Abstract
Objective:
Oxidative stress plays an important role in the development of diabetic complications including metabolic abnormality-induced diabetic micro-vascular and macro-vascular complications. Urtica dioica L. (U. dioica) has been traditionally used in Iranian medicine as an herbal remedy for hypoglycemic or due to its anti-inflammatory properties. The aim of the present study was to evaluate the effects of hydro-alcoholic extract of U. dioica on blood lipids, hepatic enzymes and nitric oxide levels in patients with type 2 diabetes mellitus.
Materials and Methods:
50 women with type 2 diabetes participated in this study and were randomly divided into two groups namely, control and intervention groups. Control group received placebo and intervention group received hydro-alcoholic extract of U. dioica. Before and after 8 weeks of continuous treatment, some biochemical serum levels including FPG, TG, SGPT, SGOT, HDL, LDL, SOD and NO were measured.
Results:
The results indicated that after 8 weeks, in the intervention group, FPG, TG, and SGPT levels significantly decreased and HDL, NO and SOD levels significantly increased as compared to the control group.
Conclusion:
Our results encourage the use of hydro-alcoholic extract of U. dioica as an antioxidant agent for additional therapy of diabetes as hydro-alcoholic extract of U. dioica may decrease risk factors of cardiovascular incidence and other complications in patients with diabetes mellitus.
Key Words: Urticadioica, Hydro-alcoholic extract, Oxidative stress, Type2 diabetes
Introduction
Diabetes mellitus is one of the most common diseases and hyperglycemia is one of the predisposing factors for oxidative stress (Manohar et al., 2013 ▶; Petal et al., 2012 ▶; Rasheed et al., 2008 ▶). In a healthy individual, there is a balance between antioxidant enzymes and free radical species in the body and an imbalance causes oxidative stress. Free radicals are atoms or molecules with unpaired electron, which are highly active and can damage different tissues in the body (Nazemi et al., 2012 ▶). Therefore, increasing oxidative stress can increase risk factors of cardiovascular incidence and other complications in patients with diabetes mellitus (Rains and Jain, 2011 ▶; Malekirad et al., 2011 ▶). Reactive oxygen species (ROS) such as hydrogen peroxide, superoxide, and hydroxyl radical are increased in diabetic patients and are commonly associated with cell damage. Moreover, it is well established that oxidative stress is an important metabolic abnormality in both cardiovascular disease and diabetic micro and macro-vascular complications (Nojima et al., 2008 ▶). As alternatives to chemical agents, plants are regarded as potential sources of antioxidants and hypoglycemic agents to control and treat diabetic patients (Patience et al., 2014 ▶; Malviya et al., 2010 ▶). Moreover, searching for new anti-diabetic drugs from natural plants is still attractive because of their low side effects, easy availability, roughly low cost, and also high effectiveness. Urtica dioica L. (U. dioica), a perennial herbaceous plant belonging to the Urticaceae family (Di Virgilio et al., 2014 ▶), is one of the medical herbs that has been traditionally consumed for a long time as medicinal plants in Iran and many parts of the world. Several studies showed beneficial effects of U. dioica against different diseases such as rheumatoid arthritis and diabetes (Nazemi et al., 2012 ▶). U. dioica has been extensively studied and has been promising in the treatment of prostate enlargement (Mamta and Preeti, 2014 ▶; Nahata et al., 2012 ▶), urinary tract infections and inflammation, nephrolithiasis, allergies, poor circulation, spleen enlargement, diabetes and other endocrine disorders. Also, U. dioica helps to lessen the swelling of hemorrhoids and stop bleeding from minor skin injuries (Ebrahimzadeh et al., 2015 ▶). It was also reported that U. dioica prevents the damage of liver tissue structure in rats (Turkdogan et al., 2003 ▶). In addition, U. dioica has been shown to have a protective effect against hepatic ischemia-reperfusion (Kandis et al., 2010 ▶), hyperglycemia (Otles and Yalcin, 2012 ▶) and hyper-cholesterolemia (Nassiri- asl et al., 2009 ▶). Supplementation of U. dioica leaves beverage has been shown to have a significant protective effect against TCA-induced liver injury (Celik and Tuluce, 2007 ▶). Moreover, some studies have shown antioxidant activity of U. dioica (Nazemi et al., 2012 ▶). Although, U. dioica is used for some in vivo and in vitro experiments, there is little evidence for the effect of U. dioica on diabetic complication and there is not any report about the effect of U. dioica on nitric oxide serum levels in diabetic patients. Therefore, the aim of the present study was to investigate the effects of hydro-alcoholic extract of U. dioica on some diabetes-related risk factors of cardiovascular incidence as well as oxidative stress biomarkers in patients with type 2 diabetes.
