Abstract
Introduction
HDAC inhibitors (HDACIs) have the potential to restore gene expression and display antitumor effects in vitro. As single agents, HDACIs have clinical activity in lymphoma. In myeloid leukemias, combinations of DNA methylation inhibitors and HDACIs are promising. Other combinations are being studied in solid tumors.
Areas covered
This article covers basic information and an update on preclinical and clinical experience with the oral isotype-selective HDACI MGCD0103 (mocetinostat) in hematological malignancies and solid tumors. It also examines data concerning MGCD0103 from recent conferences and articles through to November 2010, including new data regarding responses in lymphoma and toxicities.
Expert opinion
MGCD0103 is well-tolerated and exhibits favorable pharmacokinetic and pharmacodynamic profiles, demonstrating target inhibition and clinical responses. It induces cell death and autophagy, synergizes with proteasomal inhibitors and affects non-histone targets, such as microtubules. In 2008, new patient enrollment in trials was temporarily suspended due to potential cardiac complications. This restriction was lifted in 2009 as no correlation between MGCD0103 exposure and pericardial effusions was found. New patient enrollment in MGCD0103 clinical trials requires the exclusion of patients diagnosed with significant cardiac abnormalities prior to enrollment. Clinical and pharmacodynamic data support a three-times-weekly administration at a 90 mg fixed dose. MGCD0103 displays promising antitumor activity in several hematological diseases.
Keywords: HDAC inhibitor, Hodgkin’s lymphoma, leukemia, MDS, pharmacodynamic, pharmacokinetic
1. Introduction
Epigenetics includes important biological processes relevant to all organisms, defined as heritable changes in gene expression not accompanied by changes in DNA sequence [1]. Epigenetic changes that occur via modulation of chromatin structure are implicated in carcinogenesis and malignant transformation [2]. Acetylation of histones leads to open chromatin configuration and gene transcription, whereas deacetylation leads to a repressive state. These changes are mediated by HAT and HDAC. Histone deacetylation is an important epigenetic event involved in the development and progression of cancer, by regulating the accessibility of DNA for gene expression and transcription. It has been extensively studied over the past decade since deregulation of HDAC activity has been found in various cancers [3]. Furthermore, decreased levels of histone acetylation were demonstrated in solid tumors, such as breast, prostate and liver cancer, which correlate with clinical outcomes [2,4–6]. Thus, novel therapeutic strategies focusing on alterations in chromatin and epigenetic silencing in cancer are now being actively explored.
Although the mechanism of action of HDIs has not been completely understood, at the molecular level, inhibition of HDAC activity leads to accumulation of acetylated proteins including histones, transcription factors (including p53), tubulin and heat shock protein 90 [7,8]. These changes interfere with tumor cell proliferation, survival, and maintenance. Indeed, the mechanism of action of HDIs is complex, and includes direct antiproliferative effects via upregulation of p21 and effects on JAK-signal transducer and activator of transcription (STAT) and AKT pathways [9], extrinsic and intrinsic apoptotic pathways [10–12], induction of cell-cycle arrest [9,13], autophagic cell death [14] and senescence [15]. In addition, it has been shown to modulate microenvironment by altering secretion of of chemokines [9]. Mechanisms of cancer cells’ resistance to HDIs described in published literature are diverse since alterations of various pathways in the cells can potentially block their activity. Cellular factors that have been implicated as determinants of resistance to histone deacetylase inhibitors include drug efflux, target over-expression and desensitization, chromatin alterations, stress response mechanisms, and antiapoptotic mechanisms, as well as upregulation of pathways involved in antioxidant defense [8,16].
Inhibitors of HDACs were found to have anti-cancer function as a novel therapeutic class of drugs in various cancers [17]. Based on chemical structure, they can be divided into four different classes: hydroxamates, cyclic peptides, aliphatic acids and benzamides. Several HDACIs have shown significant antitumor activity in vitro with little toxicity in preclinical studies, suggesting selectivity for neoplastic cells. This has prompted development of additional compounds, many of which have entered clinical trials. Two HDACI have shown particular efficacy against cutaneous T cell lymphoma (CTCL), showing response rates over 30%, leading to FDAapproval of vorinostat (suberoylanilide hydroxamic acid) in 2007 and romidepsin (depsipeptide) in 2009 [18,19].
