Abstract
Objective:
To assess the influence of parental rheumatoid arthritis (RA) on risk of epilepsy.
Methods:
We performed a nationwide cohort study including all singletons born in Denmark from 1977 to 2008 (n = 1,917,723) through individual linkage to nationwide Danish registries. The children were followed for an average of 16 years. Main outcome measures were adjusted hazard ratios (HRs) for epilepsy with onset in early childhood (29 days–4 years), late childhood (5–15 years), adolescence/adulthood (≥15 years), and at any age until the end of follow-up (December 31, 2010).
Results:
Compared to unexposed children, children exposed to maternal RA had an increased risk of early and late childhood epilepsy (adjusted HRs 1.34 [95% confidence interval (CI) 1.13–1.60] and 1.26 [95% CI 1.13–1.41]), while children exposed to maternal RA had no increased risk of epilepsy in adolescence/adulthood (HR 1.15 [95% CI 0.92–1.45]). Paternal RA was not associated with an overall risk of epilepsy in the offspring (HR 0.96 [95% CI 0.81–1.15]) or at any age. Children exposed to maternal RA in utero had a more pronounced increased risk of early childhood epilepsy than children exposed to mothers who were diagnosed with RA after childbirth (HR 1.90 [95% CI 1.26–2.86] vs HR 1.26 [95% CI 1.03–1.52], respectively [p = 0.16]).
Conclusions:
Exposure to maternal RA was associated with an increased risk of childhood epilepsy, while exposure to paternal RA was not, which indicates that changes in the intrauterine environment may play a role.
Epilepsy is a neurologic disorder characterized by recurrent epileptic seizures often of unknown etiology.1 Autoimmune encephalitis is associated with epileptic seizures,2 but recently, other autoimmune and inflammatory disorders such as rheumatoid arthritis (RA), systemic lupus erythematosus, ulcerative colitis, and type 1 diabetes have been suggested as risk factors for seizures and epilepsy.3 In a population-based study, children with any autoimmune disease were found to have a 5-fold increased risk of epilepsy and RA was associated with a 3-fold increased risk of subsequent epilepsy.3
The potential autoimmune etiology of some types of epilepsy is likely to involve the production of autoantibodies that affect the CNS4 but it is also possible that the coexistence of autoimmune disease and epilepsy reflect common genetic risk factors.5
Parental autoimmune diseases including RA have been associated with increased risk of RA in the offspring as well as other autoimmune diseases including type 1 diabetes and inflammatory bowel disease.6–10 These familial association studies suggest that there may be autoimmune genes and pathways that are shared among these different diseases.6–10 Thus, RA may increase the risk of epilepsy through CNS-acting immune responses or by immuno-based genetic factors with pleotropic effects. Transgenerational effects are expected if genetic factors of importance for autoimmune disorders play a role. We hypothesized that if maternal RA but not paternal RA is associated with childhood epilepsy, then the intrauterine environment may play a role in epileptogenesis.
METHODS
Data sources.
We used data from Danish national registries. All citizens in Denmark are assigned a unique identification number, which allows linkage among various national registries. To establish a nationwide cohort, we included data from (1) the Civil Registration System, which contains information on the date of birth, immigration and emigration status, deaths, and place of residence11; (2) the Danish National Hospital Registry, including nationwide data on all admissions to any Danish Hospital since 1977 (outpatient visits have been included since 1995)12; and (3) the Medical Birth Registry, which includes information on all Danish births since 1973.13 Finally, social registries at Statistics Denmark provided data on maternal educational status at time of birth.
Study population.
