Abstract
Background
This study was aimed to reveal the role of miR-149-5p in acute myeloid leukemia (AML) cells apoptosis and the possible mechanism involved.
Material/Methods
The expression of miR-149-5p in leukemia cell lines, as well as the blood and bone marrow (BM) samples from leukemia patients, were monitored by reverse-transcription polymerase chain reaction (RT-PCR). AML cell line THP-1 was transfected with miR-149-5p mimic or inhibitor, and then cell apoptosis was determined using the APO Percentage assay kit. The target of miR-149-5p was predicted by using the microRNA.org database, and verified by RT-PCR, Western blot, and Dual-Luciferase reporter assays. Further, small interfering RNA (siRNA) against the target gene was co-transfected with miR-149-5p inhibitor, and then the cell apoptosis and the expression of apoptosis-related proteins were assessed.
Results
MiR-149-5p was significantly up-regulated in leukemia cell lines and samples from leukemia patients (P<0.01 or P<0.001), especially in THP-1 cells and samples from AML patients. Cell apoptosis was significantly decreased by miR-149-5p overexpression (P<0.01) and increased by miR-149-5p suppression (P<0.05). Fas Ligand (FASLG) was a direct target of miR-149-5p, and was negatively regulated by miR-149-5p. More importantly, the inductive effects of miR-149-5p suppression on cell apoptosis were abrogated by si-FASLG (P<0.01). Furthermore, the up-regulative effects of miR-149-5p suppression on the phosphorylated form of Fas-associated via death domain (p-FADD), caspase-8, caspase-2, caspase-3, and the cleaved forms of these caspases were abrogated by si-FASLG.
Conclusions
Inhibition of miR-149-5p can induce apoptosis in THP-1 cells. These inductive effects might be via targeting FASLG and activating FADD and caspases.
MeSH Keywords: Apoptosis; Fas Ligand Protein; Leukemia, Myeloid, Acute; MicroRNAs
Background
Leukemia is a disease of the blood or bone marrow (BM), and results in high numbers of abnormal white blood cells [1]. There are 4 main types: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), and chronic myeloid leukemia (CML) [2]. Among them, AML is the most common malignant myeloid disorder, with approximately 60% overall relapse-free survival rate [3,4]. Treatment of AML always involves some combination of chemotherapy, radiation therapy, targeted therapy, and BM transplant [5–7]. Outcomes have been greatly improved in the developed world, but remain grim [8]. Therefore, a better understanding of AML will be helpful in diagnosing and treating this disease.
MicroRNAs (miRNAs), are small, non-coding RNAs that regulate the expression of target genes by interaction with the 3′-untranslated region (3′-UTR) of their mRNAs, resulting in translation inhibition or mRNA degradation [9–11]. Multiple studies have demonstrated the versatile roles that miRNAs play in the apoptosis of cancer cells [12,13], including AML cells. For example, miR-155 has been reported as an apoptosis inducer in AML cells through activating caspase-3 [14]. Sun et al. reported that overexpression of miR-424 and miR-27a increased apoptosis of AML cells by targeting pleomorphic adenoma gene 1 (PLAG1) [9]. However, the effects of miR-149-5p on AML cells apoptosis have not been investigated.
The main objective of this study was to investigate the effects of miR-149-5p on AML cells apoptosis and its associated mechanism. The expression of miR-149-5p in leukemia cell lines and the blood and BM samples from leukemia patients were monitored. AML cell line THP-1 was used to assess the effects of miR-149-5p on the apoptosis in vitro. Furthermore, we predicted and verified the direct target of miR-149-5p in THP-1 cells, and the apoptosis-related factors were determined, revealing the underlying molecular mechanisms of miR-149-5p in AML. These findings help provide a basic understanding of miR-149-5p in AML.
Material and Methods
Patient samples and ethical statement
A total of 45 adults with newly-diagnosed AML, T cell ALL (T-ALL), and CML, and 20 healthy adults were enrolled in the current study, from January, 2012 to April, 2015. The details of these individuals are provided in Table 1. AML, T-ALL, and CML patients were diagnosed according to the provisions of the World Health Organization (WHO). The fresh blood and BM fluid samples from each individual were anticoagulated with sodium citrate, and cells from each sample were isolated by centrifugation and frozen at −80°C for further analysis. This study was approved by our local ethics committee, and written informed consent was obtained from all subjects for the use of their blood and BM samples for research.