Materials and Methods
A randomized double-blinded clinical trial was done on 50 women with type 2 diabetes (T2DM) in Diabetes Society of Shahroud affiliated to Shahroud University of Medical Sciences, Shahroud, Iran (IRCT2015031021412N1). The research was approved by the Ethics committee and Human Studies review board of Shahroud University of Medical Sciences by the identification code 920/11. The sample size was determined according to the following formula with consideration of type I error level α of % 0.05 and a test power of %90 (Khan et al., 2003 ▶).
The sample size was computed as 20 per group. Regarding a possible loss to follow-up, a safety margin of 25% was determined, and therefore, 25 patients were allocated in each group. Participants were recruited from February 2014 to May 2014. The inclusion criteria to study were as follows: Women over the age of 50 years old, HbA1c levels equal or less than 10%, using common diabetes drugs (metformin and glibenclamide), patients with triglyceride levels less than 400 mg/dl. The exclusion criteria were: patients that have cardiovascular, chronic kidney and liver diseases, allergies and who were regularly using non-steroidal anti-inflammatory drugs (NSAIDs), warfarin, alcohol and insulin injection. Participants were divided into two groups: First, 25 women with type 2 diabetes who did not receive hydro-alcoholic extract of U. dioica (control group) and second, 25 women with type 2 diabetes who received hydro-alcoholic extract of U. dioica (intervention group). After being informed about the aim of present investigation, each patient signed an informed consent form, and was advised to continue her diet and physical activity habits without any changes during the intervention. After adjusting the patients by age and duration of diabetes, they were randomly divided into control and intervention groups using computer’s random numbers (Mahluji et al., 2013 ▶).
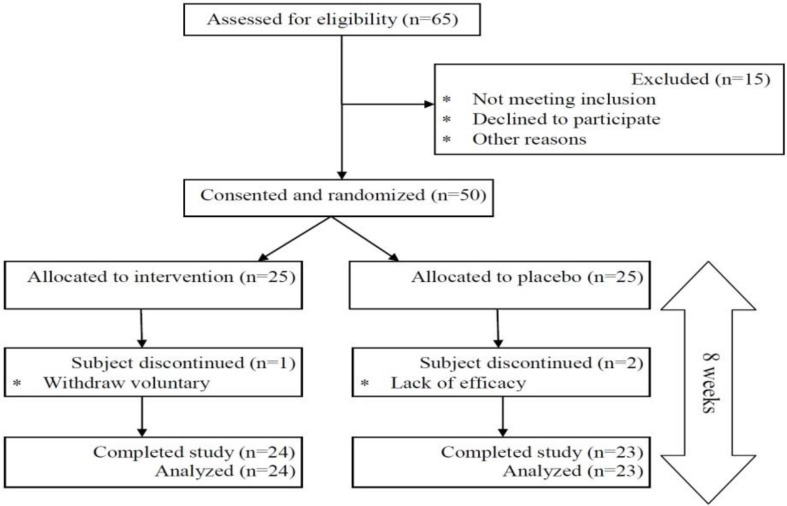
Patients received 5 ml of hydro-alcoholic extract of U. dioica or placebo (water, alcohol and chlorophyll color) in 3 portions a day (every 8 hr), after each main meal. Both experimental and placebo treatments were contained in the same bottles of liquid herbal extract with identical appearance, which were administered by a blinded research assistant. They dissolved each portion in 1 glass of water and continued for 8 weeks. The Participants in intervention and placebo groups were instructed to complete 24-hr dietary recall for three days (2 week days and 1 weekend day) at baseline and the end of study. Weight and height were measured by standard methods and the demographic characteristics (including BMI) were recorded for all patients. BMI was calculated by dividing weight (kg) by square of height (m). Patients were contacted every week and they were asked for any complaint about using U. dioica extract such as probable side effects including nausea and reflux and serious drug interactions with other drugs. Also, they were asked to return used bottles of U. dioica extract and placebo and receive new bottles. The flowchart in Figure 1 describes the progress of the trial.
Figure 1.
Study flowchart describing the progress of the trial
Extract specifications
U. dioica certified by the Pharmacogenosy department of Gorgan University of Medical Science, Gorgan, Iran. Aerial parts of U. dioica were dried and powdered and the extract was prepared with percolation method using ethanol [60% (v/v) ethanol/water]. Final hydro-alcoholic extract of U. dioica contained 45% ethanol, 55% water and 2.7 g of dry matter in 1 l of extract. Water and alcohol percentage in placebo was equal to water and alcohol percentage in U. dioica extract, chlorophyll color was added to placebo. There was not any difference in the color between U. dioica extract and placebo.
Biochemical measurements
Before and after 8 weeks of continuous treatment, blood sample was taken from forearm vein of 2 groups (at the beginning and the end of the study) after overnight fasting (12-14 hr) for biochemical analysis of fasting plasma glucose (FPG), triglycerides (TG), low density lipoprotein (LDL), high density lipoprotein (HDL), glutamic pyruvic transaminase (SGPT), glutamic oxaloacetic transaminase (SGOT), superoxide dismutase (SOD) and nitric oxide (NO) using commercially available kits of Abcam (UK). HDL and LDL levels were analyzed using a Pars Azmoon kit (Pars Azmoon Co., Tehran, Iran).
