Abstract
Objective:
To investigate the association among migraine, ischemic stroke, and stroke subtypes in the Atherosclerosis Risk in Communities (ARIC) study.
Methods:
In this ongoing, prospective, longitudinal community-based cohort study, participants were given an interview ascertaining migraine history in 1993–1995, and were followed for all vascular events, including stroke. All stroke events over the subsequent 20 years were adjudicated and classified into stroke subtypes by standard definitions. Cox proportional hazards models adjusted for stroke risk factors were used to study the relationship between migraine and ischemic stroke, overall, as well as stroke subtypes (cardioembolic, lacunar, or thrombotic).
Results:
We identified 1,622 migraineurs among 12,758 participants. Mean age of the study population at the 3rd clinical visit was 59 years. When compared to nonheadache participants, there was a significant association between migraine with visual aura and ischemic stroke (hazard ratio [HR] 1.7, 95% confidence interval [CI] 1.2–2.6, p = 0.008). Migraine without visual aura was not significantly associated with ischemic stroke (HR 1.2, CI 1.0–1.8, p = 0.28) when compared to nonheadache participants. Among the 3 subtypes of ischemic stroke evaluated, migraine with visual aura was significantly associated only with cardioembolic stroke (HR 3.7, 95% CI 1.6–8.7, p = 0.003).
Conclusion:
In participants with migraine with visual aura in late middle age, increased risk of cardioembolic stroke was observed. Migraine with visual aura was linked to increased stroke risk, while migraine without visual aura was not, over the period of 20 years. These results are specific to older migraineurs.
Epidemiologic studies have demonstrated that migraine with aura is associated with increased risk of ischemic stroke.1–6 A recent review on migraine and stroke presented substantial neuroimaging and genetic evidence supporting the link between migraine with aura and ischemic stroke.7 Furthermore, subclinical infarcts have been linked to migraine.8,9 However, there is a paucity of data on the pathogenesis of increased risk of ischemic stroke in migraine with aura. Investigation of stroke subtypes and association with migraine may shed light on the potential mechanism. Furthermore, there are still controversial reports on relationship between migraine without aura and ischemic stroke; this may be due to variations in study populations across prior published studies.10–14 In the Atherosclerosis Risk in Communities (ARIC) study, we proposed to evaluate the association between migraine with and without aura and ischemic stroke and to expand on prior work in ARIC and in other studies. While other studies have examined the relationship between migraine and stroke, we specifically aimed to examine the association between ischemic stroke subtypes, including thrombotic, cardioembolic, and lacunar strokes, and migraine with and without aura.
METHODS
Study population.
The cohort of the ARIC study was initiated in 1987 with an aim of studying the causes of atherosclerosis and clinical sequelae.15 The study enrolled 15,792 participants within the age group of 45–64 years in 4 different US communities (Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland). Baseline information was collected at the first visit from 1987 to 1989, and follow-up visits every 3 years for visits 2 (1990–1992), 3 (1993–1995), and 4 (1996–1998) were completed. Surviving participants completed visit 5 during 2011–2013. Vascular risk factors and markers as well as various health interviews were completed at each visit, and participants underwent laboratory testing at each visit.15 In addition, participants are contacted annually by telephone, and queried about any hospitalizations. Hospital medical records are obtained for all vascular hospitalizations, including stroke.
In the current study, we included all participants who completed the third clinic visit (1993–1995) in ARIC (n = 12,882). We excluded participants with first ischemic stroke event that occurred before the third clinical visit in the ARIC study (1993–1995) (n = 87) and participants with missing headache information (n = 37). A total of 12,758 participants fulfilled our criteria and were included for analysis. For analysis, only individuals without any headache history were included as controls.
Standard protocol approvals, registrations, and patient consents.
The institutional review boards of all participating institutions approved the study, and all participants provided written informed consent.
Assessment of migraine.
