Abstract
Endocannabinoids are endogenous ligands for the cannabinoid (CB) receptors which include anandamide (AEA) and 2-arachidonyl glycerol (2-AG). 2-AG has been linked to inflammation due to its elevated expression in animal models of autoimmunity and hypersensitivity. However, administration of exogenous 2-AG has been shown to suppress inflammation making its precise role unclear. In the current study, we investigated the role of 2-AG following immunization of C57BL/6 (BL6) mice with methylated BSA (mBSA) antigen, which triggers both delayed type hypersensitivity (DTH) and antibody response. We found that while naïve T cells and B cells expressed low levels of 2-AG, expression significantly increased upon activation. Furthermore, mBSA-immunized mice exhibited higher 2-AG concentration than naïve mice. Exogenous 2-AG treatment (40mg/kg) in mBSA-immunized mice led to reduced DTH response, and decreased Th1 and Th17-associated cytokines including IL-6, IL-2, TNF-α and the IgG response. Addition of 2-AG to activated popliteal lymph node (PopLN) cell cultures also inhibited lymphocyte proliferation. Together, these data show for the first time that activated T and B cells produce 2-AG, which plays a negative regulatory role to decrease DTH via inhibition of T-cell activation and proliferation. Moreover, these findings suggest that exogenous 2-AG treatment can be used therapeutically in Th1- or Th17-driven disease.
Keywords: T cell suppression, Cannabinoid receptor, Endocannabinoids, Th1, Th17, Anti-inflammatory, Delayed type hypersensitivity (DTH)
Introduction
The endocannabinoid (EC) system consists of cannabinoid (CB) receptors (CB1 and CB2) and endogenous ligands for these receptors, called endocannabinoids (EC) [1]. Anandamide (AEA) and 2-arachidonyl glycerol (2-AG) remain the most widely studied ECs [2]; [3]. 2-AG is proposed as the “true natural ligand” for CB receptors for three reasons: multiple synthesis pathways, elevated tissue expression, and agonist activity at both CB receptors [4]. 2-AG can be formed along a degradative pathway from inositol phospholipid hydrolysis [5], and synthesized from arachidonic acid-containing phosphatidylcholine [6]. 2-AG is also highly expressed in numerous tissues [7]. Furthermore, 2-AG exhibits binding affinity and efficacy for CB1 and CB2 receptors making it a full agonist for the CB receptors [8].
The EC system is well known to play a role in immune response (as reviewed throughout the years by [9], [10], [11], [12]). Moreover, CB receptor agonists such as Δ-9-tetrahydrocannabinol (THC), AEA, and 2-AG have all been connected to the regulation of lymphocyte activation. For example, Robinson et al recently found that expression of CB2 increased on murine T cells after activation in vitro [13] while activation of human primary T cells led to increased CB1 but not CB2 induction [14]. In a recent study, we found that the ability of cannabinoids to suppress T cell-mediated skin graft rejection in vivo, was regulated through CB1 but not CB2 receptors [15]. Type IV hypersensitivity (delayed type hypersensitivity, DTH) is driven by proinflammatory T helper cell subsets, Th1 and Th17 [16]; [17]. Recently, a study using 2,4-dinitroflurobenzene to induce dermatitis found increased ear weight correlated with elevated 2-AG [18]. Furthermore, mice lacking Fatty Acid Amide Hydrolase (FAAH), a degradative enzyme of ECs, showed decreased response to contact hypersensitivity [19]. Considering the fact that DTH is mediated primarily by inflammatory T cells similar to those involved in allograft rejection, and because we found CB1 to play a critical role in the latter model [15], we chose, in this study, to focus on the role of CB1 in EC-mediated suppression of DTH response in vivo.
Here, we investigated the concentration of ECs, particularly 2-AG, in naïve and activated T and B cells using the antigen mBSA which triggers DTH response via Th1 and Th17 cells and increased B cell antibody production. The findings suggest that 2-AG increases upon lymphocyte activation and systemically during DTH response. We also demonstrated that exogenous 2-AG treatment reduced DTH response via inhibition of T cell activation and proliferation, further supporting a regulatory role for 2-AG in the suppression of inflammation.
Results and Discussion
Expression or secretion of the endocannabinoid 2-AG increases upon lymphocyte activation
While ECs have been measured in secondary lymphoid organs [7, 20], concentrations in naïve and activated lymphocytes have yet to be determined. As such, we isolated naïve T and B cells from BL6 mouse spleens and using liquid chromatography with tandem mass spectrometry (LC/MS/MS) quantified AEA and 2-AG (Fig. 1A). We found that AEA, while detectable, was minimal and significantly lower than 2-AG in T and B cells (Fig. 1B). In order to quantify changes in 2-AG expression/secretion in activated T and B cells, we treated splenocytes with the mitogen Concanavalin A (ConA) or the endotoxin lipopolysaccharide (LPS), which polyclonally activate T or B cells respectively. Upon activation, significantly more 2-AG was found in T and B cell supernatants than intracellularly (Fig. 1C&D). These findings are interesting as earlier studies by Lee et al have indicated that 2-AG can inhibit T cell proliferation after stimulation with anti-CD3 [21] suggesting that 2-AG plays a role in regulating immune cell activation in vitro.