Statistical analysis
Data are reported as Mean±SD. Statistical analysis was performed by Prism (Harvey, 2007 ▶). The normality of the distribution of variables was determined by the Kolmogorov-Smirnov test. Data with Abnormal distribution were converted to normal distribution by calculating logarithmic ratio. For variables with normal distribution, a paired t-test was used and comparison quantitative variables between groups were performed by Student’s t-test. A p<0.05 was considered significant for all variables.
Results
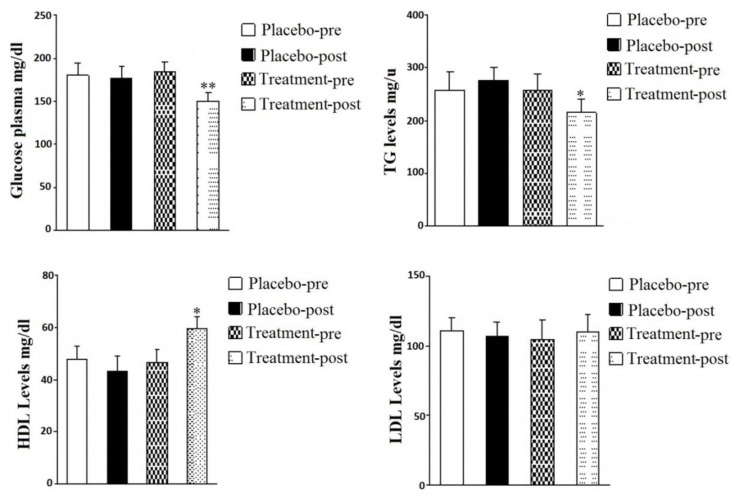
In this study, 48 patients completed the study (24 patients of intervention and 24 patients of control group). Demographic and baseline characteristics of subjects including age, body mass index (BMI), duration of diabetes, physical activity, common diabetes drugs (metformin and glibenclamide), systolic and diastolic pressure, HbA1c and triglycerides level are described in Table 1. In the beginning of study, there were no remarkable differences in means of measured factors between the intervention and control groups. After treatment with hydro-alcoholic extract of U. dioica, the results showed that hydro-alcoholic extract of U. dioica, decreased fasting plasma glucose (Figure 2A) and TG level in intervention group at the end of 8-week treatment. However, an increase in TG level was observed in the patients using placebo (Figure 2B). The results showed that hydro-alcoholic extract of U. dioica increased HDL level in the intervention group after 8 weeks as compared to patients undergoing pretreatment with U. dioica extract (Figure 2C). The result showed that there were no significant differences in LDL level of intervention and placebo groups after 8 weeks (Figure 2D). Apart from glucose, the patients with type2 debates showed abnormalities in lipid metabolism as evidenced by significantly increased LDL levels which might contribute to various cardiovascular complications.
Table1.
Demographic and biochemical characteristics of type 2 diabetic patients in intervention and control group at baseline.
| Variable |
Intervention
(n= 23) |
Control
(n=24) |
|---|---|---|
| Age (Years) | 61.96±6.44 | 59.65±6.93 |
| BMI (kg/m 2 ) | 23.56±2.22 | 23.28±1.96 |
| Duration of diabetes (Years) | 12.92±4.74 | 13.43±3.95 |
| Physical activity (%) | 65.30±9.6 | 63.12±10.5 |
| Metformin tablets (No/Day) | 2.33±1.48 | 2.52±1.51 |
| Glibenclamide tablets (No/Day) | 1.80±1.41 | 1.85±1.48 |
| Systolic pressure (mmHg) | 147.30±8.1 | 141.42±8.7 |
| Diastolic Pressure (mmHg) | 88.70±5.20 | 89.20±4.70 |
| HbA1c (%) | 7.80±1.60 | 8.10±1.9 |
| Triglycerides (mg/dl) | 256.10±29.6 | 255.63±36 |
Values are shown as Mean±SD.
Figure 2.