2. Overview of the market
Although many advances have been made in hematopoietic malignancies, the treatment of patients with relapsed and refractory disease remains challenging. Only a fraction of patients will be cured with salvage therapy and transplantation. For those that are either ineligible or relapse after transplant, treatment options are limited. Similarly, for patients with solid tumors presenting at advanced stage or developing resistance, only few therapeutic options exist. This illustrates an urgent need for new drugs and novel treatment strategies. As mentioned above, vorinostat and romidepsin have been recently approved by FDA for the treatment of relapsed or refractory CTCL. These agents are also being tested for other indications as well. Other HDACIs, such as LBH589 (panobinostat), FK228 (depsipeptide), PXD101 (belinostat), ITF2357 (givinostat), MGCD0103 (mocetinostat), MS-275 (entinostat), PCI-24781, and valproic acid have also demonstrated therapeutic potential as monotherapy or in combination with other antitumor drugs in various malignancies (Box 1). At least 80 clinical trials are currently underway, testing more than 11 HDAC inhibitory agents including both hematological and solid malignancies. This review focuses on recent progress in MGCD0103 development.
Box 1. Drug summary.
3. Introduction to the compound
HDACIs can be divided into four chemical classes including hydroxamic acid derivatives, cyclic peptides, aliphatic acids and benzamides. Most of them, except benzamides, target part or all of class I and II HDACs and are therefore referred to us pan-inhibitors. Although several HDAC inhibitors have demonstrated antiproliferative activity in vitro against a variety of tumour types, their clinical application has been limited by their in vivo toxic effects. Therefore, class-I selective HDAC inhibitors may be of interest for clinical use due to absence of significant haematological toxicity, specifically thrombocytopenia [20]. Benzamides MGCD0103 and MS-275 are isotype-selective and do not affect the class II HDACs. MGCD0103 was developed by MethylGene of Canada. It is a chemically synthesized, orally available, small-molecule HDACI, highly specific for classes I and IV HDACs. Interestingly, recent data shows that it exhibits multiple effects due to non-histone targets on cancer cells which may contribute to its activity. It induces cell death, in part via mitochondrial pathway, as well as autophagy, synergizes with proteasome inhibitors, and destabilizes microtubules [20–23]. Furthermore, a synergistic effect was recently described between pan-HDAC inhibitors and proteasome inhibitors, which was initially attributed to the ability of pan-HDAC inhibitors to inhibit HDAC6-dependent aggresome function, suggesting that class-I HDAC inhibitors may lose this potential synergistic advantage [20]. In contrast, recent data showed that MGCD0103 synergizes with proteasomal inhibitor bortezomib in vitro [20]. Collectively, it was demonstrated that HDAC6 inhibition was not required for enhancing proteasome inhibitor activity, providing rationale for future development of potentially less toxic combination regimens of the class-I HDAC inhibitors and proteasome inhibitors for the treatment of cancer.
4. Chemistry
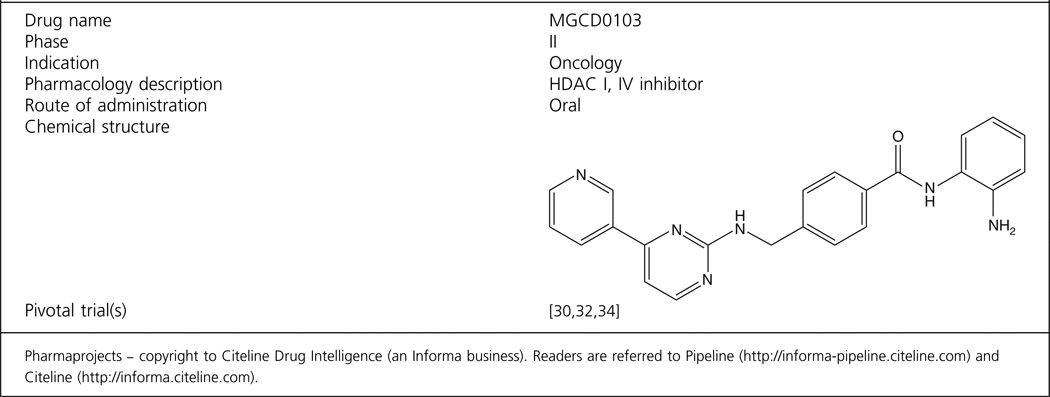
The chemical name of MGCD0103 is N-(2-Amino-phenyl)-4-[(4-pyridin-3-yl-pyrimidin-2-ylamino)-methyl] benzamide dihydrobromide. Its molecular weight is 558.27 g/mol. Its chemical structure has been previously described and is shown in the drug summary box [24]. Briefly, initial studies to identify HDACI were based on suberoylanilide hydroxamic acid (SAHA)/Trichostatin A (TSA)-like straight chain hydroxamates. These results helped the design of a series of nonhydroxamate HDACIs, and optimization of the structural features to improve selectivity, safety and efficacy led to the discovery of MGCD-0103. The details of the design, chemical synthesis and biological evaluation of MGCD-0103 have been previously published [25].
