Abstract
Background
Pediatric sepsis has high morbidity in children, may lead to acute kidney injury (AKI), and further aggravate the disease. Baicalin is a kind of flavonoid in Scutellaria baicalensis Georgi and has been reported to protect against several diseases, but its roles in septic AKI remain unclear. This study aimed to uncover the effects of baicalin in AKI during pediatric sepsis.
Material/Methods
Blood urea nitrogen (BUN) and serum creatinine (Cr) levels were detected in 50 pediatric patients, who underwent basic therapy with or without baicalin adjunctive therapy. Mouse sepsis models were constructed by cecal ligation and puncture (CLP) and treated with baicalin intragastrically, after which BUN and Cr examination, TUNEL apoptosis assay, and expression analyses of BAX and BCL2 were performed.
Results
Baicalin adjunctive therapy significantly decreased BUN and Cr levels in pediatric sepsis patients (P<0.05). CLP led to elevated BUN and Cr levels in the mouse model (P<0.01), indicating kidney injury accompanied by sepsis. Baicalin decreased BUN and Cr levels (P<0.05), and reduced the apoptotic cell percent in the renal tissue (P<0.05) of the CLP model. It inhibited BAX and promoted BCL2 in the renal tissue, which was consistent with cell apoptosis changes.
Conclusions
Baicalin is capable of suppressing renal cell apoptosis and protecting against AKI in pediatric sepsis. This study provides a potential adjunctive therapy for treating AKI in pediatric sepsis, and further research is necessary to reveal its deeper mechanisms.
MeSH Keywords: Acute Kidney Injury, Apoptosis, Pediatrics, Scutellaria baicalensis, Sepsis
Background
Sepsis is the systemic inflammation caused by infection with bacteria, viruses, fungi, parasites, or toxic products, and has become one of the leading causes of mortality in children [1]. In developed countries, the mortality rate of pediatric sepsis is about 2–10% and is as high as 50% in developing countries [2]. Pediatric sepsis usually leads to tachycardia, tachypnea, peripheral vasodilation, fever or hypothermia, and even multiple organ dysfunction syndrome (MODS) [3]. Frequently-used applications in pediatric sepsis treatment concentrate on maintaining metabolic, circulatory, and respiratory stabilities, and antimicrobial agents, as well as surgical and adjunctive therapies such as nitric oxide and corticosteroids [4,5], which show great benefits in the outcome of pediatric sepsis treatment.
Acute kidney injury (AKI) is a severe consequence of sepsis and MODS, and about 15–27% of AKI in children is generated from pediatric sepsis [6]. The pathophysiology of septic AKI is related to the altered global renal blood flow, as well as the comprehensive effects of inflammation, microvasculature blood flow, and bioenergetics factors [7]. For example, glomerular endothelial injury during AKI progression in a lipopolysaccharide-induced mouse sepsis model is closely related to tumor necrosis factor and receptor 1 [8]. Due to the increased risk of mortality caused by septic AKI, it is imperative to explore effective therapies for controlling AKI in pediatric sepsis.
Baicalin is one of the flavonoid constituents in Scutellaria baicalensis Georgi, a kind of perennial herb with protective effects against diseases like hydrogen peroxide-induced oxidative stress and cerebral ischemia injury [9–11]. Specifically, baicalin has been revealed to protect against oxidants [12], anxiety [13], acute hepatic injury [14], experimental periodontitis [15], and sepsis [16]. It also alleviates reperfusion-induced damage to the myocardium, the mechanism of which may be associated with its anti-apoptotic roles [17]. Moreover, a recent study found that baicalin can inhibit inflammation and cell apoptosis, which allows it to protect against ischemia-reperfusion injuries in the kidney [18]. However, few studies have investigated the role of baicalin in septic AKI or pediatric sepsis.
This study aimed to uncover the effects of baicalin in treating AKI in pediatric sepsis patients. The baicalin adjunctive therapy was tested in pediatric sepsis patients, after which the effect was compared based on the renal function assessment from BUN and serum creatinine (Cr). We also performed experiments in the cecal ligation and puncture (CLP)-induced mouse sepsis model to investigate the effects and potential mechanisms of baicalin in septic AKI. This study lays the foundation for future application of baicalin in adjunctive therapies for AKI in pediatric sepsis.
