument). Before starting fluorescence measurements, 30–40 regions of interest representing individual cells were selected on the field of view. Fura-2 fluorescent images were captured at 3-s intervals by alternating excitation of the cells at 340-nm and 380-nm wavelengths, reflected off a dichroic mirror with a cutoff wavelength at 510 nm and bandpass emission filtering centered at 530 nm. The real-time fluorescent images were displayed on a monitor and stored on a hard drive for subsequent detailed analysis with UltraVIEW software (version 4.0; PerkinElmer). The changes of [Ca2+]i were expressed as the F340:F380 ratio, where F340 and F380 were Fura-2 fluorescence intensities obtained at 340-nm and 380-nm excitation wavelengths, respectively (Grynkiewicz et al. 1985). For the interaction with HBMEC, the bacteria were washed twice in HBSS and resuspended in the same solution. Approximately 108 bacteria were added to each monolayer, and the variation of fluorescence was measured as described above.
Statistics
Statistical analyses throughout the study were performed by Student’s t-test. P-values less than 0.05 were considered significant.
Results
Measurement of intracellular Ca2+ in HBMEC
Fura-2-loaded HBMEC were used to monitor cytosolic Ca2+ changes in response to E. coli K1. [Ca2+]i changes were expressed as the 340:380 ratio. As shown in Fig. 1a,b, E. coli K1 strain RS218 caused transient increases of [Ca2+]i lasting approximately 5 min, and some cells exhibited Ca2+ oscillations, whereas exposure of HBMEC to a laboratory E. coli strain HB101 did not result in significant changes in [Ca2+]i (data not shown). The presence of multiple bacteria on the HBMEC monolayer is shown in Fig.1c. Thus, transient [Ca2+]i increases occurred in response to E. coli K1 on HBMEC, possibly representing one of the earlier responses of HBMEC to meningitis-causing E. coli. Next, we examined whether such [Ca2+]i changes induced by E. coli K1 played any role on the bacterial invasion of HBMEC.
Fig. 1.
Intracellular [Ca2+]i transients induced by E. coli K1. Fura-2-loaded HBMEC, grown on glass-bottomed culture dishes, were mounted on the stage of a fluorescence microscope, and 30–40 regions of interest were selected for time-lapse observation. Cells were exposed to E. coli K1 strain (5×108 CFU/ml), and real-time fluorescent images were captured by alternating the 340-nm and 380-nm excitation wavelengths. Results are representative of four independent experiments. a Addition of E. coli at time-point a (74th s) induced transient [Ca2+]i changes. To show the functional integrity of HBMEC and the refilling potential of the intracellular Ca2+ pools after E. coli treatment, 10 µM ATP was added at the end of the experiment (black arrow). [Ca2+]i changes are expressed as the 340:380 ratio (Ca2+ Ratio). b Time-lapse images of [Ca2+]i changes between the time points of 28 s and 352 s (see b in a). Increased [Ca2+]i as indicated by the color change from blue to red (white arrow at 217 s cells undergoing Ca2+ oscillations). c To show the presence of bacteria on the cell surface during Ca2+ measurements, a differential interference contrast image was taken at the time point marked by arrow 1 in a. Bacteria are represented by multiple dots on the surface of the cell monolayer. Bars 40 µm (b, c)
Sources of intracellular Ca2+ elevation and their contribution to E. coli invasion of HBMEC
By using Ca2+ modulators, we have examined the source of intracellular [Ca2+]i elevation, and whether intracellular Ca2+ is involved in the E. coli K1 invasion of HBMEC. We have previously shown that the E. coli K1 invasion of HBMEC is the result of interactions of bacterial structures with their respective HBMEC receptors (Kim 2003). For example, E. coli K1 proteins, such as IbeA and cytotoxic necrotizing factor (CNF) 1, contribute to the invasion of HBMEC and interact with specific receptors present on HBMEC, a 45-kDa protein and 69LR, respectively (Nemani et al. 1999a; Chung et al. 2003). We have also shown that FimH, one of the E. coli structures contributing to the binding to and invasion of HBMEC is able to induce Ca2+ changes in HBMEC through its interaction with the putative CD48 receptor: preincubation of HBMEC with FimH or CD48 antibody prevents subsequent CD48-induced or FimH-induced [Ca2+]i changes, respectively (Khan et al. 2007). We have, therefore, hypothesized that activation of one or all of those HBMEC receptors results in the activation of cellular Ca2+ signaling, which, in turn, facilitates bacterial invasion into HBMEC.