Table 1.
Characteristics of the individuals in this study.
| Characteristics | Normal | AML | T-ALL | CML |
|---|---|---|---|---|
| Age: median (range) | 52.6 (20–72) | 32.9 (19–59) | 46.0 (23–79) | 47.2 (25–66) |
| Gender (male/female) | 12/8 | 13/7 | 9/7 | 6/3 |
| Total number | 20 | 20 | 16 | 9 |
AML – acute myeloid leukemia; T-ALL – T cell acute lymphoblastic leukemia; CML – chronic myeloid leukemia.
Cell culture
T-ALL cell line Molt-4, CML cell line K562, and AML cell line THP-1 were purchased from the American Type Culture Collection (ATCC; Manassas, VA). Normal control cells were peripheral blood mononuclear cells derived from healthy donors. These cell lines were maintained in RPMI-1640 culture medium (Hyclone, Logan, UT) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT) [15]. All cells were cultured in humidified atmosphere with 5% CO2 at 37°C [9].
Isolation of RNA and real-time reverse transcription polymerase chain reaction (RT-PCR)
For RNA isolation and cDNA synthesis, TRIzol reagent-phenol chloroform (Invitrogen Life Technologies, Carlsbad, CA) and Transcriptor First Strand cDNA Synthesis Kit (Roche, USA) were used, according to the manufacturer’s instructions. For the RT-PCR analysis, FastSTART Universal SYBR Green Master (ROX) (Roche, USA) was used, according to the manufacturer’s instructions. Each real-time PCR was carried out in triplicate for a total of 20-μL reaction mixtures on the ABI PRISM 7500 Real-time PCR System (Applied Biosystems, Foster City, CA). Data were analyzed according to the classic 2−ΔΔCt method, and were normalized to GAPDH or U6 snRNA expression in each sample [16]. All primers were synthesized by GenePharma (Shanghai, China).
Cell transfection
For miRNA and small interfering RNA (siRNA) transfection, THP-1 cells were plated on a 60-mm dish and incubated for 24 h. Afterward, miR-149-5p mimic, inhibitor, or its negative control, and Fas Ligand (FASLG) siRNA or its negative control (GenePharma, shanghai, China) were transfected into cells, respectively. The transfection was performed by using Lipofectamine 2000 (Invitrogen, USA), according to the manual. At 48 h after transfection, the cells were collected for further investigation.
Apoptosis assay
Cell apoptosis was assessed using the APO Percentage assay kit (Biocolor Ltd., UK), according to the manufacturer’s instructions. In brief, cells were planted on 24-well plates at a density of 1×105 cells/well. After 24 h of incubation, the cells were collected and stained with the APO Percentage™ dye and analyzed by flow cytometry on a Becton Dickinson FACScan instrument (BD Pharmingen™, USA) [17].
Dual-Luciferase reporter assay
The 3′-UTR of FASLG was amplified by PCR and placed in the pmiR-Report vector (Ambion). These vectors were co-transfected with miR-149-5p mimic, inhibitor, or its negative control into cells using Lipofectamine 2000 (Invitrogen). At 48 h after transfection, firefly and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay (Promega) [18].
Western blotting
Cellular protein was extracted by lysis buffer (Beyotime, Shanghai, China) and equal amounts of protein samples were separated on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and blotted onto polyvinylidene difluoride membranes. After blocking within 5% skim milk for 1 h at room temperature, the membranes were incubated with primary antibodies: FASLG, Fas-associated via death domain (FADD), p-FADD, caspase-8, cleaved caspase-8, caspase-2, cleaved caspase-2, caspase-3, cleaved caspase-3 and GAPDH (1:1000; Santa Cruz, CA) at 4°C overnight. Then the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:1000; Santa Cruz, CA) for 1 h at room temperature. The immunoreactive protein bands were developed by enhanced chemiluminescence reagent (GE Healthcare, Little Chalfont, UK).
Statistical analysis
Multiple data are presented as mean ± standard derivations (SD) from 3 independent experiments and analyses. Statistical analyses were performed using GraphPad Prism 5 software (GraphPad, San Diego, CA), and statistical significance between different groups was analyzed by t tests (and nonparametric tests). Statistical significance was defined as P<0.05.
