Abstract
Objective:
At present, there are many antiepileptic drugs with a wide range of side effects on the human body. It was assumed that Zataria multiflora Boiss (Z. multiflora) with sedative, anti-spasmodic and anti-inflammatory activity may be effective in the treatment of epilepsy. The aim of the present study was to elucidate the effect of Z. multiflora hydroalcoholic extract and its fraction extracts on pentylenetetrazole (PTZ)-induced chemical kindling.
Materials and Methods:
In this experimental study, eight separate groups of male albino mice were used. All groups received 11 separate intraperitoneal injections of PTZ (35 mg/kg) with two-day intervals. 30 min before the injection of PTZ, mice received vehicle, Z. multiflora hydroalcoholic extract (300 and 600 mg/kg), n-hexane, acetone, methanol fraction extracts (150 mg/kg), or diazepam (10 mg/kg).
Results:
The kindled mice that were pretreated with vehicle showed a gradual increase in their seizure scores up to the end of the study. The hydroalcoholic extract of Z. multiflora (300 and 600 mg/kg) reduced seizure scores significantly. However, n-hexane, acetone and methanol extracts did not affect seizure scores significantly.
Conclusion:
The present findings demonstrate that the hydroalcoholic extract of Z. multiflora did reduce the severity of seizure attacks in PTZ-induced chemical kindling in mice.
Key Words: Seizure, Zataria multiflora Boiss, Plant extracts, Pentylenetetrazole
Introduction
Epilepsy is a common neurological disorder recognized by unpredictable and episodic repeated seizures, which are induced by abnormal discharges of cerebral neurons. Different types of seizures are identified based on their clinical features. Seizures are mostly divided into two main categories: partial and generalized (Italiano et al., 2014 ▶). In partial seizures, attacks have a localized onset in the brain, while in generalized seizures they have a distributed onset. This disorder has physical, psychological and socioeconomic side effects that further affect patients’ quality of life. According to the previous reports, 0.84 to 1.54 percent of people worldwide have epilepsy (Helmers et al., 2015 ▶ and Bell et al., 2014 ▶). Prevention of recurrent seizures is the main goal in the treatment of epilepsy. Because of the refractory epilepsies, adverse effects of the existing antiepileptic medications and inability of the current medications in correcting abnormalities that induce epilepsy, finding new medications for epilepsy is of great importance (Sucher and Carles, 2015 ▶). In recent years, interest in finding herbal-based medications for the treatment of various diseases, including epilepsy, has been growing (Sucher and Carles, 2015 ▶). Previous evidence showed that some herbal medications are useful in the treatment of epilepsy (Zhu et al., 2014 ▶).
Zataria multiflora Boiss (Z. multiflora) is a thyme-like plant. Z. multiflora grows only in southern and central Iran, Afghanistan and Pakistan (Sajed et al., 2013 ▶). The plant has phytochemicals similar to Thymus vulgaris, a well-known and widely used medicinal plant. It has low toxicity in in vivo studies, and has been largely used as a condiment in the food industry (Sajed et al., 2013 ▶). This plant has several medicinal uses including treatment of fever, premature labor pain, bone and joint pain, headache, migraine, common cold, bloating, nausea, and diarrhea (Naghibi et al., 2005 ▶). Z. multiflora extract has a prominent effect in animal models of lung diseases such as chronic obstructive pulmonary disease (Boskabady and Gholami Mahtaj, 2014 ▶).
Animal models of epilepsy have been used extensively for finding better antiepileptic drugs. Chemical kindling is a model of epileptic seizures. In this model, seizure is induced by repeated administration of an initially sub-convulsive chemical such as pentylenetetrazole (PTZ) (Hansen et al., 2004 ▶). These administrations decrease seizure threshold and culminate in a generalized seizure (Hansen et al., 2004 ▶). Kindling is a valuable experimental model for complex partial epilepsy in patients (Kupferberg, 2001 ▶), and is considered as a drug resistant model of epilepsy (Loscher et al., 1993 ▶). Z. multiflora extract is able to inhibit calcium channels (Gharib Naseri, 2003 ▶). This inhibition leads to anticonvulsant effects in vivo (Damasceno et al., 2012 ▶). Considering this finding and previous evidence regarding the anticonvulsive (Mandegary et al., 2013 ▶) and sedative effects (Sharif Rohani et al., 2008 ▶) of Z. multiflora, we aimed to assess the effect of the extract of this plant on PTZ-induced chemical kindling.
