Abstract
Background
Although pituitary adenoma is a malignant tumor, it can present as invasive growth in some cases. MicroRNA (miR)-26a has been found to be abnormally highly expressed in pituitary adenoma, indicating possible involvement in pathogenesis. As a known target gene of miR-26a, PLAG1 has abnormally low expression in pituitary adenoma. The correlation between miR-26a or PLAG1 expressional abnormality and occurrence of pituitary adenoma is still unknown, as is its association with invasiveness of pituitary adenoma.
Material/Methods
Pituitary adenoma tissues, including both invasive and non-invasive subtypes, were collected from our Neurosurgery Department, in parallel with normal pituitary tissues from postmortem autopsy. qRT-PCR was used to detect mRNA expression of miR-26a and PLAG1, while Western blotting was used to test PLAG1 protein expression. The correlation between miR-26a and PLAG1, and with pathological features, were analyzed. ROC analysis revealed the utility of miR-26a and PLAG1 in differential diagnosis of invasive/non-invasive pituitary tumors and in analyzing their effects on patient prognosis.
Results
MiR-26a was remarkably upregulated in pituitary tumors, while PLAG1 was downregulated, especially in invasive pituitary tumors. miR-26a and PLAG1 had higher diagnostic values for differentiating between invasive and non-invasive pituitary tumors (AUC=0.889 and 0.818, respectively). Those patients with miR-26 overexpression and PLAG1 downregulation had unfavorable prognosis. miR-26 and PLAG1 are independent factors affecting patient diagnosis.
Conclusions
MiR-26a can facilitate occurrence of pituitary tumor and invasiveness, probably via inhibiting PLAG1 expression.
MeSH Keywords: Antineoplastic Agents, beta 2-Microglobulin, Microradiography, Securin
Background
As one of the most common intracranial tumors in humans, pituitary adenoma is the third most common primary brain tumor after glioma and meningioma, accounting for about 15~20% of all primary intracranial tumors. The average incidence of pituitary adenoma is about 7.5~10 per 100 000 population, but has rapidly increased in recent years. Many pituitary tumors are identified by MRI or CT when diagnosing nasopharyngeal disease, brain trauma, or other head injury, with an incidence of over 20%. Although it is a benign tumor, some pituitary adenomas showed invasive growth with a malignant tendency, invading peripheral tissues, including bone, dura matter, suprasellar, parasellar, and even cavernous sinus or/and sphenoid sinus, entrenching blood vessels and nerves. Such tumors are termed invasive pituitary adenoma (IPA). IPA can severely affect patient quality of life and body growth. The radical treatment of IPA is extremely difficult because it is hard to completely remove during surgery. Even with post-operative radio-/chemo-therapy, the recurrence rate is still as high as 21~86%. Therefore, the treatment of IPA is a major challenge in neurosurgery. Basic research has been performed to explain the molecular mechanism governing the occurrence and progression of IPA from genetic or protein levels, in an attempt to obtain early diagnosis, prognostic evaluation, and guided treatment. MicroRNA (miR) is a family of endogenous, non-coding, single-stranded RNA 21~23 nucleotides in length. It can regulate gene expression at the post-transcriptional level via inhibiting translation or degrading target mRNA. miR has been found to affect a series of biological processes, including cell proliferation, apoptosis, differentiation, migration, and tumor malignant transformation. The abnormal expression and function of miR is closely correlated with the occurrence, progression, recurrence, and malignant transformation of IPA. Some scholars used high-throughput microarray technique to detect differential expression of miR across invasive and non-invasive pituitary adenoma. It has been confirmed that this differential expression of miR is closely correlated with invasive growth of pituitary adenoma. miR-26a has been demonstrated to be highly expressed in pituitary adenoma, suggesting its possible involvement in tumor occurrence. Pleomorphic adenoma gene 1 (PLAG1) is a definitive target gene of miR-26a, and is involved in multiple biological effects in regulating cell proliferation, induction of cell apoptosis, and cell cycle arrest. Under physiological conditions, PLAG1 is abundantly expressed in pituitary tissues, but is significantly downregulated in pituitary adenoma. However, the abnormal expression or function of miR-26a and PLAG1 related with occurrence of IPA is still unknown. This study aimed to investigate the relationship of pituitary tumor invasion with miR-26a and plag1.