Material and methods
Sample collection
A total of 50 pediatric sepsis patients ages 1–4 years were selected from the patients hospitalized from November 2013 to October 2015. All 50 patients were diagnosed with pediatric sepsis based on the diagnostic standard proposed in 2005 [19], and with AKI at the same time according to the criteria of AKI proposed by Acute Kidney Injury Net (AKIN) [20]. The following conditions were excluded: patients with kidney injuries caused by prerenal or postrenal factors, and patients with a history of renal diseases such as acute or chronic renal failure. The 50 patients were randomly divided into 2 groups: control and baicalin, each group containing 25 patients. No significant difference existed in the age between the 2 groups (2.58±0.17 and 2.46±0.20). Patients in the control group received basic treatment, and those in the baicalin group received basic treatment plus oral baicalin (Inner Chengzi Pharmaceutical, Chifeng, China) according to the manufacturer’s instructions. The blood samples were collected 1 day before treatment and 15 days after treatment for BUN and Cr detection by the hospital. Informed consent was obtained from parents of patients before the treatment and sampling procedures. These procedures were approved by our local ethics committee and were performed according to the instructions of the hospital.
Animal model
CLP was performed on male C57BL/6 mice (SPF, age 8–10 weeks, weight 22–26 g) (Vital River Laboratories, Beijing, China) to induce a septic AKI model based on the method in a previous report [21]. The mice were raised in controlled laboratory conditions. Thirty individuals were randomly divided into 3 groups: sham, CLP, and CLP + baicalin. The mice were anesthetized by amobarbital sodium (0.05 g/kg). A middle laparotomy was made to expose the cecum. The cecum was ligated at 1 cm to the distal end, and double punctures were made to extrude feces into the abdominal cavity. Then the cecum was relocated to the abdominal cavity, and the abdomen was closed. The mice were resuscitated by intraperitoneal injection of 0.9% saline (24 mL/kg). The sham group underwent all the procedures except cecum ligation and puncture. Mice in the CLP + baicalin group were intragastrically administrated with baicalin 200 mg/(kg·d) (PureOne Biotechnology, Shanghai, China) for 6 days [22], after which all the mice were anesthetized and killed for blood and renal tissue sampling. BUN and Cr were detected by the hospital. The animal experiment was approved by our local ethics committee.
TUNEL assay
Cell apoptosis in the mouse renal tissue was detected by TdT-mediated dUTP nick-end labeling (TUNEL) method with the In Situ Cell Death Detection Kit, POD (Roche, Basel, Switzerland) according to the manufacturer’s instructions. Briefly, the renal tissue of each mouse was embedded in paraffin and cut into 5-μm slices. Fresh TUNEL mix (50 μL) was added to the slices for 1-h incubation at 37°C in the dark. Then, 50 μL of converter-POD was added for incubation of 30 min at 37°C in the dark, after which 50 μL of diaminobenzidine was added to develop positive signals in the dark for 10 min. The slices were washed in phosphate-buffered saline (PBS) 3 times between steps, each time lasting 5 min. Slices were then dehydrated, mounted, and observed with an optical microscope (Olympus, Tokyo, Japan). Five fields were randomly chosen for each individual, and apoptotic cells were counted.
qRT-PCR
Total RNA samples of the mouse renal tissue were extracted by TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. DNA contamination was removed by DNase I (Invitrogen) and then the quality and quantity of RNA samples were examined by NanoDrop 2000 (Thermo Scientific, Carlsbad, CA). For each mouse, 1 μg of total RNA was used for reverse transcription catalyzed by SuperScript II Reverse Transcriptase (Invitrogen). Then the synthesized complementary DNA (20 ng) was used in qRT-PCR on the QuantStudio 6 Flex Realtime PCR system (Applied Biosystems, Carlsbad, CA) with the specific primers for mouse Bax (Fw: 5′-TGTTT GCTGA TGGCA ACTTC-3′ and Rv: 5′-GATGG TTCTG ATCAG CTCGG-3′) and Bcl2 (Fw: 5′-GTAGA AGAGG GTGTG ACAGC-3′ and Rv: 5′-AGCAC TGACT CTGGG ATCGC-3′). Data were analyzed by the 2−ΔΔCt method with Gapdh (Fw: 5′-TCAAC AGCAA CTCCC ACTCT TCCA-3′ and Rv: 5′-ACCCT GTTGC TGTAG CCGTA TTCA-3′) as an internal control.
