Abstract
To date, no studies have explored the effect of abnormal cerebral venous circulation on brain disorders, whereas many studies have investigated neurodegenerative brain anomalies associated with arterial diseases. The aim of our study was to demonstrate the feasibility of different surgical techniques to induce venous obstruction of cerebral brain drainage. Six C57/black mice underwent bilateral occlusion of the external jugular vein (group EJV), six underwent bilateral occlusion of the internal jugular vein (group IJV), and six underwent bilateral occlusion of both the EJV and the IJV (group EJV/IJV). Within each group, the interruption of blood flow was obtained via monopolar electro-coagulation (ME) in three mice and via surgical ligation (SL) in the remaining three mice. A “sham group” of two mice was used as the control. High-frequency ultrasound (HFUS) was used to detect the absence of blood flow in the examined vessel. The ME procedure led to successful results in two of nine (22%) mice, one in the EJV group, one in the EJV/IJV group, and zero in the IJV group, and 4 of 18 (22%) mice when considering individual veins (i.e., total number of EJVs and IJVs occluded). The SL procedure was successful in two of three (67%) mice in the EJV group, in three of three (100%) mice in the IJV and in three of four (75%) mice in the EJV/IJV group. Therefore, the overall success rate was 8/10 (80%) when considering mice, and 20/26 (77%) when considering individual veins. The monopolar electro-coagulation method exhibited a high mortality due to cardiorespiratory arrest, while the results of the bilateral surgical ligation of EJVs and IJVs show that it is technically feasible and safe.
Keywords: High-frequency ultrasound, color-Doppler, cerebral blood outflow, mice model, surgical venous occlusion
Introduction
Abnormalities in extracranial cerebral venous districts could have important consequences on the hemodynamics of intracranial cerebral circulation and consequently on the development of brain disorders. In the literature, even in recent reports, associations have been suggested between abnormal cerebral venous circulation and many disorders or diseases, such as transient global amnesia, idiopathic Parkinson’s disease, idiopathic intracranial hypertension, senile dementia, and normal pressure hydrocephalus.1
In comparison to the number of studies on the physiology and pathophysiology of cerebral arterial districts,2–8 very few studies exploring the physiology of cerebral venous circulation in normal subjects and in patients with neurological diseases are available.9–13 Therefore, the role of venous circulation in the pathophysiology of neurological diseases is largely unknown.
Cerebral venous circulation is very difficult to study because of the high anatomical variability of the vessels and the influence of the hydration status of the patient, as well as their posture, rotational movements of the neck, respiratory movements, intrathoracic pressure, heart rate, and venous compression by external structures (i.e. muscles or bony structures).14–20 Moreover, the functioning of the extracranial venous valve system is important because its incompetence has been found in some common situations that increase central venous pressure (e.g. congestive heart disease, primary pulmonary disease and chronic obstructive pulmonary disease).21,22
The use of animal models yields important information on the etiology and pathophysiology of several diseases. It is often necessary to develop multiple animal models for the study of the effects of various causal factors on the same pathology due to the heterogeneity of certain diseases. For example, in the context of neurodegenerative diseases, the study of different animal models has become necessary to evaluate the possible etiologies, such as viral, toxic and autoimmune causes.23–26 To the best of our knowledge, no animal model has been developed that enables study of the effect of changes in cerebral venous circulation on the development of neurological diseases. Even if animal models represent a unique tool for inferring human biological responses, research results will not be directly translatable to humans owing to species-specific anatomical and physiological differences in cerebral venous systems,27 which could preclude a simple translation of the results.
The aim of our study was to develop a surgical technique for the occlusion of both the external (EJVs) and internal jugular veins (IJVs) by means of monopolar electrocoagulation and/or surgical ligation to develop an animal model of obstructive alteration of cerebral blood drainage. This study is preparatory for future studies that will investigate the effects of venous obstruction on brain tissue and a possible association between venous damage and the onset of neurological diseases.
Materials and methods
Animals
Animal studies were performed in accordance with National Institutes of Health (NIH) guidelines and the Animal Research Advisory Committee (ARAC) procedure (Office of Animal Care and Use 2015) after approval by the Institutional Animal Research Committee (Institutional Animal and Care Committee of the CEINGE and the Italian Ministry of Health) Protocol no. 2, 15/01/14. All animal procedures in this study were conducted by a Diplomate in Veterinary Medicine and conformed to all regulations protecting animals used for research purposes, including national guidelines and the implementation of the 2010/63/EU Directive on the protection of animals used for scientific purposes.