5. Pharmacodynamics
In human cancer cells, MGCD0103 induces core histone H3 and H4 acetylation at micromolar doses. In past clinical trials with HDACI, pharmacodynamic effects in patients were monitored by studying induction of histone acetylation in peripheral white blood cells using immunoblotting, ELISA or flow cytometry, or in tumor tissues by immunohistochemistry [26–28]. These assays detected acetylated histone H3 with an antiacetylated H3 histone antibody, which required peripheral white blood cells to be permeabilized to allow the antibody to become fixed. Indeed, the low sensitivity of these assays and the limitation of clinical materials available made their use challenging. Recently, an HDAC assay with a cell-permeable HDAC small-molecule substrate in intact peripheral white blood cells from patients was developed [29]. Pharmacodynamic studies of MGCD0103 using this assay showed that it is inhibits HDAC enzyme activity which correlated with induction of histone acetylation [30]. These studies have shown dose-dependent increases in the inhibition of HDAC activity in clinical trials of both solid tumors and hematological malignancies. Although no evidence of clear correlation between HDAC inhibition by these assays and clinical outcome has been demonstrated, these findings are a proof of principle for MGCD0103 action.
6. Pharmacokinetics and metabolism
Pharmacokinetic data in animal models revealed good oral bioavailability [31]. In patients with solid tumors and leukemia, MGCD0103 demonstrated a favorable pharmacokinetic profile with a dose-dependent exposure and half-life was ~ 7 – 11 h [30,32]. There was little accumulation with repeated dosing, suggesting that induction of metabolic clearance pathways by the drug or its metabolites is unlikely. Pharmacokinetic analyses demonstrated interpatient variability, improved by co-administration of MGCD0103 with low pH beverages, such as carbonated soft drinks [32]. It has been suggested that MGCD0103 is cleared via biliary and fecal routes, similarly to other HDAc inhibitors [33], however there is no published data regarding drug binding in plasma and elimination.
7. Clinical efficacy
7.1 Phase I trials
In AML/MDS, two schedules of MGCD0103 were first studied in Phase I trials (Table 1). In the first, MGCD0103 was given orally three times weekly [30]. The recommended daily dose was 60 mg/m2, which corresponds to a fixed dose of 110 mg. Out of 29 patients 3 (10%) achieved complete response of the marrow blasts at recommended dose. The second schedule was MGCD0103 orally twice weekly without a rest week [34]. The established recommended Phase II daily dose was similar (66 mg/m2), but the dose intensity with this trial was lower than in the first schedule.
Table 1.
Selected recent clinical studies with MGCD0103.
| Drug | Number of patients# |
Phase | Dosage | Cancer type | Results | Ref. |
|---|---|---|---|---|---|---|
| MGCD0103 | 38 | I | 45 mg/m2 (85 mg) | Advanced solid tumors | 16% SD | [32] |
| MGCD0103 + Gemcitabine |
29 | I | 90 mg | Advanced solid tumors | 17% PR, 7% SD | [35] |
| MGCD0103 | 29 | I | 60 mg/m2 (110 mg) | MDS/AML | 10% CR | [30] |
| MGCD0103 | 27 | II | 85 – 110 mg | Relapsed or refractory HL | 10% CR, 28% SD | [41] |
| MGCD0103 | 38 | II | 110 mg, then decreased to 85 mg. |
DLBCL and FL | 15% OR for DLBCL, 11% OR for FL |
[40] |
| MGCD0103 | 51 | II | 110 or 85 mg | DLBCL | 29% OR for DLBCL | [43] |
AML: Acute myeloid leukemia; CR: Complete remission; DLBCL: Diffuse large B-cell lymphoma; FL: Follicular lymphoma; HL: Hodgkin’s lymphoma; MDS: Myelodysplastic syndrome; OR: Overall response; PR: Partial remission; SD: Stable disease.