Materials and Methods
Plant material and preparation of extracts
Z. multiflora aerial parts were collected during March 2011 from Isfahan Botany Herbarium, and were identified by Dr. Valiollah Mozaffarian at Botany Research Division, Research Institute of Forests and Rangelands, Tehran, Iran. A voucher specimen has been kept in Isfahan Botany Herbarium (voucher specimen no. F-1-8-4-21). For preparation of the hydroalcoholic extract, 250 g of dried and powdered Z. multiflora was macerated with 80% ethanol (80% ethanol – 20% water). To obtain the non-polar, semi-polar and polar extract fractions, powdered sample of Z. multiflora was extracted with n-hexane three times during 72 h with constant stirring. The remaining solid material was then extracted with acetone and then with methanol with the same procedure at room temperature (Molina-Salinas et al., 2006 ▶). The solvents were eliminated from the extracts by evaporation under vacuum in a rotary evaporator. The crude extract and its fractions were stored at -20 oC before experiments.
Animals
Male albino mice weighing 25–35 g were used in this study. Animals were housed six per cage, in a room with a 12:12 h light/dark cycle (lights on at 07:00 h) and controlled temperature (23 ± 2 oC). Animals had free access to food and water. The method of this study was approved by the Ethics and Animal Care Committee of Rafsanjan University of Medical Sciences.
Induction of kindling and experimental design
For induction of kindling, PTZ (35 mg/kg) was injected intraperitoneally every other day for 20 days (Ben et al., 2014 ▶). The vehicle (10 ml/kg), hydroalcoholic extract (300 and 600 mg/kg), fraction extracts (150 mg/kg), and diazepam (as control, 10 mg/kg), all were administered intraperitoneally 30 min before PTZ injections. The doses for hydroalcoholic extract and diazepam were selected according to studies of Arzi et al., 2003 and Crestani et al., 2000 respectively. In injection days, each mouse was placed in a Plexiglas box and its behavior was observed for 30 min to record the incidence of convulsions. The intensity of the seizure response was scored on the following scale: 0=no response; 1=vibrissae twitching, mouth and facial jerks; 2=myoclonic body jerks or head nodding; 3=forelimb clonus; 4=rearing, falling down, forelimb tonus, and hindlimb clonus; and 5=tonic extension of hindlimb, status epilepticus (Jain et al., 2011 ▶). The highest response was recorded for each animal for each day.
Statistical analysis
The means of seizure scores were recorded in experimental groups. Repeated measurement analysis of variance (RMA) followed by Dunnett post hoc test was used for comparing the seizure scores in different times in each group. For comparison scores between different groups, one-way ANOVA followed by Dunnett post hoc test was used. Data are expressed as means ± S.E.M of six animals per group. p<0.05 was considered statistically significant.
Results
The effect of Z. multiflora hydroalcoholic extract on PTZ-induced kindling
The results showed that repeated administration of PTZ for 20 days (control group) gradually decreased seizure threshold, which was manifested as increased seizure scores (p<0.01).
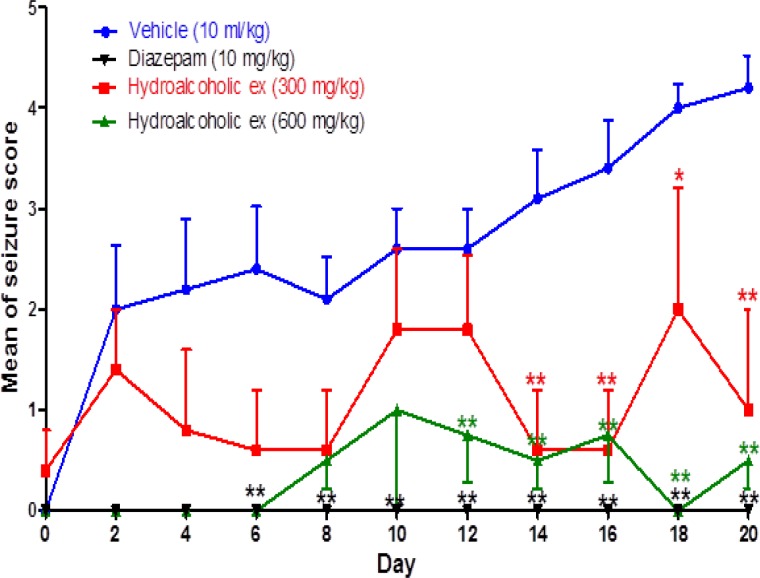
In animals that were pretreated with hydroalcoholic extract of Z. multiflora (300 and 600 mg/kg), the seizure scores were not statistically different from day 0 to 20. Moreover, comparing the seizure scores between these animals with the control group (day to day comparison) showed significant differences in seizure scores in days 14-20 for 300 mg/kg extract and days 6-20 for 600 mg/kg extract (p<0.01). These data indicated that hydroalcoholic extract of Z. multiflora inhibited the development of PTZ-induced chemical kindling (figure 1). Diazepam as control drug at the dose of 10 mg/kg had a significant effect in controlling seizure scores (p< 0.01, Figure 1).
