Abstract
Purpose
Because of the observed success of phase I/II trials, the novel anti-CD30 agent brentuximab vedotin is now being evaluated as a frontline agent in the high-risk pediatric Hodgkin lymphoma trial HLHR13. The objectives of this study were to evaluate the pharmacokinetic variability during weekly dosing of 1.2 mg/kg of brentuximab vedotin, determine factors that may explain this variability, compare our drug exposure with published data, and evaluate toxicity of brentuximab vedotin in the pediatric population.
Methods
Brentuximab vedotin, MMAE and anti-therapeutic antibody levels were measured in the serum samples of 16 pediatric patients with Hodgkin lymphoma. A compartmental pharmacokinetic model was fit to the data by using non-linear mixed-effects modeling.
Results
Clearance and volume of brentuximab vedotin were significantly correlated with weight (p<.001), which was responsible for over 60% of the parameters inter-individual variability. Clearance and volume were higher in boys compared to girls (p=0.08 and p=0.03, respectively). Brentuximab vedotin’s AUC and Cmax were lower in our pediatric study than those reported in adult studies (25% and 11% respectively). Toxicity was comparable to that of the standard-of-care backbone using vincristine instead of brentuximab vedotin. The sera of all 16 patients remained negative for anti-therapeutic antibodies during and at the end of therapy.
Conclusions
As in previous studies, weight continues to be the most significant factor explaining brentuximab vedotin’s pharmacokinetic variability in pediatric patients. Exposure to weekly dosing appears to be safe and tolerable in pediatric patients.
Keywords: Brentuximab vedotin, Pharmacokinetics, Pediatric, Hodgkin lymphoma
Introduction
Although pediatric Hodgkin lymphoma (HL) remains one of the most curable forms of cancer with 5-year survival rates greater than 90%,[1] 30% to 40% of patients have disease that is either refractory to initial therapy or relapse[2] and long-term side effects of therapy continue to cause morbidity and early mortality in survivors. To help overcome these challenges, antibody-drug conjugates (ADCs) were created to target the delivery of anticancer drugs directly to tumor cells with increased specificity and decreased toxicity.[3] The novel agent brentuximab vedotin (SGN35) is an ADC containing an anti-CD30 murine/human chimeric monoclonal antibody (cAC10; brentuximab) linked to monomethyl auristatin E (MMAE).[4, 5] After binding CD30, a transmembrane receptor highly expressed on the malignant Reed-Sternberg cells in HL, [5, 6] brentuximab vedotin is internalized and MMAE is released. Both vincristine and MMAE exert their antineoplastic effect by inhibiting tubulin polymerization, leading to M-phase arrest and apoptosis.[7] The efficacy of brentuximab vedotin was demonstrated in the treatment of patients with relapsed classical HL as well as systemic anaplastic large cell lymphoma (ALCL), which also highly expresses CD30. [2, 8–12] Because of the safety and activity observed in these trials, brentuximab vedotin is now being evaluated as a frontline agent for pediatric patients with high-risk HL.
The treatment protocol HLHR13, an investigator initiated study, was developed with the goal of preserving the vincristine, etoposide, prednisone and doxorubicin (OEPA)- cyclophosphamide, vincristine, prednisone and dacarbazine (COPDac) backbone, a well-established pediatric HL treatment regimen widely used throughout the world. This combination of agents first demonstrated success in the GPOH-HD-2002 study.[13] The HLHR13 protocol uses brentuximab vedotin (ADCETRIS®) in place of vincristine, which has a similar mechanism of action, to form the treatment regimen AEPA/CAPDac.
Despite the increasing use of brentuximab vedotin, knowledge of its pharmacokinetics is limited in pediatric patients. Published studies report the ADC and MMAE pharmacokinetics since the total antibody and the ADC have similar pharmacokinetic profiles.[3] A retrospective analysis of 9 pediatric patients treated with brentuximab vedotin provided safety and efficacy pilot data for use in pediatrics.[14] The safety and efficacy of brentuximab vedotin has been evaluated in adults with relapsed classical HL or systemic ALCL and the maximum tolerated dose (MTD) was determined to be 1.8 mg/kg (max dose of 1800 mg) every 3 weeks.[4] Although less commonly used, weekly dosing was studied in an adult phase I dose-escalation trial that determined the maximum tolerated weekly dose in 44 patients (38 of which had HL) to be 1.2 mg/kg.[2]
Given the promising results of brentuximab vedotin in previous studies, [9, 11] more data are needed to establish the drug’s pharmacokinetics and safety for use in pediatric patients. We evaluated brentuximab vedotin pharmacokinetics in 16 pediatric patients with HL. The primary goals of this study were to evaluate the variability of the pharmacokinetics, determine factors that may affect pharmacokinetics, compare pediatric pharmacokinetics and drug exposures with published data on the recommended 1.8 mg/kg every 3 week dosing, and evaluate the toxicity of this novel agent as part of a frontline regimen in the pediatric population.[9, 15]
Materials and methods
Pediatric patients with newly diagnosed high-risk Hodgkin lymphoma were eligible for enrollment on a single-arm phase II study at St. Jude Children’s Research Hospital after approval from the institutional review board. Enrollment of all patients took place after written informed consent, as well as assent when appropriate, were obtained. All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Eligibility
Eligible patients were those 18 years old or younger at the time of diagnosis who had previously untreated CD30+ classical HL with confirmed histology and an Ann Arbor stage of IIB, IIIB, IVA, or IVB. Patients were excluded if their disease was CD30−, if they had received prior therapy for HL or had inadequate organ function (glomerular filtration rate <70 ml/min/1.83m2, total bilirubin ≥1.5 × upper limit of normal for age, and aspartate aminotransferase or alanine aminotransferase ≥ 2.5 × upper limit of normal for age).
