Abstract
Ocular complications associated with diabetes mellitus are progressive and becoming one of the most important causes of morbidity worldwide. The purpose of the study is to evaluate the protective effect of Polygonatum sibiricum polysaccharide, an important component of Polygonatum sibiricum, on ocular complications in streptozotocin-induced diabetes mellitus rats. Sprague Dawley rats were made diabetic with streptozotocin(60 mg/kg, i.v.) and then the rats were treated with Polygonatum sibiricum polysaccharide 200, 400 and 800 mg/kg.d by gavage for 12 weeks. Biochemical analysis indicated that Polygonatum sibiricum polysaccharide lowered the levels of fasting blood glucose and glycated hemoglobin in blood and elevated the levels of insulin and C-peptide in plasma of diabetes mellitus rats in a dose-dependent manner. Physical measurements revealed that Polygonatum sibiricum polysaccharide improved clinical symptoms of polydipsia, polyphagia, polyuria and weight loss in diabetes mellitus rats. The content of malondialdehyde and activity of superoxide dismutase in plasma were determined, and the data showed Polygonatum sibiricum polysaccharide suppressed oxidative stress reaction. Lens opacification was observed using slit lamp illumination, and the data showed Polygonatum sibiricum polysaccharide delayed cataract progression in a dose-dependent manner. Electroretinogram showed Polygonatum sibiricum polysaccharide treatment reversed the decrease of electroretinogram b and OPs2 waves’ amplitudes. Flash-visual evoked potential test indicated that the peak time of P2 wave was prolonged, and the amplitude of N2-P2 was lowered in diabetes mellitus group, and Polygonatum sibiricum polysaccharide suppressed these changes. Fundus fluorescein angiography showed Polygonatum sibiricum polysaccharide alleviated the retinal vasculopathy in a dose-dependent manner. In conclusion, these results suggest that the administration of Polygonatum sibiricum polysaccharide slows the progression of diabetic retinopathy and cataract through alleviating hyperglycemia and reducing oxidative stress in streptozotocin-induced diabetes mellitus rats.
Keywords: Polygonatum sibiricum polysaccharide, diabetes mellitus, diabetic retinopathy, cataract, ocular complications
Introduction
With the rising of human being’s life standard, diabetes mellitus (DM) has become the most prevalent endocrine disorder around the world. A report published by the International Diabetes Federation currently indicates that DM has impact on at least 378 million people worldwide, and this figure is most likely to be doubled by 2035.1 DM alone is not horrible, but its complications are more than horrible. DM can lead to several ocular complications such as diabetic retinopathy (DR), cataract, glaucoma, etc., which remains a major cause of blindness.2 DR, one of the most common microvascular complications in DM, is the leading cause of blindness among working people in developed countries.3 For patients whose diabetic duration is less than five years, DR morbidity is approximately 38–39%, and for those whose duration is 5–10 years, the morbidity is approximately 50–56.7%, whereas for patients whose diabetic duration is more than 10 years, the morbidity is approximately 69–90%.4 Good blood glucose control and attenuating other risk factors such as oxidative stress are main goals to prevent ocular complications of DM.
In spite of presently available conventional drugs (insulin (INS), sulfonylureas, biguanides, α-glucosidase inhibitors, thiazolidinediones and dipeptidyl peptidase-4inhibitors), the treatment of DM has been a difficult task. This is due to the unwanted side-effects associated with the use of conventional drugs, including hypoglycemia, weight gain, hypersensitivity, gastrointestinal discomfort, nausea, liver and heart failure, and diarrhoea.5 However, traditional Chinese medicine can curve these problems, since fewer side-effects have been reported with the use of plants in the treatment of several diseases.6,7
Polygonatum sibiricum grows in the wild and is cultivated as a traditional medicinal herb and foodstuff in China. As a common Chinese medicine, P. sibiricum is considered to have the functions of compensating vital essence, removing dryness, promoting secretion of fluid and quenching thirst.8 Previous study also indicated P. sibiricum improved obesity conditions and INS resistance.9 In recent years, many components have been isolated from P. sibiricum and Polygonatum sibiricum polysaccharide (PSP) is the major medicinal effective ingredient. As an important component of P. sibiricum, PSP is known to have low toxicity and is considered to be suitable for long-term administration. In this study, we investigated the effect of PSP treatment on ocular lesion of streptozotocin (STZ)-induced diabetic rats so as to elucidate the therapeutical effect of PSP on DM and its ocular complications.
Materials and methods
Materials and reagents
PSP was purchased from Taian Zhonghui Plant Biochemical Co. Ltd (China), and the essence of PSP is 98% in purity. STZ was purchased from Sigma (USA). Blood glucose determination kit was provided by Shanghai Qiaodu Biotechnology Co. Ltd (China). Enzyme-linked immunosorbent assay (ELISA) kits of INS and C-peptide were purchased from Shanghai Lanji Biotechnology Co. Ltd (China). Glycated hemoglobin (HbA1c) determination kit was purchased from Shanghai Huayi Biotechnology Co. Ltd (China). Malondialdehyde (MDA) and superoxide dismutase (SOD) determination kits were obtained from Nanjing Jiancheng Bioengineering Institute (China). Tropicamide was the product of Santen Pharmaceutical Co. Ltd (Japan). Chloral hydrate (7%) was produced by Affiliated Hospital of Taishan Medical University (China). Fluorescein sodium injection was purchased from Guangzhou Baiyunshan Mingxing Pharmaceutical Co., Ltd (China). Fundus camera and slit lamp illumination tester were obtained from Shanghai New Eyes Medical Co. Ltd (China). Eye electrophysiological diagnosis system was obtained from Roland (Germany). Synchronous confocal laser fundus fluorescence angiography was purchased from Heidelberger Druckmaschinen AG (Germany).