Western blot
Protein samples of mouse renal tissue were extracted by M-PER Mammalian Protein Extraction Reagent (Thermo Scientific) and quantified by Bio-Rad Protein Assay (Bio-Rad, Hercules, CA) according to the manufacturers’ instructions. The protein samples were separated on sodium dodecyl sulfate polyacrylamide gels and transferred to polyvinylidene fluoride membranes. The blots were blocked in 5% skim milk for 2 h at room temperature and then incubated in rabbit monoclonal primary antibodies for BAX and BCL2 (ab32503 and ab32124, Abcam, Cambridge, UK) overnight at 4°C. GAPDH (ab181602) was used as an internal control. After washing in PBS 5 times, the blots were incubated in goat anti-rabbit horse-radish peroxidase-conjugated secondary antibody (ab6721) for 1 h at room temperature. ECL Plus Western Blotting Substrate (Thermo Scientific) was used to develop positive signals. Signal intensity was analyzed by ImageJ 1.49 software (National Institutes of Health).
Statistical analysis
All the experiments were repeated 5 times. Data are represented as the mean ± standard deviation. Comparison between groups was performed with one-way analysis of variance and t test in SPSS 20 (IBM, New York, NY). P<0.05 was considered to be statistically significant.
Results
Baicalin attenuates AKI in pediatric sepsis patients
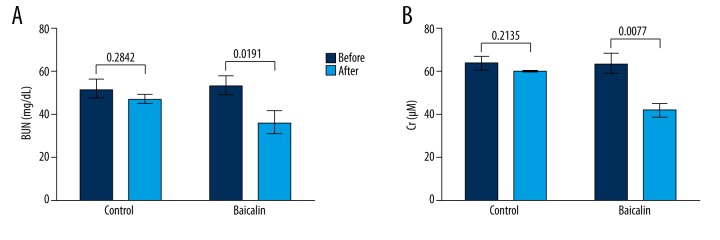
In the treatment of 50 pediatric sepsis patients, 25 received adjunctive therapy of oral baicalin for 15 days and the other 25 patients received only basic therapies. Results showed that both BUN and Cr levels in the serum of the control group did not changed greatly after basic therapies (P=0.2842 and 0.2135, Figure 1A, 1B), while those of the baicalin group were significantly decreased after baicalin adjunctive therapy (P=0.0191 and 0.0077). It could be inferred based on the 2 indexes that the renal functions of the baicalin group were improved, possibly due to the baicalin adjunctive therapy. Thus, baicalin might attenuate AKI in these pediatric sepsis patients.
Figure 1.
Baicalin improves renal functions during pediatric sepsis. Altogether, 50 pediatric patients were analyzed. Patients (n=25) in the control group received only basic therapies and patients (n=25) in the baicalin group received basic therapies plus adjunctive baicalin oral treatment for 15 days. Blood urea nitrogen (BUN) and serum creatinine (Cr) levels were detected before and after treatment to reflect renal function changes, as shown in (A) and (B), respectively. P values are indicated for each comparison.
Baicalin improves renal functions in the CLP-induced mouse model
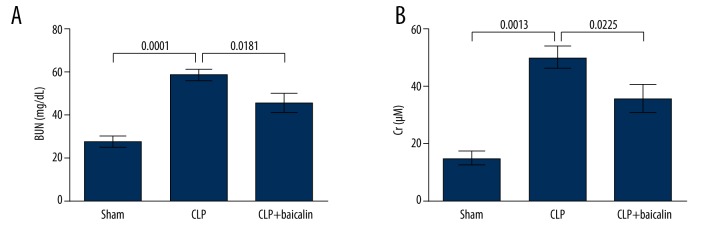
We next performed experiments in the mouse model to analyze the function of baicalin in septic AKI. Mice in the CLP group showed significantly elevated BUN and Cr levels (P=0.0001 and 0.0013, Figure 2A, 2B), suggesting that the kidney injury occurred after CLP. Both BUN and Cr levels were significantly decreased in the CLP + baicalin group (P=0.0181 and 0.0225), indicating that baicalin treatment effectively alleviated kidney injury in the CLP-induced mouse sepsis model.