We utilized several pharmacological agents known to interfere with the cellular calcium signaling resulting from various treatments. We used U73122, a specific PLC inhibitor, to prevent PLC activation and, thus, to block subsequent inositol triphosphate (IP3)-induced release of intracellular Ca2+. As we demonstrated for other pathogens (Nikolskaia et al. 2006), such pretreatment of HBMEC resulted in the abolishment of the E. coli K1-induced Ca2+ rise (data not shown), and, as shown in Fig. 2, pretreatment of HBMEC with 5 µM U-73122 reduced E. coli invasion by approximately 40%.
Fig. 2.
Inhibition by host phospholipase C (PLC) reduced the invasion of the meningitis-causing E. coli K1 strain (RS218) into HBMEC. The HBMEC were pretreated with 5 µM U73122 (+U73122), an inhibitor of PLC, for 30 min, and then E. coli invasion assays were performed. Separate control experiments showed that U73122 at the concentration used for this experiment did not affect the viability of HBMEC or the growth of E. coli. E. coli K-12 strain HB101 (HB101) was used as a negative control for the invasion assay. Means±SEM of three independent experiments performed in triplicate are presented. *P<0.05
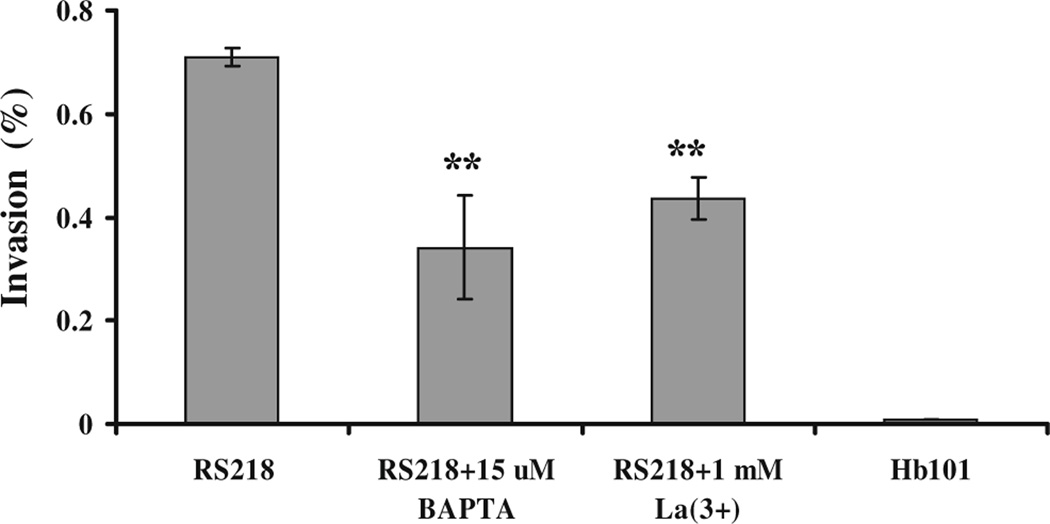
We next examined the role of intracellular Ca2+ on the E. coli K1 invasion of HBMEC by chelating intracellular Ca2+ with BAPTA/AM. BAPTA/AM is lipophilic and thus can cross the plasma membrane into the cell where it is hydrolysed by a cellular esterase to BAPTA. BAPTA is a double aromatic analog of EGTA that selectively chelates Ca2+ and completely blocks intracellular Ca2+ changes. As shown in Fig. 3, pretreatment of HBMEC with 15 µM BAPTA/AM reduced E. coli K1 invasion by approximately 50%. These findings indicated that cytosolic free Ca2+ contributed in part to the E. coli K1 invasion of HBMEC.
