Abstract
The pulmonary and intestinal systems have several characteristics in common. It is believed that these similarities somehow function to cause pulmonary–intestinal crosstalk during inflammation. Many studies have shown that pulmonary disease occurs in association with inflammatory bowel disease more often than is commonly recognized. Bambusae Caulis in Taeniam, a medicinal herb originated from the inner bark of Phyllostachys nigra var. henosis (Milford) Rendle (Poaceae), has been used to cure fever, diarrhea, and chest inflammation in Korea as well as in China. Cigarette smoke is a well-known risk factor for several inflammatory disorders. In this study, we induced pulmonary and bowel inflammation in mice using cigarette smoke and investigated whether Bambusae Caulis in Taeniam extract modulates the inflammatory response in both the lung and the bowel. C57BL/6 mice were exposed to cigarette smoke for 90 min per day for three weeks, and Bambusae Caulis in Taeniam extract was administered via oral injection 2 h before cigarette smoke exposure. The bronchoalveolar lavage cells were counted and hematoxylin and eosin staining were performed. Levels of inflammatory mediators in lung and large intestine were determined by enzyme-linked immunosorbent assay, real-time polymerase chain reaction, and Western blotting. Our results showed that Bambusae Caulis in Taeniam attenuated cigarette smoke-induced inflammatory response in both the lung and the bowel of mice by inhibiting the production of pro-inflammatory cytokines, chemokines, and protease as well as NF-κB signaling factor. Therefore, we suggest that Bambusae Caulis in Taeniam extract might be a candidate therapeutic agent for inhibiting pulmonary and intestinal inflammation.
Keywords: Bambusae Caulis in Taeniam, cigarette smoke, intestinal inflammation, NF-κB, pulmonary inflammation
Introduction
Cigarette smoke (CS) contains more than 4000 deadly chemicals, of which hundreds are toxic and nearly 70 are carcinogenic. Accordingly, CS can induce major health problems and diseases, which may lead to death in severe cases. Although it is well recognized that CS has adverse effects on the respiratory system, little is known about the harmful nature of CS in the intestinal bowel. Accumulating evidence suggests a detrimental effect of CS on the gut by showing a strong correlation between CS and bowel inflammation.1–4 Therefore, CS is thought to be a major risk factor for inflammation in both the lung and the intestine. CS affects the generation of cytokines and chemokines and induces uncontrolled protease productions, which are possible mechanisms of respiratory–intestinal crosstalk.5–7
Inflammation in the lung is prevalent worldwide and is therefore an important ongoing multinational research topic.8 Pulmonary inflammation is characterized by activated pro- and anti-inflammatory cytokines such as TNF-α, IL-6, IL-1β, and MCP-1 that cause cell damage in the lung.9,10 Intestinal inflammation is characterized by an excessive gut inflammatory response that affects the gastrointestinal tract and has shown an increasing incidence over the last 50 years.11,12 Recent studies showing a remarkable upsurge of tissue levels of TNF-α, IL-6, IL-1β, and MCP-1 during inflammatory bowel disease (IBD) have supported the significance of these factors during intestinal inflammation. The marked increase in cytokine and chemokine levels led to the hypothesis that intestinal epithelial cells might provide early signals to immune cells in the mucosa during inflammation.13–15
Although several factors such as cytokines are known to be associated with the pathogenesis of pulmonary and intestinal inflammation, no cure for this inflammation has yet been developed and continuing treatment is required for patients.5 Despite clinical acceptance that pulmonary inflammation and intestinal inflammation are linked, there are few research studies that explain their association.16–19
The pulmonary and intestinal systems have several characteristics in common. It is believed that these similarities somehow function to cause pulmonary–intestinal crosstalk during inflammation. Many clinical studies of Western populations have shown that pulmonary disease occurs in association with IBD more often than is commonly recognized. Interestingly, the Dongui-Bogam, which was published about 500 years ago in Korea, proposes a specific linkage theory between lung and large intestinal diseases and suggests several medicinal herbs for their treatment.
Bambusae Caulis in Taeniam (BC), a medicinal herb originated from the inner bark of Phyllostachys nigra var. henosis (Milford) Rendle (Poaceae), has shown many pharmacologic activities. The material media of past dynasties in Chinese history indicated that BC was used to treat fever, diarrhea, and chest inflammation.20–22 Accordingly, Ministry of Health in China approved BC as an ethnopharmacological functional food material. Other study demonstrated BC’s anti-inflammatory effects with anti-oxidative properties.22 Our previous study showed the efficacy of BC in the OVA-induced asthma model.23
In our present study, we induced and identified pulmonary and intestinal inflammatory response using a cigarette smoking murine model and investigated whether BC modulates smoke-induced inflammation in both the lung and the bowel. We also investigated both the inhibitory activities of BC on the production of pro-inflammatory cytokines and chemokines, as well as its activation of the NF-κB signal transduction pathway, which is known to be involved in the regulation of the inflammatory response.
Materials and methods
Preparation of BC extracts
The inner bark middle layer of BC was purchased from Omniherb Co. (Daegu, Korea) and its identity was confirmed by one of the authors (Y.P. Jang). The voucher specimen (KHOP00315) was deposited at Kyung Hee Korean Traditional Herbal Medicine Museum of College of Pharmacy, Kyung Hee University. Extraction was performed according to the method described previously.23
High-performance liquid chromatography analysis of BC extracts
High-performance liquid chromatography (HPLC) grade acetonitrile was purchased from J. T. Baker (NJ, USA), and analytical-reagent grade acetic acid was obtained from Wako (Osaka, Japan). Caffeic acid, ferulic acid, and p-coumaric acid were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The total extract was dissolved at a concentration of 10 mg/mL in 50% (v/v) methanol aqueous solution. As a standard, p-coumaric acid was dissolved at 1 mg/mL in methanol and then diluted 5-, 10-, 20-, and 40-fold. All samples and standard solutions were filtered through a 0.45-µm syringe filter (Millipore, MA, USA). HPLC analysis was performed on a Waters system (Milford, MA, USA) equipped with a Waters 996 photodiode array detector running Empower software. A Capcellpak (Shiseido, Tokyo, Japan) C18 column (250 mm × 4.6 mm, i.d. 5 µm) was selected for the analysis of BC extract. The UV chromatogram was monitored at 280 nm. The flow rate was 0.8 mL/min. The mobile phase consisted of 0.1% acetic acid in water (solvent A) and 0.1% acetic acid in methanol (solvent B). The gradient program started with 10% B for 5 min, followed by a linear gradient to 30% B in 15 min, isocratic elution to 45 min, and linear gradient to 50% B at 55 min. The injection volume of total extract and standard solutions was 10 µL.