Figure 2.
Baicalin improves renal functions in the cecal ligation and puncture (CLP)-induced mouse sepsis model. Altogether, 30 mice were analyzed. The sham group (n=10) went through all the surgical procedures except CLP. The CLP group (n=10) went through CLP to induce sepsis. The CLP + baicalin group (n=10) received baicalin treatment intragastrically after CLP. Blood urea nitrogen (BUN) and serum creatinine (Cr) levels were detected after 6 days, as shown in (A) and (B), respectively. P values are indicated for each comparison.
Baicalin inhibits renal cell apoptosis
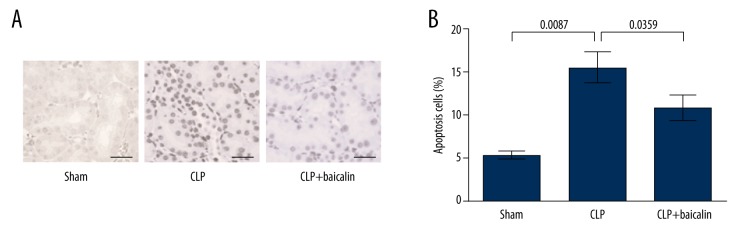
The possible mechanism of baicalin in protecting against septic kidney injury of the mouse model was investigated from changes in cell apoptosis. TUNEL assay showed more apoptotic cells in the CLP group compared to the sham group, and baicalin treatment reduced apoptotic cell numbers (Figure 3A), with significant differences between groups (P=0.0087 and 0.0359, Figure 3B). Thus, baicalin had suppressive effects on renal cell apoptosis in the CLP-induced mouse model.
Figure 3.
Baicalin inhibits renal cell apoptosis in the cecal ligation and puncture (CLP)-induced mouse sepsis model. (A) TUNEL assay was performed to assess cell apoptosis in the renal tissue of the 3 mouse groups. Apoptotic cells were dyed brown. Bar indicates 25 μm. (B) Average percent of apoptotic cells in the 3 mouse groups. P values are indicated for each comparison.
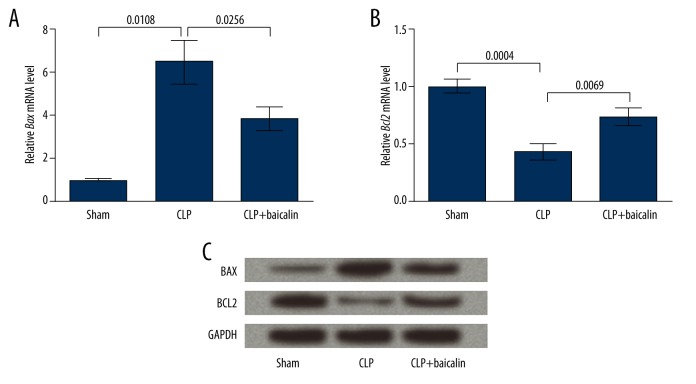
BAX and BCL2, 2 factors indicating cell apoptosis, were detected at both mRNA and protein levels to verify the changes in renal cell apoptosis. qRT-PCR results showed that Bax mRNA level was significantly elevated in the CLP group (P=0.0108) and then was down-regulated by baicalin treatment (P=0.0256, Figure 4A). Bcl2 mRNA level showed the opposite changing pattern to Bax, with significant differences between groups (P=0.0004 and 0.0069, Figure 4B). Protein level changes of the 2 factors were consistent with their mRNAs, as shown by Western blot analysis (Figure 4C). Since up-regulated BAX and down-regulated BCL2 are usually correlated with promoted cell apoptosis [23,24], these results further confirmed that CLP induced renal cell apoptosis and that baicalin suppressed apoptosis in the mouse sepsis model.