Figure 1.
The effect of different doses of Zataria multiflora Boiss hydroalcoholic extract and diazepam on the mean of seizure score in PTZ kindled rats. Values are mean ± S.E.M. *: p<0.05 and **: p<0.01 when compared with the respective day in the vehicle-injected control group
The effect of Z. multiflora fractionated extracts on PTZ-induced kindling
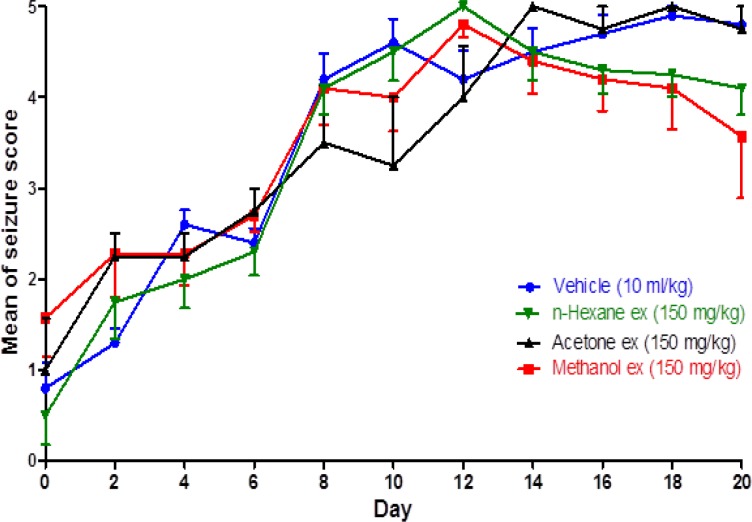
The effects of n-hexane, acetone and methanol extracts on PTZ-induced kindling are presented in Figure 2. In these animals repeated administration of PTZ for 20 days gradually increased seizure scores (p<0.001).
Figure 2.
The effect of different fractions of Zataria multiflora Boiss on the mean of seizure scores in PTZ kindled rats. Values are mean ± S.E.M
Moreover, comparing the seizure scores between these animals with the control group (day to day comparison), showed no significant difference in seizure scores in any of the 20 experimental days (p>0.05). These data indicated that in contrast to the results of the total plant extract (hydroalcoholic), n-hexane, acetone and methanol extracts did not inhibit the development of PTZ-induced chemical kindling (Figure 2).
Discussion
The main finding of the present study is that hydroalcoholic extract of Z. multiflora had an anticonvulsant effect, while unexpectedly its n-hexane, acetone and methanol fraction extracts did not show any significant effect in PTZ kindled mice. This finding suggests that a mixture of different chemical compounds in the hydroalcoholic extract was able to reduce seizure score, whereas separation of these compounds by fractionation reduced the anticonvulsant effect of the plant extract. In agreement with the present finding, the anticonvulsant effect of total plant extract (methanolic) and essential oil of Z. multiflora against PTZ- and maximal electroshock- induced convulsions was reported in a recent study (Mandegary et al., 2013 ▶). The main difference between the present study and the previous one is the animal model that was employed. Furthermore, neither of the previous studies evaluated the effect of Z. multiflora fractions on experimental models of seizure. In the present study a low and non-convulsant dose of PTZ (35 mg/kg) gradually induced seizure attacks resembling complex partial epilepsy (Kupferberg, 2001 ▶), while administration of a high dose of PTZ (100 mg/kg) in the other study induced convulsions similar to absence and myoclonic seizures in humans (Loscher and Schmidt, 1988 ▶). The protective effects of Z. multiflora against maximal electroshock in Mandegary and coworkers’ study, may imply that the total extract is also able to reduce generalized tonic-clonic seizures in clinical practice (Loscher et al., 1991 ▶). Furthermore, it has been reported that Z. multiflora hydroalcoholic extract is able to reduce nicotine-induced convulsions (Arzi et al., 2003 ▶). These findings suggest that Z. multiflora is a plant with possible broad anticonvulsant effects that needs further basic and clinical studies. However, in the present study, fractionation of the extract, as discussed previously, did not produce such anticonvulsant effects. This implies that the total extract should be considered for future studies. The main active constituents of Z. multiflora are thymol, carvacrol, p-cymene, γ-terpinene, linalool apigenin, luteolin, 6-hydroxyluteolin, and β-sitosterol (Sajed et al., 2012 ▶). Some of these natural chemicals have significant protective effects in animal models of seizure. For example, in a recent study it was demonstrated that thymol had anticonvulsant effects in PTZ-induced chemical kindling (Sancheti et al., 2014 ▶). Another study revealed that carvacrol reduced latency to seizure both in PTZ- and electroshock-induced convulsions (Quintans-Júnior et al. 2013 ▶). Such anticonvulsant effects have also been reported for apigenin (Han et al., 2012 ▶). Hence, we suggest that these compounds possibly participated, at least in part, in the anticonvulsant effect of Z. multiflora ethanolic extract.