Figure 1. Lymphocyte activation causes dysregulation of endocannabinoids.
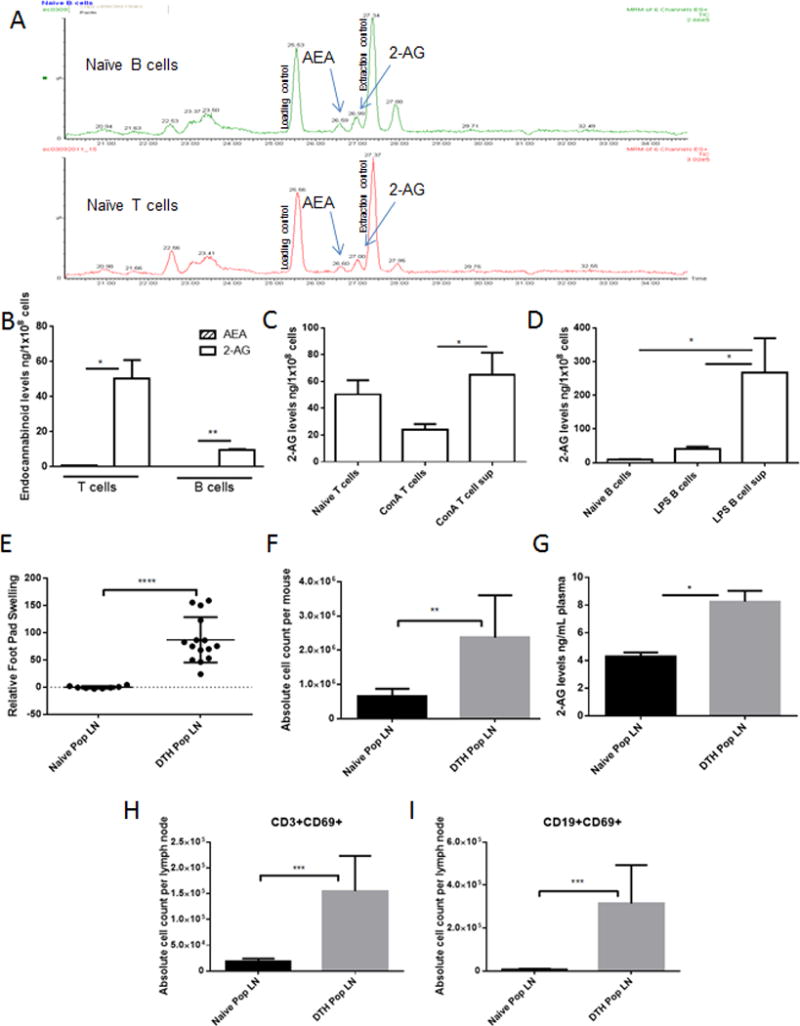
(A) Raw chromatogram for naïve C57BL/6 (BL6) T cells and B cells after LC/MS/MS detection. (B) Quantified AEA and 2-AG in naïve lymphocytes (n=2 individual samples ran in triplicate-pooled from multiple mice). (C, D) Spleens were collected from naïve BL6 mice and activated with ConA (2.5μg/mL) or LPS (5μg/mL). 2-AG was quantified after (C) ConA or (D) LPS activation using LC/MS/MS detection (n=6 individual samples ran in triplicate). Delayed type hypersensitivity (DTH) induction, 24 hours after rechallenge, was determined using footpad swelling and lymphocyte activation (n=15 per treatment group). (E) Relative percent swelling of mBSA rechallenged footpad compared to naïve mouse footpad. (F) Popliteal lymph node (PopLN) absolute cell counts (per mouse) was determined by hemocytometer viability counts. (G) Circulating 2-AG in plasma (individual samples ran in triplicate). Absolute cell counts from flow cytometer proportions applied to viability counts (H) CD3+CD69+ activated T cells and (I) CD19+CD69+ activated B cells (gated on live cells). Data shown as mean + SEM of duplicate measurements (n=5 per treatment group per experiment). Student’s t-test or ANOVA/Tukey * p<0.05 ** p<0.01 ***p<0.001 ****p>0.0001
To ensure that elevated ECs were biologically relevant, we used methylated bovine serum albumin (mBSA) to induce DTH hypersensitivity [16]; [17]. The DTH response following rechallenge with mBSA led to significant swelling in the hind footpads of mice (Fig. 1E) and increased cellularity in the draining popliteal lymph nodes (PopLN) (Fig. 1F). Interestingly, we observed a 2-fold increase in plasma 2-AG in DTH compared to naive mice (Fig. 1G), however AEA remained low (data not shown). To confirm that mBSA rechallenge elicits lymphocyte activation, PopLN cells were analyzed for T- and B-cell co-expression of CD69. We found that CD3+CD69+ and CD19+CD69+ cells were significantly enhanced in mBSA rechallenged mice compared to naïve (Fig. 1H&I).