Animal
A total of 80 male Sprague Dawley (SD) rats (200 ± 20 g, two months old) were purchased from SPF (Beijing) Experimental Animal Technology Co. Ltd. All rats were housed under controlled conditions (22–24℃, 12:12 h light/dark cycle) and provided with food and water ad libitum. All experiments were approved by the laboratory animals’ ethical committee of Taishan Medical University and followed national guidelines for the care and use of animals.
Induction of DM and experimental groups
DM was induced by intravenous injection of STZ (60 mg/kg) in citrate buffer via tail vein. Only STZ-treated rats with blood glucose levels higher than 13.9 mmol/L at day 3 and day 7 after STZ injection were considered diabetic and were included in the study. In this experiment, 56 out of 64 rats were modeled successfully, and the success rate of model making is 87.5%. The DM rats were randomly allocated into four groups: DM model group (DM), DM with low dosage of PSP group (PSP-L), DM with middle dosage of PSP group (PSP-M) and DM with high dosage of PSP (PSP-H). Every group is composed of 14 DM rats. Another 16 rats were only given sodium citrate in place of STZ and used as blank control group (BC). From the seventh day, BC and DM groups were given normal saline of 1 mL/100 g once a day by gavage. PSP-L, PSP-M and PSP-H groups were given equal amount of PSP saline solution, and the amount of PSP was 200 mg/kg.d, 400 mg/kg.d and 800 mg/kg.d, respectively. This experiment lasted for 12 weeks.
Physical measurements
After modeling, for all rats in each group their food intake, water intake and urine output were measured by metabolic cages every two weeks, and their body weight was measured by electronic balance every one week.
Biochemical analysis
After 12 h overnight fasting, blood samples were collected by angula vein. Fasting blood glucose (FBG) level was measured by glucose-oxidase method using blood glucose meter (One Touch UltraEasy, USA) every one week. HbA1c level was estimated from whole blood collected in EDTA vacutainer by immunoturbidimetry method using automatic biochemical analyzer. Plasma concentrations of INS and C-peptide were determined by ELISA kit according to the manufacturer’s instructions. The levels of HbA1c, INS and C-peptide were tested at 0th, 4th, 8th and 12th weeks.
Plasma MDA level was determined by a spectrophotometric measurement of thiobarbituric acid-reactive substances according to the manufacturer’s instruction. SOD activity was measured by the Fridovich’s method. This method uses xanthine and xanthine oxidase to produce superoxide radicals, which react with p-iodonitrotetrazolium violet to generate a red formazan measured at 505 nm.10
Cataract examination
After dilating the pupils of unanesthetized animals with 1% tropicamide, lens opacification was observed by a researcher (blinded to the treatment condition) using slit lamp illumination. To ensure equivalent assessment of cataract for each observation, the slit lamp illumination was standardized to the same slit width and light intensity for all examinations.
Electroretinogram
Rats were anesthetized by intraperitoneal injection of 7% chloral hydrate solution, pupils were dilated with 1% tropicamide, and the corneal surface was anesthetized with 0.5% tetracaine HCl. Full-field electroretinogram (ERG) was performed according to the ISCEV standard.11 Recordings were made with an electrode placed on the cornea. The reference electrode was placed on ear and the ground electrode was put on posterior fontanelle. Scotopic rod response and oscillatory potentials (OPs) were tested and analyzed eye electrophysiological diagnosis system.
Flash-visual evoked potential
This operation was followed by ERG examination. Before test, anesthetic effect was tested so as to add anesthetic dose, if necessary. Electrode was put on posterior fontanelle, and reference electrode was put at the midpoint of the line between two eyes, and ground electrode was put on the right ear. On the platform of eye electrophysiological diagnosis system, the item of flash-visual evoked potential (F-VEP) was chosen. By fine tuning contact location of every electrode, when the value of resistance was 10 Kohm or less, F-VEPs of two eyes were measured. When the eliciting stimulus was a 1.4 Hz flash, peak times of N1-3 and P1-3 were recorded as well as amplitudes of N1-P1, N2-P2 and N3-P3. Then, the eliciting stimulus was switched to 11 Hz, and peak times of N1 and P1 were recorded as well as amplitude of N1-P1.
Fundus fluorescein angiography
This operation was followed by F-VEP examination. For the rats with cataract, this process was given up. After intraperitoneal injection of fluorescein sodium (10%, 50 mg/kg), the rats were placed on a stereotactic platform, and dynamic fundus photographs were taken.
Statistical analysis
The results are presented as mean ± SD. Statistical analysis was carried out by one-way analysis of variance followed by Student–Newmann–Keuls multiple comparison tests with the SPSS 19.0 software for Windows. P-value less than 0.05 was considered statistically significant.
Results
PSP regulated glucose metabolism and improved clinical symptoms in STZ-induced DM rats
PSP lowered the levels of FBG and HbA1c and elevated the levels of INS and C-peptide in DM rats
Compared with that of BC group, FBG level of DM group was promoted by more than twice after STZ injection (P < 0.001) and continued to elevate throughout the experimental period. While FBG level in DM rats treated with PSP lowered significantly in a dose-dependent manner from first week (Figure 1(a)). Compared with DM group, middle dosage and high dosage of PSP downregulated FBG level at every experimental time starting from first week (P < 0.01); low dosage of PSP downregulated FBG level as well (P < 0.01 or P < 0.05) during experiment period (except for fifth and sixth weeks).
Figure 1.