Figure 4.
Baicalin regulates BAX and BCL2 expression in the cecal ligation and punctures (CLP)-induced mouse sepsis model. (A) qRT-PCR showing the expression of BCL2-associated X protein (Bax) mRNA in the 3 mouse groups. (B) qRT-PCR showing the expression of B-cell CLL/lymphoma 2 (Bcl2) mRNA in the 3 mouse groups. P values are indicated for each comparison. (C) Western blot analysis showing the protein levels of BAX and BCL2 in the 3 mouse groups. GAPDH is an internal control.
Discussion
This study performed investigations in pediatric sepsis patients and in a CLP-induced mouse sepsis model to reveal the role of baicalin in AKI of pediatric sepsis. Baicalin adjunctive therapy in the patients and treatment in the mouse model decreased BUN and Cr levels. Further histological and expression analyses suggested the suppressive function of baicalin in renal cell apoptosis.
The 50 pediatric patients participating in this study were chosen from pediatric sepsis patients diagnosed with AKI, and the mouse model induced by CLP was detected with elevated BUN and Cr levels, which suggested potential kidney injury. Previous studies using mouse sepsis models used various baicalin treatment methods, including topical application on the mouse skin to analyze the effect of baicalin on epidermis [25], intraperitoneal injection to investigate the role of baicalin in mammary glands [26], and baicalin-containing diets to study liver injury, inflammation in the kidney, and lung carcinoma [22,27–29]. In these studies, the dose of baicalin was usually 100–200 mg/(kg·d), and the duration ranged from 3 to 50 days. Thus, in the present study we chose to perform baicalin treatment in the model mouse via intragastric administration of 200 mg/(kg·d) for 6 days, and the significantly elevated BUN and Cr levels in the CLP group compared to the sham group indicated that this treatment method effectively induced kidney injury in the mouse sepsis model.
Clinical trials with baicalin adjunctive therapy have focused on its protective roles in ulcerative colitis, hepatic fibrosis, and diabetes mellitus [30–32], and several studies have investigated the potential of baicalin in treating patients with early diabetic nephropathy, in which baicalin inhibits aldose reductase activity and reduces urinary albumin excretion rate and the blood β2-microglobulin [33]. The present study further discovered the protective role of baicalin against AKI in pediatric sepsis patients, which was reflected in the reduced BUN and Cr levels. Moreover, the experiments in the mouse model confirmed that baicalin treatment in a sepsis animal model could improve renal functions. These findings show the potential of using baicalin to attenuate AKI in pediatric sepsis patients, but the proper dosage and possible complications need to be assessed in future research.
We also investigated the underlying mechanisms of baicalin in attenuating kidney injury in sepsis. TUNEL results showed that baicalin treatment in the mouse model significantly suppressed renal cell apoptosis, and Western blot and qRT-PCR analyses indicated consistent changes in the expression of BAX and BCL2. A similar mechanism has been reported in mouse mammary glands, where baicalin inhibits cell apoptosis via regulating BAX and BCL2 [26]. In addition, baicalin inhibits hepatocyte apoptosis in mouse kidney injury induced by concanavalin A [34]; therefore, suppressing renal cell apoptosis is likely to be one of the mechanisms by which baicalin attenuates septic kidney injury. However, the anti-proliferative role of baicalin has also been reported in studies on lung cancer and mouse embryonic stem cells [28,35], and it is converted to baicalin by intestinal gut flora to act as a pro-apoptotic and anti-proliferative substance [36]. Baicalin is capable of promoting neuronal differentiation, and thus may benefit therapies for Parkinson and Alzheimer diseases [37]. We speculate that there are multiple potential mechanisms behind the effects of baicalin in various diseases; therefore, it is necessary to uncover the detailed mechanisms in order to optimize use of baicalin to control AKI in pediatric sepsis patients.