Animals
Specific pathogen-free female C57BL/6 mice (seven weeks old) were purchased from Orient Bio Inc. (Seoul, South Korea). The animals were housed in an air-conditioned room maintained at 24℃ with 55% humidity and were provided a standard sterile rodent diet (Purina Mills, St. Louis, MO, USA) with water given ad libitum. All experimental procedures were carried out in accordance with the requirements of the Animal Care and Ethics Committee of Kyung Hee University.
CS exposure and drug treatment
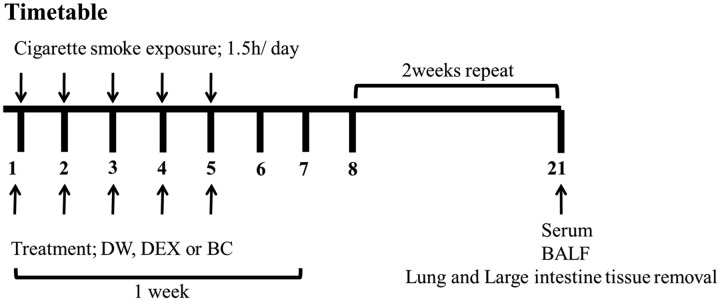
Control group mice (n = 5) were exposed to fresh air with distilled water (DW). Experimental groups (n = 5) were exposed to CS (Reference Cigarette 3R4F without a filter, University of Kentucky, Lexington, KY, USA) with either DW (CS group), dexamethasone (DEX group, treated with 1 mg/kg of dexamethasone), or BC (BC group, treated with 100 mg/kg of BC extract). Mice were subjected to whole-body exposure to CS in a smoke chamber for three 30-min periods with recovery in a fresh air environment for 1 h between each exposure for five days per week for three weeks. In our experiment, the mice were exposed to the side stream smoke, and each cigarette was completely burned out during the first 1 min due to the pressure generated from an oil-less air pump (M-technology, Incheon, South Korea). In addition, the oil-less pump was set to the inhalation rate of 30 per 1 min. Mice were orally treated with DEX or BC 2 h before exposure to CS. Individual body weights were measured twice a week, and the mice were sacrificed on day 21. These experiments were performed in duplicate. The experimental procedure of CS exposure is described in Figure 1.
Figure 1.
Time course of cigarette smoke exposure. Mice were divided into four groups (n = 5/group). CON: fresh air with DW; CS: cigarette smoke (reference cigarettes 3R4F, University of Kentucky, Lexington, Kentucky, USA) with DW; DEX: CS with dexamethasone (DEX, 1 mg/kg, p.o.); and BC: CS with BC extract (BC, 100 mg/kg, p.o.). Mice were subjected to whole-body exposure to CS for 30-min periods in the smoking chamber, after which they rested in a fresh air environment for 1 h. This process was repeated three times for 3 weeks
Analysis of bronchoalveolar lavage fluid
After the mice were sacrificed, bronchoalveolar lavage fluid (BALF) was collected by infusion and extraction of PBS. Total cell numbers were counted using a hemocytometer, and differential cell counts were performed on slides prepared by cytocentrifugation and Diff-Quick staining (Life Technologies, Auckland, New Zealand) using light microscopy. BALF samples were then centrifuged and the supernatants were stored at −80℃ for measurement of cytokines. Levels of TNF-α, IL-6, and MCP-1 in the BALF were measured by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (OptELATM Kits; BD Biosciences, San Diego, CA, USA) according to the manufacturer’s protocols.
Histological study
Lung and colon tissue was fixed in 10% formaldehyde for 24 h and embedded in paraffin. Tissue sections (4 µm) were stained with hematoxylin and eosin solution (H&E, Sigma-Aldrich, MO, USA) for inflammation score assessment. H&E-stained sections were evaluated using light microscopy at a magnification of 100×. The air space of lung was determined by digital image analysis. Micrographs were obtained using Image Pro-Plus 5.1 software (Media Cybernetics, Inc. Silver Spring, MD, USA).
Collection of colonic tissue samples and analysis of large intestine
The whole colon was pulled out, the surrounding mesentery was detached, and the weight/length ratio of the colon was measured. The large intestine tissue was washed with PBS using a gavage needle attached to a 5 mL syringe and the tissue was stored at −80℃ for analysis. Proteins were extracted using T-PER tissue protein extraction reagent (Pierce, Rockford, IL, USA) containing a protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Protein concentrations were determined using the Bradford method. The amount of TNF-α, IL-6, IL-1β, and MCP-1 in the intestinal tissue was measured by ELISA using a commercial kit.
Real-time polymerase chain reaction
Lung and intestinal tissues were lysed using TRIzol reagent (Invitrogen) and total RNA was isolated in accordance with the manufacturer’s guidance. The total RNA was reverse transcribed for cDNA synthesis using M-MuLV Reverse Transcriptase (Invitrogen), and the synthesized cDNA was used as a template for polymerase chain reaction (PCR) amplification. mRNA levels of TNF-α, IL-6, IL-1β, MCP-1, and MMP-12 were determined by performing real-time PCR using a Thermal Cycler Dice™ real-time (RT) PCR system (Takara, Katsushika, Japan). The primers used for SYBR Green real-time PCR were as follows: TNF-α, forward, 5′-CAAGGGACAAGGCTGCCCCG-3′ and reverse, 5′-TAGACCTGCCCGGACTCCGC-3′; IL-6, forward, 5′-TGCTGGTGACAACCACGGCCT-3′ and reverse, 5′-ACAGGTCTGTTGGGAGTGGTATCCT-3′; IL-1β, forward, 5′-ACCTGCTGGTGTGTGACGTT-3′ and reverse, 5′-TCGTTGCTTGGTTCTCCTTG-3′; MCP-1, forward, 5′-TCACAGTTGCCGGCTGGAGC-3′ and reverse, 5′-CAGCAGGTGAGTGGGGCGTT-3′; MMP-12, forward, 5′-GGCCATTCCTTGGGGCTGCA-3′ and reverse, 5′-GGGGGTTTCACTGGGGCTCC-3′; and GAPDH, forward, 5′-TCTCAGGTGCCGCCTGGAGA-3′ and reverse, 5′-TGGGCCCTCAGATGCCTGCT-3′ (Cosmogentech Ltd, Seoul, Korea). The specificity of amplification was confirmed by a melting curve with a single peak. Real-time PCR was performed with the following conditions: denaturation at 95℃ for 10 s, annealing at 60℃ for 10 s, and elongation at 72℃ for 12 s. GAPDH was used as the endogenous control for normalization.
Western blotting
NF-κB protein expression in the lung and large intestinal tissue was detected by Western blotting. Proteins (20 µg/lane) were separated by SDS-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. The transblotted membranes were blocked with 0.5% defatted milk in TBS for 1h and were incubated overnight with primary antibodies against β-actin and NF-κB (Santa Cruz Biotechnology, Dallas, TX, USA; diluted 1:1,000 in TBS-T). The blots were washed, and then incubated with secondary antibodies. Blots were washed again, and then developed using an enhanced chemiluminescence Western blot analysis system (AbClon Inc., Seoul, South Korea).