Similar to Z. multiflora hydroalcoholic extract, there are other plants with good anticonvulsant effects such as Valeriana officinalis (Rezvani et al., 2010 ▶) and Rosa damascena (Homayoun et al., 2015 ▶). Because of limited geographical distribution of Z. multiflora, the pharmacological effects of the extract have not been evaluated in detail. Hence, it is hard to suggest the molecular mechanisms behind the anticonvulsant effect of Z. multiflora hydroalcoholic extract. One explanation is blockade of calcium channels; previous studies showed that inhibition of L-type calcium channels can reduce seizure activity in vivo (Damasceno et al., 2012 ▶). Moreover, hydroalcoholic extract of Z. multiflora through inhibition of calcium channels had a spasmolytic effect on rat ileum (Naseri, 2003 ▶). Thus, it is a possibility that Z. multiflora extract through inhibition of these channels reduced convulsions. It has been reported that following a single injection of PTZ or PTZ-induced kindling, the brain antioxidant system changed significantly (Erakovic et al., 2003 ▶). Accordingly, recent studies are considering oxidative stress as an important factor in the pathophysiology of epilepsy (Martinc et al., 2014 ▶). Therefore, it is a possibility that Z. multiflora, through inhibition of oxidative stress (Boskabady and Gholami Mahtaj, 2015 ▶), decreased the seizure score in the present study. It may also be suggested that a combination of antioxidants in different fractions was necessary to induce such antiepileptic effects. On the other hand, Z. multiflora essential oil had beneficial effects in a mouse model of Alzheimer’s disease (Majlessi et al., 2012 ▶). The anti-cholinesterase effects of Z. multiflora essential oil have also been reported separately (Sharififar et al., 2012 ▶). Tacrine, as an important drug in the treatment of Alzheimer’s disease, is able to reduce the deteriorative effect of PTZ on memory during kindling (Getova and Dimitrova, 2000 ▶), therefore it may be suggested that Z. multiflora may also be useful in the treatment of memory failure in epileptic patients (Piazzini et al., 2001 ▶). In the present study, we did not evaluate the effect of the extract and its fractions on motor coordination and locomotor activity of the animals as potential confounding factors. It is a possibility that Z. multiflora through inhibition of locomotor activity and/or change in muscular tone changed the seizure scores. Although this hypothesis was rejected by Mandegari et al. (2013) ▶, who showed that administration of the methanolic extract of Z. multiflora up to 2 g/kg did not change the rotarod performance of the mice. In conclusion, Z. multiflora had an anticonvulsant effect in PTZ induced kindling in mice. However, fractionation of the total extract did not induce such effects.
Acknowledgment
This study was supported by a grant from Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
Conflict of interest
There is no conflict of interests.