2-AG treatment reduces inflammation associated with DTH in mice
To determine if increased 2-AG creates a negative feedback loop controlling the hypersensitivity response, we used exogenous 2-AG treatment (40mg/kg i.p.) in DTH mice (Fig. 2A). Treatment of DTH mice with 2-AG did not significantly alter spontaneous proliferation of splenocytes after mBSA sensitization (Fig. 2B) suggesting that treatment did not alter naïve T cell function. Interestingly, 2-AG treatment did decrease footpad swelling by more than 30% (Fig. 2C) and PopLN cellularity nearly 2-fold (Fig. 2D). While 2-AG is known to bind both CB1 and CB2 receptors our laboratory has recently shown that activation of peripheral CB1 receptors is vital to cannabinoid-mediated inhibition of the T cell driven proinflammatory response [15]. Moreover, we observed a significant increase in CB1 message in the PopLN cells of DTH mice compared to naïve (Fig. 3A) while no change was seen in CB2 message (Fig. 3B). To this end we assessed the importance of endogenous ligand binding of CB1 receptors in DTH response using CB1KO mice. We observed a 30% increase in footpad swelling in DTH mice upon complete loss of CB1 functionality (Fig 3C). This however does not rule out the role of CB2 as shown in some studies [13], [22], and further studies are necessary. Next, we quantified expression of the early activation marker CD69, which is present on both activated T and B cells. Interestingly we found that the total number of activated T (CD3+CD69+) and B cells (CD19+CD69+) in the PopLN were significantly decreased in 2-AG treated mice compared to vehicle controls (Fig. 2E&F). Additionally, the number/proportion of T central memory cells (Tcm) in PopLN significantly increased with 2-AG treatment compared to DTH+Veh, similar to DTH sensitized but not rechallenged mice (Supporting Information Fig. 1A–B).
Figure 2. Endocannabinoid 2-AG reduces lymphocyte driven inflammation.
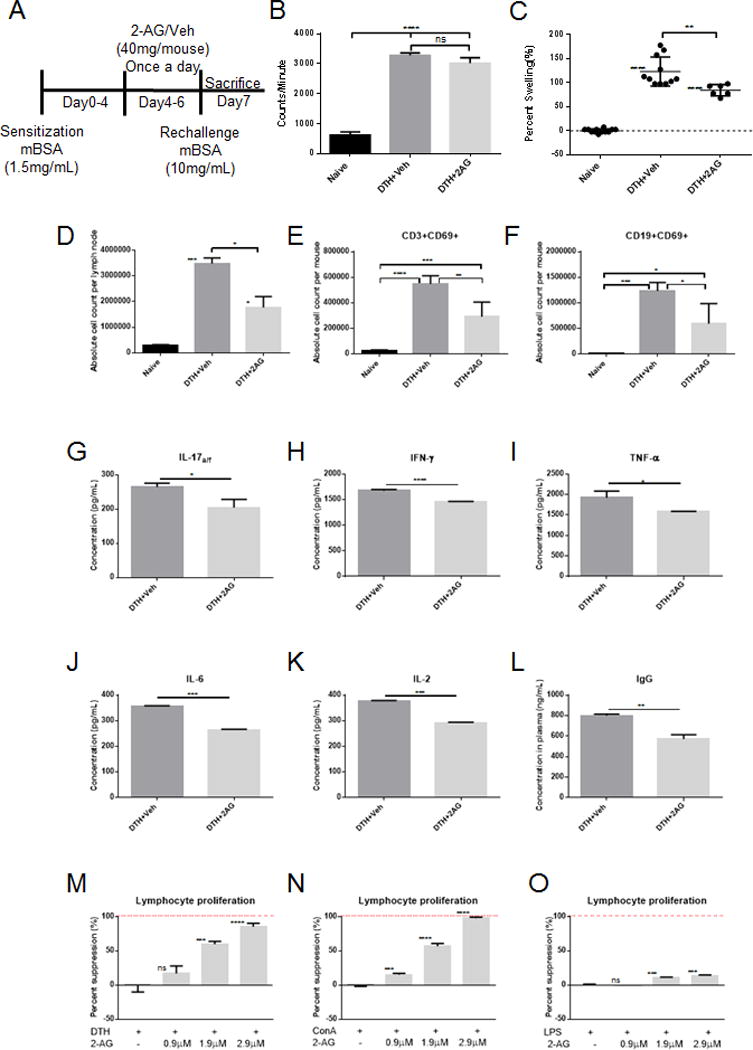
2-AG treatment (40 mg/kg n=9) or vehicle (ethanol diluted in 1×PBS n=11) was given on days 4–6 after sensitization (n=5 individual samples pooled and ran in triplicate for ELISA and proliferation assays). (A) Schematic of model and treatment regimen. (B) Splenocytes from in vivo mBSA sensitized mice (n=3 per treatment group) were pooled and co-cultured in triplicate with 2 uCi of 3H-thymidine to assess spontaneous proliferation (C) Relative percent swelling of treated mouse footpads compared to naïve mouse footpads (BL6). (D–F) PopLN absolute cell counts using hemocytometer viability (D), applied to flow cytometer proportions of CD3+CD69+ activated T cells (E), and CD19+CD69+ B cells (F). Cytokine secretion was assessed by sandwich ELISA using culture supernatants