Dosing
In this high-risk Hodgkin lymphoma trial (HLHR13), brentuximab vedotin (ADCETRIS®) was substituted for vincristine in the OEPA/COPDac backbone [13] because of its similar anti-microtubule mechanism of action, leading to the new combination AEPA/CAPDac.
Treatment
As previously described, treatment was given according to the GPOH-HD-2002 study except for the addition of a maximum prednisone dose (60mg/m2/day during the AEPA cycles and 40 mg/m2 during the CAPDac cycles) and the substitution of brentuximab vedotin for vincristine at the same schedule.[13] Brentuximab vedotin was therefore administered on days 1, 8, and 15 of each 28-day cycle for the first 2 cycles and then on days 1 and 8 of the 4 subsequent cycles. The dose was 1.2 mg/kg (maximum dose of 120 mg) and was rounded to the nearest whole number of milligrams. It was administered via outpatient IV infusion over approximately 30 minutes.[2] Routine premedication was not given prior to the first dose of brentuximab vedotin and only planned to be given with subsequent doses in patients who experienced a Grade 1 or 2 infusion-related reaction.
Toxicity
All toxicities were assessed and documented at each patient encounter, coded according to the common terminology criteria for adverse events CTCAE version 4.0 and entered prospectively into a database
Pharmacokinetic Studies
Serum samples to measure brentuximab vedotin levels were collected during the first cycle of therapy at day 1 pre-Brentuximab vedotin infusion, end of infusion, 48 hours post-infusion, and day 8 pre-infusion. Antibody-drug conjugate and MMAE levels were evaluated by an independent vendor using an ELISA assay.
Immunogenicity Samples
Serum concentrations of anti-brentuximab vedotin antibody were measured prior to the first dose of cycles 1, 2, and 3 and at the end of therapy by an ELISA-based assay, and samples were analyzed by an independent vendor.
Pharmacokinetic Analysis Methods
The population pharmacokinetic and individual post-hoc estimates of brentuximab vedotin ADC and MMAE were determined by using non-linear mixed-effects modeling via Monolix (version 4.3.0, www.monolix.org) using the Stochastic Approximation Expectation-Maximization (SAEM) approach. A linear 2-compartment model with first-order elimination was used to model ADC, a linear 1-compartment model with first-order formation from ADC and first-order elimination was used to model MMAE. The parameters estimated included brentuximab vedotin clearance (CL [L/h, mL/h/kg]), volume of brentuximab vedotin (V1 [L, mL/kg]), brentuximab vedotin intercompartmental clearance (Q [L/h, mL/h/kg]), brentuximab vedotin peripheral compartment volume (V2 [L, mL/kg]), MMAE apparent clearance (CLM [L/h, L/h/kg]), and MMAE apparent volume (VM [L, L/kg]). MMAE formation occurred via the ADC elimination rate constant (CL/V1). In addition, the individual post-hoc parameter values were used to estimate the brentuximab and MMAE area under the concentration curve (AUC) and estimated maximum concentration (Cmax). The inter-individual variability of the parameters was assumed to be log-normally distributed. A proportional residual error model was used with assumed normal distribution of the residuals.
The covariates were evaluated to determine their significance in explaining pharmacokinetic variability. A covariate was considered to be significant in a univariate analysis if their addition to the model reduced the objective function value (OFV) by at least 3.84 units (p < 0.05, based on the χ2 test for the difference in the −2 log-likelihood between 2 hierarchical models that differ by 1 degree of freedom) and the covariate term was significantly different from zero (p < 0.05, t-test).
Simulations of different doses and schedules of brentuximab vedotin and MMAE were performed by using the post-hoc individual estimates of the study population.
Results
Patients
Sixteen patients with high-risk classical HL consented to pharmacokinetic testing and had blood samples obtained prior to infusion, at the end of infusion, 48 hours post-infusion and 8 days prior to the subsequent infusion. Table 1 summarizes the characteristics of the 16 patients.
Table 1.