Twenty C57/black mice between 7 and 8 weeks of age, weighing 25–30 g, were used in this study. The mice were divided into three groups that underwent surgery for vein occlusion. Six mice underwent bilateral occlusion of the EJV (group EJV), six underwent bilateral occlusion of the IJV (group IJV), and six underwent bilateral occlusion of both the EJV and the IJV (group EJV/IJV). Within each group, the interruption of blood flow was obtained via monopolar electro-coagulation (ME) in three mice and via surgical ligation (SL) in the remaining three mice. A “sham group” of two mice underwent bilateral surgical exposure of both the EJV and IJV, with no interruption of the blood flow.
Anesthetic and pain control protocol
All procedures described below were performed under general anesthesia. Anesthesia was initially delivered in an induction chamber saturated with 5% isoflurane (Iso-Vet 1000 mg/g Inhalation Vapor, Piramal Healthcare UK Ltd., Northumberland, UK) in oxygen (2 L/min) and subsequently maintained during all procedures with a nose cone delivering isoflurane (1.5% during ultrasound examinations and titrated from 1.5 to 2.5% during surgical procedures, based on surgical stimulation) in oxygen at 2 L/min. Immediately before surgery and then every 12 h for two days after surgery, mice were administered an intraperitoneal injection of tramadol hydrochloride (Altadol 50 mg/ml, Formevet Spa, Milan, Italy) at 30 mg/kg.28
HFUS technique
Each mouse was placed in a dorsally recumbent position on a dedicated pad (VEVO Imaging Station 2, FUJIFILM VisualSonics, Inc., Toronto, Ontario, Canada), hairs were removed with a small clipper and then with the application of a depilatory cream to improve to contact between the probe and the skin. A 40 MHz transducer (MS 550 D, FUJIFILM VisualSonics, Inc., Toronto, Ontario, Canada) was mounted on the dedicated stand of the imaging station, and B-mode and color-Doppler mode images were obtained. The B-mode gain, color-Doppler gain and pulse repetition frequency (PRF) were adjusted for each examination to obtain the best image.
Ultrasonographic examinations were performed 1–2 days prior to the surgery and then every three days for one month using a dedicated equipment system (VEVO 2100, FUJIFILM VisualSonics, Inc., Toronto, Ontario, Canada). During the examinations after the surgery, the PRF was regulated in a step-by-step fashion from 4 kHz to 1 kHz to ensure the detection of even a very slow blood flow rate and to differentiate it from the absence of blood flow in the examined vessel. For each vessel, the presence or absence of flow and the blood flow velocity was recorded. Brightness (B-) mode images were obtained in both the axial and the sagittal planes, and the diameter of each EJV and IJV was recorded.
Surgical technique
The same veterinary surgeon (LA), trained in microsurgery, performed all the procedures to avoid any inter-operator variability. Each mouse was placed in a dorsally recumbent position under a dissection microscope (Leica M80, Leica Microsystems Srl, Milan, Italy). Body temperature was kept at 37.5℃ using a heat lamp. A cutaneous midline incision was made on the ventral aspect of the neck. Blunt dissection of the loose fascia was performed, and the salivary glands were separated and reflected dorsolaterally to expose the EJVs. The common trunk of the EJV, selected for blood flow interruption, lies on the most distal aspect of the neck just proximal to the thoracic inlet. This portion is covered by dense adipose tissue, and it is deeply adhered to the surrounding tissues, even after the removal of fat. To expose the IJVs, the fascia connecting the muscles overlying the trachea and the sternomastoid and omohyoid muscles was dissected. The latter muscles were retracted laterally, and the trachea was gently moved contralaterally from the sagittal plane.29 The IJV was clearly visible over the carotid artery, with the vagus nerve running alongside the IJV and the carotid artery.
ME was performed using dedicated electrosurgical equipment (Diatermo MB160, Gima s.p.a., Gessate, Milan, Italy). The reference electrode plate for the monopolar coagulation was fixed under the mice, taking care to insert thin pieces (0.5–1 cm height) of dielectric material between the head and the ears, as well as the paws of the mice and the plate, to reduce the risk of accidental burns. Coagulation was obtained by setting the power output of the electrosurgical equipment at 5 and increasing it gradually up to 12, first using the “soft coag” function and then the “forced coag” function. The vein was then cut in the middle of the coagulated tissue. The ME procedure was planned to be applied in three mice in each group (EJV, IJV and EJV/IJV).
SL was performed using a 6–0 polyfilament surgical suture (Vycril 6-0, Ethicon, Johnson & Johnson Medical S.p.A., Rome, Italy). The suture was passed around the vessel and tightened while performing a surgeon’s knot.30 The procedure was repeated twice per vessel, and the vein was then cut midway between the knots. Whichever technique was applied, either ME or SL, the procedure was considered successful when the vein could be cut easily and without bleeding. The SL procedure was applied in three mice from EJV and IJV groups and in all mice from the EJV/IJV group.