from splenocytes co-cultured with PMA and Ca Ionomycin (G) IL-17a/f, (H) IFN-γ, (I) TNF-α, (J) IL-6, and (K) IL-2. (L) Plasma was collected 24 hours after mBSA rechallenge and assessed for IgG. Splenocytes were stimulated with (M) mBSA rechallenge (10 mg/mL in vivo), (N) ConA (2.5μg/mL), or (O) LPS (5.0μg/mL), treated with 2-AG, and co-cultured with 2 uCi of 3H-thymidine to assess proliferation. Data shown as mean + SEM (individual samples ran in triplicate-pooled from n=5 mice per treatment group). Student’s t-test or ANOVA/Tukey * p<0.05 ** p<0.01 ***p<0.001 ****p<0.0001
Figure 3. Loss of CB1 receptor function increases footpad swelling.
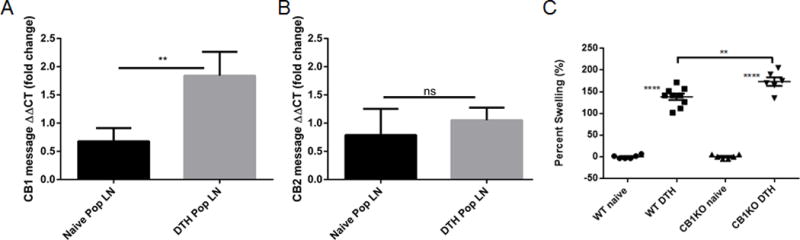
cDNA (miRNeasy-Qiagen and iScript-BioRad) was isolated form PopLN of naïve BL6 and DTH mice (n=5 mice per treatment group). mRNA was assessed for CB1 (A) and CB2 (B) mRNA message using Sso Advanced SYBR green (BioRad). CB1KO mice (BL6 background) were sensitized and rechallenged following the previously outlined protocol. Disease parameters, as described previously, were assessed (n=5 per treatment group). (C) Relative percent swelling of the treated footpad compared with that of a naïve mouse. Symbols represent individual mice and data shown as mean ± S.E.M. ANOVA/Tukey * p<0.05 ** p<0.01 ***p<0.001 ****p<0.0001
To elucidate how 2-AG reduces mBSA induced hypersensitivity, we analyzed Th1 and Th17 proinflammatory cytokine secretion from PopLN cells of DTH mice. Cytokines associated with Th17 activation, IL-17a/f (Fig. 2G), and differentiation, IL-6 (Fig. 2J), were significantly decreased with 2-AG treatment. Furthermore, Th1 secreted cytokines including IFN-γ (Fig. 2H) and TNF-α (Fig. 2I) were significantly reduced in DTH+2-AG mice, compared to controls. Additionally, IL-2 significantly decreased with 2-AG treatment, indicating reduced T cell activation and proliferation in draining lymph nodes (Fig 2K). Because mBSA also triggers Ab response [23], we measured IgG in plasma and found that 2-AG treatment caused significantly decreased IgG compared to vehicle controls (Fig. 2L). These data suggests that high 2-AG, due to exogenous treatment, is anti-inflammatory.
2-AG modulates DTH disease parameters by inhibiting T-cell proliferation
We next determined if 2-AG acts directly on T and B cells by culturing the PopLN cells from mBSA immunized mice in the presence of 2-AG or vehicle. We observed a dose- dependent suppression of lymphocyte proliferation in culture using in vivo antigen-activated cells and polyclonal activated T and B cells in the presence of 2-AG compared to vehicle controls (Fig. 2M–O). These data demonstrate that 2-AG acts directly on activated T and B cells to suppress lymphocyte activation and proliferation.