PSP lowered the levels of FBG and HbA1c and elevated the levels of INS and C-peptide in DM rats. Data are presented as mean ± SD of 14–16 animals. (a) Showing FBG level of every group during the intervention period. Compared with that of BC group, FBG level of DM group was promoted by more than twice after STZ injection (P < 0.001) and continued to elevate throughout the experimental period. Compared with DM group, middle dosage and high dosage of PSP downregulated FBG level at every experimental time starting from first week (P < 0.01); low dosage of PSP downregulated FBG level as well (P < 0.01or P < 0.05) during experiment (period except for fifth and sixth weeks). (b) Showing HbA1c level of every group during the intervention period. Compared with that of BC group, HbA1c was significantly higher in DM group (P < 0.001). Compared with DM group, HbA1c level of PSP-H group became lower at fourth week (P < 0.05); HbA1c level of PSP-M and PSP-H groups became lower at eighth week (P < 0.01); HbA1c level of PSP-L, PSP-M and PSP-H groups became lower at 12th week (P < 0.01). (c) Showing INS level of every group during the intervention period. Compared with that of BC group, induction of DM resulted in a distinct reduction of ISN (P < 0.001). Compared with DM group, INS level of PSP-L, PSP-M and PSP-H groups was increased at fourth week (P < 0.01); INS level of PSP-H group was increased at 8th and 12th weeks (P < 0.05). (d) Showing C-peptide level of every group during the intervention period. Compared with that of BC group, C-peptide level in plasma of DM group was remarkably decreased (P < 0.001) Compared with DM group, C-peptide level of PSP-L, PSP-M and PSP-H groups was increased at 12th week (P < 0.05 or P < 0.01).
INS: insulin; FBG: fasting blood glucose; HbA1c: glycated hemoglobin
HbA1c is formed by chemical reaction between hemoglobin and blood glucose. It represents a reliable and moving average of blood glucose over three months. In agreement with FBG level, HbA1c was significantly promoted in DM rats by 73%, 217% and 216% at 4th, 8th and 12th weeks, respectively, compared with BC group; however, PSP interference inhibited the increase of HbA1c level induced by STZ in a dose-dependent manner (Figure 1(b)). Compared with DM group, HbA1c level of PSP-H group became lower at 4th week (P < 0.05); HbA1c level of PSP-M and PSP-H groups became lower at 8th week (P < 0.01); HbA1c level of PSP-L, PSP-M and PSP-H groups became lower at 12th week (P < 0.01).
As shown in Figure 1(c), induction of diabetes with STZ resulted in a distinct reduction of INS by 63%, 81%, 83% at 4th, 8th and 12th weeks, respectively, compared with that of BC group. PSP treatment in DM rats led to a higher plasma INS level. Compared with DM group, INS level of PSP-L, PSP-M and PSP-H groups was increased at 4th week (P < 0.01); INS level of PSP-H group was increased at 8th and 12th weeks (P < 0.05).
C-peptide, the product of proinsulin proteolysis, is a chaperone for INS during its storage in transport vesicles of pancreatic β-cells and further is secreted into bloodstream. C-peptide level is reduced in plasma of patients with diabetes mellitus type 1 due to autoimmune destruction of β-cells.12 In this study, the level of C-peptide was also measured. In agreement with INS level, C-peptide level in plasma of DM group was remarkably decreased by 39%, 49%, 54% at 4th, 8th and 12th weeks, respectively, compared with BC group. PSP promoted C-peptide level of DM rats in a dose-dependent manner (Figure 1(d)). Compared with DM group, C-peptide level of PSP-L, PSP-M and PSP-H groups was increased at 12th week (P < 0.05 or P < 0.01).
PSP improved clinical symptom of polydipsia, polyphagia, polyuria and weight loss in DM rats
The rats in BC group were very active, swift and well proportioned during the entire experimental period. Their hair was healthy and shiny. With the constant increase of blood glucose, the body of rats in DM group became spindle-like, their hair turned dark yellow and they were in low spirits.
As shown in Tables 1 to 4, food intake, water intake and urine output of DM rats were greatly increased, whereas their body weight was lost during the experimental period compared with BC group. PSP treatment in DM rats led to a significantly improvement of diabetic typical symptoms induced by STZ injection. The effect of PSP is in a dose-dependent manner.
Table 1.
Food intake in all groups during the intervention period
| BC (g/d) | DM (g/d) | PSP-L (g/d) | PSP-M (g/d) | PSP-H (g/d) | |
|---|---|---|---|---|---|
| 0w | 26.43 ± 3.84 | 28.61 ± 3.03 | 28.11 ± 5.84 | 27.4 ± 3.05 | 26.79 ± 7.63 |
| 2w | 25.78 ± 7.07 | 36.33 ± 7.45a | 34.63 ± 7.19 | 32.57 ± 7.27 | 31.5 ± 7.63 |
| 4w | 27.39 ± 5.30 | 37.03 ± 7.04a | 36.12 ± 3.16 | 35.28 ± 6.43 | 34.71 ± 9.04 |
| 6w | 26.02 ± 10.79 | 38.23 ± 8.45a | 37.88 ± 9.15 | 36.72 ± 6.46 | 35.84 ± 6.43 |
| 8w | 24.89 ± 3.91 | 44.24 ± 7.59a | 42.98 ± 7.83 | 38.1 ± 9.32 | 37.38 ± 8.24b |
| 10w | 28.03 ± 6.88 | 45.08 ± 6.11a | 44.29 ± 8.27 | 43.7 ± 3.77 | 40.81 ± 4.93 |
| 12w | 22.14 ± 3.39 | 45.41 ± 6.89a | 44.33 ± 7.25 | 43.82 ± 5.17 | 41.51 ± 7.93 |
BC: blank control group; DM: diabetes mellitus model group; PSP-L: DM with low dosage of PSP group; PSP-M: DM with middle dosage of PSP group; PSP-H: DM with high dosage of PSP group.
Note: Data are presented as mean ± SD of 14–16 animals.
P < 0.01 vs. BC group of the same row.
P < 0.05 vs. DM group of the same row.