Effect of U. dioica hydro-alcoholic extract on plasma glucose level (A), TG level (B), HDL level (C), LDL level (D) in intervention group compared with placebo groups. *p < 0.05, **p < 0.01
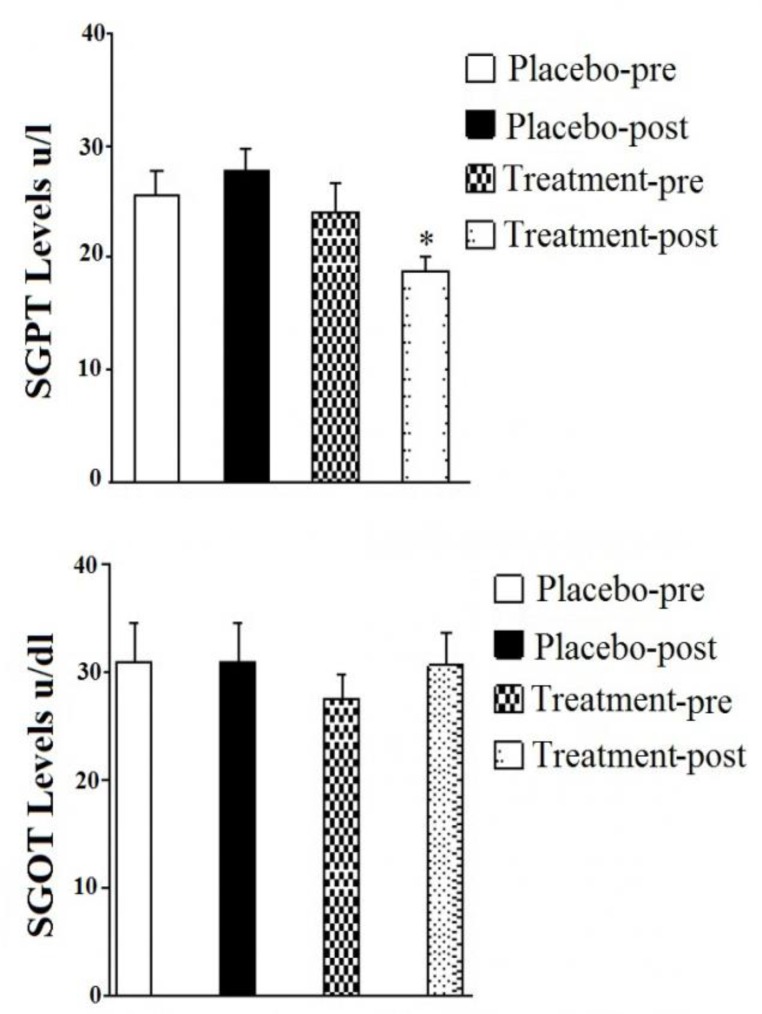
In the present study, SGPT level was decreased in the intervention group with respect to the placebo group (Figure 3A). Patients undergoing post-treatment with U. dioica extract showed significant decrease (p<0.001) in SGPT after 8 weeks as compared to patients undergoing pre-treatment with U. dioica extract and patients using placebo. Further, there was no significant decrease in SGOT level in patients undergoing pre-treatment and post-treatment with U. dioica extract and placebo group after 8 weeks (Figure 3B).
Figure 3.
Effect of U. dioica hydro alcoholic extract on serum glutamic-oxaloacetic transaminase level, SGPT (A), serum glutamic pyruvic transaminase level, SGOT (B) in intervention group compared with placebo groups. *p < 0.05, **p < 0.01
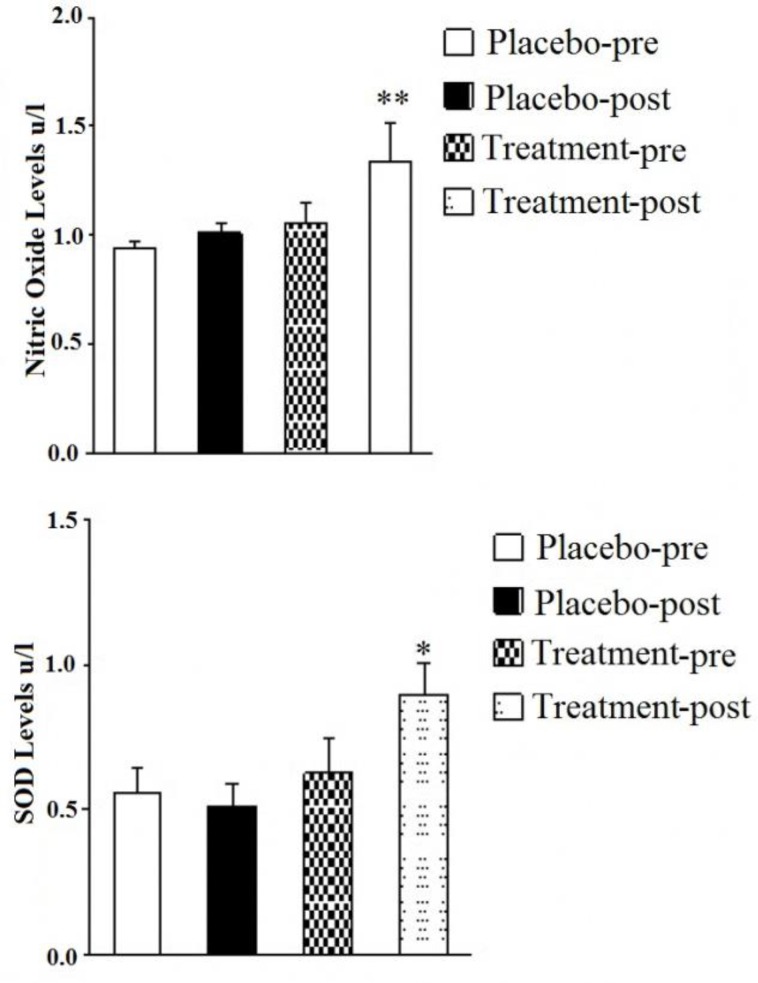
Figure 4A shows that nitric oxide level (NO) was increased significantly (p<0.001) in patients undergoing treatment with U. dioica extract after 8 weeks as compared to placebo group. This study showed statistically significant differences (p<0.001) in SOD between the intervention and the placebo groups. The result showed that extract of U. dioica increased SOD in the patients undergoing 8 weeks of post-treatment as compared to patients undergoing pre-treatment with U. dioica extract at the beginning of the study and the placebo group (Figure 4B).