Concluding Remarks
Recently, we hypothesized that ECs play a critical regulatory role in inflammation associated with autoimmune diseases [12]. Our laboratory and others found treatment of autoimmune inflammation with exogenous AEA attenuates inflammatory disease [24]; [25]; [26]. However, research on 2-AG in inflammatory models is mostly limited to increasing basal levels [27]; [28]. One exception, however, is a study wherein exogenous 2-AG increased neuroprotection and decreased mortality in a murine experimental autoimmune encephalomyelitis model [29]. However, no studies have investigated 2-AG in naïve and activated immune cells correlated with in vivo inflammation. Our findings show that basal AEA and 2-AG are found in naive T and B cells implying that basal EC concentrations are necessary for regulation of basic cell function. Furthermore, elevated 2-AG found in activated T and B cell supernatants or plasma from DTH mice suggests that 2-AG may be synthesized upon lymphocyte activation, consistent with other studies [30]; [31].
Concentration of 2-AG correlated with ear weight gain, due to dermatitis [18]. However, exogenous 2-AG inhibits the proliferation of T cells [21], and the secretion of IL-2 in vitro [32]; [33]. The role of 2-AG in B cells is complex because 2-AG stimulates chemotaxis and chemokinesis in nM concentrations [34], and decreases proliferation in μM concentrations [21]. Together the data on immune regulation by 2-AG suggests that this endocannabinoid is vital for eliciting an anti-inflammatory response. Our findings highlight the concept that anti-inflammatory properties of 2-AG are dose or threshold-dependent as shown in our proliferation studies. Together, this study substantiates our claim that endocannabinoids such as 2-AG are critical regulators of immune response. Interestingly, exogenous 2-AG treatment decreases T- and B-cell-dependent immune response in hypersensitivity and autoimmunity models. Furthermore, our findings that 2-AG suppresses DTH may be useful as a treatment modality against contact dermatitis, or therapeutically to treat various models of Th1 or Th17 driven inflammation.
Materials and Methods
Mice
Female C57BL/6 (BL6) mice, aged 6–8 weeks and average weight of 20 g, were obtained from Jackson Laboratories. Mice were housed in pathogen-free conditions and allowed filtered water and Teklad rodent diet 8604 (normal chow) ad libitum at the University of South Carolina School of Medicine Animal Research Facility. All experiments were conducted under an approved Institutional Animal Care and Use Committee animal protocol.
T and B cell isolation
Single cell suspension from BL6 mouse spleens of was plated for removal of adherent cells. Cells in suspension were resuspended in complete RPMI media (1% v/v penicillin/streptomycin, 1% v/v HEPES buffer, 10% v/v heat inactivated FBS, and 0.0002% v/v 2-mercaptoethanol). Nylon wool column, wetted with complete media, was loaded with single cell suspension. T cells were isolated based on nonadherence as elute and wash from nylon wool column [35]. B cells adhering to nylon wool were isolated by compression of incubated nylon wool, using sterile syringe plunger and complete medium. Purity (>90%) was checked using flow cytometer.
Endocannabinoid extraction
Briefly, endocannabinoids were extracted from sample with internal standards (deuterated endocannabinoids (Cayman Chemical) AEA-d4 (10ng/mL), 2-AG-d5 (20ng/mL), and PEA-d4 (200ng/mL) diluted in 50:50 (methanol:water) and (phenylmethylsulfonyl fluoride) PMSF in acetonitrile. Samples were diluted in 0.1333% TFA ultra-purified water, to 20% (v/v) acetonitrile, and ultracentrifugation to remove cellular debris. Samples were cleaned using activated SPE columns (Bond Elute C8). The endocannabinoids were eluted using 80% (v/v) acetonitrile in 0.1333% TFA ultra-purified water and evaporated to dryness. All samples were stored at −80°C.
Liquid chromatography/Tandem Mass spectrometry (LC/MS/MS)
Sample, resuspended in 100uL 80% (v/v) acetonitrile, was injected in a volume of 10 μL onto a C18 reverse phase analytical column (particle size 5 μm 2.1 × 150 mm ES Industries #06601-12-54-45318). Endocannabinoids were eluted using a linear binary gradient flowing at 200 μL/min on a Waters Acquity HPLC system. The gradient solvent composition began at 50% A: 50% B (solvent A: water/acetonitrile (95:5) with 1% ammonium acetate and 0.1% formic acid; solvent B: methanol with 1% ammonium acetate and 0.1% formic acid) and increased to 0%A: 100%B over 28 minutes. The mass spectrometer (Waters Premier XE triple quadrupole) was operated in multiple reaction monitoring (MRM) mode using positive ion electrospray ionization. The electrospray probe was held at 3 kV. Optimized mass spectrometer operating parameters for quadrupole mass spectrometer with electrospray ionization source in tandem with liquid chromatography (Micromass Quattro-LC, Waters) are listed in Supporting Information: Table I. Waters MassLynx software was used for analysis.