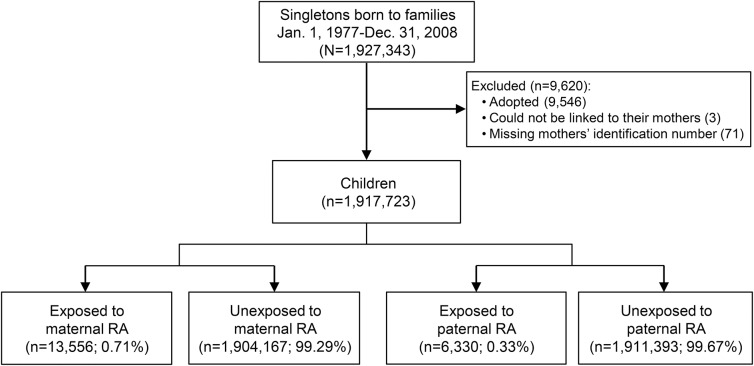
All singletons born in Denmark from January 1, 1977, to December 31, 2008 (n = 1,927,343) were identified from the Medical Birth Registry.13 Children who could not be linked to their mother (n = 3), children who had missing information on maternal identification number (n = 71), and adopted children (n = 9,546) were excluded (figure 1).
Figure 1. Identification of study population.
RA = rheumatoid arthritis.
Parental RA.
Information on maternal and paternal RA was obtained from 1977 until 2008 from the Danish National Hospital Registry.12 We used diagnoses from the ICD-8 from 1977 through 1993 (ICD 8: 712.19, 712.39, 712.59) and the ICD-10 from 1994 onwards (ICD 10: M05 and M06 [except M06.1: Still disease]) to identify persons diagnosed with RA.14
We classified the children as exposed to maternal or paternal RA if their mother or father was diagnosed with a main discharge diagnosis or a secondary discharge diagnosis of RA and we used the first hospitalization or first visit at the outpatient clinic associated with a diagnosis of RA as the date of clinically diagnosed RA. The date of first RA diagnosis was used to identify fetal exposure to clinical RA (i.e., RA diagnosed prior to birth) and preclinical RA (i.e., RA diagnosed after birth).14
Epilepsy.
We obtained information on the first diagnosis of epilepsy in the offspring from the Danish National Hospital Registry12 from January 1, 1977, until December 31, 2010 (ICD-8: 345 and ICD-10: G40-G41). Time of epilepsy onset was further stratified in 3 different age groups defined by the time of diagnosis: early childhood (from 29 days until 4 years of age), late childhood (5–15 years), and adolescence/adulthood (≥15 years).
Covariates.
Information on gestational age (at birth), sex of the child, birthweight, Apgar score at 5 minutes (available for births after 1996), maternal parity, parental age at birth, and maternal smoking (available for mothers of children born after 1996) was obtained from the Danish Medical Birth Registry.13 From Statistics Denmark, we obtained information on maternal education at the time of birth. Parents were identified in the Civil Registration System and information on RA in the other parent (father or mother respectively) and maternal epilepsy was obtained from the Danish National Hospital Registry.12
Statistical analysis.
We followed the children from the 29th day after birth (to exclude neonatal seizures) until the diagnosis of epilepsy, death, emigration, or end of follow-up (December 31, 2010), whichever came first. In age-stratified analysis, the follow-up was restricted to 3 age intervals: (1) from 29 days until <5 years, (2) 5–15 years, and (3) ≥15 years. We used Cox proportional hazards models to calculate hazard ratios (HRs) and associated 95% confidence intervals (95% CI) for risk of epilepsy among offspring exposed to parental RA (maternal or paternal) and age of the children (in days) was used as the underlying time scale. The reference group was children born in the same time period who were not exposed to maternal or paternal RA, respectively. The first adjusted hazard models included exposure variables and year of birth. Fully adjusted models also included maternal age at birth (in years), maternal education (low/middle/high), year of birth, and paternal RA (yes/no) or maternal RA (yes/no), respectively.
We made separate analyses for children exposed to maternal clinical RA in fetal life vs exposure to maternal preclinical RA in fetal life. This was to explore a potentially higher risk of epilepsy in children exposed to maternal clinically verified RA and to separate effect of potential treatment from the effect of RA in the analyses.
The analyses were stratified according to sex of the offspring in order to examine potential sex differences.