A headache questionnaire was administered to all participants who attended ARIC visit 3. Based on results from this questionnaire, modified International Classification of Headache Disorders (ICHD III beta)16 diagnostic criteria were used to characterize migraine with visual aura and migraine without visual aura. Participants were classified as having migraine if they endorsed headaches lasting at least 4 hours or longer in duration, with throbbing or pulsating pain, and if headaches were mostly on one side; headaches must have also been accompanied by photophobia and phonophobia or nausea or vomiting. This information was all identified from the headache questionnaire used in visit 3. In addition, headache was separated into migraine with visual aura if the participant reported seeing spots, jagged lines, or heat waves in one or both eyes before onset of headache. All other migraine headaches that did not qualify for migraine with visual aura were classified as migraine without visual aura. All other headaches that lasted for more than 4 hours and did not qualify for migraine headache were classified as nonmigraine headaches and those individuals who did not report a history of headaches greater than 4 hours were classified into the no headache group. Individuals with nonmigraine headaches were excluded from analysis.
Adjudication of stroke subtypes.
Neuroimaging (CT or MRI) was mandatory for stroke diagnosis and exclusion of nonstroke reasons for neurologic symptoms, although images were not reviewed directly (rather, reports were reviewed). Stroke diagnosis was based on computer-derived diagnosis and physician medical record review, with differences adjudicated by a second physician reviewer. Verified incident ischemic strokes included all the events occurring between the date of the third clinic visit (1993–1995) and December 2012. Classification required evidence of sudden or rapid onset of neurologic symptoms lasting >24 hours or leading to death, in the absence of evidence for a nonstroke cause. Ischemic strokes were further classified according