Patient Demographics
| Characteristic | n | |
|---|---|---|
| Sex | ||
| Female | 8 | |
| Male | 8 | |
| Ethnicity | ||
| White | 13 | |
| Black | 2 | |
| Hispanic | 1 | |
| Age (y) | ||
| 6–10 | 1 | |
| 11–15 | 4 | |
| 16–18 | 11 | |
| Weight (kg) | ||
| 11–20 | 1 | |
| 21–30 | 0 | |
| 31–40 | 1 | |
| 41–50 | 2 | |
| 51–60 | 4 | |
| 61–70 | 5 | |
| 71–80 | 2 | |
| >80 | 1 | |
Safety Profile
The toxicity profile of weekly dosing of brentuximab vedotin in combination with the standard backbone chemotherapy for the first 2 cycles of AEPA is reported in Table 2. The categories listed are the grades 3 and 4 toxicities found in our study compared to published toxicities from the GPOH-HD-2002 standard backbone chemotherapy as described above. No significant differences were observed between the two groups (p=0.89). [13]
Table 2.
Grade 3 or 4 Adverse Events
| Adverse Event | GPOH-HD-2002 Data for OEPA, % (n=561) |
HLHR13 Data for AEPA (n=16) |
|---|---|---|
| Anemia | 11.9% (67) | 6.3% (1) |
| Leukopenia | 70.5% (396) | 75% (12) |
| Neutropenia | 81.5% (457) | 81.3% (13) |
| Thrombocytopenia | 2.8% (16) | 0% |
| Stomatitis | 8.7% (49) | 6.3% (1) |
| Constipation | 7.3% (41) | 0% |
| Neurotoxicity, sensory | 1.3% (7) | 0% |
| Neurotoxicity, motor | 1.4% (8) | 0% |
Pharmacokinetics
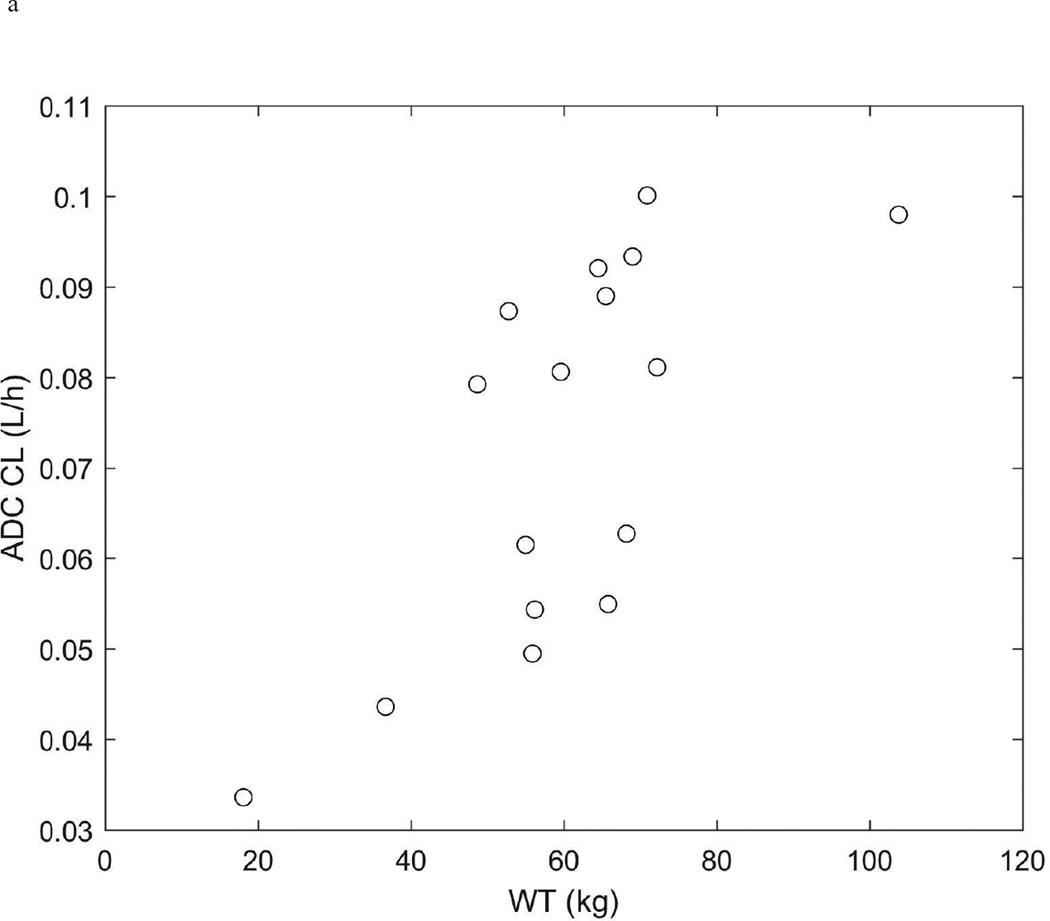
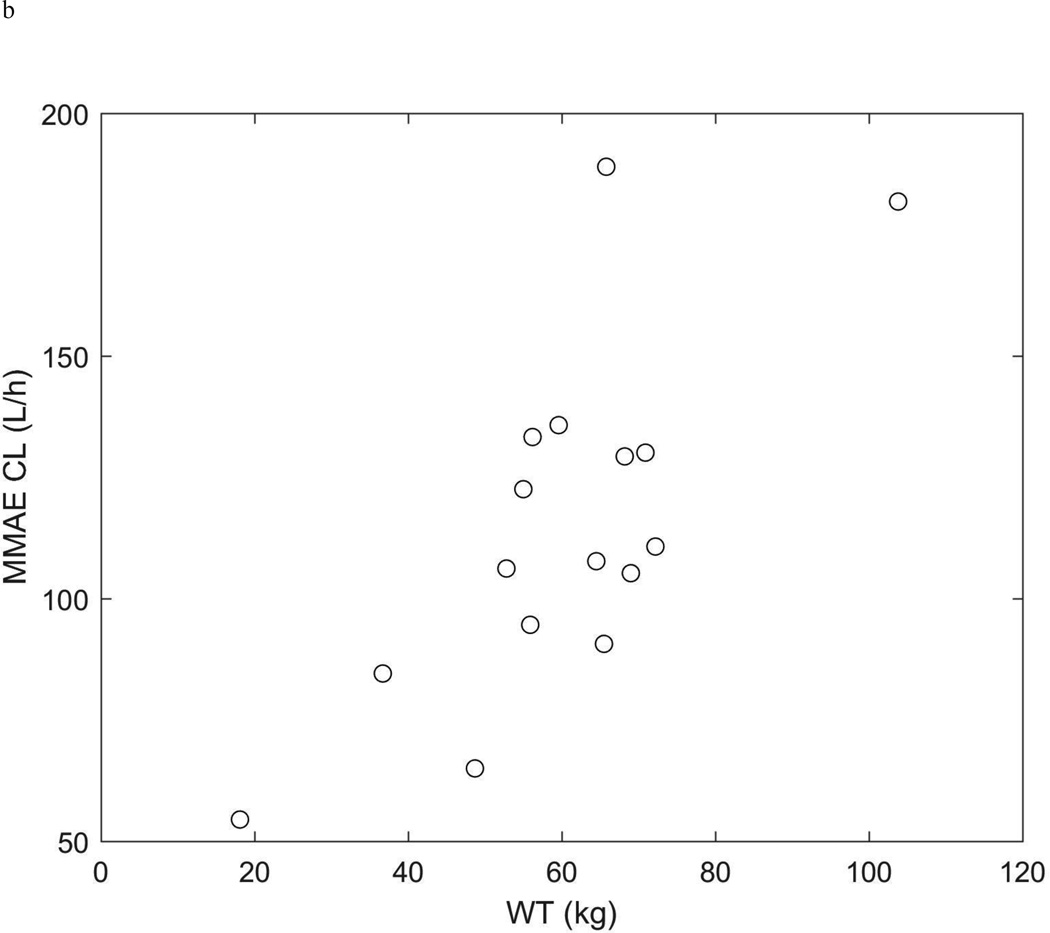
The pharmacokinetics of brentuximab vedotin and MMAE were described with the model outlined in the methods (Figure 1 and Supplemental Figures 1 and 2). The population pharmacokinetic model parameters are summarized in Table 3. Because of the strong