Fig. 3.
Intracellular Ca2+ chelator BAPTA/AM and extracellular La3+ inhibited the invasion of the meningitis-causing E. coli K1 strain (RS218) into HBMEC. The HBMEC grown in 24-well culture plates were loaded with the intracellular Ca2+ chelator BAPTA/AM (15 µM) for 30 min (RS218 + 15 µM BAPTA) or were pretreated with 1 mM La3+ for 5 min in experimental medium (RS218 + 1 mM La(3+)) prior to bacterial addition. E. coli invasion assays were then performed. Both BAPTA/AM and La3+ treatment significantly reduced the E. coli invasion of HBMEC. Separate control experiments showed that these agents at the tested concentrations did not affect the viability of HBMEC or the growth of E. coli. E. coli K-12 strain HB101 (HB101) was used as a negative control for the invasion assay. Means±SEM of three independent experiments performed in triplicate are presented. **P<0.005
Since the host cell has two potential sources of intracellular Ca2+, i.e., entry from the extracellular medium and release from the intracellular stores, we also attempted to examine the role of extracellular Ca2+ on E. coli K1 invasion of HBMEC by the pretreatment of HBMEC with EGTA, which chelates extracellular Ca2+, followed by a 90-min exposure to E. coli for bacterial invasion assays. However, the cell monolayers became detached after such a prolonged incubation in Ca2+-free medium, and it was not feasible to carry out the experiments. Since one of the mechanisms of Ca2+ entry into the cells involves calcium channels, such as store-operated calcium channels (Venkatachalam et al. 2002), we next used La3+, a known inhibitor of calcium channels, to determine the effect of extracellular Ca2+ in the E. coli K1 invasion of HBMEC. As shown in Fig. 3, 1 mM La3+ inhibited the E. coli K1 invasion of HBMEC by approximately 40%, suggesting that entry of extracellular Ca2+ into the cells, possibly via calcium channels, plays a role in the E. coli K1 invasion of HBMEC.
Taken together, these findings suggest that cytoplasmic Ca2+ increase, stemming from Ca2+ release from intracellular stores and/or influx of extracellular Ca2+, contributes to approximately 50% of the E. coli K1 invasion of HBMEC, whereas the other half of the E. coli K1 invasion of HBMEC may be attributable to calcium-independent mechanisms.
Role of CaM-related signaling in E. coli K1 invasion of HBMEC
We next examined the possible mechanisms that are involved in Ca2+-mediated E. coli K1 invasion of HBMEC. CaM, a low-molecular-mass high-affinity Ca2+-binding protein is one of the most important sensors of intracellular Ca2+ changes and has been shown to be involved in multiple Ca2+-mediated signaling pathways (Chin and Means 2000; Xia and Storm 2005). We first used the CaM antagonist, trifluoperazine, to test its effects on the E. coli K1 invasion of HBMEC. As shown in Fig. 4, trifluoperazine inhibited the E. coli K1 invasion of HBMEC in a dose-dependent manner, reaching only 14.9±3.1% at 5 µM trifluoperazine compared with untreated cells (P<0.0005). These findings illustrated the importance of CaM as a downstream effector of the Ca2+-mediated E. coli K1 invasion of HBMEC.
Fig. 4.