to pathogenic subtype as thrombotic brain infarction, lacunar infarction, or cardioembolic stroke, according to criteria adopted from the National Survey of Stroke subtype classification.17,18 Neuroimaging patterns of infarct distribution were not considered per the algorithm based on the National Survey of Stroke available at the time of this study initiation.
Statistical analysis.
We considered baseline demographics including age, sex, and race for the analysis and investigated additional covariates including hypertension, diabetes, current smoking status, regular use of nonsteroidal anti-inflammatory drugs (NSAID), physical activity, serum low-density lipoprotein (LDL), and body mass index (BMI). Low physical activity is considered significant if performed for less than 3 h/wk for at least a month. Cox proportional hazards models were used to analyze the association among migraine with visual aura, migraine without visual aura, and incidence of ischemic stroke. We used survival analysis to evaluate the incidence of each subtype of ischemic stroke in migraine with visual aura when compared to migraine without visual aura. All data analysis was carried out using SAS version 9.4 (SAS institute Inc., Cary, NC).
RESULTS
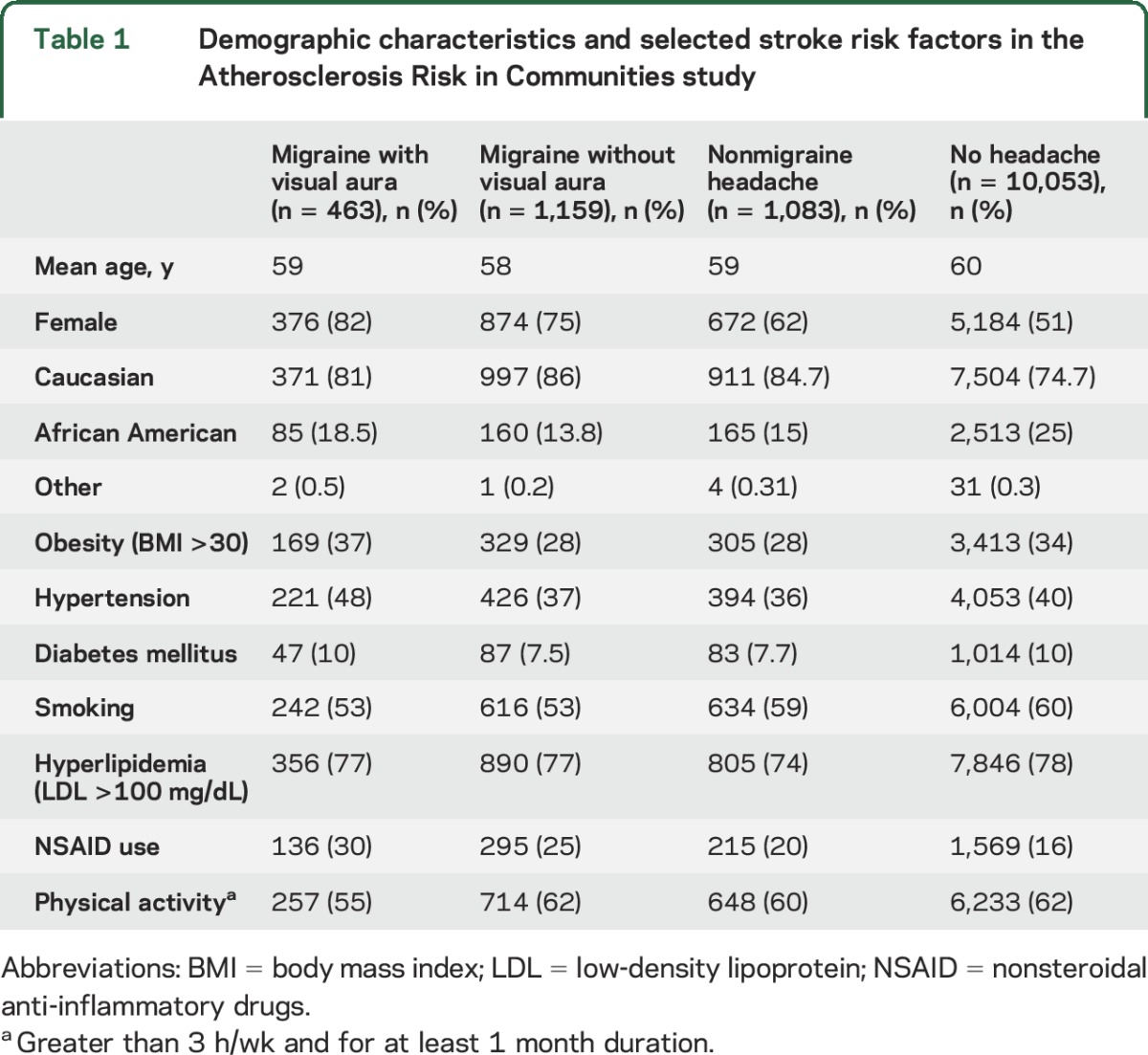
The mean age of the study participants was 59 years (range 49–73 years) at the time of start of prospective follow-up (visit 3); among included participants, 77% were white and 56% were women. Among study participants at the third clinical visit, 12.6% had a history of migraine headaches, with 3.6% having migraine with visual aura and 9% with migraine without visual aura. The average duration of migraine history in this subset of population was 19 years. Demographics and stroke risk factors of the included 12,758 participants are presented in table 1. Migraine was more prevalent among women when compared to men (3:1). Stroke risk factors including hypertension, diabetes, and obesity were slightly more prevalent in migraine with visual aura when compared to the rest of the population.
Table 1.