Results
MiR-149-5p was up-regulated during leukemia
To explore the role of miR-149-5p in leukemia, RT-PCR was performed to measure the expression of miR-149-5p in leukemia cell lines (THP-1, Molt-4, and K562), and the blood and BM samples from leukemia patients (AML, T-ALL, and CML). Results in Figure 1A–1C showed that, in leukemia cell lines and the samples from leukemia patients, the expression of miR-149-5p at mRNA level was significantly higher than in the normal group (P<0.01 or P<0.001). These results revealed that miR-149-5p might play a vital role in leukemia pathogenesis, and miR-149-5p might act as a biomarker during leukemia. Of note, THP-1 cells and the samples from AML seemed to possess the highest miR-149-5p expression; therefore, AML cell line THP-1 was selected for the in vitro investigation.
Figure 1.
Relative miR-149-5p expression at mRNA level in leukemia cell lines and the BM and blood samples from leukemia patients. The miR-149-5p expression at mRNA level in 3 leukemia cell lines, THP-1, Molt-4, and K562 was measured by RT-PCR analysis (A). The mRNA level of miR-149-5p expression was detected in the BM samples (B) and fresh blood (C) from 3 types of leukemia: AML, T-ALL, and CML. BM – bone marrow; RT-PCR – real-time reverse transcription polymerase chain reaction; AML – acute myeloid leukemia; T-ALL – T cell acute lymphoblastic leukemia; CML – chronic myeloid leukemia; ** P<0.01; *** P<0.001.
Inhibition of miR-149-5p induced cell apoptosis
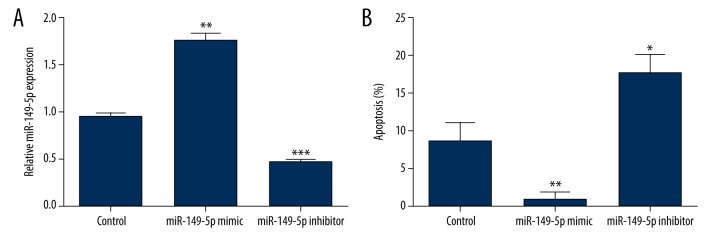
To investigate the biology functions of miR-149-5p on AML cells apoptosis, THP-1 cells were used and transfected with miR-149-5p inhibitor, mimic, or control. The transfection efficiency was verified by using RT-PCR analysis, and the detection of apoptotic cells were performed using an APO Percentage assay kit. As expected (Figure 2A), the expression of miR-149-5p was up-regulated by miR-149-5p overexpression (P<0.01), and was down-regulated by miR-149-5p inhibition (P<0.001). Results in Figure 2B showed that promotion of miR-149-5p could significantly decrease cell apoptosis (P<0.01), whereas inhibition of miR-149-5p could significantly induce cell apoptosis (P<0.05). These results demonstrated that inhibition of miR-149-5p could induce THP-1 cells apoptosis.
Figure 2.
Inhibition of miR-149-5p induced THP-1 cells apoptosis. MiR-149-5p mimic or inhibitor was transfected into THP-1 cells, and the efficiency of transfection was detected by RT-PCR analysis (A). Afterward, cell apoptosis was measured by using APO Percentage assay kit (B). RT-PCR – real-time reverse transcription polymerase chain reaction; * P<0.05; ** P<0.01; *** P<0.001.