We performed a number of sensitivity analyses. To evaluate the robustness of the exposure definition, we stratified the analyses according to the period before and after 1995, since from 1995 outpatients were included in the Danish National Hospital Registry. Exposed children were restricted to those of a parent recorded in the Danish National Hospital Registry more than once with a diagnosis of RA. Analyses of exposure to preclinical RA were further stratified by time from birth until a diagnosis of RA: <5 years after birth vs ≥5 years after birth. Additional analyses were also conducted in which all children were followed from the day of birth (instead of from 29 days).
To explore potential confounding by pregnancy history and to take dependency between siblings into account, analyses were also conducted including only firstborn children. Additional adjustments for maternal parity (1, 2, ≥3), paternal age (in years), maternal epilepsy (yes/no), and maternal smoking (smoker/nonsmoker) were performed. To explore a potential influence on estimates of mediating variables, gestational age at birth (in weeks), birthweight (in grams), and Apgar score at 5 minutes (<7/≥7) were also included in the models. In additional analyses, children diagnosed with cerebral palsy in the Danish National Hospital Registry (ICD-8: 343–344 and ICD-10: G80) were excluded from the analyses. Complete case analyses were conducted including children with complete information in the fully adjusted models (i.e., 94% of the children). Statistical analyses were performed using SAS (Cary, NC) statistical software (version 9.2) and a 5% level of significance was used. The study was performed using encrypted identification numbers on servers located at Statistics Denmark.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Danish Data Protection Agency (Jr. No. 2010-41-5535).
RESULTS
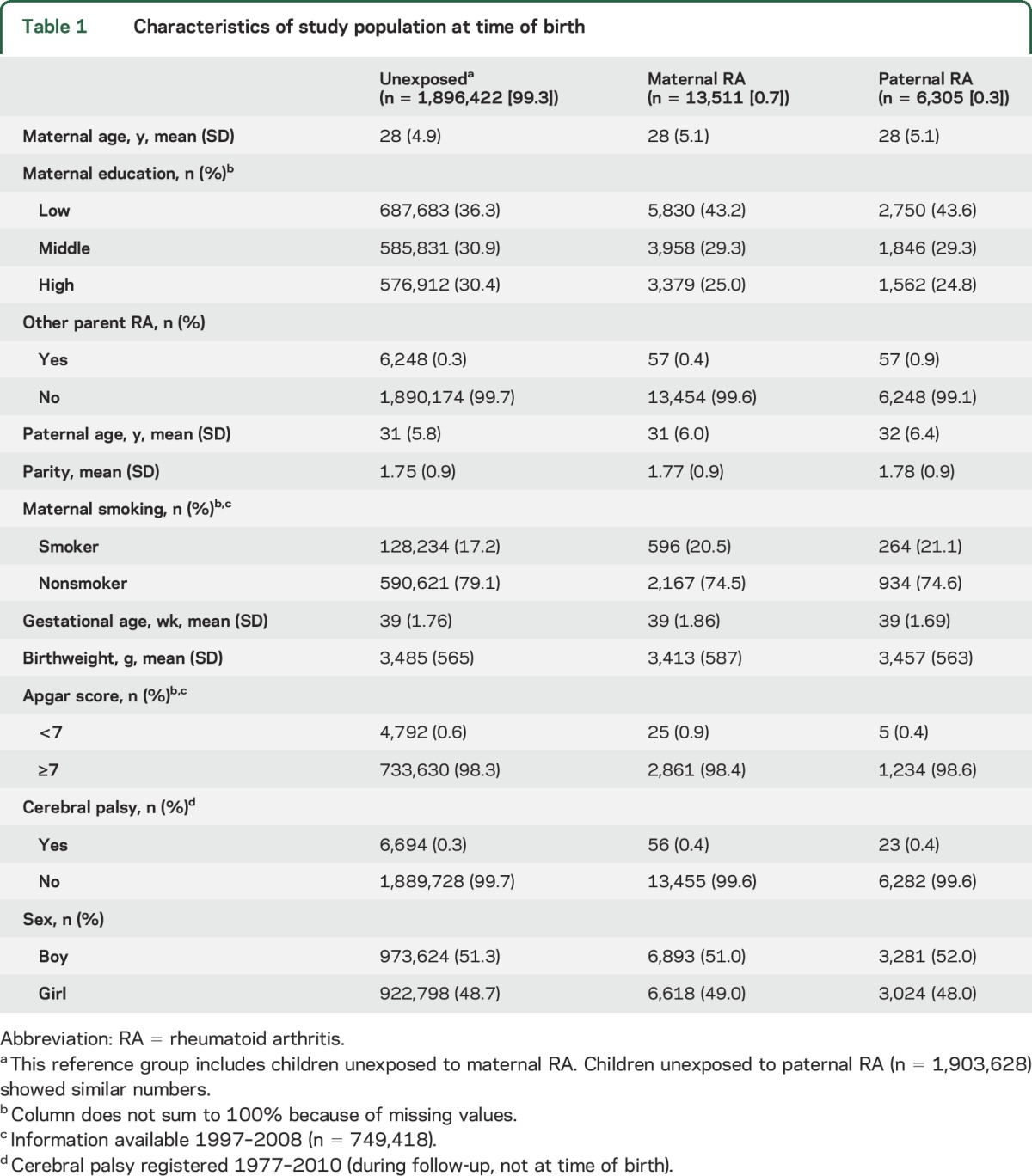