relationship between patient weight and clearance (Figure 2), we considered weight as a covariate using weight-normalized dosing. The inclusion of weight significantly improved the model fit (decrease in −2 log-likelihood=91.7; p<.001) and explained 75%, 84%, 61%, and 94% of the inter-individual variability in the ADC clearance, ADC volume, MMAE apparent clearance, and MMAE apparent volume, respectively.
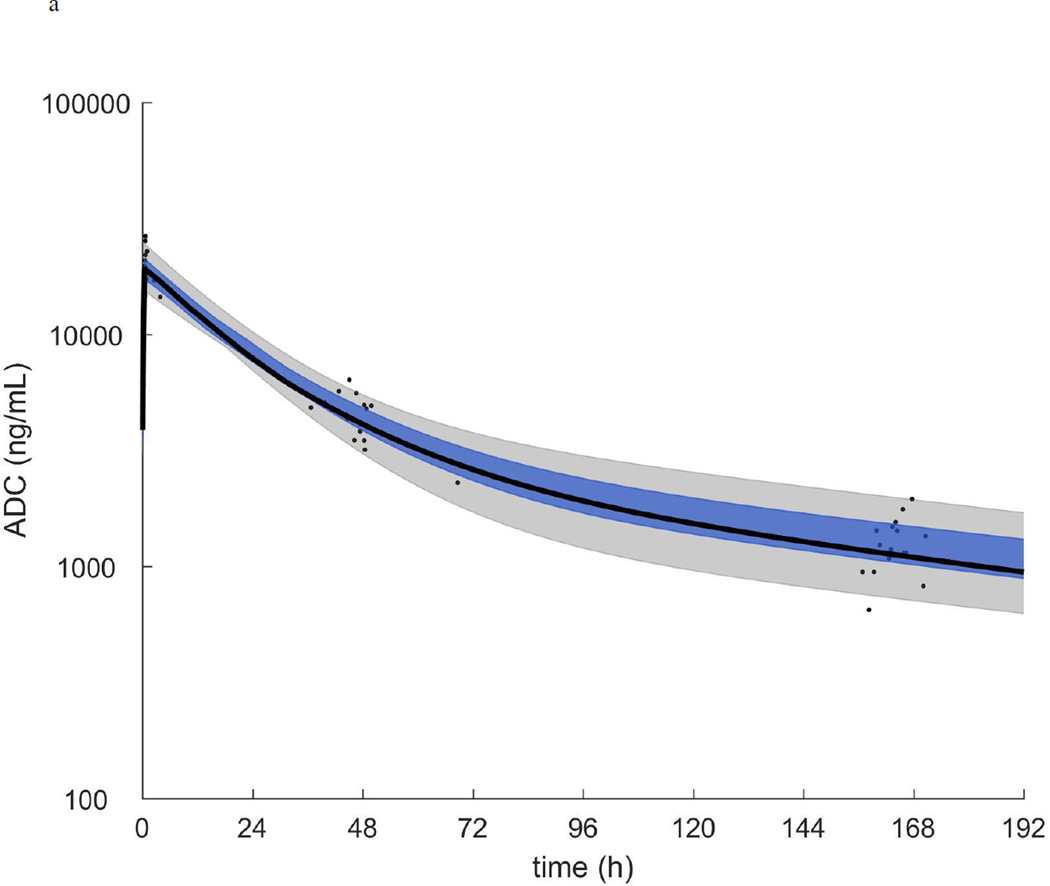
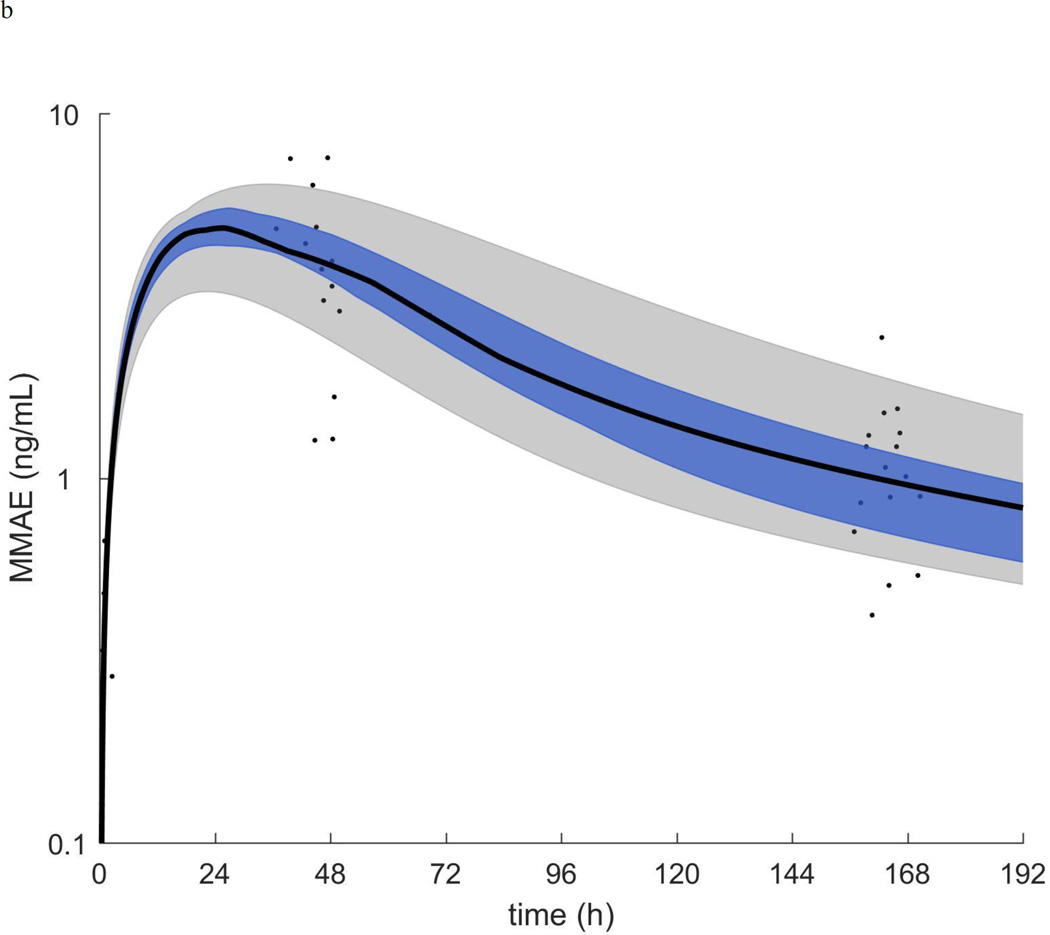
Figure 1.
a.) ADC plasma concentration vs time. b.) MMAE plasma concentration vs time. In each plot the symbols are the individual concentrations and the black curve, blue shaded region, and grey shaded region are the median, quartiles, and range of the post-hoc estimated individual concentration vs time curves respectively.