Material and Methods
Reagent and materials
RNA extraction reagent Trizol was purchased from Invitrogen (USA). Reverse transcription and fluorescent quantitative PCR kit were purchased from Takara (China). Primers for reverse transcription of miR-26a and PCR were synthesized by Ruibo Bio (China). PCR primers for PLAG1 were synthesized by Sangon (China). Rabbit anti-human PLAG1 antibody was purchased from Abcam (USA).
Clinical information
A total of 70 pituitary adenoma patients who received treatment in Yantaishan Hospital from December 2009 to July 2013 were recruited. All patients had been diagnosed by imaging and post-surgery pathology. No treatment was applied before the surgery. There were 50 males and 20 females, with an age range of 23–67 years (average age, 46.8 years). There were 22 macroadenomas (tumor size larger than 1 cm) and 48 microadenomas (smaller than 1 cm) cases. In pathological subtyping, there were 14 cases of ACTH adenomas (5 cases of invasion of sphenoid sinus, 6 cases of invasion of the cavernous sinus, 3 cases of invasion of the hypothalamus, 3 cases of < 1-cm microadenoma, 4 cases of 1–2-cm small adenomas, 5 cases of 2–3-cm large adenoma, 2 cases of the > 3-cm huge adenoma; ACTH of blood > 46 ng/ml, average 156.41+45.65 ng/ml; UFC/24 h >100 μg, average 387.51±95.36 μg), 16 cases of prolactin adenomas (7 cases of invasion of sphenoid sinus, 4 cases of invasion of the cavernous sinus, 5 cases of invasion of the hypothalamus; 4 cases of < 1-cm microadenomas, 8 cases of 1–3-cm large adenoma, 4 cases of >3-cm huge adenomas; PRL of serum >200 ng/mL, average 436.88±66.38 ng/ml), 15 cases of GH adenoma (6 cases of invasion of sphenoid sinus, 4 cases of invasion of cavernous sinus, 5 cases of invasion of the hypothalamus; 5 cases of <1-cm micro adenomas, 7 cases of 1–2-cm small adenomas, 3 cases of 2–3-cm large adenomas; serum growth hormone by oral glucose tolerance test >1 μg/L, average 4.52±0.58 μg/L) and 25 cases of non-functional adenoma (pituitary tumor was found in sella by CT and MRI, no other abnormal increase of pituitary hormone and clinical manifestations were not found except for PRL; serum prolactin levels <100 ng/ml, average 76.56+12.18 ng/ml). There were 31 IPA tumors, and 39 non-IPA tumors. The criteria of IPA included: (1) Grade III~V of Hardy-Wilson guideline or stage C, D, or E; (2) Imaging implication before surgery, as shown by destruction of cavernous sinus, parasellar or hypothalamus regions; (3) Pathological examination indicated tumor infiltration in sellar diaphragm, or sellar floor bones; and (4) Perforation of inner wall of cavernous sinus during endoscopic surgery. The criteria of non-invasive pituitary adenoma included focal tumor within sellar region, without compression on peripheral structure as shown by imaging and surgery field. Exclusive criteria were: primary pituitary diagnosis from initially clinical and imaging information, but shown as other tumors such as canalis pharyngeal canal carcinoma, sellar nodular meningioma or Rathke’s cyst. In the control group, a total of 12 normal pituitary tissues were removed from postmortem autopsy of patients without pituitary endocrine disorder. All sample collections were performed with patient consent, and the study was approved by the Ethics Committee of Yantaishan Hospital. Detailed medical history and follow-ups were pursued in all patients via telephone or mail. The survival time of patients was calculated from the day of confirmed diagnosis. Post-operative follow-up was terminated on July 2015, with a range of 1–65 months (average, 23.7±18.6 months).