References
- Arzi A, Zahedi Asl S, Fallah Zadeh D. Study of the preventive effect of Hydroalcoholic extract of Thymus Vulgaris (TV) on Nicotine induced convulsion. Jundishapur Sci Med J. 2003;37:61–72. [Google Scholar]
- Bell GS, Neligan A, Sander JW. An unknown quantity--the worldwide prevalence of epilepsy. Epilepsia. 2014;55:958–962. doi: 10.1111/epi.12605. [DOI] [PubMed] [Google Scholar]
- Ben J, de Oliveira PA, Goncalves FM, Peres TV, Matheus FC, Hoeller AA, Leal RB, Walz R, Prediger RD. Effects of pentylenetetrazole kindling on mitogen-activated protein kinases levels in neocortex and hippocampus of mice. Neurochem Res. 2014;39:2492–2500. doi: 10.1007/s11064-014-1453-5. [DOI] [PubMed] [Google Scholar]
- Boskabady MH, Gholami Mahtaj L. Effect of the Zataria multiflora on systemic inflammation of experimental animals model of COPD. Biomed Res Int. 2014;2014:802189. doi: 10.1155/2014/802189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskabady MH, Gholami Mahtaj L. Lung inflammation changes and oxidative stress induced by cigarette smoke exposure in guinea pigs affected by Zataria multiflora and its constituent, carvacrol. BMC Complement Altern Med. 2015;15:39. doi: 10.1186/s12906-015-0574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Martin JR, Mohler H, Rudolph U. Mechanism of action of the hypnotic zolpidem in vivo. Br J Pharmacol. 2000;131:1251–1254. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasceno DD, Ferreira AJ, Doretto MC, Almeida AP. Anticonvulsant and antiarrhythmic effects of nifedipine in rats prone to audiogenic seizures. Braz J Med Biol Res. 2012;45:1060–1065. doi: 10.1590/S0100-879X2012007500119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erakovic V, Zupan G, Varljen J, Simonic A. Pentylenetetrazol-induced seizures and kindling: changes in free fatty acids, superoxide dismutase, and glutathione peroxidase activity. Neurochem Int. 2003;42:173–178. doi: 10.1016/s0197-0186(02)00070-0. [DOI] [PubMed] [Google Scholar]
- Getova DP, Dimitrova DS. Effects of the anticholinesterase drug tacrine on the development of PTZ kindling and on learning and memory processes in mice. Folia Med (Plovdiv) 2000;42:5–9. [PubMed] [Google Scholar]
- Gharib Naseri MK. Effect of Zataria multiflora Boiss leaf hydroalchoholic extract on rat ileum. Behbood J. 2003;7:18–26. [Google Scholar]
- Han JY, Ahn SY, Kim CS, Yoo SK, Kim SK, Kim HC, Hong JT, Oh KW. Protection of apigenin against kainate-induced excitotoxicity by anti-oxidative effects. Biol Pharm Bull. 2012;35:1440–1446. doi: 10.1248/bpb.b110686. [DOI] [PubMed] [Google Scholar]
- Hansen SL, Sperling BB, Sanchez C. Anticonvulsant and antiepileptogenic effects of GABAA receptor ligands in pentylenetetrazole-kindled mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:105–113. doi: 10.1016/j.pnpbp.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Helmers SL, Thurman DJ, Durgin TL, Pai AK, Faught E. Descriptive epidemiology of epilepsy in the US population: A different approach. Epilepsia. 2015;56:942–8. doi: 10.1111/epi.13001. [DOI] [PubMed] [Google Scholar]
- Homayoun M, Seghatoleslam M, Pourzaki M, Shafieian R, Hosseini M, Ebrahimzadeh Bideskan A. Anticonvulsant and neuroprotective effects of Rosa damascena hydro-alcoholic extract on rat hippocampus. Avicenna J Phytomed. 2015;5:260–270. [PMC free article] [PubMed] [Google Scholar]
- Italiano D, Ferlazzo E, Gasparini S, Spina E, Mondello S, Labate A, Gambardella A, Aguglia U. Generalized versus partial reflex seizures: a review. Seizure. 2014;23:512–520. doi: 10.1016/j.seizure.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Jain S, Bharal N, Khurana S, Mediratta PK, Sharma KK. Anticonvulsant and antioxidant actions of trimetazidine in pentylenetetrazole-induced kindling model in mice. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:385–392. doi: 10.1007/s00210-011-0606-1. [DOI] [PubMed] [Google Scholar]
- Kupferberg H. Animal models used in the screening of antiepileptic drugs. Epilepsia. 2001;42:7–12. [PubMed] [Google Scholar]
- Loscher W, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs II Maximal electroshock seizure models. Epilepsy Res. 1991;8:79–94. doi: 10.1016/0920-1211(91)90075-q. [DOI] [PubMed] [Google Scholar]