Table 3.
Population pharmacokinetic parameters obtained from the base (no covariates and no dose normalization), weight normalized dosing, and the final covariate model with sex and weight normalized dosing.
| Parameter | Base Estimate |
RSE (%) |
WT Normalized Estimate |
RSE (%) |
p-value | Sex WT Normalized Estimate |
RSE (%) |
p- value |
| CL (L/hr; mL/hr/kg) |
0.071 | 10 | 1.27 | 6 | 1.20 | 10 | ||
| β: Sex* | 0.16 | 58 | 0.08 | |||||
| V1 (L; mL/kg) | 3.51 | 10 | 61.61 | 4 | 57.05 | 5 | ||
| β: Sex* | 0.16 | 46 | 0.03 | |||||
| Q (L/hr; mL/hr/kg) |
0.075 | 8 | 1.12 | 5 | 1.07 | 5 | ||
| V2 (L; mL/kg) | 6.21 | 26 | 100.61 | 20 | 91.54 | 33 | ||
| CLM (L/hr; L/hr/kg) |
106.30 | 13 | 1.96 | 9 | 2.01 | 12 | ||
| VM (L; L/kg) | 2626.61 | 13 | 48.37 | 8 | 48.41 | 10 | ||
| ADC σ prop (CV%) |
4.6 | 264 | 6.9 | 52 | 7.6 | 41 | ||
| MMAE σ prop (CV%) |
31 | 19 | 29 | 18 | 30 | 18 | ||
| −2 Log- likelihood |
888.5 | 796.8 | <1e-10 | 790.2 | <0.04 | |||
| IIV | (CV%) | RSE (%) |
(CV%) | RSE (%) |
(CV%) | RSE (%) |
||
| CL | 34 | 21 | 17 | 24 | 16 | 24 | ||
| V1 | 38 | 18 | 15 | 24 | 12 | 28 | ||
| Q | 14 | 99 | 6 | 339 | 5 | 478 | ||
| V2 | 74 | 23 | 44 | 34 | 27 | 105 | ||
| CLM | 40 | 24 | 25 | 30 | 26 | 29 | ||
| VM | 38 | 32 | 9 | 248 | 8 | 373 |
Covariate model: θ*exp(β*covariate) where covariate=[0−Female or 1−Male]
Figure 2.
ADC (a.) and MMAE (b.) clearance (L/h) vs weight (kg).
In addition, the ADC clearance and volume also differed by sex (Supplemental Figures 3 and 4). Specifically, clearance and volume were both 17% higher (p=0.08 and p=0.03, respectively) in boys compared to girls. These differences explained an additional 11% and 36% of the inter-individual variability in the ADC clearance and volume, respectively, between these patients and the weight-normalized model.
We compared the exposures in this study (observed at 1.2 mg/kg and simulated at 1.8 mg/kg) to those reported in adult studies.[9, 15] Overall, the ADC AUC and Cmax in our pediatric study were lower than those reported in adult studies (25% and 11%, respectively at 1.2 mg/kg and 35% and 16%, respectively, at 1.8 mg/kg) (Table 4). These results correspond with a higher ADC clearance (46%, p<.001) in children than in adults.[15]
Table 4.
First Dose exposure measures. Geometric Mean (coefficient of variation).
| ADC | MMAE | ||||||
|---|---|---|---|---|---|---|---|
| Study; Dose | Dose (mg/kg) | n | AUC (days µg/mL) |
Cmax (µg/mL) |
n | AUC (days ng/mL) | Cmax (ng/mL) |
| Current Study (actual) |
1.2 | 16 | 37.97 (18) | 18.47 (14) | 16 | 24.08 (24) | 4.85 (16) |
| Current Study (simulated) |
1.8 | 16 | 54.84 (18) | 28.82 (14) | 16 | 34.57 (24) | 7.29 (16) |
| Fanale et al. 2012[2] |
1.2 | 12 | 40.52 (9)* | --- | 12 | 15.93 (60)* | 3.12 (60) |
| Younes et al. 2010[9] |
1.2 1.8 |
4 12 |
46.14 (62) 79.41 (30) |
18.89 (27) 31.98 (29) |
4 12 |
20.29 (212) 37.03 (47) |
2.72 (272) 4.97 (43) |
| Han et al. 2012 (Study 5)[15] |
1.2 1.8 |
11 11 |
52.77 (28) 89.84 (25) |
22.57 (23) 36.74 (34) |
14 14 |
26.65 (71) 40.06 (53) |
4.11 (71) 4.98 (67) |
AUC from day 0–7.