Inhibitors of host calmodulin (CaM) and Ca2+/CaM-dependent myosin light-chain kinase (MLCK) block the invasion of the meningitis-causing E. coli K1 strain (RS218) into HBMEC. The HBMEC grown in 24-well plates were pretreated for 30 min with the indicated concentrations of the CaM antagonist, trifluoperazine (TFP), or ML-7, an inhibitor of MLCK, followed by E. coli invasion assays. The CaM antagonist and MLCK inhibitor induced a concentration-dependent decrease of the E. coli invasion of HBMEC. Separate control experiments showed that these agents at the tested concentrations did not affect the viability of HBMEC or the growth of E. coli. Means±SEM of two independent experiments performed in triplicate are presented. *P<0.05, **P<0.005
Ca2+/CaM associate with other downstream kinases including MLCK, which, once activated by binding with the Ca2+/CaM complex, has been shown to regulate various functions in mammalian cells, including cytoskeleton rearrangements (Kamm and Stull 2001). We therefore examined the effect of ML-7, a pharmacological inhibitor of MLCK, on the E. coli K1 invasion of HBMEC. As shown in Fig. 4, pretreatment with ML-7 reduced E. coli K1 invasion of HBMEC in a dose-dependent manner, and at 5 µM ML-7, the invasion was only 39.1±3.2% compared with untreated cells (P<0.001). These findings suggested that Ca2+/CaM activation was probably involved in the E. coli K1 invasion of HBMEC and utilized MLCK activation.
Discussion
The E. coli K1 binding and invasion of HBMEC has been shown to be a prerequisite for E. coli penetration into the CNS (Huang et al. 1995; Kim 2001, 2003), but the mechanisms that are involved in the E. coli K1 interaction with HBMEC remain incompletely understood. Several microbial determinants, such as FimH, the Ibe proteins and CNF1, have been identified as contributing to the E. coli K1 binding to and invasion of HBMEC via ligand-receptor interactions involving specific receptors and signal transduction pathways (Kim 2003). The interactions between bacterial ligands and host cell surface receptors are often associated with a transient rise of the intracellular free Ca2+ concentration, the major regulator of cellular functions (Berridge et al. 2000). However, the role of intracellular Ca2+ in the E. coli K1 invasion of HBMEC remains unclear.
Intracellular free calcium ions, [Ca2+]i, serve as a second messenger, and [Ca2+]i has been shown to link cell surface receptors with intracellular effectors in many mammalian cells (Berridge et al. 2000; Tsien and Tsien 1990). The intracellular concentration of free Ca2+ is tightly controlled and is extremely low inside the cytosol (0.1 µM), whereas the extracellular concentration of Ca2+ is approximately 10,000-fold higher (1 mM). Various stimuli can affect the intracellular concentration of Ca2+ by the opening of calcium channels residing in the plasma membrane and by the release of Ca2+ from intracellular stores, but whether [Ca2+]i plays a role in the microbial internalization of nonprofessional phagocytes such as endothelial and epithelial cells remains unclear.
In the present study, we have shown that the addition of E. coli K1 causes a transient increase of [Ca2+]i in HBMEC, and that these bacteria-induced [Ca2+]i transients are in part involved in the E. coli K1 invasion of HBMEC, an important step for E. coli penetration into the CNS. This has been revealed by our demonstration that the loading of host cells with BAPTA/AM, an intracellular Ca2+ chelator, partly prevents the bacterial invasion of HBMEC. The E. coli K1 invasion of HBMEC is also blocked by U73122, a specific inhibitor of host PLC, implying an involvement of the IP3-sensitive intracellular Ca2+ pool. An increased intracellular Ca2+ has been implicated in mediating host cell invasion by several microorganisms, such as Salmonella typhimurium, Porphomonas gingivalis, enteropathogenic E. coli, Camphylobacter jejuni, Listeria monocytogenes, and Pseudomonas aeruginosa (Belton et al. 2004; Bierne et al. 2000; Dramsi and Cossart 2003; Hu et al. 2005; Koschinski et al. 2006; Pace et al. 1993; Ratner et al. 2001; Shiner et al. 2006; Tran Van Nhieu et al. 2004). In most cases, the [Ca2+]i elevation has been attributed to the formation of calcium-permeable pores by bacterial toxins, such as hemolysin, vacuolating cytotoxin A, or listeriolysin, and the subsequent