Induction of delayed type hypersensitivity (DTH) and 2-AG treatment
DTH was induced in BL6 mice using a sensitization/rechallenge method [26]. Briefly, mice were sensitized with a subcutaneous injection (100 μL/hind flank) of 1.5 mg/mL methylated BSA (mBSA- Sigma Aldrich) emulsified in complete Freunds adjuvant (CFA- Sigma Aldrich). Six days later, the mice were rechallenged with footpad injection (20 μL/footpad) of 10 mg/mL mBSA in 1×PBS. 2-Arachidonoyl glycerol (2-AG- Cayman Chemical) evaporated under a stream of nitrogen was dissolved in ethanol (20mg/mL), diluted in 1×PBS, and used in mice at a concentration of 40mg/kg. 2-AG or vehicle (ethanol diluted in 1×PBS) was administered intraperitoneally (i.p.) at 0.1 mL/mouse into DTH mice daily Day 4 – Day 6. Percent swelling was measured as [(Thickness(mBSA rechallenged footpad) − Thickness(1×PBS rechallenged footpad)) / Thickness(1×PBS rechallenged footpad)] * 100.
Monoclonal antibodies, reagents, and flow cytometer
Antibodies used for flow cytometric analysis (BioLegend) included: Fc block (93), anti-CD3 (145-2C11), anti-CD19 (6D5), anti-CD69 (H1.2F3), anti-CD4 (GK1.5), anti-CD25 (PC61), and anti-CD62L (MEL-14). Popliteal lymph node cells (PopLN- 106 cells) from DTH BL6 mice were incubated with Fc receptor antibodies (10 min) and incubated with conjugated antibodies (20–30 minutes at 4°C). After incubation with conjugated antibodies, cells were washed twice with 1×PBS/2% FBS buffer. Stained cells were assessed by flow cytometer (FC500; Beckman Coulter) and resulting data analyzed by Cytomics CXP software (Beckman Coulter). Three-color flow cytometric analysis (gated on live cells) was used to profile lymphocyte activation and four-color analysis (gated on CD3+CD4+ cells) was used to assess T central memory cells.
Cytokine analysis in cell culture supernatants
ELISA MAX sandwich enzyme-linked immunosorbent assay (ELISA) kits (BioLegend) were used to measure IL-2, IL-6, IL-17a/f, IFN-γ, and TNF-α cytokines. Cells were isolated from spleen and draining lymph nodes of DTH mice cultured 24 hours for cytokine secretion in complete RPMI media with phorbol 12-myristate 13-acetate (PMA) and calcium ionophore ionomycin (50 ng/mL and 10 μg/mL respectively). Cytokine production was quantified from cell supernatants (stored at −20°C). Plasma was assessed for IgG using sandwich ELISA (Abcam). Absorbance was measured at 450 nm using a Victor2 1420 Multilable counter (Wallac).
Cell proliferation assays
Spleen cells from naïve mice were co-cultured in triplicate (0.2 mL/well in a round bottom 96-well plate) with ConA (2.5μg/mL) or LPS (5μg/mL) to stimulate T and B cell proliferation, respectively. Splenocytes from DTH mice sensitized alone or with rechallenge in vivo were assessed for spontaneous proliferation and dose response of 2-AG respectively. In some experiments, 2-AG was added at the time of cell seeding at increasing doses (0.9, 1.9, and 2.9 μM). Sixteen hours before collection and analysis, [3H]thymidine (2 μCi/well) was added to the cell cultures. Radioactivity was measured using a liquid scintillation counter (MicroBeta Trilux).
qPCR analysis of cannabinoid receptor expression
Total RNA was isolated from PopLN and purified using miRNeasy kit (Qiagen), following manufacturer’s procedure. iScript cDNA synthesis kit (BioRad) was used according to manufacturer’s specifications to reverse transcribe cDNA. qPCR was performed using Sso Advanced SYBR Green (BioRad) on a CFX Connect (BioRad). Samples were assessed for expression of β actin (Forward- 5′GGCTGTATTCCCCTCCATCG3′ Reverse-5′CCAGTTGGTAACAATGCCATGT3′), CB1 (cannabinoid receptor 1: Forward- 5′CTGGCCTATAAGAGGATCGTCA3′ Reverse- 5′GAGAGGCAACACAGCAATTACTA3′), and CB2 (cannabinoid receptor 2: Forward- 5′CTACAAAGCTCTAGTCACCCGT3′ Reverse-5′CCATGAGCGGCAGGTAAGAAA3′). Primers, as found on PrimerBank (Harvard Medical School), were synthesized from IDT DNA technologies with annealing temperatures of 60°C.
Statistical analysis
Data were shown as mean ± S.E.M. Student’s t-test was used to compare data between two groups. One-way ANOVA with Tukey post-hoc test was used to compare three or more groups. Experimental groups were compared to controls, and p < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank the University of South Carolina Mass Spectrometry Laboratory Core facility, especially Dr. Walla and Dr. Cotham, for help optimizing LC/MS/MS parameters. This work was supported in part by the National Institute of Health Grants R01 MH094755, P01 AT003961 and P20 GM103641 to PN, R01 ES019313 to PN and MN, and R01 AT006888 and the Veterans Administration Merit Award BX001357 to MN.