We also simulated the expected exposure for several different doses/schedules in our pediatric population. The median ADC AUC (day 0–21, normalized per week) for 1.2 mg/kg given weekly for 3 doses was 48% higher (p<.001) than a single dose of 1.8 mg/kg (Supplemental Figure 5). In addition, we observed a 7% higher ADC AUC (day 0–21, normalized per week) with 3 weekly 1.2 mg/kg doses (Supplemental Figure 6) and a 23% lower ADC AUC (day 0–21, normalized per week) with 2 weekly 1.2 mg/kg doses compared to a single dose of 1.8 mg/kg in adults (Supplemental Figure 7).[15]
Immunogenicity
Of the 16 patients, 1 patient had a positive ATA test prior to the start of therapy that was negative on all subsequent testing. All 16 patients’ tests remained negative for anti-therapeutic antibodies during therapy and at the end of therapy (i.e., after 14 doses of brentuximab vedotin). The patient with a positive ATA test prior to the start of therapy had the same drug metabolism as did baseline patients.
Discussion
Our results demonstrate a constant relationship between age and brentuximab vedotin clearance when normalized for weight, as seen in the adult data, suggesting that the drug is safe to administer to younger patients at the same dosing (1.2 mg/kg weekly dosing or 1.8 mg/kg every 3 week dosing).[15] This relationship was also validated in a pediatric phase I/II study that included 12 patients treated in the phase I portion and 16 patients in the phase II portion for the treatment of relapse or refractory HL or systemic ALCL.[16, 17] Patients received either 1.4 mg/kg every 3 weeks or 1.8 mg/kg every 3 weeks for a median of 7 cycles.[16, 17] The pharmacokinetic analysis was combined with five phase 1 and 2 studies that included adult patients and found the pharmacokinetics of ADC and MMAE to be linear, MMAE exposures to be lower than ADC exposure, and body weight to be the only significant covariate.[15]
The clearance and volumes were found to be 17% higher in boys than in girls, and no difference was detected for age or race. There are no confounding factors inherent in the study, as both sexes received the same chemotherapy at identical per-kilogram dosing. This difference was also observed in a phase 1 and 2 analysis in which female subjects were found to have a 14% lower volume than male subjects.[18]
None of the patients in our cohort developed an anti-therapeutic antibody to brentuximab vedotin with a weekly dosing schedule, much lower than published data.[4] Specifically, the development of anti-therapeutic antibodies has been demonstrated in up to 37% of patients, but the majority, 30%, were transiently positive, only 7% were persistently positive and the development of antibodies had minimal effect on pharmacokinetics.[2], [4, 11, 18] In our cohort, the development of antibodies may have been suppressed by daily steroid doses given concomitantly with brentuximab vedotin. Although there has been no correlate between the formation of anti-therapeutic antibodies and outcomes, there has been a correlation with increased infusion reactions and decreased exposure.[2] Therefore, it is promising that anti-therapeutic antibodies did not develop in any of the 16 patients in our cohort. Of note, one individual had a low level of anti-therapeutic antibody prior to the start of therapy but did not have detectable anti-therapeutic antibody in any subsequent samples. The pharmacokinetics of this patient’s sample were comparable to those of all other patient samples. This same scenario was observed in the SG035-003 and SG035-004 studies, in which 2 patients had anti-therapeutic antibodies at baseline, but none were detected after initiation of brentuximab vedotin.[18]
When brentuximab vedotin was administered as a monotherapy, some of the most common observed adverse events were peripheral sensory neuropathy, fatigue, nausea, diarrhea, arthralgia, and pyrexia; and the majority of events were mild to moderate in severity.[2, 4, 9, 11],[19] A small pediatric cohort demonstrated similar results with the most frequent treatment-related adverse events being fatigue, nausea, and peripheral neuropathy.[14] Three of 9 patients experienced ≥ Grade 3 toxicity (hyperesthesia, leukopenia, and neutropenia).[14] Although the toxicities observed in this study cannot be compared to published toxicities for treatment with brentuximab vedotin as a single agent, they can be compared to those of the OEPA/COPDac backbone used in the GPOH-HD-2002 study.[13] The HLHR13 protocol used the same dosing as OEPA/COPDac, and our toxicity should reflect a single change in this backbone of therapy from vincristine to brentuximab vedotin. Our AEPA toxicity results are not statistically different (p=0.89) than the OEPA results published from GPOH-HD-2002 and demonstrate a 4.5% increase in leukopenia; an equivalent percent of neutropenia; and a decrease in anemia, thrombocytopenia, stomatitis, constipation, and neurotoxicity (see Table 2).[13] There are several explanations for decreased side effects; our regimen was given upfront to patients with newly diagnosed disease who had not received prior chemotherapy rather than to patients with relapsed disease who had already been given many toxic medications prior to their enrollment.[2, 19] Additionally, daily steroid dosing on HLHR13 may not only suppress the formation of anti-therapeutic antibodies, but may also contribute to the decreased nausea and systemic symptoms.