Statistical analysis
Statistical analysis of the data was carried out using Prism 4 software (GraphPad Software Inc., CA, USA). Data are presented as mean ± standard error of the mean (SEM), and multiple comparisons were performed using one-way ANOVA. Results with P < 0.05 were considered statistically significant.
Results
HPLC analysis of BC extracts
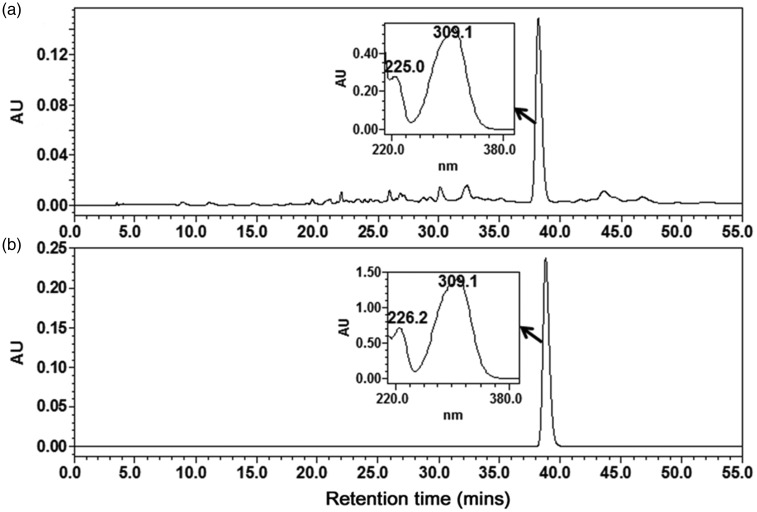
The HPLC chromatogram of total BC extract is shown in Figure 2. The main peak in the chromatogram (Rt = 38 min) showed an UV maximum absorption at 310 nm. This peak was assumed to be a phenylpropanoid because a simple phenylpropanoid was reported to be a major component of bamboo extract.24 To identify the peak, standard compounds containing ferulic acid, caffeic acid, and p-coumaric acid were injected into the HPLC under exactly the same conditions and their retention time and UV spectra were compared with those of the major peak. In this way, the main peak was identified as p-coumaric acid, which was previously reported to be a component of the leaves of this plant.25
Figure 2.
High-performance liquid chromatography (HPLC) chromatogram of the water extract of Bambusae Caulis (BC) (a) and p-coumaric acid (b). UV-visible absorption spectra of major peak in the water extract of BC (a) and p-coumaric acid (b) are also shown on the chromatogram
To quantify the p-coumaric acid in the BC extract, a calibration curve was established using a stock solution of p-coumaric acid serially diluted to specified concentrations. The coefficients value (R2) was 0.9997, demonstrating that the linearity in this range was sufficient to provide a highly accurate value of the content in each sample. Precision was determined using a triplicate measurement of each standard, and the relative standard deviations (RSD) were less than 2.4% (Table 1). Using the established calibration curves, the content of p-coumaric acid in the extract was calculated to be 4.19 ± 0.46 µg/mg.
Table 1.
The regression data, precision and quantification of p-coumaric acid from the total extract of Bambusae Caulis.
| Compound | Regression equation | R2 | Linear range (µg/mL) | RSD (%) (n = 3) | Contents of p-coumaric acid in the water extract (µg/mg) |
|---|---|---|---|---|---|
| p-coumaric acid | y = 7 × 107 × + 401,809 | 0.9997 | 35–290 | 0.07–2.40 | 4.19 ± 0.46 |
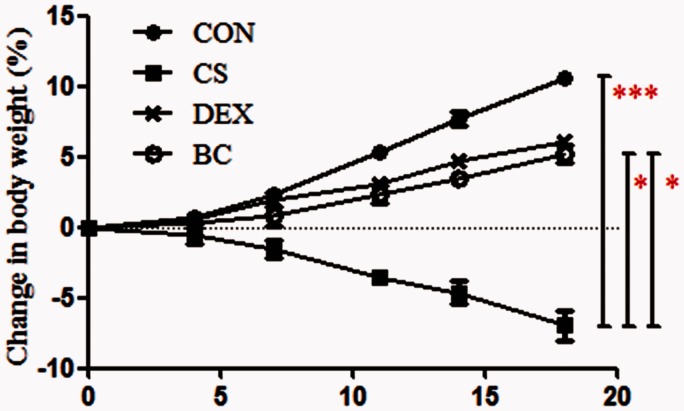
BC alleviated weight loss in mice exposed to CS
The body weights of the control (CON) group increased from 17.3 ± 0.5 g to 19.2 ± 0.3 g (10.6% increase) over three weeks. In comparison, the weight of the CS (CS)-exposed group decreased to 16 ± 0.4 g (7% decrease). Compared with the CS group, the body weights of the DEX and BC group were higher at 18.1 ± 0.3 g (6.1% increase) and 18.2 ± 0.72 g (5.2% increase), respectively (Figure 3). The DEX group was used as a positive control.
Figure 3.
Effect of BC on variation in body weight. Body weights were determined for each group of mice twice a week. Statistical analysis was performed on day 19 after CS exposure. Values are mean ± SEM. Statistical analysis was by one-way ANOVA; *P < 0.05 and ***P < 0.001. (A color version of this figure is available in the online journal.)
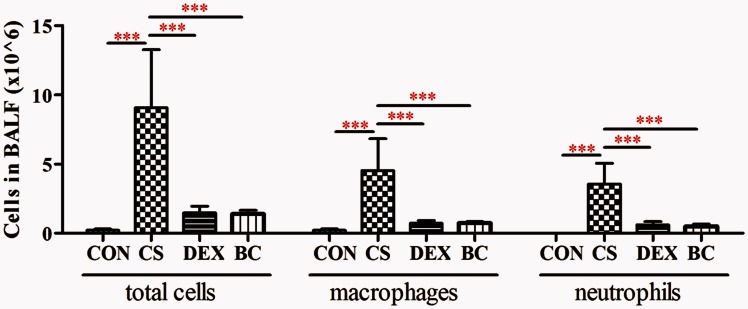
BC inhibited the infiltration of inflammatory cells into the lung
The number of inflammatory cells in the BALF was examined. The CS group showed a significant increase in the number of total cells, neutrophils, and macrophages compared to the CON group. Compared with the CS group, the DEX group (as a positive control) showed a significant reduction in total cells (84%), macrophages (85%), and neutrophils (84%). Treatment with BC reduced the amount of total cells (85%), macrophages (85%), and neutrophils (86%), respectively (Figure 4). These results demonstrated that CS inhalation induced lung inflammation and treatment with BC inhibited lung inflammation in the CS group.