Demographic characteristics and selected stroke risk factors in the Atherosclerosis Risk in Communities study
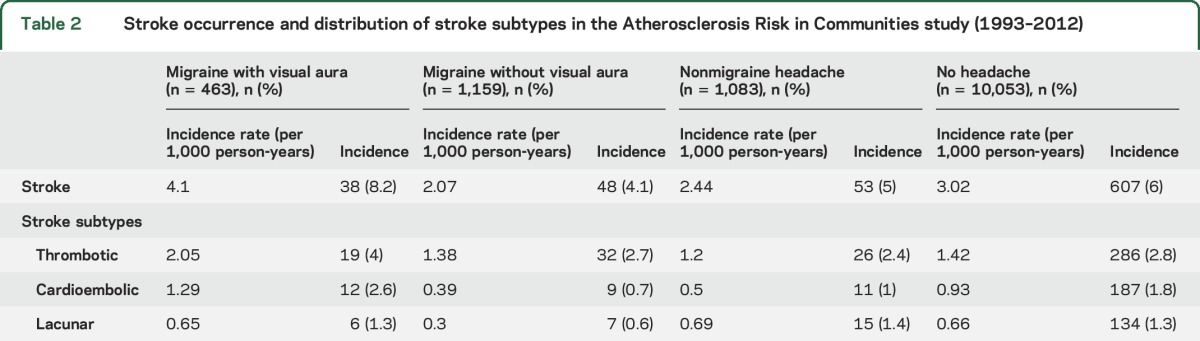
In this cohort, 6% of the total population had a first-time ischemic stroke since the third clinic visit; during the same period, 5.3% of all migraineurs (with or without visual aura) developed ischemic stroke. Furthermore, the incidence rate of stroke in migraine with visual aura was 4.1 per 1,000 persons per year, and was 2.07 per 1,000 persons per year in migraine without visual aura. Table 2 summarizes the incidence rate of ischemic stroke stratified by migraine/headache type.
Table 2.
Stroke occurrence and distribution of stroke subtypes in the Atherosclerosis Risk in Communities study (1993–2012)
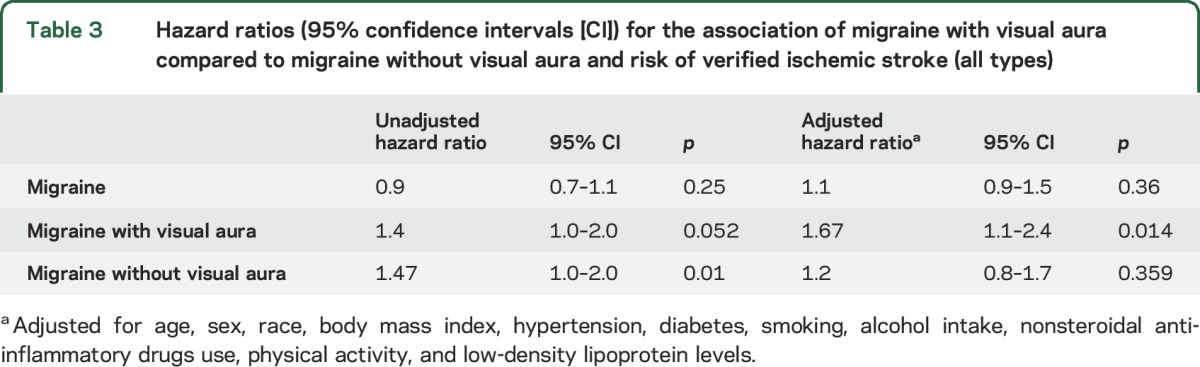
The unadjusted association between migraine with visual aura and ischemic stroke as compared to the risk of ischemic stroke among those without any headache did not reach statistical significance (hazard ratio [HR] 1.4, confidence interval [CI] 1.0–2.0, p = 0.052). After adjustment for covariates, the association between migraine with visual aura and ischemic stroke was significant (HR 1.67, CI 1.1–2.4, p = 0.014). We observed an association between migraine without visual aura and ischemic stroke when compared to the general population in ARIC without any history of headache (HR 1.47, CI 1.0–2.0, p = 0.01); however, after adjusting for age, sex, race/center, hypertension, diabetes, current smoking status, regular use of NSAID, physical activity, serum LDL, and BMI, the association was not statistically significant (HR 1.2, CI 0.87–1.75, p = 0.28). These results are summarized in table 3.
Table 3.