In conclusion in pediatric patients, weight continues to be the most significant factor explaining brentuximab vedotin pharmacokinetic variability. A higher clearance and lower exposure was demonstrated in our study compared to published adult data. Sex continues to be a statistically significant, though minor, factor explaining pharmacokinetic variability. Further studies are warranted to delineate whether the discrepancy between volumes and clearance in male and female subjects is substantiated across a large cohort and to investigate whether these differences correlate with outcomes or toxicity. There was no development of anti-therapeutic antibodies during or after therapy and no increase in toxicity seen with the substitution of brentuximab vedotin for vincristine in the standard OEPA/COPDac backbone. While further studies are warranted to continue to evaluate pharmacokinetics and demonstrate safety, our results suggest that brentuximab vedotin is safe to administer to pediatric patients at the same dosing (1.2 mg/kg weekly dosing or 1.8 mg/kg every 3 week dosing) as used in adults.
Supplementary Material
Acknowledgments
This study was supported in part by the NIH Cancer Center Support Grant CA21765 and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
- 1.Kelly KM, et al. Children's Oncology Group's 2013 blueprint for research: Hodgkin lymphoma. Pediatr Blood Cancer. 2013;60(6):972–978. doi: 10.1002/pbc.24423. [DOI] [PubMed] [Google Scholar]
- 2.Fanale MA, et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res. 2012;18(1):248–255. doi: 10.1158/1078-0432.CCR-11-1425. [DOI] [PubMed] [Google Scholar]
- 3.Teicher BA, Chari RV. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17(20):6389–6397. doi: 10.1158/1078-0432.CCR-11-1417. [DOI] [PubMed] [Google Scholar]
- 4.Seattle Genetics, I. Adcetris (brentuximab vedotin) Product Information. Bothell, WA: 2012. [Google Scholar]
- 5.Katz J, Janik JE, Younes A. Brentuximab Vedotin (SGN-35) Clin Cancer Res. 2011;17(20):6428–6436. doi: 10.1158/1078-0432.CCR-11-0488. [DOI] [PubMed] [Google Scholar]
- 6.Younes A. CD30-targeted antibody therapy. Curr Opin Oncol. 2011;23(6):587–593. doi: 10.1097/CCO.0b013e32834bb8a7. [DOI] [PubMed] [Google Scholar]
- 7.Jackson D, Stover D. Using the Lessons Learned From the Clinic to Improve the Preclinical Development of Antibody Drug Conjugates. Pharm Res. 2015;32(11):3458–3469. doi: 10.1007/s11095-014-1536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogura M, et al. Phase I / II study of brentuximab vedotin in Japanese patients with relapsed or refractory CD30-positive Hodgkin's lymphoma or systemic anaplastic large-cell lymphoma. Cancer Sci. 2014;105(7):840–846. doi: 10.1111/cas.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younes A, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 10.Gopal AK, et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood. 2012;120(3):560–568. doi: 10.1182/blood-2011-12-397893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Younes A, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gopal AK, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(8):1236–1243. doi: 10.1182/blood-2014-08-595801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauz-Korholz C, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin's lymphoma: the GPOH-HD-2002 study. J Clin Oncol. 2010;28(23):3680–3686. doi: 10.1200/JCO.2009.26.9381. [DOI] [PubMed] [Google Scholar]
- 14.Fanale M, F A, Radhakrishnan R, Termuhklen A, Gopal AK, Shustiv A, et al. Complete Remissions Observed in a Subset of Pediatric Patients With CD30-expressing Malignant Lymphomas Treated in Clinical Studies of Brentuximab Vedotin (SGN-35). European Multidisciplinary Cancer Congress; 2011; Stockhold, Sweden. [Google Scholar]
- 15.Han TH, K D, Hayes S, Lynch CM. The Pharmacokinetics of Brentuximab Vedotin (SGN-35), An Antibody-Drug Conjugate (ADC). American Society for Clinical Pharmacology and Therapeutics Annual Meeting; 2012; Washington, DC. [Google Scholar]
- 16.Locatelli F, N K, Rosolen A, Landman-Parker J, Aladjidi N, Beishuizen A, Daw S, Gore L, Franklin ARK, Fansanmade A, Wang J, Sachs J, Mauz-Korholz C. Phase 1/2 Study of Brentuximab Vedotin in Pediatric Pts with Relapsed/Refractory (R/R) Hodgkin Lymphoma (HL) or Systemic Anaplastic Large-Cell Lymphoma (sALCL): Preliminary Phase 2 HL Data. Klin Padiatr. 2014;226:O-20. [Google Scholar]
- 17.Neville K, G L, Mauz-Körholz C, Rosolen A, Landman-Parker J, Sanchez de Toledo J, Beishuizen A, Keating Franklin AR, Fasanmade A, Wang J, Huebner D, Locatelli F. Phase I/II study of brentuximab vedotin in pediatric patients (pts) with relapsed or refractory (RR) Hodgkin lymphoma (HL) or systemic anaplastic large-cell lymphoma (sALCL): Interim phase (ph) I safety data. J Clin Oncol. 2013;(suppl) abstr 10028, 2013. [Google Scholar]
- 18. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/125388Orig1s000ChemR.pdf.
- 19.de Claro RA, et al. U.S. Food and Drug Administration approval summary: brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large-cell lymphoma. Clin Cancer Res. 2012;18(21):5845–5849. doi: 10.1158/1078-0432.CCR-12-1803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.