Once the surgery was completed, all the tissues dissected were apposed with a simple continuous pattern using a 6–0 polyfilament surgical suture. A double-antibiotic ointment (Cicatrene®, Istituto biochimico nazionale Savio S.r.l., Rome, Italy) was placed over the skin incision, and the mice were left to recover under the heat lamp. The medication was applied every day for five consecutive days. The mice were checked daily for the presence of suture dehiscence, suture line drainage and/or infection, and poor health.
Results
Surgical exposure of the EJV and IJV
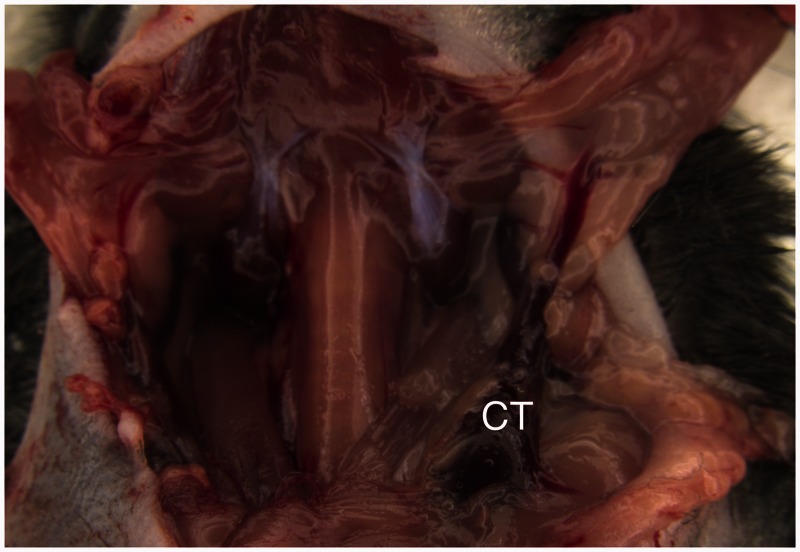
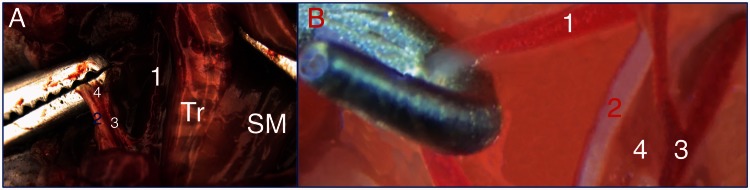
The EJV runs from the caudal aspect of the intermandibular region on both sides of the sagittal plane. It receives the common facial vein and a large branch from the homolateral salivary gland. The EJV is clearly visible subcutaneously as soon as the salivary glands are reflected dorsolaterally (Figure 1). The common trunk of the EJV was easily selected for blood flow interruption (Figure 1). The vagus nerve, the IJV and the carotid artery are contained within a thin tissue fold (Figure 2(a) and (b)), and dissection of the IJV over its length was difficult because of its fragility.
Figure 1.
Surgical exposure of the left external jugular vein (EJV) after dorsolateral reflection of the salivary gland. Note the large branch running from the salivary gland into the vein. CT: the common trunk of the EJV, which was selected for the blood flow interruption and subsequent division of the vein
Figure 2.
Surgical exposure of the right internal jugular vein (IJV). (A) Ex vivo visualization of the IJV after the contralateral traction of the strap muscles (SM) overlying the trachea (Tr). (B) In vivo high-magnification image of the neurovascular bundle in the neck. (1) Carotid artery, (2) vagus nerve, (3) IJV, and (4) the tissue fold that includes the bundle
Monopolar electro-coagulation
The ME procedure led to successful results in two of nine (22%) mice, one in the EJV group, one in the EJV/IJV group, and zero in the IJV group, and in 4 of 18 (22%) cases when considering individual veins (i.e. total number of EJVs and IJVs occluded). Because all IJV mice that received ME died during or immediately after the procedure due to cardio-respiratory arrest, the EJV/IJV mice underwent ME only for EJV blood flow interruption, whereas the IJVs were always occluded with the SL technique. The four unsuccessful procedures were due: in one case in the EJV group, to incomplete EJV coagulation, which resulted in a fatal hemorrhage when attempting to divide the vein; in one case in the EJV/IJV group, to a complete necrosis of the tissues with the creation of a drainage tract through the skin; and in two cases, one in the EJV group and one in the EJV/IJV group, to the inability to cut one EJV due to the massive retraction of the surrounding tissues as a result of the thermoelectric stimulus. The success rate of the ME technique within groups is summarized in Table 1 for mice and Table 2 for individual veins.