Of the total study population (n = 1,917,723 singletons), 1,909,933 children were alive and resident in Denmark 29 days after birth. Among these children, 13,511 were exposed to maternal RA (clinical or preclinical) and 6,305 to paternal RA (table 1). During a mean follow up of 16 years (range 29 days–34 years), epilepsy was diagnosed in 31,491 children. Children exposed to parental RA had mothers with shorter education at time of birth compared with unexposed children. Maternal smoking was more frequent among the exposed children. Other characteristics were similar among exposed and unexposed children (table 1).
Table 1.
Characteristics of study population at time of birth
Early childhood epilepsy.
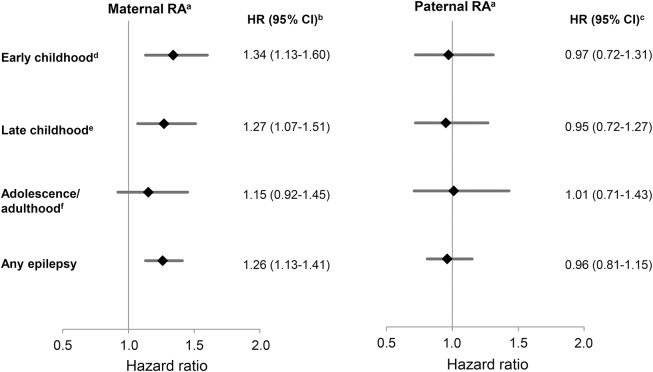
Compared to offspring of mothers without RA, children exposed to maternal RA had an increased risk of early childhood epilepsy (HR 1.34 [95% CI 1.13–1.60]). In contrast, children exposed to paternal RA were not at increased risk of early childhood epilepsy (HR 0.97 [95% CI 0.72–1.31]) compared with children of fathers without RA (figure 2).
Figure 2. Age-specific hazard ratios (HRs) of epilepsy in offspring of parents with rheumatoid arthritis (RA).
CI = confidence interval. aNo. of children followed: 1,909,933; no. of events: any epilepsy, 31,491; early childhood, 12,838; late childhood, 12,384; adolescence/adulthood, 6,269. bAdjusted for age of the child, maternal age, maternal education, birth year, and paternal RA. cAdjusted for age of the child, maternal age, maternal education, birth year, and maternal RA. d29 days–<5 years. e5–15 years. f≥15 years. The simple adjusted estimates were similar to the presented fully adjusted estimates.
Late childhood epilepsy.
Maternal RA was also associated with an increased risk of late childhood epilepsy (HR 1.27 [95% CI 1.07–1.51]), while paternal RA was not, when compared to unexposed children (HR 0.95 [95% CI 0.72–1.27]) (figure 2).
Adolescence/adulthood epilepsy.