Different schedules have been studied in patients with advanced solid tumors. The first was a daily schedule with 14 days of consecutive administration followed by a 7-day rest, every 3 weeks [35]. MGCD0103 was not well tolerated due to dose-limiting fatigue and this trial was closed prematurely, since less frequent administration was better tolerated. In a second Phase I study, MGCD0103 was given orally three times weekly for 14 days followed by 7 days rest every 3 weeks, the recommended daily dose was established at 45 mg/m2, which corresponds to a fixed dose of ~ 90 mg [32]. No objective tumor responses were observed among 32 patients. Five patients with progressive colon, renal cell and lung cancers had stable disease > 4 cycles. DLTs consisting of fatigue, nausea, vomiting, anorexia, and dehydration were observed in 3 out of 11 (27%) and 2 out of 3 (67%) patients treated at the 45 and 56 mg/m2/day dose levels, respectively. MGCD0103 synergizes with gemcitabine, inhibiting pancreatic cell growth both in vitro and in vivo [36]. A Phase I/II study with MGCD0103 alone or combination with gemcitabine was initiated in patients with solid tumors recently [37]. Phase I part of the trial studied adults with refractory solid tumors, while Phase II part of the trial was limited to patients with locally advanced or metastatic pancreatic cancer. Patients received MGCD0103 3 times a week in 28-day cycles at sequential ascending doses. Gemcitabine was administered at 1000 mg/m2, weekly three times per cycle. DLTs included fatigue, vomiting, abdominal pain, thrombocytopenia and anemia. Inhibition of HDAC activity was observed in patients’ PBMCs. The MTD and recommended Phase II dose was 90 mg. Among 14 response-evaluable Phase I patients, there were two PRs out of five pancreatic cancer patients, two PRs in patients with nasopharyngeal cancer and one in a patient with CTCL. Two patients were observed with SD after receiving more than two cycles (one lung and one pancreatic). The results of Phase II, with 90 mg of MGCD0103, have not been reported.
7.2 Phase II trials
Based on preclinical data, Phase I/II study in AML/MDS was initiated with 5-azacitidine administered subcutaneously at a standard dose (75 mg/m2) daily for 7 days, and with MGCD0103 started on day 5 of 5-azacitidine on a three-times-weekly schedule, without rest periods between the treatment cycle [38,39]. The recommended Phase II dose of MGCD0103 was determined as a 90-mg fixed dose. Among 52 patients, 19 (36%) had an objective response.
In preclinical studies, MGCD0103 exhibited significant biological activity in lymphoma models [31]. Thus, safety and efficacy of MGCD-0103 given orally three times per week was evaluated in a Phase II study in patients with relapsed and refractory Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL) which are summarized in Table 1 [40,41]. Patients were to continue therapy for up to 1 year in the absence of heavy toxicity or disease progression. Of 21 evaluable patients who received the 110 mg starting dose, 38% (eight patients) showed objective responses – two CRs and six PRs. The overall disease control rate (CR + PR + stable disease) was 43%. The two CR patients had preliminary progression-free survival of 14 and 9 months, respectively, at the time of analysis. Among patients with relapsed or refractory follicular lymphoma (FL) or diffuse large B-cell lymphoma (DLBCL), enrollment in the DLBCL cohort was 41 patients, and 28 patients have been enrolled in the FL cohort for a total of 69 patients; 59 patients were evaluable for efficacy. In the DLBCL cohort, the objective response rate was 15% (one CR and five PRs). The objective response rate for enrolled patients in the FL cohort was 11% (three PRs). Grade 3 and 4 toxicities were mainly fatigue, while hematological toxicity was rare. Several patients developed pericardial effusion, but no QT interval (QTc) changes were observed [42]. Furthermore, after 1 week of therapy, serum thymus and activation-regulated chemokine (TARC) levels measured by ELISA assay were decreased by at least 40% in five patients, and all these patients achieved major clinical responses, suggesting that an early decline of serum TARC levels may serve as a predictive marker for treatment response [41]. An update of a Phase II enrolling refractory/ relapsed Hodgkin’s Lymphoma was presented at the American Society of Hematology (ASH) last year [43]. A total of 51 patients were enrolled; 2 CR, 12 PR and 1 SD were reported with overall response rate of 29%. Finally, the ongoing study of FL patients has been recently re-opened to recruitment [44]. MGCD0103 was given 3times per week starting at 85 or 110 mg. The updated interim efficacy evaluation indicates that 3 out of 28 patients experienced PR (11%). The most common toxicities of grade ≥ 3 were fatigue, neutropenia, anemia, nausea, anorexia and thrombocytopenia. In addition, one patient experienced a pericardial serious adverse event (SAE) (grade 2 pericarditis associated with a grae1 pericardial effusion). Further details regarding toxicities are described below.