Table 1.
Success rate in mice within groups for the two surgical techniques
| Surgical technique Group | ME | SL |
|---|---|---|
| EJV | 1/3–33% | 2/3–67% |
| IJV | 0/3–0% | 3/3–100% |
| EJV/IJV | 1/3–33%* | 3/4†–50% |
| Total | 2/9–22% | 8/10–80% |
ME was applied only for EJV occlusion.
The SL procedure was also applied in one of the three mice from the ME EJV/IJV subgroup for IJV occlusion.
Table 2.
Success rate for the use of single veins for the two surgical techniques
| Surgical technique Vein | ME | SL |
|---|---|---|
| EJV (n = 24) | 4/12–33% | 8/12–67% |
| IJV (n = 24) | 0/6–0% | 12/14–67% |
| Total | 4/18–22% | 20/26–77% |
Surgical ligation
The SL procedure was successful in two of three (67%) mice in the EJV group, in three of three (100%) mice in the IJV and in three of four (75%) mice in the EJV/IJV group. Therefore, the overall success rate was 8/10 (80%) when considering mice and 20/26 (77%) when considering individual veins. In the EJV group, the single unsuccessful procedure was related to the partial perforation of the right EJV during the application of the proximal ligature, but the bleeding was promptly controlled and an additional ligature was effectively applied; nonetheless, the procedure was considered unsuccessful. The one unsuccessful procedure in the EJV/IJV group was due to the formation of a large hematoma during the manipulation of the left IJV. The success rate of the SL technique within groups is summarized in Table 1 for mice and Table 2 for individual veins.
Complications of the surgical techniques
Among the mice whose surgery was considered successful, three of three (100%) from the EJV/IJV group and two of three (67%) from the EJV group presented diffuse swelling of the face and head as soon as one day post-surgery, independent of the surgical technique. The swelling tended to subside beginning at day 7.
HFUS evaluations
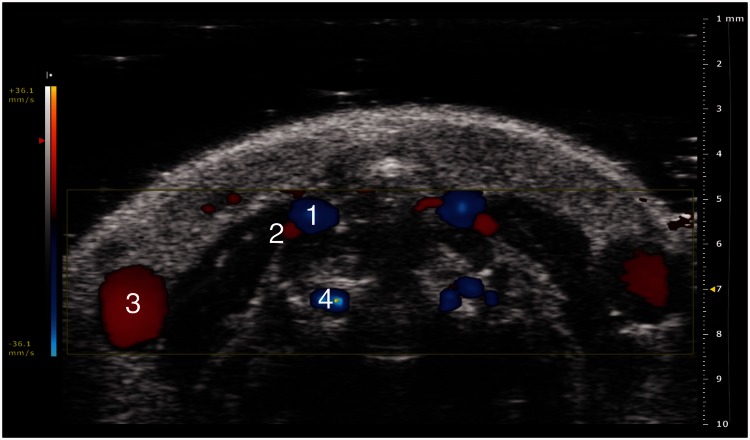
In all the subjects examined, HFUS enabled evaluation of vessel patency. Blood flow was assessed pre-operatively in EJVs using the standard color-Doppler settings (i.e. PRF of 4 kHz). Pre-operatively, in 12/40 (30%) total IJVs (i.e. including all groups plus the two sham mice), blood flow was detectable with a PRF of 4 kHz. In 10 of 40 (25%) IJVs, the PRF had to be adjusted to 3 kHz (Figure 3), and in 18 of 40 (45%) IJVs, the PRF had to be adjusted to 2 kHz. The PRF values required to identify the IJVs pre-operatively are summarized in Table 3.
Figure 3.
Trans-axial color-Doppler HFUS image at a PRF of 3 kHz (36.1 mm/s blood velocity). (1) Carotid artery, (2) internal jugular vein, (3) external jugular vein and (4) vertebral artery
Table 3.