Figure 4.
Effects of BC on inflammatory cell infiltration in bronchoalveolar lavage fluid. The amount of inflammatory cells was determined three weeks after CS exposure. Total cells, macrophages, and neutrophils in BALF were counted with light microscopy after Diff-Quick staining. Values are mean ± SEM. Statistical analysis was by one-way ANOVA; ***P < 0.001. (A color version of this figure is available in the online journal.)
BC reduced the production of inflammatory cytokines and chemokines in BALF
The amount of TNF-α, IL-6, and MCP-1 in the BALF was evaluated using ELISA. Compared to the CON group, the CS group showed significant increase in the productions of TNF-α, IL-6, and MCP-1. However, in the DEX group, the levels of TNF-α, IL-6, and MCP-1 were significantly reduced by 40%, 50%, and 77%, respectively, compared with the CS group. Similarly, the BC group showed significantly reduced levels of TNF-α (38%), IL-6 (46%), and MCP-1 (49%) (Figure 5). These results further demonstrated that treatment with BC inhibited lung inflammation in the CS group.
Figure 5.
Effects of BC on proteins production of inflammatory cytokines and chemokines in bronchoalveolar lavage fluid. The amount of TNF-α (a), IL-6 (b), and MCP-1 (c) was measured by enzyme-linked immunosorbent assay. Values are mean ± SEM. Statistical analysis was by one-way ANOVA; *P < 0.05 and ***P < 0.001. (A color version of this figure is available in the online journal.)
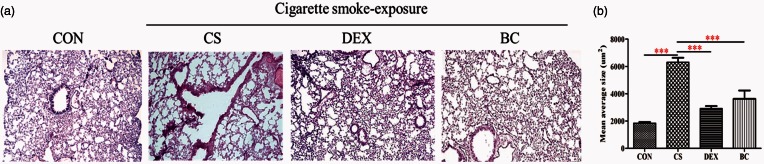
BC alleviated the CS-induced emphysematous changes
To determine the effects of BC on pathologic changes in lung tissues, we performed histologic examination by H&E staining. The CS group showed mild emphysematous changes with cells infiltration into surrounding the bronchi regions compared to the CON group. In contrast, treatment with DEX or BC significantly alleviated the CS-induced emphysematous changes and cell infiltration in the lungs of CS-exposed mice (Figure 6). The DEX group was used as a positive control. These results provided further evidence that CS inhalation induces lung inflammation that is ameliorated by treatment with BC.
Figure 6.
Effects of BC on emphysematous changes. Lung tissue sections (4 µm thickness) were stained with H&E solution (magnification × 100) (a). The air space size was evaluated by digital image analysis (b). Values are mean ± SEM. Statistical analysis was by one-way ANOVA; ***P < 0.001. (A color version of this figure is available in the online journal.)
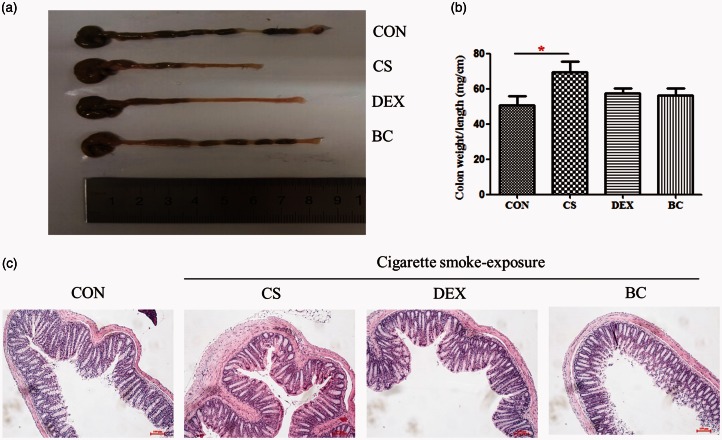
BC attenuated CS-induced macroscopic and histopathological changes in the intestine
The colon weight/length ratio was used as a marker of disease-related intestinal wall thickening and intensity of inflammation. Thus, we evaluated colonic inflammation index by measuring the weight/length ratio of the colon.26 As shown in Figure 7, compared with CON group, CS group colon weight/length ratio was increased, and BC group showed reduction. These results demonstrated that CS exposure induces intestinal wall thickening and BC inhibited CS-induced intestinal wall thickening. Furthermore, the histologic study data supported these results. We performed histologic examination by H&E staining to determine the effects of BC on pathologic changes in colon tissues. The CS group showed a marked thickening of the colonic wall with cells infiltration compared to the CON group. In contrast, treatment with DEX or BC significantly alleviated the CS-induced edematous swelling of the colonic wall and cell infiltration in the colon of CS-exposed mice. The DEX group was used as a positive control. These results provided further evidence that CS inhalation induces colonic inflammatory response that is ameliorated by treatment with BC.
Figure 7.
Effect of BC on CS-induced macroscopic and histopathological changes in the colon. Mice were exposed to CS to induce inflammation. The colon length was measured with a ruler under macroscopic examination (a), and the weight/length ratio was depicted in a graph (b). Colon tissue sections (4 µm thickness) were stained with H&E solution (magnification×100) (c). Values are mean ± SEM. Statistical analysis was by one-way ANOVA; *P < 0.05. (A color version of this figure is available in the online journal.)
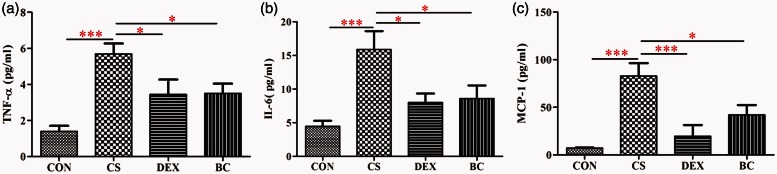
BC decreased the secretion of inflammatory cytokines and chemokines in the large intestine tissue
The protein productions of TNF-α, IL-6, IL-1β, and MCP-1 in the intestine were determined by ELISA. As shown in Figure 8, the CS group showed significantly increased levels of these cytokines and chemokine compared to the CON group. In contrast, treatment with DEX as a positive control reduced the levels of TNF-α (74%), IL-6 (91%), IL-1 β (45%), and MCP-1 (85%) compared with the CS group. The BC group similarly showed a reduction in TNF-α (69%), IL-6 (91%), IL-1 β (80%), and MCP-1 (95%) compared to the CS group. These results further demonstrated that CS induces large intestine inflammation and treatment with BC inhibits this inflammatory response.
Figure 8.