From preclinical data [21], compelling evidence supporting the role of epigenetic silencing in CLL and feasibility of intermittent dosing in patients with various tumors, a multi-centre Phase II trial of MGCD103 was conducted in patients with relapsed and refractory CLL to determine the overall response rate. A total of 21 patients received a median of two cycles of MGCD0103 (range, 0 – 12) with no responses observed [45]. Grade 3 – 4 toxicity consisted of infections, thrombocytopenia, anemia, diarrhea and fatigue. HDAC inhibition was observed in six out of nine patients on day 8. Therefore, only limited single-agent activity was observed with MGCD0103 in CLL.
8. Safety and tolerability
As described above, fatigue was the most common toxicity, followed by gastrointestinal toxicities (nausea, diarrhea and vomiting), whereas hematological ones are rare. Since initiation of clinical trials, several events of pericardial toxicity were reported with MGCD0103. Based on this on July 22, 2008, all new patient enrollment was suspended due to observation of pericarditis and pericardial effusion. To date, 437 patients have been treated with MGCD0103 [44]. There were 19 patients with a SAE where one of the listed terms involved the pericardium [46]. Patients with HL were more likely (9.5%) to experience a pericardial SAE as compared with other diagnoses, while patients with solid tumors had an incidence of only 0.9%. The mechanism of these effusions and reasons behind increase of those specifically in hematological malignancies is unclear: although the frequency of pleural effusion is 20 – 30% in NHL and HL, the involvement of pericardial cavities is uncommon [47]. Most pericardial SAEs occurred during cycle 1 of treatment. Statistically significant associations were found with patients who had a history of pericardial disease, the presence of lung lesions and on-study reports of chest pain or pleural effusion. The partial enrollment restriction was lifted on September 22, 2009, since no clear correlation between MGCD0103 exposure and pericardial effusions were found. The conditions agreed to between MethylGene and the FDA for new patient enrollment in MGCD0103 clinical trials is the exclusion of patients diagnosed with significant cardiac abnormalities (chronic heart failure, myocardial infacrtion or pericardial effusion) prior to starting MGCD0103 therapy.
9. Regulatory affairs
MGCD0103 was patented in March of 2003 by MethylGene, Inc. MGCD0103 has received orphan drug designation from the FDA and has been designated an orphan medicinal product by the European Medicines Agency (EMEA).
10. Conclusions
MGCD0103 is generally well tolerated at doses ≤ 90 mg fixed dose administered two to three times weekly, and shows antitumor activity in several hematological malignancies and to a lesser extent in solid tumors. A convenient fixed recommended dose has been established, replacing dosage based on body surface area. Most common side effects are manageable such as fatigue, nausea and vomiting. Consistently, hematological toxicities have been more common in patients with hematological malignancies. The serious adverse effects (pericardial effusion) were observed in 19 out of 437 patients and was most commonly seen among HL patients and during the first cycle. This led to temporary enrollment restriction of clinical trials which was lifted at the end of 2009. Patients diagnosed with significant cardiac abnormalities (chronic heart failure, myocardial infacrtion, pericardial effusion) should now be excluded prior to starting MGCD0103 therapy. Rest weeks incorporated in some schedules led to decreased dose intensity and appeared ineffective. A novel peripheral blood HDAC enzyme assay has been successfully applied in these studies of MGCD0103, and appears to be quite sensitive. However, no correlation between HDAC inhibition and clinical results was demonstrated.
11. Expert opinion
MGCD0103 is a novel HDAC inhibitor, highly specific for classes I and IV HDACs. Interestingly, it has been recently shown to exhibit various effects on non-histone targets on cancer cells. The latest preclinical studies have demonstrated that it can induce both cell death and autophagy [21,22], is able to synergize with proteasome inhibitors [20], and interestingly, can destabilize microtubules which may have further implications for its therapeutic use [23].