Figure 4.
Effect of U. dioica hydro-alcoholic extract on serum levels of nitric oxide (A) and superoxide dismutase (B) in intervention group compared with placebo groups. *p<0.05 and **p < 0.01
Discussion
Diabetes Mellitus is a clinical syndrome, characterized by hyperglycemia caused by a relative or absolute deficiency of insulin at the cellular level. It is the most common endocrine disorder, affecting mankind all over the world with an increasing prevalence (Karim et al., 2011 ▶; Tong and Cockrum, 2003 ▶). Traditional preparations from plant sources are widely used almost everywhere in the world to treat this disease (Patel and Udayabanu, 2013 ▶). U. dioica has shown a protective effect against hyperglycemia (Otles and Yalcin, 2012 ▶). Our results clearly indicate that the hydro-alcoholic extracts of U. dioica, decreases FPG level and potentially controls the hyperglycemic state of the patients with type2 diabetes after 8 weeks of treatment. The blood sugar lowering effect of U. dioica as a medicinal plant has been introduced before in animal models. Moreover, Farzami et al. (2003) ▶ showed that the increase in insulin level was associated with a decrease in FPG level. They reported that U. dioica extract can increase insulin secretion by Langerhans island and cause anti-diabetic and blood glucose-reducing effects. Current findings also showed that anti-diabetic property of U. dioica was due to its inhibitory effect on alpha-glucosidase. Furthermore, flavonoids can be effective in improving the blood glucose indexes via their antioxidant activity. Hydro-alcoholic extract of U. dioica could lead to rebuild beta cells in pancreas via its antioxidant characteristics (Golalipour and Khori., 2007 ▶). Moreover, tannin and carotenoids, as constituents of U. dioica could be effective in improving blood glucose indexes (Bahmani et al., 2014 ▶). Das et al. (2011) ▶ showed that U. dioica has an anti-hyperglycemic and anti-hyperlipidemic activity in type 2 diabetic rats. They also have shown that in the insulin deficient subjects, aqueous extract of U. dioica fails to activate the lipoprotein lipase enzyme, causes hypertriglyceridemia and lowers the cholesterol levels in type 2 diabetes model in rats. Therefore, a drug that is found to be active in type 2 diabetes models may have some role in decreasing cholesterol and triglycerides levels (Das et al., 2012 ▶). However, in our study similar to earlier studies on U. dioica (Pourahmadi et al., 2014 ▶), triglyceride levels deceased significantly whereas the decrease in concentrations of plasma cholesterol was not significant as compared with placebo group which may due to insufficient number of individuals. Pourahmadi et al. (2014) ▶ reported that U. dioica root extract decreased the HMG-COA reductase activity, resulting in lower plasma LDL levels in rats. Comparably, in our study, LDL level was increased in hydro-alcoholic U. dioica extract-treated group after 8 weeks. Moreover, high blood glucose causes fat deposits in the liver which is likely to increase SGOT and SGPT activities (Ahangarpour et al., 2014 ▶). Therefore, significant increase in SGOT/SGPT and decrease in HDL levels is observed in patients with type 2 diabetes. Similarly, in the present study, treatment of diabetic patients with U. dioica, reduced SGPT levels. Therefore, it is supposed that U. dioica could prevent liver damage in patients with type 2 diabetes. It is widely known that one of the most important vasorelaxing mechanisms depends on nitric oxide (NO), released from endothelium and acting through the stimulation of the soluble enzyme guanylate cyclase and elevation of the cGMP (Marazioti et al., 2011 ▶). In the present study, a decrease in NO level was observed in the patients using placebo and serum levels of NO were increased in patients after 8 weeks of treatment with U. dioica extract. Comparably, Tessari et al., (2010) ▶ and Ghosh et al., (2012) ▶ have shown that NO synthesis is reduced in subjects with type 2 diabetes and nephropathy. Moreover, Testai et al., (2002) ▶ concluded that U. dioica can produce hypotensive responses through a vasorelaxing effect mediated by the release of endothelial NO, opening of potassium channels, and a negative inotropic action in animal models. Golalipour and Khori, (2007) ▶ concluded that U. dioica has protective effect against oxidative stress in hyperglycemic rats.