Table 2.
Water intake in all groups during the intervention period
| BC (ml/d) | DM (ml/d) | PSP-L (ml/d) | PSP-M (ml/d) | PSP-H (ml/d) | |
|---|---|---|---|---|---|
| 0w | 42.92 ± 6.13 | 46.83 ± 7.01 | 45.42 ± 9.68 | 43.78 ± 8.51 | 43.25 ± 8.49 |
| 2w | 45.04 ± 7.78 | 66.04 ± 10.17a | 53.83 ± 8.21b | 51.75 ± 5.70b | 47.46 ± 11.56b |
| 4w | 38.33 ± 18.22 | 98.92 ± 6.58a | 92.92 ± 7.95 | 78.58 ± 7.45b | 66.83 ± 10.82b |
| 6w | 39.17 ± 9.33 | 111.5 ± 6.63a | 102 ± 8.44b | 95.08 ± 9.76b | 84.17 ± 8.17b |
| 8w | 44 ± 14.32 | 131.17 ± 11.78a | 123.92 ± 10.55 | 112.43 ± 7.85b | 102.67 ± 6.60b |
| 10w | 42.5 ± 4.22 | 144.83 ± 9.66a | 133.92 ± 12.73b | 123.33 ± 6.95b | 118.58 ± 8.23b |
| 12w | 38.67 ± 10.46 | 162.25 ± 12.13a | 159.92 ± 11.30 | 146.33 ± 13.29b | 132.5 ± 9.60b |
BC: blank control group; DM: diabetes mellitus model group; PSP-L: DM with low dosage of PSP group; PSP-M: DM with middle dosage of PSP group; PSP-H: DM with high dosage of PSP group.
Note: Data are presented as mean ± SD of 14–16 animals.
P < 0.01 vs. BC group of the same row.
P < 0.01 vs. DM group of the same row.
Table 3.
Urine output in all groups during the intervention period
| BC (ml/d) | DM (ml/d) | PSP-L (ml/d) | PSP-M (ml/d) | PSP-H (ml/d) | |
|---|---|---|---|---|---|
| 0w | 7.42 ± 3.18 | 6.25 ± 3.17 | 7.25 ± 3.38 | 8.08 ± 2 | 8.42 ± 3.59 |
| 2w | 7.13 ± 1.92 | 25.25 ± 6.41a | 23.67 ± 6.58 | 22.17 ± 7.58 | 21.42 ± 7.78 |
| 4w | 10.17 ± 4.6 | 58.58 ± 7.14a | 56.09 ± 7.82 | 55.17 ± 8.08 | 50.33 ± 7.09b |
| 6w | 8.92 ± 2.66 | 89.5 ± 5.66a | 83.92 ± 7.01c | 75.83 ± 6.03b | 70.67 ± 8.2b |
| 8w | 11.67 ± 3.2 | 93.32 ± 9.85a | 87.33 ± 8.84 | 77.67 ± 9.52b | 72.08 ± 7.59b |
| 10w | 11.75 ± 3.11 | 102.92 ± 8a | 90.75 ± 9.41b | 82.75 ± 6.07b | 78.75 ± 8.41b |
| 12w | 11.08 ± 2.35 | 121.08 ± 6.91a | 119.58 ± 6.75 | 115.83 ± 5.92 | 86.33 ± 9.70b |
BC: blank control group; DM: diabetes mellitus model group; PSP-L: DM with low dosage of PSP group; PSP-M: DM with middle dosage of PSP group; PSP-H: DM with high dosage of PSP group.
Note: Data are presented as mean ± SD of 14–16 animals.
P < 0.01 vs. BC group of the same row.
P < 0.01 vs. DM group of the same row.
P < 0.05.
Table 4.
Body weight in all groups during the intervention period
| BC (g) | DM (g) | PSP-L (g) | PSP-M (g) | PSP-H (g) | |
|---|---|---|---|---|---|
| 0w | 265.87 ± 3.83 | 264.63 ± 5.90 | 267.45 ± 3.33 | 267.48 ± 3.32 | 267.29 ± 2.97 |
| 1w | 280.92 ± 3.91 | 270.74 ± 5.92a | 275.63 ± 3.27b | 277.49 ± 3.27b | 279.54 ± 2.93b |
| 2w | 295.98 ± 3.96 | 276.73 ± 5.94a | 283.68 ± 3.22b | 287.51 ± 3.25b | 291.50 ± 2.98b |
| 3w | 310.93 ± 3.82 | 282.69 ± 6.00a | 291.58 ± 3.37b | 297.54 ± 3.27b | 303.45 ± 2.92b |
| 4w | 325.76 ± 3.94 | 288.67 ± 5.96a | 299.54 ± 3.25b | 307.46 ± 3.26b | 315.49 ± 2.93b |
| 5w | 341.05 ± 3.93 | 294.63 ± 5.89a | 307.54 ± 3.27b | 317.47 ± 3.31b | 327.37 ± 2.87b |
| 6w | 355.98 ± 3.91 | 300.76 ± 5.83a | 315.58 ± 3.35b | 327.41 ± 3.33b | 339.45 ± 3.02b |
| 7w | 370.93 ± 3.94 | 306.80 ± 5.99a | 323.55 ± 3.16b | 337.46 ± 3.32b | 351.36 ± 2.97b |
| 8w | 385.83 ± 3.91 | 312.72 ± 5.97a | 331.54 ± 3.30b | 347.57 ± 3.22b | 363.45 ± 3.05b |
| 9w | 400.83 ± 3.90 | 318.53 ± 5.91a | 339.48 ± 3.31b | 357.44 ± 3.30b | 375.31 ± 2.95b |
| 10w | 415.88 ± 3.87 | 324.66 ± 5.95a | 347.50 ± 3.24b | 367.39 ± 3.27b | 387.48 ± 2.94b |
| 11w | 430.86 ± 3.90 | 330.63 ± 5.95a | 355.47 ± 3.33b | 377.39 ± 3.30b | 399.34 ± 2.95b |
| 12w | 446.02 ± 3.99 | 336.74 ± 5.97a | 363.61 ± 3.27b | 387.42 ± 3.27b | 411.40 ± 2.97b |
BC: blank control group; DM: diabetes mellitus model group; PSP-L: DM with low dosage of PSP group; PSP-M: DM with middle dosage of PSP group; PSP-H: DM with high dosage of PSP group.