MGCD0103 has clearly shown clinical activity, observed even among patients with refractory disease, especially in HL/DLBCL. It is still unclear whether MGCD0103 has clinical advantages over other HDACI. Initially, MGCD0103 was thought to have increased therapeutic index over other broad-spectrum HDACI. Unfortunately, toxicity profiles do not seem to differ significantly between MGCD0103 and other HDACI. Furthermore, increased incidence of pericardial effusions reported with the drug resulted in a hold in new patient enrollment, which has been recently lifted. Nevertheless, despite the setbacks, class I isotype-selective HDACI such as MGCD0103 may display advantages over broad-spectrum HDACI due to longer half-life and the fact that an oral formulation is available. Finally, MGCD0103 has shown most promise in refractory Hodgkin’s lymphoma which has limited effective salvage therapy. Current ongoing and planned studies of MGCD0103 will provide further evidence on the role of this HDACI in cancer therapeutics. Indeed, future studies should include predictive biomarkers, for instance serum TARC levels, in patients treated with MGCD0103, to determine which subset of patients would get the most benefit from this compound. Recent in vitro data with MGCD0103 indicate that it synergizes with proteasomal inhibitors in lymphoma [20] as well as demethylating agents [48], BCL-2 antagonists [22] and inhibitors of antioxidant pathway [16] in leukemic cells. Further development of clinical trials of MGCD0103 in combination with these agents is therefore warranted.
Footnotes
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
- 1.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraga MF, Ballestar E, Villar-Garea A, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37:391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 3.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 4.Seligson DB, Horvath S, Shi T, et al. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262–1266. doi: 10.1038/nature03672. [DOI] [PubMed] [Google Scholar]
- 5.Tryndyak VP, Kovalchuk O, Pogribny IP. Loss of DNA methylation and histone H4 lysine 20 trimethylation in human breast cancer cells is associated with aberrant expression of DNA methyltransferase 1, Suv4-20h2 histone methyltransferase and methyl-binding proteins. Cancer Biol Ther. 2006;5:65–70. doi: 10.4161/cbt.5.1.2288. [DOI] [PubMed] [Google Scholar]
- 6.Pogribny IP, Ross SA, Tryndyak VP, et al. Histone H3 lysine 9 and H4 lysine 20 trimethylation and the expression of Suv4-20h2 and Suv-39h1 histone methyltransferases in hepatocarcinogenesis induced by methyl deficiency in rats. Carcinogenesis. 2006;27:1180–1186. doi: 10.1093/carcin/bgi364. [DOI] [PubMed] [Google Scholar]
- 7.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 8.Fantin VR, Richon VM. Mechanisms of resistance to histone deacetylase inhibitors and their therapeutic implications. Clin Cancer Res. 2007;13:7237–7242. doi: 10.1158/1078-0432.CCR-07-2114. [DOI] [PubMed] [Google Scholar]
- 9.Buglio D, Georgakis GV, Hanabuchi S, et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008;112:1424–1433. doi: 10.1182/blood-2008-01-133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlisi D, Lauricella M, D’Anneo A, et al. The histone deacetylase inhibitor suberoylanilide hydroxamic acid sensitises human hepatocellular carcinoma cells to TRAIL-induced apoptosis by TRAIL-DISC activation. Eur J Cancer. 2009;45:2425–2438. doi: 10.1016/j.ejca.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Ruefli AA, Ausserlechner MJ, Bernhard D, et al. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci USA. 2001;98:10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peart MJ, Tainton KM, Ruefli AA, et al. Novel mechanisms of apoptosis induced by histone deacetylase inhibitors. Cancer Res. 2003;63:4460–4471. [PubMed] [Google Scholar]
- 13.Nimmanapalli R, Fuino L, Stobaugh C, et al. Cotreatment with the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) enhances imatinib-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Blood. 2003;101:3236–3239. doi: 10.1182/blood-2002-08-2675. [DOI] [PubMed] [Google Scholar]