Neither maternal nor paternal RA was associated with adolescence/adulthood epilepsy in the offspring when compared to unexposed adolescence/adulthood epilepsy (HR 1.15 [95% CI 0.92–1.45] vs HR 1.01 [95% CI 0.71–1.43]) (figure 2).
Epilepsy at any age.
The HR of epilepsy diagnosed at any age in children of mothers with RA was 1.26 (95% CI 1.13–1.41), while the HR was 0.96 (95% CI 0.81–1.15) in children of fathers with RA when compared to unexposed children (figure 2).
Maternal clinical RA vs preclinical RA.
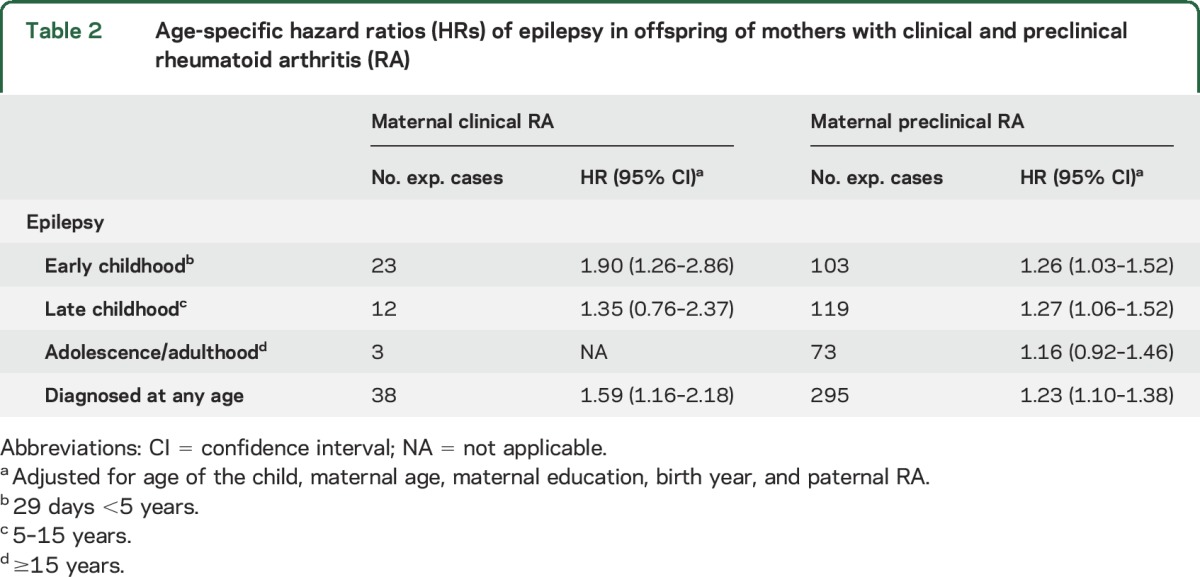
Children exposed to maternal clinical RA in fetal life seemed to have a higher risk of early childhood epilepsy compared to children exposed to maternal preclinical RA (HR 1.90 [95% CI 1.26–2.86] vs HR 1.26 [95% CI 1.03–1.52]) (p = 0.16). The corresponding HRs for late childhood epilepsy were 1.35 (95% CI 0.76–2.37) and 1.27 (95% CI 1.06–1.52) (p = 0. 65), respectively (table 2).
Table 2.
Age-specific hazard ratios (HRs) of epilepsy in offspring of mothers with clinical and preclinical rheumatoid arthritis (RA)
Sex-related differences.
There were no sex-related differences in risk of epilepsy among offspring exposed to maternal or paternal RA (data not shown).
Sensitivity analyses.
The risk of epilepsy among children of parents diagnosed with RA while hospitalized (i.e., born before 1995) was similar to that of all children and to that of children born after 1995 (hospitalized or outpatient) (data not shown). Results were unchanged when analyses were restricted to children having a parent with more than one diagnosis of RA recorded (data not shown).