Note: Data are presented as mean ± SD of 14–16 animals.
P < 0.01 vs. BC group of the same row.
P < 0.01 vs. DM group of the same row.
PSP attenuated oxidative stress in STZ-induced DM rats
Oxidative stress is a serious condition that may result in ocular complications associated with DM.13 MDA is one of the most reliable and widely used indices of oxidative stress. The toxic effects of reactive oxygen species can be reduced by antioxidants such as SOD.14 In our study, we determined SOD activity and MDA level in plasma. As shown in Figure 2, compared with that of BC group, SOD activity in plasma was significantly decreased by 21%, 28%, 29% at 4th, 8th and 12th weeks, respectively, in DM group. Meanwhile MDA level was significantly increased by 95%, 119%, 121% at 4th, 8th and 12th weeks, respectively, in DM group. Compared with that of DM group, PSP significantly promoted SOD activity and lowered MDA level in a dose-dependent manner (Figure 2).
Figure 2.
PSP attenuated oxidative stress in STZ-induced DM rats. (a) Showing the content of MDA in plasma during the intervention period. (b) Showing the activity of SOD in plasma during the intervention period. Data are presented as mean ± SD of 14–16 animals. ##P < 0.01 vs. BC group; *P < 0.05, **P < 0.01 vs. DM group.
MDA: malondialdehyde; SOD: superoxide dismutase; BC: blank control; DM: diabetes mellitus; PSP-H: Polygonatum sibiricum polysaccharide high; PSP-L: Polygonatum sibiricum polysaccharide low; PSP-M: Polygonatum sibiricum polysaccharide middle
PSP slowed the progression of ocular complications in STZ-induced DM rats
PSP had anticataract effect on STZ-induced DM rats
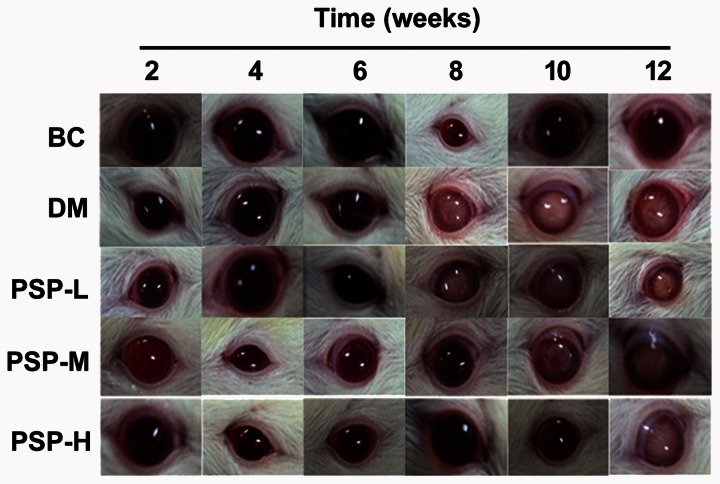
The effect of PSP on lens opacity was shown in Figure 3. During 12-week study period, all lens from BC rats were clear and normal. The eyes in DM group showed lens opacity. In DM group cataract occurred on both left and right eyes of one rat at 6th week, both eyes of two rats at 8th week and both eyes of another two rats during 10–12 weeks. In PSP-L group, cataract was shown on single eye of two rats at 8th week and both eyes of one rat at 10th week. In PSP-M group, cataract was found on both eyes of two rats at 10th week. In PSP-H group, cataract was discovered on single eye of one rat at 12th week.
Figure 3.
PSP had anticataract effect on STZ-induced DM rats. After dilating the pupils of animals with 1% tropicamide, lens opacification was observed using slit lamp illumination. This figure shows the representative photos from each group during the intervention period. Cataract in DM group developed rapidly, while those in PSP groups progressed slowly in a dose-dependent manner. (A color version of this figure is available in the online journal.)
BC: blank control; DM: diabetes mellitus; PSP-H: Polygonatum sibiricum polysaccharide high; PSP-L: Polygonatum sibiricum polysaccharide low; PSP-M: Polygonatum sibiricum polysaccharide middle
PSP delayed the procession of DR in STZ-induced DM rats
Effect of PSP on ERG of DM rats
The effect of PSP on ERG was determined and the results were shown in Figure 4. Compared with that of BC group, decrease of b-wave amplitude under scotopic 0.01 ERG was observed in vehicle-treated DM group at 4th, 8th and 12th weeks. Compared with that of DM group, the amplitude of b-wave was significantly elevated at 8th week. As to OPs2, its amplitude under scotopic 3.0 ERG in DM group was lowered by 37% at 12th week compared with that of BC group. At the same time, OPs2 amplitude of PSP-M and PSP-H groups was increased by 31% and 37%, respectively, compared with DM group. There were no differences in peak times of b and OPs2 waves among these five groups at 4th, 8th and 12th weeks.
Figure 4.
Effect of PSP on ECG of DM rats. Full-field ERG was performed using eye electrophysiological diagnosis system. (a) Showing peak time of ERG b wave. (b) Showing amplitude of ERG b wave. (c) Showing peak time of ERG OPs2 wave. (d) Showing amplitude of ERG OPs2 wave. Data are presented as mean ± SD of 14–16 animals. ##P < 0.01 vs. BC group; *P < 0.05, **P < 0.01 vs. DM group.