- Loscher W, Rundfeldt C, Honack D. Pharmacological characterization of phenytoin-resistant amygdala-kindled rats, a new model of drug-resistant partial epilepsy. Epilepsy Res. 1993;15:207–219. doi: 10.1016/0920-1211(93)90058-f. [DOI] [PubMed] [Google Scholar]
- Loscher W, Schmidt D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988;2:145–181. doi: 10.1016/0920-1211(88)90054-x. [DOI] [PubMed] [Google Scholar]
- Majlessi N, Choopani S, Kamalinejad M, Azizi Z. Amelioration of amyloid beta-induced cognitive deficits by Zataria multiflora Boiss essential oil in a rat model of Alzheimer's disease. CNS Neurosci Ther. 2012;18:295–301. doi: 10.1111/j.1755-5949.2011.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandegary A, Sharififar F, Abdar M. Anticonvulsant effect of the essential oil and methanolic extracts of Zataria multiflora Boiss. Cent Nerv Syst Agents Med Chem. 2013;13:93–97. doi: 10.2174/1871524911313020001. [DOI] [PubMed] [Google Scholar]
- Martinc B, Grabnar I, Vovk T. Antioxidants as a preventive treatment for epileptic process: a review of the current status. Curr Neuropharmacol. 2014;12:527–550. doi: 10.2174/1570159X12666140923205715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Salinas GM, Ramos-Guerra MC, Vargas-Villarreal J, Mata-Cardenas BD, Becerril-Montes P, Said-Fernandez S. Bactericidal activity of organic extracts from Flourensia cernua DC against strains of Mycobacterium tuberculosis. Arch Med Res. 2006;37:45–49. doi: 10.1016/j.arcmed.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Naghibi F, Mosaddegh M, Mohammadi Motamed S, Ghorbani A. Labiatae family in folk medicine in Iran: from ethnobotany to pharmacology. Iran J Pharm Res. 2005;2:63–79. [Google Scholar]
- Naseri MKG. Effect of Zataria multiflora Boiss leaf hydroalcoholic extract on rat ileum. J Kermanshah Univ Med Sci. 2003;7:1–4. [Google Scholar]
- Piazzini A, Canevini MP, Maggiori G, Canger R. The perception of memory failures in patients with epilepsy. Eur J Neurol. 2001;8:613–620. doi: 10.1046/j.1468-1331.2001.00287.x. [DOI] [PubMed] [Google Scholar]
- Quintans-Júnior L J, Guimarães AG, Araújo BE, Oliveira GF, Santana MT, Moreira FV, Santos MRV, Cavalcanti WD, Lucca Júnior WD, Botelho M, Ribeiro LA, Nóbrega FF, Almeida RN. Carvacrol,(-)-borneol and citral reduce convulsant activity in rodents. Afr J Biotech. 2013;9:6566–6572. [Google Scholar]
- Rezvani ME, Roohbakhsh A, Allahtavakoli M, Shamsizadeh A. Anticonvulsant effect of aqueous extract of Valeriana officinalis in amygdala-kindled rats: possible involvement of adenosine. J Ethnopharmacol. 2010;127:313–318. doi: 10.1016/j.jep.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Sajed H, Sahebkar A, Iranshahi M. Zataria multiflora Boiss (Shirazi thyme) an ancient condiment with modern pharmaceutical uses. J Ethnopharmacol. 2013;145:686–698. doi: 10.1016/j.jep.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Sancheti J, Shaikh MF, Chaudhari R, Somani G, Patil S, Jain P, Sathaye S. Characterization of anticonvulsant and antiepileptogenic potential of thymol in various experimental models. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:59–66. doi: 10.1007/s00210-013-0917-5. [DOI] [PubMed] [Google Scholar]
- Sharif rohani M, Assaeian H, Leshtoo aghaee G. A study of anesthetic effect of Zataria multiflora Boiss (Labiatae) essence on Oncorhynchus mykiss and cultured Salmo trutta caspius. Iran Sci Fish J. 2008;16:99–106. [Google Scholar]
- Sharififar F, Mirtajadini M, Azampour MJ, Zamani E. Essential oil and methanolic extract of Zataria multiflora Boiss with anticholinesterase effect. Pak J Biol Sci. 2012;15:49–53. doi: 10.3923/pjbs.2012.49.53. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Carles MC. A pharmacological basis of herbal medicines for epilepsy. Epilepsy Behav. 2015;52:308–318. doi: 10.1016/j.yebeh.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Zhu HL, Wan JB, Wang YT, Li BC, Xiang C, He J, Li P. Medicinal compounds with antiepileptic/anticonvulsant activities. Epilepsia. 2014;55:3–16. doi: 10.1111/epi.12463. [DOI] [PubMed] [Google Scholar]