Conclusions
The investigations of baicalin in pediatric sepsis patients and the CLP-induced mouse sepsis model reveal the protective role of baicalin against AKI in pediatric sepsis. Baicalin can inhibit renal cell apoptosis, which may be one of its functional mechanisms. This study provides a potential adjunctive therapy for treating AKI in pediatric sepsis, and future research will concentrate on more detailed mechanisms of baicalin.
Footnotes
Source of support: Departmental sources
References
- 1.Khilanani A, Mazwi M, Paquette ET. Pediatric sepsis in the global setting. Clinical Pediatric Emergency Medicine. 2014;15:193–203. [Google Scholar]
- 2.Oliveira CF, Nogueira de Sá FR, Oliveira DSF, et al. Time- and fluid-sensitive resuscitation for hemodynamic support of children in septic shock: Barriers to the implementation of the American College of Critical Care Medicine/Pediatric Advanced Life Support Guidelines in a Pediatric Intensive Care Unit in a Developing World. Pediatr Emerg Care. 2008;24:810–15. doi: 10.1097/PEC.0b013e31818e9f3a. [DOI] [PubMed] [Google Scholar]
- 3.Despond O, Proulx F, Carcillo JA, Lacroix J. Pediatric sepsis and multiple organ dysfunction syndrome. Curr Opin Pediatr. 2001;13:247–53. doi: 10.1097/00008480-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Kirkebøen KA, Strand OA. The role of nitric oxide in sepsis – an overview. Acta Anaesthesiol Scand. 1999;43:275–88. doi: 10.1034/j.1399-6576.1999.430307.x. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman JJ. A history of adjunctive glucocorticoid treatment for pediatric sepsis: Moving beyond steroid pulp fiction toward evidence-based medicine. Pediatr Crit Care Med. 2007;8:530–39. doi: 10.1097/01.PCC.0000288710.11834.E6. [DOI] [PubMed] [Google Scholar]
- 6.Pundzienė B, Dobilienė D, Rudaitis S. Acute kidney injury in pediatric patients: Experience of a single center during an 11-year period. Medicina (Kaunas) 2010;46:511–55. [PubMed] [Google Scholar]
- 7.Mickells GE, Moga M-A, Smith CM. Acute kidney injury in pediatric sepsis. Clinical Pediatric Emergency Medicine. 2014;15:185–92. [Google Scholar]
- 8.Xu C, Chang A, Hack BK, et al. TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney Int. 2014;85:72–81. doi: 10.1038/ki.2013.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu S, Sun A, Liu R. Separation and purification of baicalin and wogonoside from the Chinese medicinal plant Scutellaria baicalensis Georgi by high-speed counter-current chromatography. J Chromatogr A. 2005;1066:243–47. doi: 10.1016/j.chroma.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 10.Gao Z, Huang K, Xu H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol Res. 2001;43:173–78. doi: 10.1006/phrs.2000.0761. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Wang X, Wang X, et al. Protective effect of flavonoids from Scutellaria baicalensis Georgi on cerebral ischemia injury. J Ethnopharmacolo. 2006;108:355–60. doi: 10.1016/j.jep.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20:2861–65. [PubMed] [Google Scholar]
- 13.Liao JF, Hung WY, Chen CF. Anxiolytic-like effects of baicalein and baicalin in the Vogel conflict test in mice. Eur J Pharmacol. 2003;464:141–46. doi: 10.1016/s0014-2999(03)01422-5. [DOI] [PubMed] [Google Scholar]
- 14.Park S-W, Lee C-H, Kim YS, et al. Protective effect of baicalin against carbon tetrachloride-induced acute hepatic injury in mice. J Pharmacol Sci. 2008;106:136–43. doi: 10.1254/jphs.fp0071392. [DOI] [PubMed] [Google Scholar]