BC: blank control; DM: diabetes mellitus; PSP-H: Polygonatum sibiricum polysaccharide high; PSP-L: Polygonatum sibiricum polysaccharide low; PSP-M: Polygonatum sibiricum polysaccharide middle
Effect of PSP on F-VEP of DM rats
Compared with that of BC group, peak time of P2 wave under frequency of 1.4 HZ was prolonged by 16% and 28% at 8th and 12th weeks, respectively, in DM group, while treatment of PSP with 400 mg/kg.d and 800 mg/kg.d doses reversed the prolongation at 12th week. Compared with that of BC group, the amplitude of N2-P2 was lowered in DM group at 4th, 8th and 12th weeks and PSP treatment with 800 mg/kg.d dose reversed this change at 8th and 12th weeks (Figure 5).
Figure 5.
Effect of PSP on F-VEP of DM rats. F-VEP was recorded using eye electrophysiological diagnosis system. (a) Showing peak time of F-VEP P2 wave. (b) Showing amplitude of N2-P2 wave. Data are presented as mean ± SD of 14–16 animals. #P < 0.05, ##P < 0.01 vs. BC group; *P < 0.05 vs. DM group.
BC: blank control; DM: diabetes mellitus; PSP-H: Polygonatum sibiricum polysaccharide high; PSP-L: Polygonatum sibiricum polysaccharide low; PSP-M: Polygonatum sibiricum polysaccharide middle
Effect of PSP on FFA of DM rats
The normal rats exhibited complete retinal vascular net with uniform thickness of retinal capillary diameter and straight direction on optic disc. Macula lutea retinae, leakage and hemorrhage were not found on optic disc. Different degrees of retinal vascular morphology changes were found in DM rats, including disordered arrangement of capillary net, twisted capillaries, uneven thickness of capillary tube cavities and leaked fluorescence from sixth week. PSP treatment alleviated the retinal vasculopathy in a dose-dependent manner.
Discussion
DM is an important health problem which causes significant morbidity and mortality due to specific microvascular complications (retinopathy, nephropathy and neuropathy) and macrovascular complications (ischemic heart disease and peripheral vasculopathy). The eye is one of the principal organs affected by this disease, and ocular complications associated with DM are progressive rapidly and becoming one of the most important causes of morbidity worldwide. DR is a frequent and serious complication of DM resulting from damage to retinal microvasculature. The retinal cells primarily involved in DR are both endothelial and neuronal cells. The progress of DR is as follows: firstly, when the control of blood glucose is inadequate, DM leads to the weakening of smaller vessel walls, which results in the formation of microaneurysms and then edema, bleeding, and microinfarcts (ischemia). Next, neovascularization occurs which is called “proliferative stage.” The new vessels grow in a chaotic way by damaging nervous tissue, causing more serious bleeding and promoting retinal detachment. Therefore, DR is classified into two types: non-proliferative diabetic retinopathy and proliferative diabetic retinopathy. Besides DR, cataract characterized by opacification of crystalline lens is also a complication of DM,15,16 which can affect patients at younger age.17 In general, diabetic cataract attacks in both eyes and develops very swiftly and within several months, weeks or even several days, it will progress to complete opacification of crystalline lens.18
As an important component of P. siibiricum included in Chinese Pharmacopoeia (ChP),19 PSP has a variety of medical effects such as anti-inflammation,20 antioxidation, antiaging,21 lipid-lowering22 and blood sugar control.23 Because PSP has remarkable pharmacological function, cheap price and few side-effects, it has greater potential in application. In our study, the purity of PSP we applied is 98% and it consists of three polysaccharides. The molecular weights of these three polysaccharides are all more than 200 kDa. Polysaccharide I is mainly composed of fructose with β-(1→2) linked backbone, and the glucose is linked to the main chain by α-bond; polysaccharide II is mainly composed of mannose and galactose with 1→6 linkage; polysaccharide III is pectin with partial ethyl esterification. Our results showed that PSP treatment significantly decreased the concentrations of FBG and HbA1c in blood, increased the levels of INS and C-peptide in plasma and lessened the symptom of polydipsia, polyphagia, polyuria and weight loss in STZ-induced DM rats. These data indicate PPS had the effect of regulating glucose metabolism and improving clinical symptoms of DM. More importantly, we discovered for the first time that PSP delayed the progress of cataract and DR and promoted visual function in experimental diabetic rats. These data indicate that PSP has preventive effect on diabetic eye disease.
In our study, we applied ERG and F-VEP to assess retinal and nervous (optic tract) function of rats and used Fundus fluorescein angiography (FFA) technique to observe pathologic change of retinal microvessel. These techniques are safe, repeatable, quick and objective and have been proved to be successful tools for early diagnosis of DR and, potentially, for ophthalmologic follow-up of diabetic patients. ERG is applied to test the operation of the entire surface of neuroretina, only on photoreceptor and outer plexiform layers. The potentials recorded reflect many events are related to different types of cells: photoreceptors, bipolar cells, amacrine cells and Müller cells. ERG a-wave response reflects photoreceptor cell function, and b-wave reflects the bioelectrical activities of bipolar and Müller cells.24 The amplitude of ERG b-wave is low in DM without DR patients, and the amplitude is lower in DM with DR patients.24 However, OP waves are currently considered the most relevant electroretinographic index for DR diagnosis.25 OP waves might originate from the inhibitory feedback circuits of the inner retinal layers, and they are more sensitive to the changes of blood circulation in retina. The amplitudes of OPs2 and OPs3 have obvious correlation with the severity of DR, i.e. the lower the amplitude is, the more severe DR becomes.26 In this study, the amplitudes of ERG b and OPs2 waves were decreased in DM group (Figure 4), which indicated the damage of retinal function and an animal model of DM with DR had been successfully established. PSP treatment reversed the decrease of the two waves, which indicated that PSP had preventive and therapeutic effect on DR by reducing cellular damage and improving retinal blood circulation.