influx of extracellular Ca2+ (Belton et al. 2004; Dramsi and Cossart 2003; Koschinski et al. 2006; Marlink et al. 2003; Repp et al. 2002; Soderblom et al. 2005; Tran Van Nhieu et al. 2004). Much less is known about the role of the receptor-induced [Ca2+]i rise that can result from bacterial interaction with host cell surface receptor(s). Our data with an inhibitor of PLC, the activity of which is dependent on G-protein-coupled or tyrosine kinase receptor activation, suggest that E. coli K1 determinant(s) involved in the invasion of HBMEC activates a Ca2+-dependent receptor on the surface of HBMEC. Investigations to identify this bacterial interaction with HBMEC receptor are in progress. Additionally, our demonstration of the inhibition of the E. coli K1 invasion of HBMEC with La3+ (a nonspecific calcium channel inhibitor) suggests an involvement of an extracellular calcium influx in the E. coli K1 invasion of HBMEC; this influx most probably occurs as the result of the activation of a store-operated Ca2+ influx (Venkatachalam et al. 2002). Studies are needed to determine whether the kinetics of Ca2+ changes induced by E. coli K1, e.g., a continuous or oscillatory Ca2+ rise, have different outcomes on the E. coli K1 invasion of HBMEC.
Elevation of [Ca2+]i results in the activation of numerous intracellular proteins, including CaM, the major intracellular sensor of Ca2+ changes (Berridge et al. 2000; Chin and Means 2000; Xia and Storm 2005). CaM is a small 16.7-kDa ubiquitous intracellular protein belonging to a small group of EF-hand Ca2+-binding proteins (Chin and Means 2000). Upon Ca2+ binding, CaM undergoes dramatic conformational changes that allow Ca2+/CaM complexes to bind to specific hydrophobic domains of various proteins, such as protein kinases and phosphatases and proteins involved in the regulation of ion transport and the cytoskeleton (Chin and Means 2000; Xia and Storm 2005). Our data show that the inhibition of CaM activity by the specific CaM antagonists, trifluoperazine or calmidazolium, results in a markedly reduced E. coli K1 invasion of HBMEC (~80% reduction).
We have previously demonstrated that the invasion of HBMEC by meningitis-causing bacteria such as E. coli K1 requires cytoskeleton rearrangements in the host cells (Kim 2001). Since the binding of the Ca2+/CaM complex causes dramatic activation of MLCK, one of the key proteins involved in cytoskeleton rearrengements (Kamm and Stull 2001), we have examined the role of MLCK in the E. coli K1 invasion of HBMEC. We have found that the inhibition of MLCK activity by ML-7 results in a significant reduction of the E. coli K1 invasion of HBMEC (~60% reduction). Taken together, our findings suggest that the E. coli K1 induction of [Ca2+]i elevation activates CaM and subsequently Ca2+/CaM-activated MLCK, resulting in the actin cytoskeleton changes necessary for bacterial invasion into HBMEC.
Of note, the rearrangements in the actin cytoskeleton of the host cell required for the E. coli K1 invasion of HBMEC can be induced by both calcium-dependent and calcium-independent mechanisms in HBMEC, e.g., by activation of MLCK, cPLA2, FAK, PI3K, and/or RhoA-associated kinase ROCK (Kim 2003). We have shown that E. coli K1 utilizes multiple microbial-host interactions for efficient internalization into HBMEC (Kim 2003), interactions that may involve both calcium-dependent and calcium-independent mechanisms. Therefore, our observations that the inhibition of the intracellular Ca2+ elevation results in a partial (~50%) reduction of the E. coli K1 invasion of HBMEC are unsurprising. It would be interesting to determine whether a more pronounced inhibition of the E. coli K1 invasion of HBMEC with CaM antagonists and the MLCK inhibitor might be attributable to their effect on the HBMEC cytoskeleton rearrangements that are required for E. coli K1 invasion, compared with BAPTA, U73187 and La3+, which inhibit Ca2+ transients. However, the inhibitory effects of CaM antagonists and MLCK inhibitor on the E. coli K1 invasion of HBMEC is unlikely to be related to their effect on intercellular tight/adherens junctions and/or paracellular penetration into HBMEC. For example, we have previously documented transcellular penetration of E. coli K1 into HBMEC without any disruption of intercellular tight/adherens junctions (Kim 2003; Nemani et al. 1999b).