FASLG was a direct target of miR-149-5p
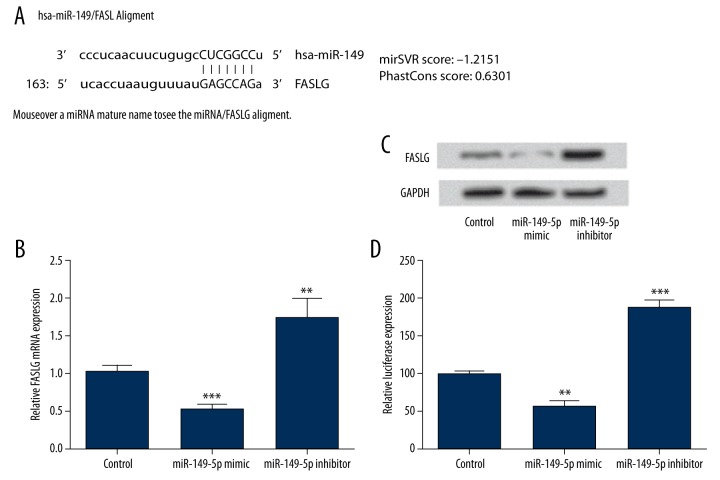
To predict the target gene of miR-149-5p in THP-1 cells, microRNA.org (www.microrna.org) database was used. As the analysis results in Figure 3A show, FASLG might be a target gene of miR-149-5p. To confirm this prediction, the mRNA and protein level expression of FASLG were measured by RT-PCR (Figure 3B) and Western blot after dysregulation the expression of miR-149-5p (Figure 3C). We found that the expression of FASLG at mRNA and protein level was significantly decreased by miR-149-5p overexpression (P<0.001), but was significantly increased by miR-149-5p suppression (P < 0.01). In addition, to determine whether miR-149-5p directly targeted the 3′-UTR of FASLG, the Dual-Luciferase reporter assay was performed (Figure 3D). In THP-1 cells, the FASLG 3′-UTR luciferase expression was significantly decreased by miR-149-5p overexpression (P<0.01), whereas was significantly increased by miR-149-5p suppression (P<0.001). Taken together, these data support that FASLG is a direct and specific target of miR-149-5p and is negatively regulated by miR-149-5p.
Figure 3.
FASLG was predicted and verified as a direct target of miR-149-5p. The microRNA.org database was used to predict the target gene of miR-149-5p (A). The mRNA and protein level of FASLG expression in miR-149-5p overexpressed cells or miR-149-5p suppressed cells were detected by RT-PCR (B) and Western blot (C). Dual-Luciferase reporter assay was performed to confirm miR-149-5p targeted the 3′-UTR of FASLG (D). FASLG – Fas ligand; RT-PCR – real-time reverse transcription polymerase chain reaction; 3′-UTR – 3′-untranslated region; ** P<0.01; *** P<0.001.
Inhibition of miR-149-5p induced cell apoptosis via targeting FASLG
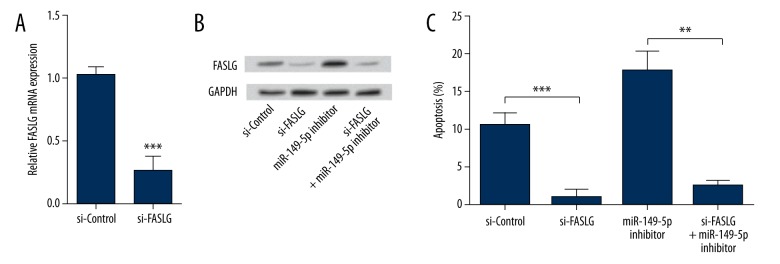
To investigate the relationship between miR-149-5p and FASLG in THP-1 cells apoptosis, cells were transfected with si-FASLG and/or miR-149-5p inhibitor, and then evaluated for changes in apoptosis rate. As we expected, the mRNA level of FASLG was significantly down-regulated by si-FASLG (P<0.001) (Figure 4A). Further, the protein expression of FASLG was notable down-regulated by si-FASLG, and the up-regulative impact of miR-149-4p suppression on the protein expression of FASLG was abrogated by si-FASLG (Figure 4B). In cell apoptosis assays (Figure 4C), cells transfected with si-FASLG showed significantly decreased apoptotic rate (P<0.001) compared with its control group, suggesting that FASLG acted as an apoptotic inductor in THP-1 cells. More important, si-FASLG abrogated the inductive effects of miR-149-5p suppression on cell apoptosis (P<0.01). Thus, we speculated that inhibition of miR-149-5p induced cell apoptosis via targeting FASLG.
Figure 4.
Inhibition of miR-149-5p induced cell apoptosis via targeting FASLG. si-FASLG or its negative control was transfected into THP-1 cells, and then the mRNA level of FASLG expression was measured by RT-PCR (A). si-FASLG or/and miR-149-5p inhibitor were transfected into THP-1 cells, and the expression of FASLG protein was detected by Western blot (B). si-FASLG or/and miR-149-5p inhibitor were transfected into THP-1 cells, and the cell apoptosis was determined using the APO Percentage assay kit (C). FASLG – Fas ligand; RT-PCR – real-time reverse transcription polymerase chain reaction; ** P<0.01; *** P<0.001.