VEPs are defined as changes in bioelectric potentials of occipital cortex evoked by visual stimuli. They are generated by complex neurosensory events related to the translation and transmission of nerve impulses along optic tract from photoreceptors to occipital cortex. They can be elicited with pattern or flash stimuli. The objects of our experiment are rats, and they cannot cooperate well; therefore, F-VEP was applied to carry out the experiment. DM affects both electrophysiological and psychophysical aspects of visual function. A significant reduction in amplitude and increased latency of VEPs were found in DM without signs of retinopathy,27 which denotes a functional neuronal loss before anatomical abnormalities can be detected. In this study, we also found that the peak time of P2 wave was prolonged, and the amplitude of N2-P2 was lowered in DM group. However, PSP treatment reversed these changes (Figure 5).
In the present study, FFA was performed by injecting intraperitoneally sodium fluorescein and angiograms obtained showed retinal arterial–venous cycle. Subtle retinal vascular abnormalities, capillary angiography and vascular leakage can be detected only using this procedure. In our study, the retina from DM group showed capillaries’ deformation and vascular leakage as evidence on fluorescein angiograms (Figure 6). Other experimental studies have also revealed similar findings in DR.28–30 However, the retina treated by PSP was not detected to have clear vascular leakage, which indicated retinal vasculopathy was weakened. Therefore, it can be referred that PSP treatment has offered a protective effect on diabetes induced vasculopathy from results of present study.
Figure 6.
Effect of PSP on FFA of DM rats. After intraperitoneal injection of fluorescein sodium, the dynamic fundus photographs were taken. This figure shows the representative photos of each group during the intervention period. DM rats had different degrees of retinal vascular morphology changes from sixth week. PSP treatment alleviated the retinal vasculopathy in a dose-dependent manner.
BC: blank control; DM: diabetes mellitus; PSP-H: Polygonatum sibiricum polysaccharide high; PSP-L: Polygonatum sibiricum polysaccharide low; PSP-M: Polygonatum sibiricum polysaccharide middle
Several different pathogenetic mechanisms precipitate formation of diabetic eye disease. Besides the duration and control of DM, oxidative stress also contributes to the development of DR and cataract.31,32 Hyperglycemia causes overproduction of O2−, which leads to increased lipid peroxidation level and decreased antioxidase activity, and thereby enhances oxidative stress in subjects with DM. On one hand, oxidative stress can cause the activation of four biochemical pathways, including polyol pathway, advanced glycation end products pathway, protein kinase C pathway and hexosamine pathway.33 On the other hand, oxidative stress results in an increase in thrombotic tendency and a reduction in prostacyclin-stimulating factors in diabetics.34 The two aspects contribute to the progress of diabetic eye disease. Our study showed an increased lipid peroxidation in terms of MDA and a decreased antioxidase activity in terms of SOD in DM rats (Figure 2). However, PSP attenuated oxidative stress in the study, which might be one of the mechanisms of preventive effect on ocular complications in DM rats.
In summary, our study revealed that PSP slows the progression of DR and cataract in STZ-induced DM rats. Alleviating hyperglycemia and reducing oxidative stress may be the mechanisms contributing to preventing and delaying the procession of ocular complications associated with DM. This study suggests that PSP is a valuable adjuvant therapy for DM and its ocular complications.
Acknowledgements
This study was financially supported by Natural Science Foundations of China (81370381 and 91539114), Taishan Scholars Foundation of Shandong Province (ts201511057), Shandong Provincial Key Research and Development Program (2015GSF119008) and Taian science and technology development plan (2015NS1119).
Authors’ contribution
All authors participated in the design and laboratory experiments of the study. YW and SQ analyzed data and wrote the manuscript. All authors approved the final version of the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Yisahak SF, Beagley J, Hambleton IR, Narayan KM. Diabetes in North America and the Caribbean: an update. Diabetes Res Clin Pract 2014; 103: 223–30. [DOI] [PubMed] [Google Scholar]
- 2.Threatt J, Williamson JF, Huynh K, Davis RM. Ocular disease, knowledge and technology applications in patients with diabetes. Am J Med Sci 2013; 345: 266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010; 376: 124–36. [DOI] [PubMed] [Google Scholar]
- 4.Santiago JG, Walia S, Sun JK, Cavallerano JD, Haddad ZA, Aiello LP, Silva PS. Influence of diabetes and diabetes type on anatomic and visual outcomes following central rein vein occlusion. Eye (Lond) 2014; 28: 259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hung HY, Qian K, Morris-Natschke SL, Hsu CS, Lee KH. Recent discovery of plant-derived anti-diabetic natural products. Nat Prod Rep 2012; 29: 580–606. [DOI] [PubMed] [Google Scholar]
- 6.Singh RS. Plant disease management. New York: Science Publishers, Inc. 2001.