Among children exposed to an early state of maternal preclinical RA (RA diagnosed 5 or more years after childbirth), the HRs for early or late childhood epilepsy were 1.23 (95% CI 1.10–1.38) and 1.22 (95% CI 1.00–1.49), respectively; i.e., almost similar to the HRs for exposure to preclinical RA, indicating that subdividing exposure to preclinical RA did not affect the risk estimates.
Results remained similar when all children (n = 1,917,723) were followed from the day of birth, i.e., including children 0–28 days old.
The analyses restricted to the firstborn children revealed estimates similar to the analyses including all children. Additional adjustment for parity, paternal age, birthweight, gestational age at birth, Apgar score, maternal epilepsy, and maternal smoking, respectively, did not change the estimates. Finally, the results were similar after excluding children with cerebral palsy (data not shown).
DISCUSSION
Children exposed to maternal RA had an increased risk of early and late childhood epilepsy compared to children unexposed to maternal RA. In contrast, paternal RA was not associated with epilepsy in the offspring, and a lower HR was found among children exposed to maternal preclinical RA. These findings indicate mechanisms involved in epileptogenesis that operate via changes in the intrauterine environment or via specific treatment for RA.
Recently, children with RA were found to have a 3-fold increased risk of subsequent epilepsy.3 Epilepsy may follow other autoimmune disorders; i.e., a higher prevalence of epilepsy will be expected in patients with autoimmune diseases that may directly involve the brain, such as systemic lupus erythematosus,15 antiphospholipid syndrome,16 or multiple sclerosis. However, it is new knowledge that other autoimmune diseases, such as RA, that are not known to directly affect the brain may increase the risk of epilepsy.3 The potential biological mechanism behind the association is unknown, but is likely to involve the production of antibodies, the increased synthesis and release of specific cytokines and chemokines with increased inflammatory microglial response in the brain, or the results of vascular complications including stroke and hemorrhage.17 It is also possible that the coexistence of autoimmune diseases and epilepsy reflects genetic predisposition that is common to the primary disease and epilepsy (pleiotropic effects).3,5,18 However, in the current study, additional adjustment for maternal epilepsy did not influence the results, indicating that maternal epilepsy did not explain the association between maternal RA and epilepsy in the offspring.
Epidemiologic studies have consistently shown strong associations between parental RA and other autoimmune diseases.6–10 Among the same cohort of children as in the current study, we also found a moderate to highly increased risk of autoimmune disease among offspring of parents with RA.14 Thus, it could be speculated that the risk of epilepsy on an autoimmune basis is higher in children of parents with RA compared to children of parents without RA.
Preclinical RA is recognized as a period with elevations of disease-related biomarkers, including autoantibodies, prior to the development of clinically overt RA.19 Exposure to a preclinical state of RA in fetal life also increased the risk of early and late childhood epilepsy. These findings may point towards an effect of the disease rather than a strong effect of specific RA treatment, since women with preclinical RA are not assumed to receive specific RA treatment. However, we did not have information on exposure to specific RA treatment during pregnancy.
The increased risk of childhood epilepsy in children exposed to preclinical RA could also indicate genetic factors as a plausible biologic mechanism. This hypothesis is supported by the approximately similar increased risk of childhood epilepsy in children of mothers with an early state of preclinical RA (i.e., mothers diagnosed with RA more than 5 years after childbirth).
We were able to follow a nationwide cohort of 1,909,859 children for a mean follow-up of 16 years. During this time, 31,491 children were diagnosed with epilepsy and more than 19,000 children were exposed to parental RA. Because of the nationwide design, selection bias of study participants is less likely. We included children exposed to both maternal and paternal RA, as well as maternal clinical and preclinical RA. Thus we were able to elucidate on potential underlying biological mechanisms. Furthermore, adjustment for a large number of potential confounders (including maternal educational level and maternal smoking) did not affect the associations, indicating no important confounding by the adjustment variables. Confounder adjustments also applicable to this study have been discussed further in a prior publication.14 Finally, we explored if differences in maternal epilepsy, gestational age, low birthweight, low Apgar score, or cerebral palsy in children exposed to parental RA could explain the increased risk of childhood epilepsy, and found that they did not.