Effects of BC on the protein production of inflammatory cytokines and chemokines in the large intestine. Protein levels of TNF-α (a), IL-6 (b), IL-1β (c), and MCP-1 (d) were evaluated by ELISA. Values are mean ± SEM. Statistical analysis was by one-way ANOVA; *P < 0.05, **P < 0.01 and ***P < 0.001. (A color version of this figure is available in the online journal.)
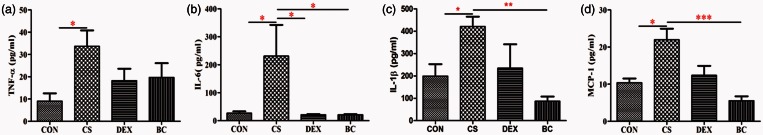
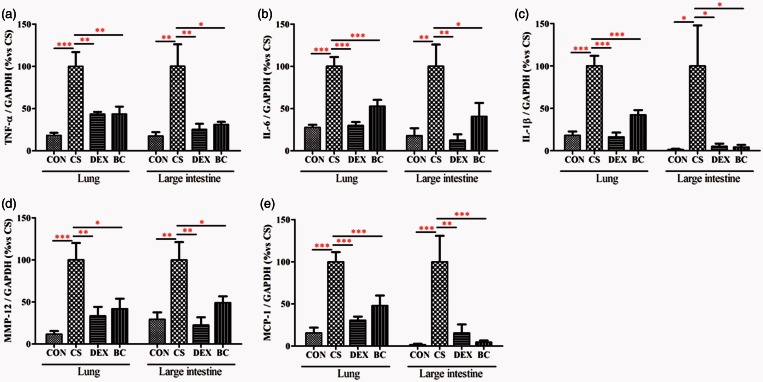
BC reduced TNF-α, IL-6, IL-1β, MCP-1, and MMP-12 mRNA expression in lung tissue and large intestinal tissue
ELISA results showed that BC reduced inflammatory proteins in the BALF and colon. After that, we confirmed the inhibitory effect of BC on the mRNA expression of inflammatory mediators in the lung and colon tissue together. We evaluated TNF-α, IL-6, IL-1β, MCP-1, and MMP-12 mRNA expression in lung and large intestine by real-time PCR. In the lung tissue, the CS group showed significantly increased mRNA expressions of TNF-α, IL-6, IL-1β, MCP-1, and MMP-12 compared to the control group. Compared with the CS group, the DEX group showed a significant reduction in these mRNAs (56%, 50%, 84%, 69%, and 66%, respectively.) The BC group showed significant decrease in mRNA expression (56%, 47%, 58%, 52%, and 58%, respectively). Similarly, the CS group showed significant increase in the levels of TNF-α, IL-6, IL-1β, MCP-1, and MMP-12 mRNA in the large intestinal tissue compared with the CON group. Compared to the CS group, the levels of TNF-α, IL-6, IL-1β, MCP-1, and MMP-12 mRNA were decreased by treatment with DEX (75%, 87%, 95%, 85%, and 77%, respectively) or BC (69%, 63%, 96%, 95%, and 51%, respectively) (Figure 9). Thus, BC ameliorated the expression of inflammatory cytokines and chemokines in the lung and large intestine following exposure to CS.
Figure 9.
Effects of BC on cytokine and chemokine mRNA production in the lung and the large intestine. mRNA levels of TNF-α (a), IL-6 (b), IL-1β (c), MCP-1 (d), and MMP-12 (e) were determined by real-time PCR. GAPDH was used as the endogenous control for normalization. Values are mean ± SEM. Statistical analysis was by one-way ANOVA; *P < 0.05, **P < 0.01, and ***P < 0.001. (A color version of this figure is available in the online journal.)
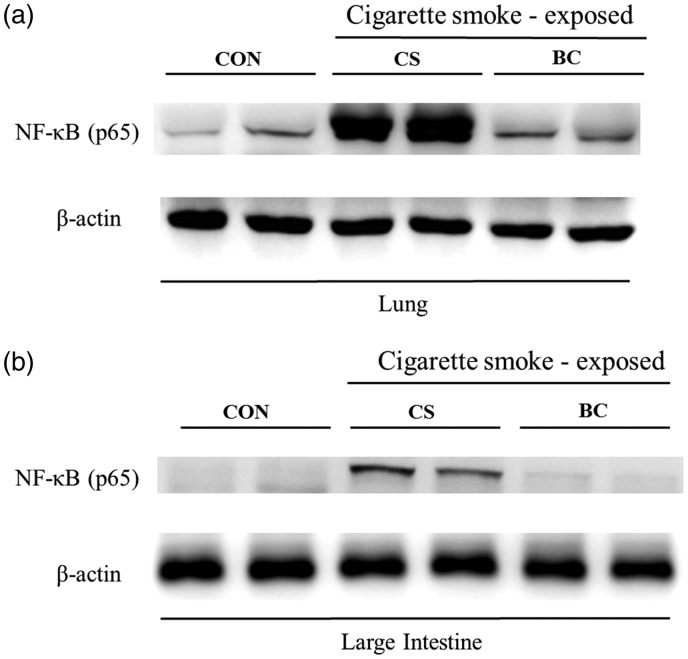
BC inhibited expression of NF-kB in lung and the large intestinal tissues
The effects of BC on NF-κB protein expression in the lung and large intestine of CS-exposed mice model were examined by Western blotting. Compared to the normal group, the CS group showed a significant increase in NF-κB expression in the lung and large intestine, and BC treatment significantly reduced CS-induced NF-κB production in both tissues (Figure 10).
Figure 10.
Effects of BC on NF-κB activation in the lung and the large intestine tissue. Tissue proteins were extracted by using T-PER tissue protein extraction reagent containing protease inhibitor cocktail and total protein concentration was measured using Bradford assay. NF-κB expression in lung (a) and large intestine (b) tissue was determined by Western blotting
Discussion
Current body of scholarship strongly demonstrates the link between the lung and the intestine. It is supported by numerous studies that organs sharing common embryological origins may communicate with each other even if there is no anatomical association.27–36 The primitive foregut has been shown from a growing number of literatures as the embryological origin of respiratory and gastrointestinal epithelia.37,38 Wang et al.39 indicated the existence of the lung–intestine communication by presenting the lung’s duplication of inflammatory reactions in the bowel. Recently, Western medical theory recognized that the pulmonary and intestinal systems have much in common.40 The respiratory and intestinal tracts both have a sophisticated luminal surface area covered by protective epithelia barrier and a mucus gel layer,5 and connective and lymphoid tissue under the surface that plays a crucial role in immune defenses.41,42 It is believed that these similarities somehow function to cause pulmonary–intestinal crosstalk during inflammation.5
Although the mechanism is unknown, the association between pulmonary and intestinal inflammation is supported by many clinical studies. Comparison of two representative inflammatory diseases, chronic obstructive pulmonary disease (COPD) and IBD, reveals the stark correlation between pulmonary and intestinal inflammation. Indeed, exacerbation of pulmonary and intestinal inflammation leads to COPD and IBD, respectively. COPD is characterized by gradual air blockage in the lung and accounts for the third most common cause of death and the fifth most common cause of disability.43 IBD has two phenotypes, ulcerative colitis (UC) and Crohn’s disease (CD), characterized by inflammatory damage to the intestinal area.44 A population-based cohort study reported a higher chance of having CD in COPD patients than in healthy controls, and indicated an increased susceptibility to CD in first-degree relatives of COPD patients.16 Another study showed that the small intestine of COPD patients shows aberrations in absorbing essential nutrients such as fats, protein, and carbohydrates.17 Conversely, the study by Jess et al.18 shows an increased risk of dying from COPD in CD patients. Black et al.19 reported the similar finding that thoracic abnormalities are common in IBD patients.