- 7.Calixto BJ. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz J Med Biol Res 2000; 33: 179–89. [DOI] [PubMed] [Google Scholar]
- 8.Xu DP, Hu CY, Zhang Y. Two new steroidal saponins from the rhizome of Polygonatum sibiricum. J Asian Nat Prod Res 2009; 11: 1–6. [DOI] [PubMed] [Google Scholar]
- 9.Ko JH, Kwon HS, Yoon JM, Yoo JS, Jang HS, Kim JY, Yeon SW, Kang JH. Effects of Polygonatum sibiricum rhizome ethanol extract in high-fat diet-fed mice. Pharm Biol 2015; 53: 563–70. [DOI] [PubMed] [Google Scholar]
- 10.Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol 1983; 23: 239–57. [DOI] [PubMed] [Google Scholar]
- 11.Marmor MF, Holder GE, Seeliger MW, Yamamoto S. Standard for clinical electroretinography (2004 update). Doc Ophthalmol 2004; 108: 107–14. [DOI] [PubMed] [Google Scholar]
- 12.Eisenbarth GS, Buse JB. Type 1 diabetes mellitus. In: Melmed S, Polonsky KS, Larsen PR. (eds). Williams textbook of endocrinology, Philadelphia: Elsevier, 2011, pp. 1436–1453. [Google Scholar]
- 13.Chen WP, Wang YD, Ma Y, Zhang ZY, Hu LY, Lin JL, Lin BQ. Danhong Huayu Koufuye combined with metformin attenuated diabetic retinopathy in Zuckerdiabetic fatty rats. Int J Ophthalmol 2015; 8: 1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 2007; 93: 903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geloneck MM, Forbes BJ, Shaffer J, Ying GS, Binenbaum G. Ocular complications in children with diabetes mellitus. Ophthalmology 2015; 122: 2457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato A, Yasuko H, Goto H, Hollinshead J, Nash RJ, Adachi I. Inhibitory effect of rhetsinine isolated from Evodia rutaecarpa on aldose reductase activity. Phytomedicine 2009; 16: 258–61. [DOI] [PubMed] [Google Scholar]
- 17.Falck A, Laatikainen L. Diabetic cataract in children. Acta Ophthalmol Scand 1998; 76: 238–40. [DOI] [PubMed] [Google Scholar]
- 18.Thangaraju P, Chakrabarti A, Banerjee D, Hota D, Tamilselvan, Bhatia A, Gupta A. Dual blockade of renin angiotensin system in reducing the early changes of diabetic retinopathy and nephropathy in a diabetic rat model. N Am J Med Sci 2014; 6: 625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Pharmacopoeia Committee. Chinese pharmacopoeia. Beijing: China Medical Science Press, 2010.
- 20.Ni Wen-peng, Zhu Xuan-xuan, Wang Hai-dan, Li Qi-yi, Wan Meng Research of polygonatum polysaccharide on the protective mechanism of LPS-induced HUVEC injury. Chin Arch Traditional Chin Med 2012; 30: 593–4. [Google Scholar]
- 21.Ko JH, Kwon HS, Yoon JM, Yoo JS, Jang HS, Kim JY, Yeon SW, Kang JH. Effects of Polygonatum sibiricum rhizome ethanol extract in high-fat diet-fed mice. Pharm Biol 2015; 53: 563–70. [DOI] [PubMed] [Google Scholar]
- 22.Yang JX, Wu S, Huang XL, Hu XQ, Zhang Y. Hypolipidemic activity and antiatherosclerotic effect of polysaccharide of Polygonatum sibiricum in rabbit model and related cellular mechanisms. Evid Based Complement Alternat Med 2015; 2015: 391065.–391065.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li You-yuan, Deng Hong-bo, Zhang Ping, Chen Long-fong Effect of polygonati polysaccharide on glucose metabolism in diabetic mice. Chin J Clin Rehabil 2005; 9: 90–1. [Google Scholar]
- 24.Segura F, Sánchez-Cano A, Jarabo S, López de la Fuente C, Cuenca N, Villegas-Pérez MP, Pinilla I. Assessment of visual and chromatic functions in a rodent model of retinal degeneration. Invest Ophthalmol Vis Sci 2015; 56: 6275–83. [DOI] [PubMed] [Google Scholar]
- 25.Cobb WA, Morton HB. A new component of the human electroretinogram. J Physiol 1954; 123: 36P–37P. [Google Scholar]
- 26.Chrysostomou V, Crowston JG. The photopic negative response of the mouse electroretinogram: reduction by acute elevation of intraocular pressure. Invest Ophthalmol Vis Sci 2013; 54: 4691–7. [DOI] [PubMed] [Google Scholar]
- 27.Pescosolido N, Barbato A, Stefanucci A, Buomprisco G. Role of electrophysiology in the early diagnosis and follow-up of diabetic retinopathy. J Diabetes Res 2015; 2015: 319692–319692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maharjan S, Lee S, Agrawal V, Choi HJ, Maeng YS, Kim K, Kim NJ, Suh YG, Kwon YG. Sac-0601 prevents retinal vascular leakage in a mouse model of diabetic retinopathy. Eur J Pharmacol 2011; 657: 35–40. [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Kim JH, Jun HO, Yu YS, Kim KW. Inhibition of protein kinase C delta attenuates blood-retinal barrier breakdown in diabetic retinopathy. Am J Pathol 2010; 176: 1517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Kim JH, Yu YS, Min BH, Kim KW. Protective effect of clusterin on blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci 2010; 51: 1659–65. [DOI] [PubMed] [Google Scholar]
- 31.Zong H, Ward M, Stitt AW. AGEs, RAGE, and diabetic retinopathy. Curr Diab Rep 2011; 11: 244–52. [DOI] [PubMed] [Google Scholar]
- 32.Cui Y, Xu X, Bi H, Zhu Q, Wu J, Xia X, Qiushi Ren, Ho PC. Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Exp Eye Res 2006; 83: 807–16. [DOI] [PubMed] [Google Scholar]
- 33.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005; 54: 1615–25. [DOI] [PubMed] [Google Scholar]
- 34.Jennings PE, McLaren M, Scott NA, Saniabadi AR, Belch JJ. The relationship of oxidative stress to thrombotic tendency in type 1 diabetic patients with retinopathy. Diabet Med 1991; 8: 860–5. [DOI] [PubMed] [Google Scholar]