MiR-149-5p targeted FASLG and activated FADD and caspases
To further explore the possible mechanisms of miR-149-5p in AML cells apoptosis, we detected the expression of FADD, p-FADD, and 6 caspases by Western blot (Figure 5). We found that the expression of FADD was little changed by si-FASLG or miR-149-5p suppression. However, protein expressions of p-FADD, caspase-8, cleaved caspase-8, caspase-2, cleaved caspase-2, caspase-3, and cleaved caspase-3 were down-regulated by si-FASLG, but were remarkably up-regulated by miR-149-5p suppression. Of note, si-FASLG abrogated the up-regulative impacts of miR-149-5p on the expression of these 7 proteins. Thus, we inferred that inhibition of miR-149-5p induced cell apoptosis via targeting FASLG, and was accompanied by activation of these factors.
Figure 5.

MiR-149-5p targeted FASLG and activated FADD and caspases. si-FASLG or/and miR-149-5p inhibitor were transfected into THP-1 cells, and then the protein expressions of p-FADD, FADD, caspase-8, cleaved caspase-8, caspase-2, cleaved caspase-2, caspase-3, and cleaved caspase-3 were determined by Western blot. FASLG – Fas ligand; FADD – Fas-associated via death domain.
Discussion
In the current study we aimed to investigate the role of miR-149-5p in AML cells apoptosis, and explored its possible mechanisms. We found that miR-149-5p level was up-regulated in leukemia cell lines and the samples from leukemia patients, especially in THP-1 cells and AML samples. FASLG was a direct target gene of miR-149-5p, and was negatively regulated by miR-149-5p. More importantly, miR-149-5p suppression induced cell apoptosis via targeting FASLG, and was accompanied by activation of FADD and caspases.
Recent studies have demonstrated the versatile roles of miRNAs in cancer. Dysregulation of miRNAs has been proposed to be a rising feature in cancer [14]. The role of miR-149-5p in cancer is complex because miR-149-5p has been identified as both oncogene and anti-oncogene, depending on the category of tumor. To date, miR-149-5p has been found to be down-regulated in tongue squamous cell carcinoma [19], renal clear cell carcinoma [20], and prostate carcinoma [21]. In addition, Li et al. found that miR-149-5p was down-regulated in grade I-IV of astrocytomas, and in vitro studies revealed miR-149-5p acts as an anti-oncogene by controlling proliferation and migration [22]. However, Fan et al. demonstrated that miR-149-5p was highly expressed in T-ALL, and that miR-149-5p functioned as an oncogene via regulating proliferation, cell cycle, and apoptosis [23]. Our findings in this study were partly consistent with the study of Fan et al., which reported miR-149-5p was up-regulated in leukemia, especially in AML. Moreover, miR-149-5p acted as an oncogene via regulating THP-1 cells apoptosis.
To investigate the underlying mechanism by which miR-149-5p affects THP-1 cells apoptosis, the target of miR-149-5p was predicted and verified in vitro. We found that FASLG was a direct target of miR-149-5p. To date, several miRNAs, such as miR-21 [24], miR-23a [25], and miR-25 [26], have shown the ability to target FASLG in different cancers. However, our study is the first to find that FASLG is a direct target of miR-149-5p and that FASLG could be negatively regulated by miR-149-5p. FASLG is a type-II membrane protein that naturally binds and activates Fas-receptors, resulting in cellular apoptosis [27]. Studies have shown that FASLG also induces apoptosis in leukemia cells [28], and FASLG has been proposed as a potential therapeutic target for the treatment of leukemia [29,30]. In the current study, we found that the inductive impacts of miR-149-5p suppression on cell apoptosis were abrogated by knockdown of FASLG. Taken together, we speculated that the inductive effects of miR-149-5p suppression on THP-1 cells apoptosis might be via targeting FASLG.
To further explore the deeply associated mechanism of miR-149-5p affecting cell apoptosis via targeting FASLG, the expression changes of apoptosis related factors were measured. We found that knockdown of FASLG abrogated the up-regulative effects of miR-149-5p on p-FADD, caspase-8, caspase-2, caspase-3, and the cleaved forms of these caspases. Usually, the combination of FASLG with Fas death receptor, induces the polymerization of Fas to form death-inducing signaling, and is complicated by recruiting the death domain adapter protein FADD, caspase-8, and caspase-2 [31,32]. Caspase-8 and caspase-2 are activated through autoproteolytic cleavage and subsequently cleave caspase-3, and then result in cytochrome C release from the mitochondria, ultimately triggering apoptosis [33,34]. Thus, we inferred that miR-149-5p suppression induced THP-1 cells apoptosis via targeting FASLG, subsequently regulating the expression of p-FADD and caspases.