Hazard ratios (95% confidence intervals [CI]) for the association of migraine with visual aura compared to migraine without visual aura and risk of verified ischemic stroke (all types)
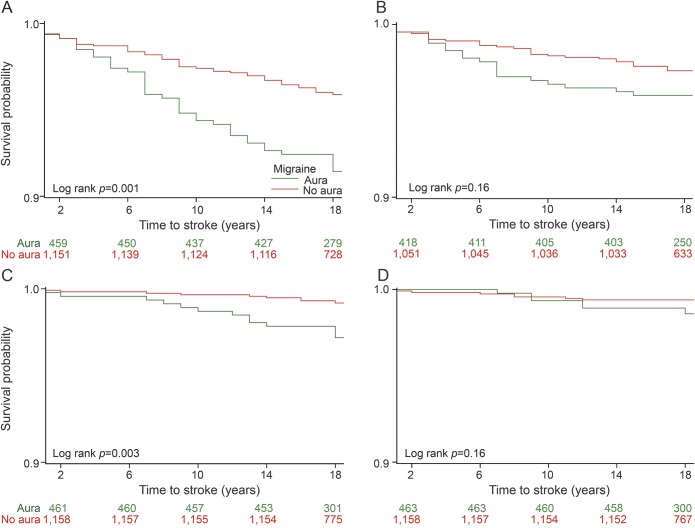
Among the 3 stroke subtypes, there was an increased hazard of cardioembolic stroke in participants with migraine with visual aura when compared to migraine without visual aura (HR 3.7, 95% CI 1.6–8.7, p = 0.003), but not of lacunar (HR 2.6, 95% CI 0.9–7.4, p = 0.07) or nonlacunar thrombotic stroke (HR 1.6, 95% CI 0.9–2.8, p = 0.09). The association of thrombotic, cardioembolic, or lacunar subtype of stroke and migraine with or without visual aura is demonstrated in Kaplan-Meier survival graphs (figure).
Figure. The association of thrombotic, cardioembolic, and lacunar subtype of stroke and migraine with/without visual aura (limited data available).
A) Kaplan-Meier survival distribution for stroke in patients with migraine with aura and migraine without aura. (B) Kaplan-Meier survival distribution for large artery atherosclerosis in patients with migraine with aura and migraine without aura. (C) Kaplan-Meier survival distribution for cardioembolic stroke in patients with migraine with aura and migraine without aura. (D) Kaplan-Meier survival distribution for lacunar stroke in patients with migraine with aura and migraine without aura.
DISCUSSION
We investigated the association between various stroke subtypes in a large prospective cohort, which has rarely been reported on historically. An earlier study in ARIC11 suggested that migraine with visual aura was associated with an increased occurrence of stroke/TIA, and since that time, an additional 546 new adjudicated ischemic stroke events were identified in the subsequent 12-year period (2001–2012). This allowed us to study temporal relationship of migraine with and without visual aura and ischemic stroke, and stroke subtypes over 2 decades (1993–2012). This study demonstrated a significant increased risk of cardioembolic stroke in older migraineurs with visual aura as compared to participants with migraine without visual aura, suggesting that clinicians might consider initiation of workup for assessment of factors predisposing to cardioembolism in migraine with visual aura patients, especially among those who have other coexisting stroke risk factors. It is possible that an increased risk of cardioembolic stroke is secondary to undiagnosed paroxysmal atrial fibrillation or patent foramen ovale (PFO) in this study population.