Number and percentage of internal jugular veins identified pre-operatively at each pulse repetition frequency (PRF, in kHz) setting
| PRF (kHz) | 4 | 3 | 2 |
|---|---|---|---|
| IJV (n = 40) | 12/40–30% | 10/40–25% | 18/40–45% |
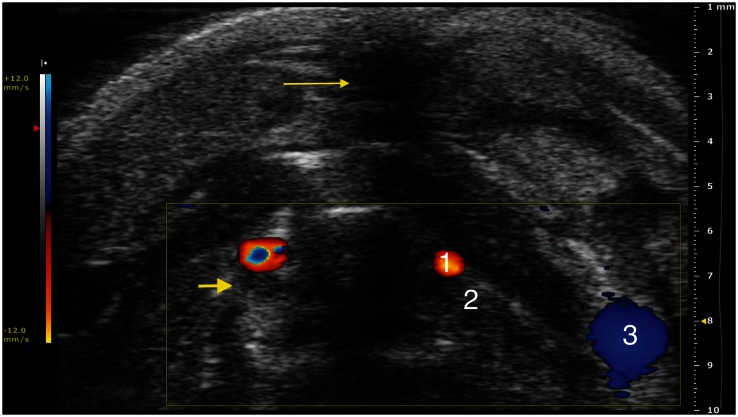
To assess the absence of blood flow after the surgical procedures, the PRF was adjusted from 4 kHz to 1 kHz in a step-by-step fashion. A diagnosis of occlusion of the vessel was made when there was no detectable flow at the lowest PRF level. In the two mice in which it was not possible to cut one EJV, the HFUS revealed occlusion of the vein at two days post-surgery and recanalization with low flow after 10 days (Figure 4). In the only case without ligature of the IJV, HFUS evaluation did not reveal any flow at both 2 and 10 days post-surgery, with the PRF set at 1 kHz.
Figure 4.
Trans-axial color-Doppler HFUS image at a PRF of 1 kHz (12.0 mm/s blood velocity). (1) Carotid artery, (2) internal jugular vein, (3) external jugular vein. Note: Acoustic shadowing due to surgical access (thin arrow); moderately hyperechoic material is visible within the internal jugular vein, in addition to the absence of flow, which is compatible with thrombotic phenomena following the interruption of the blood flow (large arrow)
The mean vessel diameters were 0.36 mm for IJVs and of 0.93 mm for EJVs.
Discussion
In our study, surgical ligation had a higher percentage of a success than electrocoagulation in the occlusion of jugular veins. We speculate that the 100% rate of failure recorded for IJV blood flow interruption using ME might be due to a vagus nerve reflex evoked by the thermoelectric impulse applied to the IJV via the tissue connection between the two structures.31 The bilateral surgical ligation of EJVs and IJVs was shown to be technically feasible and to result in low mortality when performed by a trained operator. The HFUS evaluation allowed us to follow up on the post-procedural progression of each technique employed, increasing the cost-effectiveness of the procedure and reducing the number of mice required.
Yonekawa and colleagues have previously discussed the importance of microsurgical training when operating on small-diameter vessels.29 The techniques they described (i.e. vascular anastomoses and dissections of the major arteries) were performed on rats with vessels between 0.8 and 1.5 mm in diameter, although they underline that in pediatric microsurgery, vessels of 0.5 mm diameter might be encountered.29 In our study, we performed surgery on veins as small as 0.4 mm in diameter. The same veterinary surgeon (LA) performed all procedures to avoid any operator variability. IJV dissection and exteriorization were found to be the most difficult part of the microsurgical procedure. Its deep location, small size, fragility, and contiguity with the vagus nerve and the carotid artery all justified the time spent on the accurate and delicate manipulation of this vein. The manipulations led to the formation of a hematoma in only one procedure due to the rupture of the vein wall before the tissue fold that contains the IJV had been dissected. After this event, we chose to end the procedure on the affected vein because of the high likelihood of producing a fatal hemorrhage. Although the HFUS evaluation showed no blood flow in the described vein, we considered it as an unsuccessful procedure.
In our study, the overall efficiency of ME was 22%, which is lower than that reported by Diamantis and colleagues in a comparative study between the use of ME, bipolar electro-coagulation, Ligasure™ (radiofrequency-mediated fusion) and Ultracision (ultrasound harmonic scalpel) in rabbits.32
The sensitivity of the color-Doppler method used permitted us to recognize even the minimum blood flow in the veins that underwent the occlusion, both with SL and ME. When using color-Doppler on small vessels, thoroughly understanding and precisely adjusting the PRF, Doppler gain, and angle of insonation are crucial to obtain optimal images of blood flow.33
This study could be of help to researchers interested in exploring the relationship between cerebrovascular anomalies and neuroinflammatory diseases. Even if the chronic cerebrospinal venous insufficiency (CCSVI) theory represents an intriguing pathogenic explanation for neuroinflammation linked to venous alterations, clinical studies have not yet reached complete agreement on the relationship between ultrasonographic signs, symptoms, and venous blood flow anomalies observed.10,34–40 In this regard, the demonstration of a direct causal effect and the pathophysiological mechanisms that lead to neuroinflammation through blood flow disruption are deemed essential. To the best of our knowledge, only one other attempt has been made to develop a mouse model of cerebral venous outflow deficiency.41 In this study, bilateral ligation of EJVs did not produce neuroinflammation or demyelination.41 The cerebral drainage pathway is quite different between humans and mice. In fact, cerebral venous outflow also depends on IJV patency in mice.27 Therefore, we decided to develop a bilateral EJV and IJV blood flow interruption model. It could be interesting in future studies to examine the effect of mono- and bilateral EJV and IJV occlusion on the hemodynamics of cerebral venous outflow and the neuroinflammatory and/or degenerative effects of such hemodynamic changes. Furthermore, once the complete occlusion model will be fully characterized, it will be interesting to consider developing a model of sub-occlusion. This surgical approach may have two advantages: it could create a stenosis effect rather an acute occlusion and it could permit to understand if removal of stenosis may change something in brain pathophysiology.