Abbreviations used
- 2-AG
2-arachidonyl glycerol
- AEA
anandamide
- CB
cannabinoid
- CFA
Complete freund’s adjuvant
- DTH
Delayed type hypersensitivity
- EC or Endocannabinoid
endogenous cannabinoid receptor ligand
- LC/MS/MS
liquid chromatography with tandem mass spectrometer
- mBSA
Methylated bovine serum albumin
- miRNA or miR
microRNA
- PopLN
Popliteal lymph node
- Tcm cells
T central memory cells
Footnotes
Conflict of Interest:
The authors declare no commercial or finical conflict of interest.
References
- 1.Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: an overview. Immunobiology. 2010;215:588–597. doi: 10.1016/j.imbio.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 3.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 4.Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, Waku K. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J Biol Chem. 2000;275:605–612. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- 5.Prescott SM, Majerus PW. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J Biol Chem. 1983;258:764–769. [PubMed] [Google Scholar]
- 6.Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- 7.Kondo S, Kondo H, Nakane S, Kodaka T, Tokumura A, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist: identification as one of the major species of monoacylglycerols in various rat tissues, and evidence for its generation through CA2+-dependent and -independent mechanisms. FEBS Lett. 1998;429:152–156. doi: 10.1016/s0014-5793(98)00581-x. [DOI] [PubMed] [Google Scholar]
- 8.Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol. 2000;57:1045–1050. [PubMed] [Google Scholar]
- 9.Robson PJ. Therapeutic potential of cannabinoid medicines. Drug Test Anal. 2014;6:24–30. doi: 10.1002/dta.1529. [DOI] [PubMed] [Google Scholar]
- 10.Leleu-Chavain N, Desreumaux P, Chavatte P, Millet R. Therapeutical potential of CB(2) receptors in immune-related diseases. Curr Mol Pharmacol. 2013;6:183–203. doi: 10.2174/1874467207666140219122337. [DOI] [PubMed] [Google Scholar]
- 11.Massi P, Vaccani A, Parolaro D. Cannabinoids, immune system and cytokine network. Curr Pharm Des. 2006;12:3135–3146. doi: 10.2174/138161206777947425. [DOI] [PubMed] [Google Scholar]
- 12.Sido JM, Nagarkatti PS, Nagarkatti M. Role of Endocannabinoid Activation of Peripheral CB1 Receptors in the Regulation of Autoimmune Disease. Int Rev Immunol. 2014 doi: 10.3109/08830185.2014.921165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson RH, Meissler JJ, Breslow-Deckman JM, Gaughan J, Adler MW, Eisenstein TK. Cannabinoids inhibit T-cells via cannabinoid receptor 2 in an in vitro assay for graft rejection, the mixed lymphocyte reaction. J Neuroimmune Pharmacol. 2013;8:1239–1250. doi: 10.1007/s11481-013-9485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borner C, Hollt V, Kraus J. Activation of human T cells induces upregulation of cannabinoid receptor type 1 transcription. Neuroimmunomodulation. 2007;14:281–286. doi: 10.1159/000117809. [DOI] [PubMed] [Google Scholar]
- 15.Sido JM, Nagarkatti PS, Nagarkatti M. Delta(9)-Tetrahydrocannabinol attenuates allogeneic host-versus-graft response and delays skin graft rejection through activation of cannabinoid receptor 1 and induction of myeloid-derived suppressor cells. J Leukoc Biol. 2015;98:435–447. doi: 10.1189/jlb.3A0115-030RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fong TA, Mosmann TR. The role of IFN-gamma in delayed-type hypersensitivity mediated by Th1 clones. J Immunol. 1989;143:2887–2893. [PubMed] [Google Scholar]
- 17.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 18.Mimura T, Oka S, Koshimoto H, Ueda Y, Watanabe Y, Sugiura T. Involvement of the endogenous cannabinoid 2 ligand 2-arachidonyl glycerol in allergic inflammation. Int Arch Allergy Immunol. 2012;159:149–156. doi: 10.1159/000336167. [DOI] [PubMed] [Google Scholar]
- 19.Karsak M, Gaffal E, Date R, Wang-Eckhardt L, Rehnelt J, Petrosino S, Starowicz K, Steuder R, Schlicker E, Cravatt B, Mechoulam R, Buettner R, Werner S, Di Marzo V, Tuting T, Zimmer A. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–1497. doi: 10.1126/science.1142265. [DOI] [PubMed] [Google Scholar]