In summary, the E. coli K1 invasion of HBMEC is an important, but complex, step in the pathogenesis of E. coli meningitis. Our findings presented here suggest that both calcium-dependent and calcium-independent mechanisms are likely to contribute to the efficient E. coli K1 invasion of HBMEC. Further characterization of the mechanisms that are involved in the contribution of [Ca2+]i to the E. coli invasion of HBMEC should help in the elucidation of how E. coli K1 is able to cross the blood-brain barrier.
Acknowledgments
This work was supported by the American Heart Association (grant SDG 0435177N to Y.K.) and by NIH grants (to K.S.K.).
References
- Belton CM, Goodwin PC, Fatherazi S, Schubert MM, Lamont RJ, Izutsu KT. Calcium oscillations in gingival epithelial cells infected with Porphyromonas gingivalis. Microbes Infect. 2004;6:440–477. doi: 10.1016/j.micinf.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bierne H, Dramsi S, Gratacap MP, Randriamampita C, Carpenter G, Payrastre B, Cossart P. The invasion protein InIB from Listeria monocytogenes activates PLC-gamma1 downstream from PI 3-kinase. Cell Microbiol. 2000;2:465–476. doi: 10.1046/j.1462-5822.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- Chung JW, Hong SJ, Kim KJ, Goti D, Stins MF, Shin S, Dawson VL, Dawson TM, Kim KS. 37 kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J Biol Chem. 2003;278:16857–16862. doi: 10.1074/jbc.M301028200. [DOI] [PubMed] [Google Scholar]
- Dramsi S, Cossart P. Listeriolysin O-mediated calcium influx potentiates entry of Listeria monocytogenes into the human Hep-2 epithelial cell line. Infect Immun. 2003;71:3614–3618. doi: 10.1128/IAI.71.6.3614-3618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Raybourne RB, Kopecko DJ. Ca2+ release from host intracellular stores and related signal transduction during Campylobacter jejuni 81–176 internalization into human intestinal cells. Microbiology. 2005;151:3097–3105. doi: 10.1099/mic.0.27866-0. [DOI] [PubMed] [Google Scholar]
- Huang SH, Wass CA, Fu Q, Nemani PV, Stins M, Kim KS. E. coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of E. coli invasion gene Ibe10. Infect Immun. 1995;63:4470–4475. doi: 10.1128/iai.63.11.4470-4475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- Khan NA, Kim Y, Shin S, Kim KS. FimH-mediated Escherichia coli invasion of human brain microvascular endothelial cells. Cell Microbiol. 2007;9:169–178. doi: 10.1111/j.1462-5822.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Elliott SA, DiCello F, Stins MF, Kim KS. The K1 capsule modulates trafficking of E. coli-containing vacuoles and enhances intracellular bacterial survival in human brain microvascular endothelial cells. Cell Microbiol. 2003;5:245–252. doi: 10.1046/j.1462-5822.2003.t01-1-00271.x. [DOI] [PubMed] [Google Scholar]
- Kim KS. E. coli translocation at the blood-brain barrier. Infect Immun. 2001;69:5217–5222. doi: 10.1128/IAI.69.9.5217-5222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS. Neurological diseases: pathogenesis of bacterial meningitis: from bacteremia to neuronal injury. Nat Rev Neurosci. 2003;4:376–385. doi: 10.1038/nrn1103. [DOI] [PubMed] [Google Scholar]
- Kim YV, DiCello F, Hillaire CS, Kim KS. Protease-activated receptors of human brain microvascular endothelial cells: expression and differential Ca2+ signaling induced by thrombin and protease-activated receptor-1 activating peptide. Am J Physiol Cell Physiol. 2004;286:C31–C42. doi: 10.1152/ajpcell.00157.2003. [DOI] [PubMed] [Google Scholar]