The present study has some limitations. We used only monopolar electro-coagulation, and we do not actually know whether the use of bipolar electro-coagulation would have led to different results. Moreover, the ability of HFUS to detect even low rates of flow in uncut veins might be influenced by the hemodynamic level of mice posed in a supine position and under deep anesthesia.
Finally, we cannot exclude the formation of collateral circulation that allows cerebral outflow soon after permanent venous ligation, and repeated HFUS examinations and magnetic resonance studies of mice would be desirable to determine whether any changes occurred in blood outflow from the brains of ligated mice. A well-trained surgeon and repeated HFUS Doppler measurements after the surgical procedure are desirable to avoid the drawbacks of these procedures. Further studies will be developed by our team to investigate whether any brain damage occurred after jugular vein ligation. Furthermore, to fully characterize the cerebral venous ligation model, we would like to plan behavioral, cognitive and motor tests to understand which functions may result compromised by the venous outflow impairment. In order to evaluate the cerebral blood volume and flow, and the development of collateral circulation, HFUS Doppler could be helpful. Finally, the consequences of brain ligation could be investigated both with imaging studies (like Magnetic Resonance Imaging to ascertain the eventual disruption of the blood-brain barrier) and both with histological and immunohystochemical techniques.
Acknowledgements
This work was supported by a research grant from the Italian Ministry for Education, University and Research in the framework of PRIN (2010XE5L2R_004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors’ contributions
AG, MS, and MM participated in the design, interpretation of the studies, analysis of the data, and review of the manuscript; LA and SA conducted the experiments; LM critically reviewed the results and the whole manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Müller LO, Toro EF, Haacke EM, Utriainen D. Impact of CCSVI on cerebral haemodynamics: a mathematical study using MRI angiographic and flow data. Phlebology 2015; 0: 1–20. [DOI] [PubMed] [Google Scholar]
- 2.Okuyama S, Okuyama J, Okuyama J, Tamatsu Y, Shimada K, Hoshi H, Iwai J. The arterial circle of Willis of the mouse helps to decipher secrets of cerebral vascular accidents in the human. Med Hypotheses 2004; 63: 997–1009. [DOI] [PubMed] [Google Scholar]
- 3.Yuan L, Li Y, Lu H, Zhao L, Tong S. Early artery blood flow is more prognostic in rodent model of middle cerebral artery occlusion. Conf Proc IEEE Eng Med Biol Soc 2014; 2014: 2845–8. [DOI] [PubMed] [Google Scholar]
- 4.Balbi M, Ghosh M, Longden TA, Jativa Vega M, Gesierich B, Hellal F, Lourbopoulos A, Nelson MT, Plesnila N. Dysfunction of mouse cerebral arteries during early aging. J Cereb Blood Flow Metab 2015; 35: 1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ, Goeggel-Simonetti B, Engelter ST, Pezzini A, Bijlenga P1, Southerland AM, Naggara O, Béjot Y, Cole JW, Ducros A, Giacalone G, Schilling S, Reiner P, Sarikaya H, Welleweerd JC, Kappelle LJ, de Borst GJ, Bonati LH, Jung S, Thijs V, Martin JJ, Brandt T, Grond-Ginsbach C, Kloss M, Mizutani T, Minematsu K, Meschia JF, Pereira VM, Bersano A, Touzé E, Lyrer PA, Leys D, Chabriat H, Markus HS, Worrall BB, Chabrier S, Baumgartner R, Stapf C, Tatlisumak T, Arnold M, Bousser MG. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol 2015; 14: 640–54. [DOI] [PubMed] [Google Scholar]
- 6.El Amki M, Clavier T, Perzo N, Bernard R, Guichet PO, Castel H. Hypothalamic, thalamic and hippocampal lesions in the mouse MCAO model: Potential involvement of deep cerebral arteries? J Neurosci Methods 2015; 254: 80–5. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura A, Saito S, Maki T, Oishi N, Ayaki T, Hattori Y, Yamamoto Y, Urushitani M, Kalaria RN, Fukuyama H, Horsburgh K, Takahashi R, Ihara M. Gradual cerebral hypoperfusion in spontaneously hypertensive rats induces slowly evolving white matter abnormalities and impairs working memory. J Cereb Blood Flow Metab 2015; 0: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menshawi K, Mohr JP, Gutierrez J. A functional perspective on the embryology and anatomy of the cerebral blood supply. J Stroke 2015; 17: 144–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvis-Miranda HR, Castellar-Leones SM, Alcala-Cerra G, Moscote-Salazar LR. Cerebral sinus venous thrombosis. J Neurosci Rural Pract 2013; 4: 427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zivadinov R, Chung C.