Toldy et al., (2005 ▶) showed that U. dioica (30 mg/kg) decreased ROS. Also in agreement with (Coskun et al., 2005 ▶) the present study showed that significant increase in the antioxidant enzyme activity of SOD in intervention group as compared with placebo group at the end of study. Phytochemical analyses of hydro-alcoholic extract of U. dioica showed that the polyphenols that are found in U. dioica include tannin, anthocyanin, chlorogenic acid, cafe oil malic, syringic, myricetin, quercetin, kaempferol, rutin, ellagic, isorhamnetin, p-coumaric, ferulic, naringin, fumaric, and vanillic (Otles and Yalcin, 2012 ▶). Phenolic compounds and especially a group of flavonoids which are present in U. dioica seem to be responsible for the antioxidant activity (Golalipour et al., 2011 ▶; Joshi et al., 2014 ▶; Khare et al., 2012 ▶) and play an important role in stabilizing lipid peroxidation (Bahmani et al., 2014 ▶). Quercetin 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one, is dedicated to the greatest extent of flavonoids in U. dioica. These properties caused antioxidative characteristic of U. dioica in patients with type 2 diabetes (Jeong et al., 2012 ▶). Jeong et al., (2012) ▶ reported that consumption of quercetin can decrease plasma glucose levels and total cholesterol and increase HDL-cholesterol, hepatic GSH-Px activity and plasma adiponectin which is a hormone produced from adipose tissue and reduces insulin resistance. Our results showed that hydro-alcoholic extract of U. dioica is an interesting source of bioactive compounds and may decrease the diabetes-related risk factors of cardiovascular incidence and other complications in patients with diabetes mellitus. The main limitations of this study were the relatively small sample size, lack of precise control of diet and exercise of patients who participated in our study. Also, due to the relatively short duration of our study, we may have missed late beneficial effects and side effects of U. dioica extract; so, our results are suggestive rather than conclusive. Finally, similar studies with higher number of patients and longer duration of intervention are suggested for a better observation of the anti-oxidative effects of U. dioica in improving diabetic patient status.
Acknowledgment
We gratefully acknowledge the logistic support to this study provided by Shahroud Diabetes Society and School of Medicine, Shahroud University of Medical Sciences, Shahroud, Iran and we would like to thanks the Giah Esanse Company for preparing the hydro alcoholic extract of U. dioica and placebo.
Conflict of interest
The authors declare that they have no conflicts of interest concerning this manuscript.
References
- Abo-elmatty D M, Essawy SS, Badr JM, Sterner O. Antioxidant andanti-inflammatoryeffectsof Urtica pilulifera extracts in type 2 diabetic rats. J Ethnopharmacol. 2013;145:269–277. doi: 10.1016/j.jep.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Ahangarpour A, Heidari H, Ramezani Ali Akbari F, Pakmehr M, Hajeye S, Ahmadi I, Mombeini Z, Babadi Hajani M. Effect of Boswellia serrata supplementation on blood lipid, hepatic enzymes and fructosamine levels in type2 diabetic patients. J Diabetes Metab Disord. 2014;13:1–5. doi: 10.1186/2251-6581-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmani M, Zargaran A, Rafieian Kopaei, Saki K. Bahmani. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac J Trop Med. 2014;7:348–354. doi: 10.1016/S1995-7645(14)60257-1. [DOI] [PubMed] [Google Scholar]
- Celik I, Tuluce Y. Elevation protective role of Camellia sinensis and Urtica dioica infusion against trichloroacetic acid-exposed in rats. Phytother Res. 2007;21:1039–1044. doi: 10.1002/ptr.2204. [DOI] [PubMed] [Google Scholar]
- Coskun O, Kanter M, Korkmaz A, Oter S, Quercetin a. Quercetin, a flavonoid antioxidant prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res. 2005;51:117–23. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Das M, Sarma BP, Rokeya B, Parial R, Nahar N, Mosihuzzaman M, Khan A, Ali L. Antihyperglycemic and antihyperlipidemic activity of Urtica dioica on type 2 diabetic model rats. J Diabetol. 2012;2:1–6. [Google Scholar]
- Di Virgilio N, Papazogloub EG, Jankauskienec Z, Di Lonardod S, Praczyke M, Wielgusze K. The potential of stinging UD (Urtica dioica L) as a crop with multiple uses. INDCRO. 2014 No of Pages 8. [Google Scholar]