Diagnoses of RA and epilepsy were obtained from the Danish National Hospital Registry. RA diagnosis is more often confirmed according to the American College of Rheumatology criteria for RA20 in patients who have been diagnosed while hospitalized (i.e., RA diagnosis confirmed in up to 80%) or in patients who have had more than one diagnosis of RA recorded (i.e., confirmed in up to 91%).21 In our study, estimates remained similar after restricting to those of a parent diagnosed with RA while hospitalized, and after restricting to children of a parent diagnosed with RA more than once.
Outpatient data were included from 1995 forward and the coming of a private health care sector in Denmark in the 2000s also necessitated notification from private hospitals and clinics to the Danish National Hospital Registry. Thus, some patients with RA were not captured in this study; i.e., those who were diagnosed and treated as outpatients before 1995 or at private practitioners exclusively. However, associations were similar before and after 1995, indicating no effect on results of some children falsely classified as unexposed, which is not surprising since the unexposed group comprises almost 2 million unexposed children, giving limited room for false-negatives.
When assessing an epilepsy diagnosis according to the International League Against Epilepsy criteria in the Danish National Hospital Registry, the positive predictive value was 81% (95% CI 75%–87%).22,23 However, 40% of the patients who did not meet the criteria for epilepsy (more than 1 unprovoked seizure on separate days) had 1 unprovoked seizure reported.23 Thus, the true validity may be higher.23 It is also possible that some children with epilepsy were not hospitalized or seen in outpatient clinics, while no information on the completeness of the register was available.23 The number of children with unregistered epilepsy is expected to be small, since hospitalization and assessment in outpatient clinics is tax paid and free of charge for all citizens in Denmark.
A number of studies have tried to identify clinical indicators of autoimmune epilepsy, and personal24,25 and possibly also family history of autoimmunity is among the clinical features suggestive of autoimmune epilepsy.25 The validity of the epilepsy subtype diagnosis is low,23 but we used age at diagnosis of epilepsy as proxy for different types of epilepsy.22,23,26,27 The risk of epilepsy following exposure to maternal RA was different in the individual age groups, suggesting that risk of specific epilepsy subtypes may be differently influenced by maternal RA. Further studies are therefore needed to address the influence of parental autoimmune and rheumatic diseases on the risk of epilepsy and specific types of epilepsy.
This study suggests a 90% increased risk of early childhood epilepsy in children of mothers with clinical RA and a 30% increased risk of early childhood epilepsy in children of mothers with preclinical RA, while late childhood epilepsy was increased 30% in children of mothers with clinical or preclinical RA. Our findings indicate shared immunologic and genetic mechanisms underlying the association between maternal RA and epilepsy in their children.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Charlotte Skovlund for assistance with data management.
GLOSSARY
- CI
confidence interval
- HR
hazard ratio
- ICD-8
International Classification of Diseases, 8th revision
- ICD-10
International Classification of Diseases, 10th revision
- RA
rheumatoid arthritis
Footnotes
Editorial, page 2502
AUTHOR CONTRIBUTIONS
Study concept and design: Drs. Rom, Olsen, and Mørch. Acquisition, analysis, or interpretation of data: All authors. Drafting the manuscript: Drs. Rom, Olsen, and Mørch. Critical revision of the manuscript for important intellectual content: All authors.