-P. Potential involvement of the extracranial venous system in central nervous system disorders and aging. BMC Med 2013; 11: 260–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raz E, Pontecorvo S, Barra V, Marincola BC, Morreale M, Tinelli E, Saba L, Di Paolo PL, Aceti A, Catalano C, Francia A, Caramia F. MR venography in patients with multiple sclerosis and correlation with clinical and MRI parameters. J Neuroimag 2014; 24: 492–7. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Chen Y, Xu L, Matei N, Tang J, Feng H, Zhang JH. Venous system in acute brain injury: Mechanisms of pathophysiological change and function. Exp Neurol 2015; 272: 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeshima Y, Miyake H, Nakagawa I, Motoyama Y, Park YS, Nakase H. Visualization of regional cerebral blood flow dynamics during cortical venous occlusion using laser speckle contrast imaging in a rat model. J Stroke Cerebrovasc Dis 2015; 24: 2200–06. [DOI] [PubMed] [Google Scholar]
- 14.Wilkins RW, Halperin MH, Litter J. The effect of the dependent position upon blood flow in the limbs. Circulation 1950; 2: 373–9. [DOI] [PubMed] [Google Scholar]
- 15.Wilson EM, Halsey JH., Jr Bilateral jugular venous blood flow by thermal dilution. Stroke 1970; 1: 348–55.. [DOI] [PubMed] [Google Scholar]
- 16.Watson GH. Effect of head rotation on jugular vein blood flow. Arch Dis Child 1974; 49: 237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willeput R, Rondeux C, De Troyer A. Breathing affects venous return from legs in humans. J Appl Physiol Respir Environ Exerc Physiol 1984; 57: 971–6. [DOI] [PubMed] [Google Scholar]
- 18.Virolainen J, Ventilä M, Turto H, Kupari M. Influence of negative intrathoracic pressure on right atrial and systemic venous dynamics. Eur Heart J 1995; 16: 1293–9. [DOI] [PubMed] [Google Scholar]
- 19.Alperin N, Lee SH, Sivaramakrishnan A, Hushek SG. Quantifying the effect of posture on intracranial physiology in humans by MRI flow studies. J Magn Reson Imaging 2005; 22: 591–6. [DOI] [PubMed] [Google Scholar]
- 20.Diaconu CI, Fox RJ, Grattan A, Rae-Grant A, Lu M, Gornik HL, Kim ESH. Hydration status substantially affects chronic cerebrospinal venous insufficiency assessments. Neurol Clin Pract 2013; 3: 386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akkawi NM, Agosti C, Borroni B, Rozzini L, Magoni M, Vignolo LA, Padovani A. Jugular valve incompetence: a study using air contrast ultrasonography on a general population. J Ultrasound Med 2002; 21: 747–51. [DOI] [PubMed] [Google Scholar]
- 22.Doepp F, Bähr D, John M, Hoernig S, Valdueza JM, Schreiber SJ. Internal jugular vein valve incompetence in COPD and primary pulmonary hypertension. J Clin Ultrasound 2008; 36: 480–4. [DOI] [PubMed] [Google Scholar]
- 23.Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson's disease. Bioessays 2002; 24: 308–18. [DOI] [PubMed] [Google Scholar]
- 24.Wang LH, Qin ZH. Animal models of Huntington's disease: implications in uncovering pathogenic mechanisms and developing therapies. Acta Pharmacol Sin 2006; 27: 1287–302. [DOI] [PubMed] [Google Scholar]
- 25.Ramaswamy S, McBride JL, Kordower JH. Animal models of Huntington's disease. ILAR J 2007; 48: 356–73. [DOI] [PubMed] [Google Scholar]
- 26.Mitra NK, Bindal U, Eng Hwa W, Chua CL, Tan CY. Evaluation of locomotor function and microscopic structure of the spinal cord in a mouse model of experimental autoimmune encephalomyelitis following treatment with syngeneic mesenchymal stem cells. Int J Clin Exp Pathol 2015; 8: 12041–52. [PMC free article] [PubMed] [Google Scholar]
- 27.Mancini M, Greco A, Tedeschi E, Palma G, Ragucci M, Bruzzone MG, Coda ARD, Torino E, Scotti A, Zucca I, Salvatore M. Head and neck veins of the mouse. A magnetic resonance, micro computed tomography and high frequency color Doppler ultrasound study. PlosOne 2015; 10: e0129912–e0129912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aydin ON, Ek RO, Temoçin S, Ugǧur B, Alaçam B, Sşen S. The antinociceptive effects of systemic administration of tramadol, gabapentin and their combination on mice model of acute pain. Agǧri 2012; 24: 49–55. [DOI] [PubMed] [Google Scholar]
- 29.Yonekawa Y, Frick R, Roth P, Taub E, Imhof HG. Laboratory training in microsurgical techniques and microvascular anastomosis. Operative Techniques in Neurosurgery 1999; 2: 149–58. [Google Scholar]
- 30.Toombs JP, Clarke KM. Basic operative techniques. In: Slatter D (ed.) Textbook of small animal surgery. 3rd ed. Philadelphia, PA: Saunders Elsevier Science, 2003, pp.199-221.