CS is a major possible risk factor for IBD and COPD and is considered to have a significant influence on the possible mechanisms of respiratory–intestinal crosstalk inflammation. Crosstalk between these two organs is induced by uncontrolled inflammatory cytokine secretion when immune-inflammatory responses occur.5–7 Reactive oxygen species (ROS) from CS degrade epithelial cells through peroxidation of cell membrane components and other constituents activate signaling pathways to activate inflammatory factors such as TNF-α and NF-κB.45
C57BL/6 mice are good responders to CS and present a well-formed injurious airway or intestinal states.46–48 In our experiment, we exposed C57BL/6 mice to CS using the 3R4F reference cigarette. After three weeks of CS inhalation, we confirmed a significant weight loss in the CS-exposed mice. These data are meaningful since CS is reported to decrease body weight by affecting the amount of caloric intake,49 and chronic disorders such as IBD and COPD are characterized by body weight loss. Next we identified infiltration of inflammatory cells, including macrophages and neutrophils, in the BAL fluid. Furthermore, we observed abnormal airspace enlargement, a pathologic change in the lung parenchyma caused by severe inflammatory response, and macroscopic changes in the large intestine. In particular, colonic shortening in CS-exposed mice provides evidence that a CS-induced inflammatory response occurred in the large intestine.26 We also confirmed an increase in the concentration of several cytokines, including TNF-α and IL-6, the chemokine MCP-1, the protease MMP-12, and the transcription factor NF-κB in both the lung and the large intestine tissues of the CS group compared to the control group (Figure 9).
TNF-α is an important cytokine that plays a crucial role in inflammatory and immune reactions50 and has been implicated to show a strong correlation with COPD51 and the progression of CD.52 TNF-α is believed to activate not only pro-inflammatory cytokines such as IL-6 and IL-853 but also the transcription factor NF-κB.54 IL-6 is necessary for regulation of local or systemic acute inflammatory responses55 and is thought to have a direct role in pulmonary–intestinal crosstalk by driving cross-organ inflammation. To our knowledge, we are the first to present experimental evidence for an elevated level of IL-6 during pulmonary–intestinal crosstalk inflammation in an animal model. MCP-1 is a CC chemokine that fights infection by attracting macrophages to areas of inflammation.56,57 Indeed, the expression of MCP-1 in lung and intestinal inflammation has been reported in many studies.58–61 MMP-12 is a member of the metalloproteinase (MMP) family and plays a role in tissue remodeling.62 MMP-12 is reported to be involved in recruiting neutrophils, macrophages, and pro-inflammatory cytokines.63 Also, the lack of MMP-12 neutralization resulting from A1AT deficiency exacerbates tissue damage during mucosal inflammation.64,65 MMP-12 has been associated with the pathogenesis of both COPD,66–68 and IBD.69–72 On the basis of these results, we confirmed that CS-exposure induces inflammatory response in the lung and large intestine together. However, there is still a possibility where intestinal inflammation occurred from the licking behavior of CS-exposed mice rather than from the lung and intestinal crosstalk. Thus, further study about the mechanism of pulmonary–intestinal crosstalk inflammation is needed.
In this study, BC treatment attenuated the CS-induced weight loss, airspace enlargement, and colonic shortening. Also, the BC-treated group had significantly lower numbers of total cells, macrophages, and neutrophils in BALF compared to the CS group. Furthermore, the increased levels of the cytokines TNF-α and IL-6 and the chemokine MCP-1 induced by CS were significantly ameliorated by BC treatment. Real-time PCR and ELISA analyses indicated a decrease in MMP-12 levels in both the lung and the large intestine in the BC-treated group. Lastly, we observed a reduction in the production of NF-κB after pro-inflammatory cytokine stimulation in lung and large intestine of the BC-treated mice.
In conclusion, we showed that BC is involved in the regulation of CS-induced lung and intestinal inflammation through inhibition of pro-inflammatory cytokines, chemokines, and protease as well as downregulation of the NF-κB signaling factor in both the lungs and large intestine tissues of mice exposed to CS. Therefore, we suggest that BC extract may be a candidate resource for the development of a therapeutic that inhibits pulmonary and intestinal inflammation.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (No. NRF-2014R1A1A3050811) and Traditional Korean Medicine R&D Project, Ministry of Health & Welfare, Republic of Korea (HI15C0171)
Authors’ contributions
DL and YC were involved in drafting the manuscript and made substantial contributions to the animal experiments, acquisition of data, analysis and interpretation of data. WK made substantial contributions to the animal experiments. JS and YJ made substantial contributions to the HPLC analysis and acquisition of data, analysis and interpretation of data. JK made substantial contributions to the conception and design, revising it critically for important intellectual content and giving final approval of the version to be published. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Russel MG, Volovics A, Schoon EJ, van Wijlick EH, Logan RF, Shivananda S Inflammatory bowel disease: is there any relation between smoking status and disease presentation? European collaborative IBD study group. Inflamm Bowel Dis 1998; 4: 182–6. [DOI] [PubMed] [Google Scholar]
- 2.Biedermann L, Fournier N, Misselwitz B, Frei P, Zeitz J, Manser CN High rates of smoking especially in female crohn's disease patients and low use of supportive measures to achieve smoking cessation-data from the Swiss IBD cohort study. J Crohn's Colitis 2015; 9: 819–29. [DOI] [PubMed] [Google Scholar]
- 3.Lunney PC, Kariyawasam VC, Wang RR, Middleton KL, Huang T, Selinger CP, Andrews JM, Katelaris PH, Leong RW Smoking prevalence and its influence on disease course and surgery in Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther 2015; 42: 61–70. [DOI] [PubMed] [Google Scholar]