- Ebrahimzadeh MA, Gharekhani M, Ghorbani M, Dargany P. Effect of extract of aerial parts of Urtica dioica (Urticaceae) on the stability of soybean oil. Trop J Pharm Res. 2015;14:125–131. [Google Scholar]
- Farzami B, Ahmadvand D, Vardasbi S, Majin FJ, Khaghani S. Induction of insulin secretion by a component of Urtica dioica leave extract in perifused Islets of Langerhans and it’s in vivo effects in normal and streptozotocin diabetic rats. J Ethnopharmacol. 2003;89:47–53. doi: 10.1016/s0378-8741(03)00220-4. [DOI] [PubMed] [Google Scholar]
- Golalipour MJ, Khori V. The protective activity of Urtica dioica leaves on blood glucose concentration and β-cells in streptozotocin-diabetic rats. Pak J Biol Sci. 2007;10:1200–1204. doi: 10.3923/pjbs.2007.1200.1204. [DOI] [PubMed] [Google Scholar]
- Golalipour MJ, Kabiri Balajadeh B, Ghafari S, Azarhosh R, Khori V. Protective effect of Urtica dioica L (Urticaceae) on morphometric and morphologic alterations of seminiferous tubules in STZ diabetic Rats. Iran J Basic Med Sci. 2011;14:472–477. [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Bhattacharya R, Bhattacharya G, Sinha AK. The control of stress induced type I diabetes mellitus in humans through the hepatic synthesis of insulin by the stimulation of nitric oxide production. Int J Biomed Sci. 2012;8:171–182. [PMC free article] [PubMed] [Google Scholar]
- Gupta SK. Intention to treat concept. Perspect Clin Res. 2011;2:109–112. doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harput US, Saracoglu I, Ogihara Y. Stimulation of Lymphocyte Proliferation and Inhibition of Nitric Oxide Production by Aqueous Urtica dioica Extract. Phytother Res. 2005;19:346–348. doi: 10.1002/ptr.1686. [DOI] [PubMed] [Google Scholar]
- Harvey M. Prism version 5.0 statistics guide. GraphPad Software, Inc; 2007. 255 pages. [Google Scholar]
- Jeong SM, Kang MJ, Choi HN, Kim JH, Kim JI. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr Res Pract. 2012;6:201–207. doi: 10.4162/nrp.2012.6.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi BC, Mukhija M, Kalia AN. Pharmacognostical review of Urtica dioica L. Int J of G Pharmacy. 2014;8:201–209. [Google Scholar]
- Kandis H, Karapolat S, Yildirim U, Saritas A, Gezer R. Memisogullari, Effects of Urtica dioica on hepatic ischemia-reperfusion injury in rats. Clinics (SaoPaulo) 2010;65:1357–1361. doi: 10.1590/S1807-59322010001200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- karim A, Sohail MN, Munir S, Sattar S. Pharmacology and Phytochemistry of Pakistani Herbs and Herbal Drugs Used for Treatment of Diabetes. Int J Pharmacol. 2011;7:419–439. [Google Scholar]
- Khan A, Safdar M, Khan A, Anderson R. Cinnamon Improves Glucose and Lipids of people With Type 2 Diabetes. Diabetes Care. 2003;26:3115–8. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- Khare V, Kushwaha P, Verma S, Gupta A, Srivastava S, Singh Rawat AK. Pharmacognostic evaluation and antioxidant activity of Urtica dioica L. Chin Med. 2012;3:128–135. [Google Scholar]
- Mahluji S, Ostadrahimi AR, Mobasseri M, Ebrahimzade Attari V, Payahoo L. Anti-inflammatory effects of Zingiber Officinale in type 2 diabetic patients. Adv. Pharm. Bull. 2013;3:273–276. doi: 10.5681/apb.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AH, Motawa HM, Wahba He, Ebrahim EY. Study of Some Antioxidant Parameters in Mice Livers Affected with Urtica pilulifera Extracts. Asian J of Bioch. 2006;1:67–74. [Google Scholar]
- Malekirad AA, Hosseini N, Bayrami M, Hashemi T, Rahzani K, Abdollahi M. Benefit of Lemon Verbena in Healthy Subjects; Targeting Diseases Associated with Oxidative Stress. Asian J Anim Vet Adv. 2011;6:953–957. [Google Scholar]
- Malviya N, Jain S, Malviya S. Antidiabetic potential of medicinal plants. Acta Pol Pharm. 2010;67:113–118. [PubMed] [Google Scholar]
- Mamta S, Preeti k. Urtica dioica (Stinging nettle): A review of its chemical, pharmacological, toxicological and ethnomedical properties. Int J Pharm. 2014;4:270–277. [Google Scholar]
- Manohar SM, Vaikasuvu SR, Deepthi K, Sachan A, Pemmaraju SR, Narasimha VL. An association of hyperglycemia with plasma malondialdehyde and atherogenic lipid risk factors in newly diagnosed Type 2 diabetic patients. J Res Med Sci. 2013;18:89–93. [PMC free article] [PubMed] [Google Scholar]