The link between cardioembolic stroke and migraine with visual aura suggests that visual aura symptoms may be related to distal emboli, and there may be shared pathogenesis for migraine with visual aura and cardiac emboli. To determine possible cause for stroke, we used a computerized algorithm based on the National Survey of Stroke,17 along with expert physician review. Cardioembolism was considered if the medical records revealed evidence of valvular heart disease, atrial fibrillation or flutter, cardiac or arterial procedure, acute or recent (within 4 weeks) intracardiac thrombus, or bacterial endocarditis. Data on PFO or cryptogenic stroke subtype are not available in ARIC. Data from the Women's Health Study (WHS) showed that most of the ischemic strokes in patients with migraine with aura were due to “infarct of unknown mechanism”14; however, no conclusive results regarding stroke subtypes from WHS can be obtained due to lack of statistical significance. While PFO is a common condition, found on autopsy in 25% of the population, approximately 40%–50% of individuals with cryptogenic stroke have PFO on transesophageal echocardiography.19 Increased prevalence of PFO in patients with migraine with aura has been well-documented,20–22 and PFO has been shown to be as a source of microembolism.23 However, PFO cannot be considered as a definite cause for cardioembolic stroke. Taken together, it is possible that migraine with visual aura may promote vasospasm, activate platelet aggregation,24–26 and increased concentration of procoagulant factors.27–30 Directly or indirectly in the setting of PFO, these vasoactive substances could gain access to coronary artery as well as cerebral vasculature, where they induce visual aura symptoms, and subsequently may result in brain ischemic injury if larger emboli are formed.
Our data suggest that migraine without visual aura is not an isolated risk factor for stroke, in our sample, when compared to healthy controls without any headache history; any association identified can be explained by the traditional stroke risk factors (it is also possible that the number of participants with migraine without visual aura is too low, and the study is underpowered to detect an association with stroke). Fewer studies have reported migraine without aura as an isolated risk factor for stroke,10,11 and the majority of epidemiologic evidence supports no link between ischemic stroke and migraine without aura.12–15
Our findings are consistent with several studies that reported a link between migraine with aura and increased risk of ischemic stroke. In our study population, the mean age of participants was 59 years, and the age range varied from 49 to 73 at the time of start of prospective follow-up; an average duration of migraine history in this subset of population was 19 years. This suggests that on average, the onset of migraine in this study population started between ages 30 and 54. The results of our study are limited to this specific age group. It is possible that in younger individuals (35–45), regardless of aura symptoms, migraine is associated with ischemic stroke, as previously reported.
Interestingly, calcitonin gene-related peptide (CGRP), one of the most important neuropeptides involved in an acute migraine attack, is also found to have a potent effect on coronary vasodilation.31 Although speculative, it is possible that fluctuation of CGRP level throughout a migraine attack may trigger coronary blood flow/velocity changes, and therefore increase risk of atrial fibrillation in patients with recurrent migraine. While it may be true in general that migraineurs, regardless of their status of visual aura, have elevated CGRP levels, we do not know if there is differential expression of CGRP levels in older migraineurs with or without visual aura. Furthermore, it is possible that there are other important neuropeptides/proinflammatory cytokines involved, which may only affect those with visual aura and not those without visual aura. As individuals younger than 45 were excluded from the study population, these findings cannot be generalized to younger populations.
There are several limitations of our study. First, the stroke subtype algorithm requires the presence of a possible cardioembolic source for classification of cardioembolic stroke; however, this may not necessarily mean cardioembolism is the etiology of the ischemic stroke. In addition, artery-to-artery embolic stroke (e.g., dislodged carotid plaque) is classified in ARIC as atherothrombotic. Lacunar stroke in ARIC is based on imaging features, regardless of the presence or absence of a lacunar stroke syndrome, and therefore may miss lacunar strokes with negative scans. In addition, some lacunar strokes may be cardioembolic in etiology. Even though current classification does not allow for clear distinction between these subtypes, we do not expect misclassification to differ on the basis of migraine with visual aura history. Second, the migraine classification used in ARIC is based on questionnaires, administered in late midlife, and subject to recall bias. Migraine with aura symptoms only included visual aura and may have inadvertently excluded migraine with sensory, motor (hemiplegic migraine), or brainstem aura due to the 1995 design of the headache questionnaire. We do not expect this to significantly alter our findings as between 82% and 90% of patients who have migraine with aura experience visual aura.32 In addition, the modified ICHD III-beta criteria we used were more stringent and it is possible that we may have classified a few migraineurs who presented with bilateral headache or lasted less than 1 year as nonmigraine headache group. However, we do not expect this to be biased in respect to association with stroke, and thus it would not necessarily change results. Finally, unlike stroke diagnosis, headache diagnosis in the ARIC cohort has not been adjudicated by a headache specialist.