- 14.Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2004;101:18030–18035. doi: 10.1073/pnas.0408345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu WS, Perez G, Ngo L, et al. Induction of polyploidy by histone deacetylase inhibitor:a pathway for antitumor effects. Cancer Res. 2005;65:7832–7839. doi: 10.1158/0008-5472.CAN-04-4608. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Lu W, Chen G, et al. Overcoming resistance to histone deacetylase inhibitors in human leukemia with the redox modulating compound beta-phenylethyl isothiocyanate. Blood. 2010;116:2372–2741. doi: 10.1182/blood-2009-11-256354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batty N, Malouf GG, Issa JP. Histone deacetylase inhibitors as anti-neoplastic agents. Cancer Lett. 2009;280:192–200. doi: 10.1016/j.canlet.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Duvic M, Vu J. Vorinostat: a new oral histone deacetylase inhibitor approved for cutaneous T-cell lymphoma. Expert Opin Investig Drugs. 2007;16:1111–1120. doi: 10.1517/13543784.16.7.1111. [DOI] [PubMed] [Google Scholar]
- 19.Grant C, Rahman F, Piekarz R, et al. Romidepsin: a new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev Anticancer Ther. 2010;10:997–1008. doi: 10.1586/era.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buglio D, Mamidipudi V, Khaskhely NM, et al. The class-I HDAC inhibitor MGCD0103 induces apoptosis in Hodgkin lymphoma cell lines and synergizes with proteasome inhibitors by an HDAC6-independent mechanism. Br J Haematol. 2010;151:387–396. doi: 10.1111/j.1365-2141.2010.08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Khoury V, Moussay E, Janji B, et al. The histone deacetylase inhibitor MGCD0103 induces apoptosis in B-cell chronic lymphocytic leukemia cells through a mitochondria-mediated caspase activation cascade. Mol Cancer Ther. 2010;9:1349–1360. doi: 10.1158/1535-7163.MCT-09-1000. [DOI] [PubMed] [Google Scholar]
- 22.Wei Y, Kadia T, Tong W, et al. The combination of a histone deacetylase inhibitor with the Bcl-2 homology domain-3 mimetic GX15-070 has synergistic antileukemia activity by activating both apoptosis and autophagy. Clin Cancer Res. 2010;16:3923–3932. doi: 10.1158/1078-0432.CCR-10-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chia K, Beamish H, Jafferi K, Gabrielli B. The histone deacetylase inhibitor MGCD0103 has both deacetylase and microtubule inhibitory activity. Mol Pharmacol. 2010;78:436–443. doi: 10.1124/mol.110.065169. [DOI] [PubMed] [Google Scholar]
- 24.Le Tourneau C, Siu LL. Promising antitumor activity with MGCD0103, a novel isotype-selective histone deacetylase inhibitor. Expert Opin Investig Drugs. 2008;17:1247–1254. doi: 10.1517/13543784.17.8.1247. [DOI] [PubMed] [Google Scholar]
- 25.Zhou N, Moradei O, Raeppel S, et al. Discovery of N-(2-aminophenyl)-4-[(4-pyridin-3-ylpyrimidin-2-ylamino)methyl] benzamide (MGCD0103), an orally active histone deacetylase inhibitor. J Med Chem. 2008;51:4072–4075. doi: 10.1021/jm800251w. [DOI] [PubMed] [Google Scholar]
- 26.Kelly WK, O’Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gojo I, Jiemjit A, Trepel JB, et al. Phase 1 and pharmacologic study of MS-275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood. 2007;109:2781–2790. doi: 10.1182/blood-2006-05-021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan QC, Headlee D, Acharya M, et al. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J Clin Oncol. 2005;23:3912–3922. doi: 10.1200/JCO.2005.02.188. [DOI] [PubMed] [Google Scholar]
- 29.Bonfils C, Kalita A, Dubay M, et al. Evaluation of the pharmacodynamic effects of MGCD0103 from preclinical models to human using a novel HDAC enzyme assay. Clin Cancer Res. 2008;14:3441–3449. doi: 10.1158/1078-0432.CCR-07-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Manero G, Assouline S, Cortes J, et al. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008;112:981–989. doi: 10.1182/blood-2007-10-115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kell J. Drug evaluation: MGCD-0103, a histone deacetylase inhibitor for the treatment of cancer. Curr Opin Investig Drugs. 2007;8:485–492. [PubMed] [Google Scholar]