Conclusions
Our results suggest that inhibition of miR-149-5p might be a potential therapeutic strategy for the treatment of AML by induction of apoptosis. Moreover, miR-149-5p suppression induced the apoptosis by negatively regulating its target gene, FASLG, as well as activating and regulating FADD and caspases. Nonetheless, more studies are needed to confirm the role of miR-149-5p in AML.
Footnotes
Source of support: Departmental sources
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gocek E, Ewa M. Differentiation therapy of acute myeloid leukemia. Cancers. 2011;3:2402–20. doi: 10.3390/cancers3022402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffbrand A, Moss P, Pettit J. Essential Haematolog. 5th ed. Oxford, United Kingdom, UK: Blackwell Publishing; 2006. [Google Scholar]
- 3.O’Donnell MR, Tallman MS, Abboud CN, et al. Acute myeloid leukemia, version 2.2013. J Natl Compr Canc Netw. 2013;11:1047–55. doi: 10.6004/jnccn.2013.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malinowska I, Stelmaszczyk-Emmel A, Wasik M, Rokicka-Milewska R. Apoptosis and pH of blasts in acute childhood leukemia. Med Sci Monit. 2002;8(6):CR441–47. [PubMed] [Google Scholar]
- 5.Walter RB, Othus M, Burnett AK, et al. Resistance prediction in AML: Analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia. 2015;29:312–20. doi: 10.1038/leu.2014.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahreddine HA, Culjkovic-Kraljacic B, Assouline S, et al. The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature. 2014;511:90–93. doi: 10.1038/nature13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shafer D, Grant S. Update on rational targeted therapy in AML. Blood Rev. 2016 doi: 10.1016/j.blre.2016.02.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saultz JN, Garzon R. Acute myeloid leukemia: A concise review. J Clin Med. 2016;5 doi: 10.3390/jcm5030033. pii: E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun YP, Lu F, Han XY, et al. MiR-424 and miR-27a increase TRAIL sensitivity of acute myeloid leukemia by targeting PLAG1. Oncotarget. 2016 doi: 10.18632/oncotarget.8252. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seca H, Almeida GM, Guimaraes JE, Vasconcelos MH. miR signatures and the role of miRs in acute myeloid leukaemia. Eur J Cancer. 2010;46:1520–27. doi: 10.1016/j.ejca.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Ma X, Yao Y, et al. miR-155 regulates the proliferation and invasion of clear cell renal cell carcinoma cells by targeting E2F2. Oncotarget. 2016 doi: 10.18632/oncotarget.7951. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Z, Tang J, Wang J, et al. MiR-138 acts as a tumor suppressor by targeting EZH2 and enhances cisplatin-induced apoptosis in osteosarcoma cells. PLoS One. 2016;11:e0150026. doi: 10.1371/journal.pone.0150026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan LT, Sheung Y, Guo WP, et al. Hedyotis diffusa plus Scutellaria barbata induce bladder cancer cell apoptosis by inhibiting akt signaling pathway through downregulating miR-155 expression. Evid Based Complement Alternat Med. 2016;2016:9174903. doi: 10.1155/2016/9174903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palma CA, Al Sheikha D, Lim TK, et al. MicroRNA-155 as an inducer of apoptosis and cell differentiation in Acute Myeloid Leukaemia. Mol Cancer. 2014;5:79. doi: 10.1186/1476-4598-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Zhou M, Zhang Q, et al. Anticancer effect and apoptosis induction of gambogic acid in human leukemia cell line K562 in vitro. Med Sci Monit. 2015;21:1604–10. doi: 10.12659/MSM.893004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Omoyeni OA, Hussein A, Meyer M, et al. Pleiocarpa pycnantha leaves and its triterpenes induce apoptotic cell death in Caco-2 cells in vitro. BMC Complement Altern Med. 2015;15:224. doi: 10.1186/s12906-015-0767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi W, Bruce J, Lee M, et al. MiR-449a promotes breast cancer progression by targeting CRIP2. Oncotarget. 