- 31.Chapleau MW, Sabharwal R. Methods of assessing vagus nerve activity and reflexes. Heart Fail Rev 2011; 16: 109–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diamantis T, Kontos M, Arvelakis A, Syroukis S, Koronarchis D, Papalois A, Agapitos E, Bastounis E, Lazaris AC. Comparison of monopolar electrocoagulation, bipolar electrocoagulation, ultracision, and ligasure. Surg Today 2006; 36: 908–13. [DOI] [PubMed] [Google Scholar]
- 33.Gill RW. Measurement of blood flow by ultrasound: accuracy and sources of error. Ultrasound Med Biol 1985; 7: 625–42. [DOI] [PubMed] [Google Scholar]
- 34.Zamboni P. The Big Idea: Iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J R Soc Med 2006; 99: 589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicolaides AN, Morovic S, Menegatti E, Viselner G, Zamboni P. Screening for chronic cerebrospinal venous insufficiency (CCSVI) using ultrasound. Recommendations for a protocol. Funct Neurol 2011; 26: 229–48. [PMC free article] [PubMed] [Google Scholar]
- 36.Chambers B, Chambers J, Cameron H, Macdonell R. Chronic cerebrospinal venous insufficiency is not more prevalent in patients with mild multiple sclerosis: a sonographer-blinded, case-control ultrasound study. Mult Scler 2012; 19: 749–56. [DOI] [PubMed] [Google Scholar]
- 37.Zamboni P, Galeotti R, Weinstock-Guttman B, Kennedy C, Salvi F, Zivadinov R. Venous angioplasty in patients with multiple sclerosis: results of a pilot study. Eur J Vasc Endovasc 2012; 43: 116–22. [DOI] [PubMed] [Google Scholar]
- 38.Leone MA, Raymkulova O, Lucenti A, Stecco A, Bolamperti L, Coppo L, Liboni W, Rivadossi G, Zaccala G, Maggio M, Melis F, Giaccone C, Carriero A, Lochner P. A reliability study of colour-Doppler sonography for the diagnosis of chronic cerebrospinal venous insufficiency shows low inter-rater agreement. BMJ Open 2013; 3: e003508–e003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onida S, Davies AH. The chronic cerebrospinal venous insufficiency debate. Eur J Vasc Endovasc 2014; 48: 1–3. [DOI] [PubMed] [Google Scholar]
- 40.Zivadinov R, Bastianello S, Dake MD, Ferral H, Haacke EM, Haskal ZJ, Hubbard D, Liasis N, Mandato K, Sclafani S, Siddiqui AH, Simka M, Zamboni P. Recommendations for multimodal noninvasive and invasive screening for detection of extracranial venous abnormalities indicative of chronic cerebrospinal venous insufficiency: a position statement of the International Society for Neurovascular Disease. J Vasc Interv Radiol 2014; 25: 1785–94. [DOI] [PubMed] [Google Scholar]
- 41.Atkinson, Forghani R, Wojtkiewicz GR, Pulli B, Iwamoto Y, Ueno T, Waterman P, Truelove J, Oklu R, Chen JW. Ligation of the jugular veins does not result in brain inflammation or demyelination in mice. PlosOne 2012; 7: e33671–e33671. [DOI] [PMC free article] [PubMed] [Google Scholar]