- 4.Ott C, Takses A, Obermeier F, Schnoy E, Muller M Smoking increases the risk of extraintestinal manifestations in Crohn's disease. World J Gastroenterol 2014; 20: 12269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keely S, Talley NJ, Hansbro PM Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol 2012; 5: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martey CA, Pollock SJ, Turner CK, O'Reilly KM, Baglole CJ, Phipps RP Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: implications for lung inflammation and cancer. Am J Physiol Lung Cell Mol Physiol 2004; 287: L981–91. [DOI] [PubMed] [Google Scholar]
- 7.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc 2006; 81: 1462–71. [DOI] [PubMed] [Google Scholar]
- 8.Ford JG, Rennick D, Donaldson DD, Venkayya R, McArthur C, Hansell E Il-13 and IFN-gamma: interactions in lung inflammation. J Immunol 2001; 167: 1769–77. [DOI] [PubMed] [Google Scholar]
- 9.Rahman I, MacNee W Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 2000; 16: 534–54. [DOI] [PubMed] [Google Scholar]
- 10.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir JSuppl 2001; 34: 50s–9s. [PubMed] [Google Scholar]
- 11.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 2006; 12(Suppl 1): S3–9. [DOI] [PubMed] [Google Scholar]
- 12.Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol 2006; 12: 6102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimm MC, Elsbury SK, Pavli P, Doe WF Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J Leukoc Biol 1996; 59: 804–12. [DOI] [PubMed] [Google Scholar]
- 14.Reinecker HC, Loh EY, Ringler DJ, Mehta A, Rombeau JL, MacDermott RP. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology 1995; 108: 40–50. [DOI] [PubMed] [Google Scholar]
- 15.Eckmann L, Jung HC, Schurer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff MF. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology 1993; 105: 1689–97.. [DOI] [PubMed] [Google Scholar]
- 16.Ekbom A, Brandt L, Granath F, Lofdahl CG, Egesten A. Increased risk of both ulcerative colitis and Crohn's disease in a population suffering from COPD. Lung 2008; 186: 167–72. [DOI] [PubMed] [Google Scholar]
- 17.Beloborodova EI, Akimova LA, Burkovskaia BA, Asanova AV, Semenenko EV. [Activity of systemic inflammatory reaction in patients with chronic obstructive pulmonary disease in regard to small intestinal absorption function]. Ter Arkh 2009; 81: 19–23. [PubMed] [Google Scholar]
- 18.Jess T, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ 3rd, Munkholm P, Sandborn WJ. Survival and cause specific mortality in patients with inflammatory bowel disease: a long term outcome study in Olmsted County, Minnesota, 1940–2004. Gut 2006; 55: 1248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black H, Mendoza M, Murin S. Thoracic manifestations of inflammatory bowel disease. Chest 2007; 131: 524–32. [DOI] [PubMed] [Google Scholar]
- 20.Kim A, Im M, Yim NH, Jung YP, Ma JY Aqueous extract of Bambusae Caulis in Taeniam inhibits PMA-induced tumor cell invasion and pulmonary metastasis: suppression of NF-kappaB activation through ROS signaling. PloS One 2013; 8: e78061–e78061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao J, Zhang Y, Lou D, Wu X, Zhang Y Antihyperlipidemic and antihypertensive effect of a triterpenoid-rich extract from bamboo shavings and vasodilator effect of friedelin on phenylephrine-induced vasoconstriction in thoracic aortas of rats. Phytother Res 2007; 21: 1135–41. [DOI] [PubMed] [Google Scholar]
- 22.Jung SH, Lee JM, Lee HJ, Kim CY, Lee EH, Um BH Aldose reductase and advanced glycation endproducts inhibitory effect of Phyllostachys nigra. Biol Pharm Bull 2007; 30: 1569–72. [DOI] [PubMed] [Google Scholar]
- 23.Ra J, Lee S, Kim HJ, Jang YP, Ahn H, Kim J Bambusae Caulis in Taeniam extract reduces ovalbumin-induced airway inflammation and T helper 2 responses in mice. J Ethnopharmacol 2010; 128: 241–7. [DOI] [PubMed] [Google Scholar]
- 24.Shi X, Zhang H-t, Xing X-p, Zhang Y-l, Ye J-n Determination of active components in bamboo leaves and bamboo-leaf tea by capillary electrophoresis with electrochemical detection. JAnal Sci 2009; 2: 006–006. [Google Scholar]
- 25.Xin-xing X, Shu-chai L, Wei-xian R Studies on the structural properties and the chromophore groups of MWL isolated from bamboo and its SCMP. TransacChina Pulp Paper 1995; 10: 14–9–14–9. [Google Scholar]
- 26.Lin XH, Wang HC, Wei DD, Wang B, Ge QX, Bai CY, et al. [Study of the change and role of protein C system in ulcerate colitis]. Sheng li xue bao: [Acta Physiol Sin] 2015; 67: 214–24. [PubMed] [Google Scholar]
- 27.Luo JM, Liu ZQ, Eugene CY Overexpression of pulmonary surfactant protein A like molecules in inflammatory bowel disease tissues. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2008; 33: 979–86. [PubMed] [Google Scholar]
- 28.Liu Y, Wang XY, Yang X, Jing S, Zhu L, Gao SH Lung and intestine: a specific link in an ulcerative colitis rat model. Gastroenterol Res Pract 2013; 2013: 124530–124530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao L, Wang J, Li F, Gao S, Deng Y Analysis on clinically drug-used law for lung-intestine related diseases. J Tradit Chin Med 2012; 32: 523–8. [DOI] [PubMed] [Google Scholar]
- 30.Liu P, Wang P, Tian D, Liu J, Chen G, Liu S Study on traditional Chinese medicine theory of lung being connected with large intestine. J Tradit Chin Med 2012; 32: 482–7. [DOI] [PubMed] [Google Scholar]
- 31.Ni JX, Gao SH Understanding the viscera-related theory that the lung and large intestine are exterior-interiorly related. J Tradit Chin Med 2012; 32: 293–8. [DOI] [PubMed] [Google Scholar]
- 32.Luo JM, Wan YS, Liu ZQ, Wang GR, Floros J, Zhou HH Regularity of distribution of immunoreactive pulmonary surfactant protein A in rat tissues. Int J Mol Med 2004; 14: 343–51. [PubMed] [Google Scholar]
- 33.Luo J, Li Y, Gong R The mechanism of atopic march may be the ‘social' event of cells and molecules (Review). Int J Mol Med 2010; 26: 779–85. [DOI] [PubMed] [Google Scholar]
- 34.Luo J, Wan Y Tightly regulated distribution of family members of proteins is related to social property in the open body system (Review). Int J Mol Med 2006; 17: 411–8. [PubMed] [Google Scholar]
- 35.Bhagat S, Das KM A shared and unique peptide in the human colon, eye, and joint detected by a monoclonal antibody. Gastroenterology 1994; 107: 103–8. [DOI] [PubMed] [Google Scholar]
- 36.Oshitani N, Watanabe K, Nakamura S, Higuchi K, Arakawa T [Extraintestinal complications in patients with ulcerative colitis]. Nihon Rinsho. Jpn J Clin Med 2005; 63: 874–8. [PubMed] [Google Scholar]
- 37.Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development 2007; 134: 1991–2000. [DOI] [PubMed] [Google Scholar]