The strength of this study includes its prospective design, longer follow-up period, large population cohort, and availability of covariates that may confound the study results. Our ascertainment of migraine and ischemic stroke is closely aligned with the current diagnostic criteria.
Despite the above limitations, this study demonstrates that there is significant increased risk of cardioembolic stroke, as defined in this study, in this sample of individuals with migraine with visual aura. In addition, we found that increased risk of ischemic stroke is not associated with migraine without visual aura, although this could be due to limited power to detect an association. Further prospective clinical studies focusing on cardioembolic and cryptogenic stroke in migraine with visual aura are needed to better understand the pathophysiology linking these 2 entities.
Supplementary Material
GLOSSARY
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CGRP
calcitonin gene-related peptide
- CI
confidence interval
- HR
hazard ratio
- ICHD
International Classification of Headache Disorders
- LDL
low-density lipoprotein
- NSAID
nonsteroidal anti-inflammatory drugs
- PFO
patent foramen ovale
- WHS
Women's Health Study
Footnotes
Editorial, page 2504
AUTHOR CONTRIBUTIONS
Dr. Androulakis: study concept and design, data analysis, interpretation, manuscript preparation, critical revision of the manuscript for important intellectual content. N. Kodumuri: data analysis and interpretation. L.D. Giamberardino: critical revision of the manuscript for important intellectual content. Dr. Rosamond: study supervision, data analysis, interpretation, critical revision of the manuscript for important intellectual content. Dr. Gottesman: study concept and design, data analysis, interpretation, critical revision of the manuscript for important intellectual content. E. Yim: data analysis and interpretation. Dr. Sen: study concept and design, data analysis, interpretation, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
X. Androulakis received research support from Tian Medical. N. Kodumuri, L. Giamberardino, and W. Rosamond report no disclosures relevant to the manuscript. R. Gottesman is Associate Editor for Neurology®. E. Yim reports no disclosures relevant to the manuscript. S. Sen is funded by NIH grants NS062754 and MD009738. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Spector JT, Kahn SR, Jones MR, Jayakumar M, Dalal D, Nazarian S. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med 2010;123:612–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology 2005;64:1020–1026. [DOI] [PubMed] [Google Scholar]
- 3.Kurth T. The association of migraine with ischemic stroke. Curr Neurol Neurosci Rep 2010;10:133–139. [DOI] [PubMed] [Google Scholar]
- 4.MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke 2007;38:2438–2445. [DOI] [PubMed] [Google Scholar]
- 5.Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ 2005;330:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donaghy M, Chang CL, Poulter N; European Collaborators of the World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatry 2002;73:747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mawet J, Kurth T, Ayata C. Migraine and stroke: in search of shared mechanisms. Cephalalgia 2015;35:165–181. [DOI] [PubMed] [Google Scholar]
- 8.Tietjen GE. Stroke and migraine linked by silent lesions. Lancet Neurol 2004;3:267. [DOI] [PubMed] [Google Scholar]
- 9.Kruit MC, van Buchem MA, Hofman PA, et al. Migraine as a risk factor for subclinical brain lesions. JAMA 2004;291:427–434. [DOI] [PubMed] [Google Scholar]
- 10.Tzourio C, Tehindrazanarivelo A, Iglésias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ 1995;310:830–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stang PE, Carson AP, Rose KM, et al. Headache, cerebrovascular symptoms, and stroke: the Atherosclerosis Risk in Communities study. Neurology 2005;64:1573–1577. [DOI] [PubMed] [Google Scholar]