- Koschinski A, Repp H, Unver B, Dreyer F, Brockmeier B, Valeva A, Bhakdi S, Walev I. Why Escherichia coli α-hemolysin induces calcium oscillations in mammalian cells—the pore is on its own. FASEB J. 2006;20:973–975. doi: 10.1096/fj.05-4561fje. [DOI] [PubMed] [Google Scholar]
- Marlink KL, Bacon KD, Sheppard BC, Ashktorab H, Smoot DT, Cover TL, Deveney CW, Rutten MJ. Effects of Helicobacter pylori on intracellular Ca2+ signaling in normal human gastric mucous epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G163–G176. doi: 10.1152/ajpgi.00257.2002. [DOI] [PubMed] [Google Scholar]
- Nemani PV, Huang SH, Wass CA, Kim KS. Identification and characterization of a novel Ibe10 binding protein contributing to E. coli invasion of brain microvascular endothelial cells. Infect Immun. 1999a;67:1131–1138. doi: 10.1128/iai.67.3.1131-1138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani PV, Stins M, Wass CA, Shimada H, Kim KS. Outer membrane A promoted cytoskeletal rearrangement of brain microvascular endothelial cells is required for E. coli invasion. Infect Immun. 1999b;67:5775–5783. doi: 10.1128/iai.67.11.5775-5783.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolskaia OV, Lima AP de, Kim YV, Lonsdale-Eccles JD, Fukuma T, Scharfstein J, Grab DJ. Blood-brain barrier traversal by African trypanosomes requires calcium signaling induced by parasite cysteine protease. J Clin Invest. 2006;116:2739–2747. doi: 10.1172/JCI27798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace J, Hayman MJ, Galan JE. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell. 1993;72:505–514. doi: 10.1016/0092-8674(93)90070-7. [DOI] [PubMed] [Google Scholar]
- Ratner AJ, Bryan R, Weber A, Nguyen S, Barnes D, Pitt A, Gelber S, Cheung A, Prince A. Cystic fibrosis pathogens activate Ca2+ dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J Biol Chem. 2001;276:19267–19275. doi: 10.1074/jbc.M007703200. [DOI] [PubMed] [Google Scholar]
- Repp H, Pamukçi Z, Koschinski A, Domann E, Darji A, Birringer J, et al. Listeriolysin of Listeria monocytogenes forms Ca2+ permeable pores leading to intracellular Ca2+ oscillations. Cell Microbiol. 2002;4:483–491. doi: 10.1046/j.1462-5822.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- Shiner EK, Terentyev D, Bryan A, Sennoune S, Martinez-Zaguilan R, Li G, Gyorke S, Williams SC, Rumbaugh KP. Psedomonas aeuruginosa autoinducer modulates host cell responses through calcium signaling. Cell Microbiol. 2006;8:1601–1610. doi: 10.1111/j.1462-5822.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- Soderblom T, Oxhamre C, Wai SN, Uhlen P, Aperia A, Uhlin BE, Richter-Dahlfors A. Effects of the Escherichia coli toxin cytolysin A on mucosal immunostimulation via epithelial Ca2+ signalling and Toll-like receptor 4. Cell Microbiol. 2005;7:779–788. doi: 10.1111/j.1462-5822.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- Stins MF, Gilles F, Kim KS. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Tsien RY. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Tran Van Nhieu G, Clair C, Grompone G, Sansonetti P. Calcium signalling during cell interactions with bacterial pathogens. Biol Cell. 2004;96:93–101. doi: 10.1016/j.biolcel.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Rossum DB van, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002;4:263–E272. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci. 2005;6:267–276. doi: 10.1038/nrn1647. [DOI] [PubMed] [Google Scholar]