- 20.Schmid PC, Schwartz KD, Smith CN, Krebsbach RJ, Berdyshev EV, Schmid HH. A sensitive endocannabinoid assay. The simultaneous analysis of N-acylethanolamines and 2-monoacylglycerols. Chem Phys Lipids. 2000;104:185–191. doi: 10.1016/s0009-3084(99)00124-3. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, Yang KH, Kaminski NE. Effects of putative cannabinoid receptor ligands, anandamide and 2-arachidonyl-glycerol, on immune function in B6C3F1 mouse splenocytes. J Pharmacol Exp Ther. 1995;275:529–536. [PubMed] [Google Scholar]
- 22.Eisenstein TK, Meissler JJ, Wilson Q, Gaughan JP, Adler MW. Anandamide and Delta9-tetrahydrocannabinol directly inhibit cells of the immune system via CB2 receptors. J Neuroimmunol. 2007;189:17–22. doi: 10.1016/j.jneuroim.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Getahun A, Dahlstrom J, Wernersson S, Heyman B. IgG2a-mediated enhancement of antibody and T cell responses and its relation to inhibitory and activating Fc gamma receptors. J Immunol. 2004;172:5269–5276. doi: 10.4049/jimmunol.172.9.5269. [DOI] [PubMed] [Google Scholar]
- 24.Correa F, Hernangomez-Herrero M, Mestre L, Loria F, Docagne F, Guaza C. The endocannabinoid anandamide downregulates IL-23 and IL-12 subunits in a viral model of multiple sclerosis: evidence for a cross-talk between IL-12p70/IL-23 axis and IL-10 in microglial cells. Brain Behav Immun. 2011;25:736–749. doi: 10.1016/j.bbi.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Hegde VL, Hegde S, Cravatt BF, Hofseth LJ, Nagarkatti M, Nagarkatti PS. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: involvement of regulatory T cells. Mol Pharmacol. 2008;74:20–33. doi: 10.1124/mol.108.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson AR, Nagarkatti P, Nagarkatti M. Anandamide attenuates Th-17 cell-mediated delayed-type hypersensitivity response by triggering IL-10 production and consequent microRNA induction. PLoS One. 2014;9:e93954. doi: 10.1371/journal.pone.0093954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alhouayek M, Lambert DM, Delzenne NM, Cani PD, Muccioli GG. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011;25:2711–2721. doi: 10.1096/fj.10-176602. [DOI] [PubMed] [Google Scholar]
- 28.Costola-de-Souza C, Ribeiro A, Ferraz-de-Paula V, Calefi AS, Aloia TP, Gimenes-Junior JA, de Almeida VI, Pinheiro ML, Palermo-Neto J. Monoacylglycerol lipase (MAGL) inhibition attenuates acute lung injury in mice. PLoS One. 2013;8:e77706. doi: 10.1371/journal.pone.0077706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lourbopoulos A, Grigoriadis N, Lagoudaki R, Touloumi O, Polyzoidou E, Mavromatis I, Tascos N, Breuer A, Ovadia H, Karussis D, Shohami E, Mechoulam R, Simeonidou C. Administration of 2-arachidonoylglycerol ameliorates both acute and chronic experimental autoimmune encephalomyelitis. Brain Res. 2011;1390:126–141. doi: 10.1016/j.brainres.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Kurabayashi M, Takeyoshi I, Yoshinari D, Matsumoto K, Maruyama I, Morishita Y. 2-Arachidonoylglycerol increases in ischemia-reperfusion injury of the rat liver. J Invest Surg. 2005;18:25–31. doi: 10.1080/08941930590905189. [DOI] [PubMed] [Google Scholar]
- 31.Quercioli A, Pataky Z, Vincenti G, Makoundou V, Di Marzo V, Montecucco F, Carballo S, Thomas A, Staub C, Steffens S, Seimbille Y, Golay A, Ratib O, Harsch E, Mach F, Schindler TH. Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. Eur Heart J. 2011;32:1369–1378. doi: 10.1093/eurheartj/ehr029. [DOI] [PubMed] [Google Scholar]
- 32.Rockwell CE, Raman P, Kaplan BL, Kaminski NE. A COX-2 metabolite of the endogenous cannabinoid, 2-arachidonyl glycerol, mediates suppression of IL-2 secretion in activated Jurkat T cells. Biochem Pharmacol. 2008;76:353–361. doi: 10.1016/j.bcp.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang Y, Hwang SG, Han SH, Kaminski NE. Suppression of interleukin-2 by the putative endogenous cannabinoid 2-arachidonyl-glycerol is mediated through down-regulation of the nuclear factor of activated T cells. Mol Pharmacol. 1998;53:676–683. doi: 10.1124/mol.53.4.676. [DOI] [PubMed] [Google Scholar]
- 34.Jorda MA, Verbakel SE, Valk PJ, Vankan-Berkhoudt YV, Maccarrone M, Finazzi-Agro A, Lowenberg B, Delwel R. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood. 2002;99:2786–2793. doi: 10.1182/blood.v99.8.2786. [DOI] [PubMed] [Google Scholar]
- 35.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