- 12.Carolei A, Marini C, De Matteis G. History of migraine and risk of cerebral ischaemia in young adults: the Italian National Research Council Study Group on Stroke in the Young. Lancet 1996;347:1503–1506. [DOI] [PubMed] [Google Scholar]
- 13.Tzourio C, Iglesias S, Hubert JB, et al. Migraine and risk of ischaemic stroke: a case-control study. BMJ 1993;307:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rist PM, Buring JE, Kase CS, Schürks M, Kurth T. Migraine and functional outcome from ischemic cerebral events in women. Circulation 2010;122:2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ARIC Investigators. The Atherosclerosis Risk in Community (ARIC) study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 16.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, beta version. Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 17.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 18.Autenrieth CS, Evenson KR, Yatsuya H, Shahar E, Baggett C, Rosamond WD. Association between physical activity and risk of stroke subtypes: the Atherosclerosis Risk in Communities study. Neuroepidemiology 2013;40:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wessler BS, Thaler DE, Ruthazer R, et al. Transesophageal echocardiography in cryptogenic stroke and patent foramen ovale: analysis of putative high-risk features from the risk of paradoxical embolism database. Circ Cardiovasc Imaging 2014;7:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sztajzel R, Genoud D, Roth S, Mermillod B, Le Floch-Rohr J. Patent foramen ovale, a possible cause of symptomatic migraine: a study of 74 patients with acute ischemic stroke. Cerebrovasc Dis 2002;13:102–106. [DOI] [PubMed] [Google Scholar]
- 21.Diener HC, Kurth T, Dodick D. Patent foramen ovale and migraine. Curr Pain Headache Rep 2007;11:236–240. [DOI] [PubMed] [Google Scholar]
- 22.Schwedt TJ, Demaerschalk BM, Dodick DW. Patent foramen ovale and migraine: a quantitative systematic review. Cephalalgia 2008;28:531–540. [DOI] [PubMed] [Google Scholar]
- 23.De Ceuster L, van Diepen T, Koehler PJ. Migraine with aura triggered by cardiac myxoma: case report and literature review. Cephalalgia 2010;30:1396–1399. [DOI] [PubMed] [Google Scholar]
- 24.Elliott D. Migraine and stroke: current perspectives. Neurol Res 2008;30:801–812. [DOI] [PubMed] [Google Scholar]
- 25.Tietjen GE. Migraine as a systemic disorder. Neurology 2007;68:1555–1556. [DOI] [PubMed] [Google Scholar]
- 26.D'Andrea G, Hasselmark L, Alecci M, Cananzi A, Perini F, Welch KM. Platelet secretion from dense and alpha-granules in vitro in migraine with or without aura. J Neurol Neurosurg Psychiatry 1994;57:557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeller JA, Frahm K, Baron R, Stingele R, Deuschl G. Platelet–leukocyte interaction and platelet activation in migraine: a link to ischemic stroke? J Neurol Neurosurg Psychiatry 2004;75:984–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallai V, Sarchielli P, Firenze C, et al. Endothelin 1 in migraine and tension-type headache. Acta Neurol Scand 1994;89:47–55. [DOI] [PubMed] [Google Scholar]
- 29.Tietjen GE, Al-Qasmi MM, Athanas K, Dafer RM, Khuder SA. Increased von Willebrand factor in migraine. Neurology 2001;57:334–336. [DOI] [PubMed] [Google Scholar]
- 30.Hering-Hanit R, Friedman Z, Schlesinger I, Ellis M. Evidence for activation of the coagulation system in migraine with aura. Cephalalgia 2001;21:137–139. [DOI] [PubMed] [Google Scholar]
- 31.Doggrell SA. Migraine and beyond: cardiovascular therapeutic potential for CGRP modulators. Expert Opin Investig Drugs 2001;10:1131–1138. [DOI] [PubMed] [Google Scholar]
- 32.Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain 1996;119:355–361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.