2016;7:18906–18. doi: 10.18632/oncotarget.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong TS, Liu XB, Wong BY, et al. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin Cancer Res. 2008;14:2588–92. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Brannon AR, Reddy AR, et al. Identifying mRNA targets of microRNA dysregulated in cancer: With application to clear cell Renal Cell Carcinoma. BMC Syst Biol. 2010;4:51. doi: 10.1186/1752-0509-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaefer A, Jung M, Mollenkopf HJ, et al. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer. 2010;126:1166–76. doi: 10.1002/ijc.24827. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Chen P, Li XY, et al. Grade-specific expression profiles of miRNAs/mRNAs and docking study in human grade I–III astrocytomas. OMICS. 2011;15:673–82. doi: 10.1089/omi.2011.0064. [DOI] [PubMed] [Google Scholar]
- 23.Fan SJ, Li HB, Cui G, et al. miRNA-149* promotes cell proliferation and suppresses apoptosis by mediating JunB in T-cell acute lymphoblastic leukemia. Leuk Res. 2016;41:62–70. doi: 10.1016/j.leukres.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Hatley ME, Patrick DM, Garcia MR, et al. Modulation of K-ras-dependent lung tumorigenesis by microRNA-21. Cancer Cell. 2010;18:282–93. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng H, Li W, Wang Y, et al. miR-23a inhibits E-cadherin expression and is regulated by AP-1 and NFAT4 complex during Fas-induced EMT in gastrointestinal cancer. Carcinogenesis. 2014;35:173–83. doi: 10.1093/carcin/bgt274. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JF, Shi LL, Zhang L, et al. MicroRNA-25 negatively regulates cerebral ischemia/reperfusion injury-induced cell apoptosis through Fas/FasL pathway. J Mol Neurosci. 2016;58(4):507–16. doi: 10.1007/s12031-016-0712-0. [DOI] [PubMed] [Google Scholar]
- 27.Aronin A, Amsili S, Prigozhina TB, et al. Highly efficient, in-vivo Fas-mediated apoptosis of B-cell lymphoma by Hexameric CTLA4-FasL. J Hematol Oncol. 2014;7:64. doi: 10.1186/s13045-014-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su JL, Lin MT, Hong CC, et al. Resveratrol induces FasL-related apoptosis through Cdc42 activation of ASK1/JNK-dependent signaling pathway in human leukemia HL-60 cells. Carcinogenesis. 2005;26:1–10. doi: 10.1093/carcin/bgh220. [DOI] [PubMed] [Google Scholar]
- 29.Xiao ZX, Yin XC, Tan YF, Peng YH. Effects of diallyl disulfide on apoptosis of human leukemia K562 cells and expression of Fas, FasL and caspase-8. Chinese Journal of Contemporary Pediatrics. 2011;13:53–56. [PubMed] [Google Scholar]
- 30.Lee W, Chen YR, Tseng TH. Quercetin induces FasL-related apoptosis, in part, through promotion of histone H3 acetylation in human leukemia HL-60 cells. Oncol Rep. 2011;25:583–91. doi: 10.3892/or.2010.1097. [DOI] [PubMed] [Google Scholar]
- 31.Lee K-H, Feig C, Tchikov V, et al. The role of receptor internalization in CD95 signaling. EMBO J. 2006;25:1009–23. doi: 10.1038/sj.emboj.7601016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le’Negrate G, Selva E, Auberger P, et al. Sustained polymorphonuclear leukocyte transmigration induces apoptosis in T84 intestinal epithelial cells. J Cell Biol. 2000;150:1479–88. doi: 10.1083/jcb.150.6.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufmann SH, Hengartner MO. Programmed cell death: Alive and well in the new millennium. Trends Cell Biol. 2001;11:526–34. doi: 10.1016/s0962-8924(01)02173-0. [DOI] [PubMed] [Google Scholar]
- 34.Li JJ, Chan WH, Leung WY, et al. MicroRNA-21 promotes proliferation of rat hepatocyte BRL-3A by targeting FASLG. Genet Mol Res. 2015;14:4150–60. doi: 10.4238/2015.April.27.30. [DOI] [PubMed] [Google Scholar]