STUDY FUNDING
This study was supported by grants from the NIH (grant 5R21AR059931-02), the Danish Council for Independent Research, and the Augustinus Foundation.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Palace J, Lang B. Epilepsy: an autoimmune disease? J Neurol Neurosurg Psychiatry 2000;69:711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the International Encephalitis Consortium. Clin Infect Dis 2013;57:1114–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong MS, Kohane IS, Cai T, Gorman MP, Mandl KD. Population-level evidence for an autoimmune etiology of epilepsy. JAMA Neurol 2014;71:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valencia I. Epilepsy in systemic autoimmune disorders. Semin Pediatr Neurol 2014;21:226–231. [DOI] [PubMed] [Google Scholar]
- 5.Vincent A, Crino PB. Systemic and neurologic autoimmune disorders associated with seizures or epilepsy. Epilepsia 2011;52(suppl 3):12–17. [DOI] [PubMed] [Google Scholar]
- 6.Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum 2009;60:661–668. [DOI] [PubMed] [Google Scholar]
- 7.Hemminki K, Li X, Sundquist J, Sundquist K. Familial association between type 1 diabetes and other autoimmune and related diseases. Diabetologia 2009;52:1820–1828. [DOI] [PubMed] [Google Scholar]
- 8.Hemminki K, Li X, Sundquist J, Sundquist K. The epidemiology of Graves disease: evidence of a genetic and an environmental contribution. J Autoimmun 2010;34:J307–J313. [DOI] [PubMed] [Google Scholar]
- 9.Hemminki K, Li X, Sundquist K, Sundquist J. Familial association of inflammatory bowel diseases with other autoimmune and related diseases. Am J Gastroenterol 2010;105:139–147. [DOI] [PubMed] [Google Scholar]
- 10.Hemminki K, Li X, Sundquist K, Sundquist J. Shared familial aggregation of susceptibility to autoimmune diseases. Arthritis Rheum 2009;60:2845–2847. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System: a cohort of eight million persons. Dan Med Bull 2006;53:441–449. [PubMed] [Google Scholar]
- 12.Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register: a valuable source of data for modern health sciences. Dan Med Bull 1999;46:263–268. [PubMed] [Google Scholar]
- 13.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull 1998;45:320–323. [PubMed] [Google Scholar]
- 14.Rom AL, Wu CS, Olsen J, et al. Parental rheumatoid arthritis and long-term child morbidity: a nationwide cohort study. Ann Rheum Dis 2015;75:1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanly JG, Urowitz MB, Su L, et al. Seizure disorders in systemic lupus erythematosus: results from an international, prospective, inception cohort study. Ann Rheum Dis 2012;71:1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoenfeld Y, Lev S, Blatt I, et al. Features associated with epilepsy in the antiphospholipid syndrome. J Rheumatol 2004;31:1344–1348. [PubMed] [Google Scholar]
- 17.Devinsky O, Schein A, Najjar S. Epilepsy associated with systemic autoimmune disorders. Epilepsy Curr 2013;13:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandolfo M. Pediatric epilepsy genetics. Curr Opin Neurol 2013;26:137–145. [DOI] [PubMed] [Google Scholar]
- 19.Deane KD. Preclinical rheumatoid arthritis (autoantibodies): an updated review. Curr Rheumatol Rep 2014;16:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–1588. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen M, Klarlund M, Jacobsen S, Svendsen AJ, Frisch M. Validity of rheumatoid arthritis diagnoses in the Danish National Patient Registry. Eur J Epidemiol 2004;19:1097–1103. [DOI] [PubMed] [Google Scholar]
- 22.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30:389–399. [DOI] [PubMed] [Google Scholar]
- 23.Christensen J, Vestergaard M, Olsen J, Sidenius P. Validation of epilepsy diagnoses in the Danish National Hospital Register. Epilepsy Res 2007;75:162–170. [DOI] [PubMed] [Google Scholar]
- 24.Suleiman J, Brilot F, Lang B, Vincent A, Dale RC. Autoimmune epilepsy in children: case series and proposed guidelines for identification. Epilepsia 2013;54:1036–1045. [DOI] [PubMed] [Google Scholar]
- 25.Greco A, Rizzo MI, De Virgilio A, et al. Autoimmune epilepsy. Autoimmun Rev 2016;15:221–225. [DOI] [PubMed] [Google Scholar]
- 26.Christensen J, Vestergaard M, Pedersen MG, Pedersen CB, Olsen J, Sidenius P. Incidence and prevalence of epilepsy in Denmark. Epilepsy Res 2007;76:60–65. [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Health and Care Excellence. NICE guidelines: the epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. 2012. Available at: https://www.nice.org.uk/guidance/cg137?unlid=9290908212016213144416. Accessed June 5, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.