- 32.Siu LL, Pili R, Duran I, et al. Phase I study of MGCD0103 given as a three-times-per-week oral dose in patients with advanced solid tumors. J Clin Oncol. 2008;26:1940–1947. doi: 10.1200/JCO.2007.14.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27:5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 34.Lancet JE, Nichols G, Assouline S, et al. A phase I study of MGCD0103 given as a twice weekly oral dose in patients with advanced leukemias or myelodysplastic syndromes (MDS) [abstract 2516] J Clin Oncol. 2007;25:101s. [Google Scholar]
- 35.Gelmon K, Tolcher A, Carducci M, et al. Phase I trial of the oral histone deacetylase (HDAC) inhibitor MGCD0103 given either daily or 3× weekly for 14 days every 3 weeks in patients (pts) with advanced solid tumors. J Clin Oncol. 2005;23:3147. [Google Scholar]
- 36.Nguyen H, Gravel S, MacLeod AR. Synergistic antitumor activity of the isotype-selective histone deacetylase inhibitor MGCD0103 in combination with gemcitabine [abtsract] Clin Cancer Res. 2005;11(24):9153s. [Google Scholar]
- 37.Hurwitz H, Nelson B, O’Dwyer PJ, et al. The oral isotype-selective HDAC inhibitor MGCD0103 in combination with gemcitabine in patients with refractory solid tumors. J Clin Oncol. 2008;26 abstract 4625. [Google Scholar]
- 38.Garcia-Manero G, Yang AS, Giles F, et al. Phase I/II Study of the Oral Isotype-Selective Histone Deacetylase (HDAC) Inhibitor MGCD0103 in Combination with Azacitidine in Patients (pts) with High-Risk Myelodysplastic Syndrome (MDS) or Acute Myelogenous Leukemia (AML) Blood. 2006;108:1954. [Google Scholar]
- 39.Garcia-Manero G, Yang AS, Klimek V, et al. Phase I/II Study of MGCD0103, an Oral Isotype-Selective Histone Deacetylase (HDAC) Inhibitor, in Combination with 5-Azacitidine in Higher-Risk Myelodysplastic Syndrome (MDS) and Acute Myelogenous Leukemia (AML) Blood. 2007;110:444. [Google Scholar]
- 40.Younes A, Wedgwood A, McLaughlin P, et al. Treatment of relapsed or refractory lymphoma with the oral isotype-selective histone deacetylase inhibitor MGCD0103: interim results from a Phase II study [abstract] Blood. 2007;110:2571. [Google Scholar]
- 41.Younes A, Pro B, Fanale M, et al. The isotype-selective HDAC inhibitor MGCD0103 decreases serum TARC concentrations and produces clinical responses in heavily pretreated patients with relapsed classical Hodgkin lymphoma (HL) [abstract] Blood. 2007;110:2566. [Google Scholar]
- 42.Martell RE, Garcia-Manero G, Younes A, et al. Clinical development of MGCD0103, an isotype-selective HDAC inhibitor: pericarditis/pericardial effusion in the context of overall safety and efficacy [abstract] Blood. 2009;114:4756. [Google Scholar]
- 43.Younes A, Bociek RG, Kuruvilla J, et al. Mocetinostat (MGCD0103), An Isotype-Selective Histone Deacetylase (HDAC) Inhibitor, Produces Clinical Responses In Relapsed/Refractory Hodgkin Lymphoma (HL): update from a Phase II clinical study. Blood. 2010;116:1763. [Google Scholar]
- 44.Martell RE, Younes A, Assouline SE, et al. Phase II study of MGCD0103 in patients with relapsed follicular lymphoma (FL): study reinitiation and update of clinical efficacy and safety. J Clin Oncol. 2010;28(Suppl 15s) abstract 8086. [Google Scholar]
- 45.Blum KA, Advani A, Fernandez L, et al. Phase II study of the histone deacetylase inhibitor MGCD0103 in patients with previously treated chronic lymphocytic leukaemia. Br J Haematol. 2009;147:507–514. doi: 10.1111/j.1365-2141.2009.07881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sampat K, Rossi A, Garcia-Gutierrez V, et al. Characteristics of pericardial effusions in patients with leukemia. Cancer. 2010;116:2366–2371. doi: 10.1002/cncr.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Das DK. Serous effusions in malignant lymphomas: a review. Diagn Cytopathol. 2006;34:335–347. doi: 10.1002/dc.20432. [DOI] [PubMed] [Google Scholar]
- 48.Liu HB, Mayes PA, Perlmutter P, et al. The anti-leukemic effect and molecular mechanisms of novel hydroxamate and benzamide histone deacetylase inhibitors with 5-aza-cytidine. Int J Oncol. 2011;38:1421–1425. doi: 10.3892/ijo.2011.914. [DOI] [PubMed] [Google Scholar]