- 38.Ramalho-Santos M, Melton DA, McMahon AP Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 2000; 127: 2763–72. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Liu JS, Peng SH, Deng XY, Zhu DM, Javidiparsijani S Gut-lung crosstalk in pulmonary involvement with inflammatory bowel diseases. World J Gastroenterol 2013; 19: 6794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol 1987; 7: 265–76. [DOI] [PubMed] [Google Scholar]
- 41.Holt PG. Development of bronchus associated lymphoid tissue (BALT) in human lung disease: a normal host defence mechanism awaiting therapeutic exploitation? Thorax 1993; 48: 1097–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forchielli ML, Walker WA The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr 2005; 93(Suppl 1): S41–8. [DOI] [PubMed] [Google Scholar]
- 43.Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med 2014; 35: 71–86. [DOI] [PubMed] [Google Scholar]
- 44.Baumgart DC, Carding SR Inflammatory bowel disease: cause and immunobiology. Lancet 2007; 369: 1627–40. [DOI] [PubMed] [Google Scholar]
- 45.Lee J, Taneja V, Vassallo R Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dental Res 2012; 91: 142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wandu WS, Tan C, Ogbeifun O, Vistica BP, Shi G, Hinshaw SJ Leucine-rich repeat kinase 2 (Lrrk2) deficiency diminishes the development of experimental autoimmune uveitis (EAU) and the adaptive immune response. PloS One 2015; 10: e0128906–e0128906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trebicka E, Shanmugam NK, Chen K, Su CW, Shi HN, Cherayil BJ Intestinal inflammation leads to a long-lasting increase in resistance to systemic salmonellosis that requires macrophages but not B or T lymphocytes at the time of pathogen challenge. Inflamm Bowel Dis 2015; 21: 2758–65–2758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim D, Lee E, Jeong E, Jang YP, Kim J Stemona tuberosa prevented inflammation by suppressing the recruitment and the activation of macrophages in vivo and in vitro. J Ethnopharmacol 2015; 160: 41–51. [DOI] [PubMed] [Google Scholar]
- 49.Wack JT, Rodin J Smoking and its effects on body weight and the systems of caloric regulation. Am J Clin Nutr 1982; 35: 366–80. [DOI] [PubMed] [Google Scholar]
- 50.Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 1994; 25: 1481–8. [DOI] [PubMed] [Google Scholar]
- 51.Sevenoaks MJ, Stockley RA Chronic obstructive pulmonary disease, inflammation and co-morbidity – a common inflammatory phenotype? Respir Res 2006; 7: 70–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D A role for TNF-Αalpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn's disease. J Immunol 1997; 159: 6276–82. [PubMed] [Google Scholar]
- 53.Drost EM, MacNee W Potential role of IL-8, platelet-activating factor and TNF-Αalpha in the sequestration of neutrophils in the lung: effects on neutrophil deformability, adhesion receptor expression, and chemotaxis. Eur J Immunol 2002; 32: 393–403. [DOI] [PubMed] [Google Scholar]
- 54.Osborn L, Kunkel S, Nabel GJ Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci USA 1989; 86: 2336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 1998; 101: 311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antoniades HN, Neville-Golden J, Galanopoulos T, Kradin RL, Valente AJ, Graves DT Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 1992; 89: 5371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deshmane SL, Kremlev S, Amini S, Sawaya BE Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009; 29: 313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rose CE, Jr., Sung SS, Fu SM Significant involvement of CCL2 (MCP-1) in inflammatory disorders of the lung. Microcirculation 2003; 10: 273–88. [DOI] [PubMed] [Google Scholar]
- 59.Banks C, Bateman A, Payne R, Johnson P, Sheron N Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn's disease. J Pathol 2003; 199: 28–35. [DOI] [PubMed] [Google Scholar]
- 60.McCormack G, Moriarty D, O'Donoghue DP, McCormick PA, Sheahan K, Baird AW Tissue cytokine and chemokine expression in inflammatory bowel disease. Inflamm Res 2001; 50: 491–5. [DOI] [PubMed] [Google Scholar]
- 61.Conti P, DiGioacchino M MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc 2001; 22: 133–7. [DOI] [PubMed] [Google Scholar]
- 62.Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol 1998; 161: 6845–52. [PubMed] [Google Scholar]
- 63.Nenan S, Boichot E, Lagente V, Bertrand CP Macrophage elastase (MMP-12): a pro-inflammatory mediator? Mem Inst Oswaldo Cruz 2005; 100(Suppl 1): 167–72. [DOI] [PubMed] [Google Scholar]
- 64.Sandford AJ, Weir TD, Spinelli JJ, Pare PD Z and S mutations of the alpha1-antitrypsin gene and the risk of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 1999; 20: 287–91. [DOI] [PubMed] [Google Scholar]
- 65.Sandford AJ, Weir TD, Pare PD Genetic risk factors for chronic obstructive pulmonary disease. Eur Respir J 1997; 10: 1380–91. [DOI] [PubMed] [Google Scholar]
- 66.Vlahos R, Bozinovski S, Jones JE, Powell J, Gras J, Lilja A Differential protease, innate immunity, and NF-kappaB induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. Am J PhysiolLung Cell Mol Physiol 2006; 290: L931–45. [DOI] [PubMed] [Google Scholar]
- 67.Churg A, Wang R, Wang X, Onnervik PO, Thim K, Wright JL Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax 2007; 62: 706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vernooy JH, Lindeman JH, Jacobs JA, Hanemaaijer R, Wouters EF Increased activity of matrix metalloproteinase-8 and matrix metalloproteinase-9 in induced sputum from patients with COPD. Chest 2004; 126: 1802–10. [DOI] [PubMed] [Google Scholar]
- 69.Ohkawara T, Nishihira J, Takeda H, Hige S, Kato M, Sugiyama T Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice. Gastroenterology 2002; 123: 256–70. [DOI] [PubMed] [Google Scholar]
- 70.Garg P, Vijay-Kumar M, Wang L, Gewirtz AT, Merlin D, Sitaraman SV Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol 2009; 296: G175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Medina C, Santana A, Paz MC, Diaz-Gonzalez F, Farre E, Salas A Matrix metalloproteinase-9 modulates intestinal injury in rats with transmural colitis. J Leukocyte Biol 2006; 79: 954–62. [DOI] [PubMed] [Google Scholar]
- 72.Pender SL, Li CK, Di Sabatino A, MacDonald TT, Buckley MG Role of macrophage metalloelastase in gut inflammation. Ann N Y Acad Sci 2006; 1072: 386–8. [DOI] [PubMed] [Google Scholar]