- 15.Cai X, Li C, Du G, Cao Z. Protective effects of baicalin on ligature-induced periodontitis in rats. J Periodontal Res. 2008;43:14–21. doi: 10.1111/j.1600-0765.2007.00989.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Wang J, Sheng Y, et al. Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis. PLoS One. 2012;7:e35523. doi: 10.1371/journal.pone.0035523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong F, Luan Y, Zhang ZH, et al. Baicalin protects the myocardium from reperfusion-induced damage in isolated rat hearts via the antioxidant and paracrine effect. Exp Ther Med. 2014;7:254–59. doi: 10.3892/etm.2013.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin M, Li L, Li L, et al. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complement Altern Med. 2014;14:19–27. doi: 10.1186/1472-6882-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein B, Giroir B, Randolph A International Consensus Conference on Pediatric S. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 20.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Yang J. [Protective effect of baicalin on acute and chronic liver injury model rats and mice]. China Pharmacy. 2014;25:1374–76. [in Chinese] [Google Scholar]
- 23.Dewson G, Kluck RM. Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J Cell Sci. 2009;122:2801–8. doi: 10.1242/jcs.038166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 25.Bing-Rong Z, Song-Liang J, Xiao-E C, et al. Protective effect of the baicalin against DNA damage induced by ultraviolet B irradiation to mouse epidermis. Photodermatol Photoimmunol Photomed. 2008;24:175–82. doi: 10.1111/j.1600-0781.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- 26.Guo M, Cao Y, Wang T, et al. Baicalin inhibits Staphylococcus aureus-induced apoptosis by regulating TLR2 and TLR2-related apoptotic factors in the mouse mammary glands. Eur J Pharmacol. 2014;723:481–88. doi: 10.1016/j.ejphar.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Li H, Gao Z, Xu H. Effects of dietary baicalin supplementation on iron overload-induced mouse liver oxidative injury. Eur J Pharmacol. 2005;509:195–200. doi: 10.1016/j.ejphar.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 28.Du G, Han G, Zhang S, et al. Baicalin suppresses lung carcinoma and lung metastasis by SOD mimic and HIF-1alpha inhibition. Eur J Pharmacol. 2010;630:121–30. doi: 10.1016/j.ejphar.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Lim HA, Lee EK, Kim JM, et al. PPARgamma activation by baicalin suppresses NF-kappaB-mediated inflammation in aged rat kidney. Biogerontology. 2012;13:133–45. doi: 10.1007/s10522-011-9361-4. [DOI] [PubMed] [Google Scholar]
- 30.Yu FY, Huang SG, Zhang HY, et al. Effects of baicalin in CD4 + CD29 + T cell subsets of ulcerative colitis patients. World J Gastroenterol. 2014;20:15299–309. doi: 10.3748/wjg.v20.i41.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li F, He M, Li R. Influence of baicalin and telbivudine on chronic hepatitis B cirrhosis and early serum indexes of liver fibrosis. Med J West China. 2011;23:2112–14. [Google Scholar]
- 32.Dong S, Sun L. [Traditional Chinese drug baicalin and insulin therapy on pancreatic beta-cell function innewly diagnosed type 2 diabetes]. China Medicine. 2013;8:348–50. [in Chinese] [Google Scholar]
- 33.Liu C, Li P. [Effects of baicalin on erythrocyte aldose reductase activity and early diabetes nephropathy]. Chinese Journal of Gerontology. 2001;21:334–35. [in Chinese] [Google Scholar]
- 34.Liu LL, KGL, Wang H, et al. Baicalin protects mouse from Concanavalin A-induced liver injury through inhibition of cytokine production and hepatocyte apoptosis. Liver Int. 2007;27:582–91. doi: 10.1111/j.1478-3231.2007.01450.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Masika J, Zhou J, et al. Traditional Chinese medicine baicalin suppresses mESCs proliferation through inhibition of miR-294 expression. Cell Physiol Biochem. 2015;35:1868–76. doi: 10.1159/000373997. [DOI] [PubMed] [Google Scholar]
- 36.Parekh HS, Liu G, Wei MQ. A new dawn for the use of traditional Chinese medicine in cancer therapy. Mol Cancer. 2009;8:21. doi: 10.1186/1476-4598-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Zhuang P, Shen B, et al. Baicalin promotes neuronal differentiation of neural stem/progenitor cells through modulating p-stat3 and bHLH family protein expression. Brain Res. 2012;1429:36–42. doi: 10.1016/j.brainres.2011.10.030. [DOI] [PubMed] [Google Scholar]