- Marazioti A, Bucci M, Coletta C, Vellecco V, Baskaran P, Szabo C, Cirino G, Marques AN, Guerreiro B, Goncalves AML, Seixas JD, Beuve A, Romao CC, Papapetropoulos A. Inhibition of nitric oxide–stimulated vasorelaxation by carbon monoxide-releasing molecules. Arterioscler Thromb Vasc Biol. 2011;31:2570–2575. doi: 10.1161/ATVBAHA.111.229039. [DOI] [PubMed] [Google Scholar]
- Nassiri-Asl M, Zamansoltani F, Abbasi E, Daneshi MM, Zangivand AA. Effectsof Urtica dioica extract on lipid profile in hypercholesterolemic rats. Zhong XiYi Jie He Xue Bao. 2009;7:428–433. doi: 10.3736/jcim20090506. [DOI] [PubMed] [Google Scholar]
- Nahata A, Dixit AK. Ameliorative effects of stinging U. dioica (Urtica dioica) ontestosterone-induced prostatic hyperplasia in rats. Andrologia. 2012;44:396–409. doi: 10.1111/j.1439-0272.2011.01197.x. [DOI] [PubMed] [Google Scholar]
- Nazemi N, Tarighat A, Bahrami A. The effect of hydro-alcoholic UD (Urtica dioica) extract on oxidative stress in patients with type 2 diabetes. A randomized double-blind clinical trial. Pak J Bio Sci. 2012;15:98–102. doi: 10.3923/pjbs.2012.98.102. [DOI] [PubMed] [Google Scholar]
- Nojima H, Watanabe H, Yamane K, Kitahara Y, Sekikawa K, Yamamoto H, Yokoyama A, Inamizu T, Asahara T, Kohno N. Effect of aerobic exercise training on oxidative stress in patients with type 2 diabetes mellitus. Metabo Clini Exp. 2008;57:170–176. doi: 10.1016/j.metabol.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Otles S, Yalcin B. Phenolic compounds analysis of root, stalk, and leaves of U. dioica. Sci World J. 2012;1:64–67. doi: 10.1100/2012/564367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen T, Korkmaz H. Modulatory effect of Urtica dioica L (Urticaceae) leaf extract on biotransformation enzyme systems, antioxidant enzymes, lactate dehydrogenase and lipid peroxidation in mice. Phytomedicine. 2003;10:405–15. doi: 10.1078/0944-7113-00275. [DOI] [PubMed] [Google Scholar]
- Patience OO, Estella UO, Philip FU. The search for new hypoglycemic agents from plants. Afr. J. Pharm. Pharmacol. 2014;8:292–303. [Google Scholar]
- Petal DK, Prasad SK, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012;2:320–330. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SH, Udayabanu M. Effect of Urtica dioica on memory dysfunction and hypoalgesia in anexperimental model of diabetic neuropathy. Neuroscience Letters. 2013;552:114–119. doi: 10.1016/j.neulet.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Pourahmadi M, Karimi Jashni H, Bagheri Mand, Sotoodeh Jahromi A. The effect of hydro-alcoholic extract of Urtica dioica Root on testes in adult rats. Life Sci. J. 2014;11:420–424. [Google Scholar]
- Rains JL, Jain SK. Oxidative stress, insulin signaling and diabetes. Free Radical Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed Z, Ahmad R, Rasheed N, Tripathi T, Ali R. Reactive oxygen species damaged hemoglobin present unique epitopes for type 1 diabetes autoantibodies. Int J Biol Chem. 2008;2:1–13. [Google Scholar]
- Tessari P, Cecchet D, Cosma A, Vettore A, Coracina A, Millioni R, Iori E, Puricelli L, Avogaro A, Vedovato M. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes. 2010;59:2152–2159. doi: 10.2337/db09-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testai L, Chericoni S, Calderone V, Nencioni G, Nieri P, Morelli I, Martinotti E. Cardiovascular effects of Urtica dioica L (Urticaceae) roots extracts: in vitro and in vivo pharmacological studies. J. Ethnopharmacol. 2002;81:105–109. doi: 10.1016/s0378-8741(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Tong PCY, Cockrum CS. Diabetes and its historical and social context: The epidemiology of type2 diabetes. Textbook of Diabetes. Vol. 3. Massachusetts, USA: Blackwell Science Ltd. ; 2003. pp. 1–14. [Google Scholar]
- Toldy A, Stadler K, Sasvari M, Jakus J, Jung KJ, Chung HY, Berkes I, Nyakas C, Radak Z. The effect of exercise and U dioica supplementation on oxidative stress markers in the rat brain. Brain Res Bull. 2005;65:487–493. doi: 10.1016/j.brainresbull.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Turkdogan MK, Ozbek H, Yener Z, Tuncer I, Uygan I, Ceylan E. The role of Urtica dioica and Nigella sativa in the prevention of carbon tetrachloride induced hepatotoxicity in rats. IJPR. 2003;17:942–946. doi: 10.1002/ptr.1266. [DOI] [PubMed] [Google Scholar]