qRT-PCR for gene expression
Fresh tissue samples collected from the surgery were immediately frozen in liquid nitrogen and were kept at −80°C until further use. Trizol was used to extract total RNA from tissue samples. UV spectrometry was employed to detect A260/A280 ratio of total RNA, which should have the average ratio between 1.8 and 2.0. We used 1% agarose gel electrophoresis, which revealed clear and sharp bands of 28S and 28S RNA, suggesting high purity, no degradation, and high quality of extracted RNA samples. miR-26a-specific stem-loop primer and random primer were used to synthesize cDNA for miR-26a. In a total 20 μL reverse transcription system, there were 2 μg total RNA, 2 μL dNTP (2.5 mmol/L), 4 μL RT buffer (5X), 2 μL RT primers (1 μmol/L), 2 μL reverse transcriptase, and 0.5 μL RNase inhibitor. The reverse transcription reaction was performed at 16°C for 30 min, followed incubation at 42°C for 15 min and 85°C for 5min. Products were stored at −20°C. Using cDNA as the template, PCR amplification was performed with TaqdNA polymerase. PCR primers were: miR-26aPF: 5′-TTGGA TCCGT CAGAA ATTCT CTCCC GAGG-3′; miR-26aPR: 5′-GGTCT AGATG TGAAC TCTGG TGTTG GTGC-3′; U6PF: 5′-CTCGC TTCGG CAGCA CA-3′; U6PR: 5′-AACGC TTCAC GAATT TGCGT-3′; PLAG1PF: 5′-ATCAC CTCCA TACAC ACGAC C-3′; PLAG1PR: 5′-AGCTT GGTAT TGTAG TTCTT GCC-3′; β-actinPF: 5′-GCACT CTTCC AGCCT TCC-3′; and β-actinPR: 5′-AGAAA GGGTG TAACG CAACT AAG-3′. In a total of 10 μL reaction system, we added 4.5 μL 2X SYBR Green Mixture; 0.5 μL of forward and reverse primers (5 μm/L each), 1 μL cDNA, and 3.5 μL ddH2O. The reaction conditions were: 95°C for 5 min, followed by 40 cycles each of 95°C for 15 s and 60°C for 1 min. Data were collected on an ABI ViiA7 fluorescent quantitative PCR cycler for comparative Ct method (2−ΔΔCt) analysis. The level of microRNA and mRNA was normalized against U6 or β-actin as the internal reference gene. Each sample was tested in triplicate (N=3).
Western blotting
Total cellular proteins were extracted and separated by SDS-PAGE. Proteins were then transferred to membrane, which was first blocked in 5% defatted milk powder, and then incubated in rabbit anti-human PLAG1 antibody at 4°C overnight. Unbounded primary antibody was washed, followed by secondary antibody for 60-min incubation at room temperature. ECL reagent was finally added to develop the membrane, which was exposed and captured for data analysis.
Statistical analysis
SPSS18.0 was used for data collection and analysis. Measurement data are presented as mean ± standard deviation (SD) and enumeration data are presented as percentages. Between-group comparison of enumeration data was performed with the chi-square test. Expression level of miR-26a was compared by the Mann-Whitney U rank-sum test, while the correlation between miR-26a and PLAG1 was analyzed using the Spearman rank-sum test. The receiver operating characteristic (ROC) curve was used to evaluate the diagnostic value of miR-26a and PLAG1 on invasive/non-invasive pituitary adenoma. Kaplan-Meier analysis was used to construct a patient survival curve. The log-rank test was used for compare survival rates. The Cox regression model was used in multi-variant analysis of related prognostic factors. Statistical significance was defined when p<0.05.
Results
MiR-26a and PLAG1 mRNA expression in pituitary adenoma patients
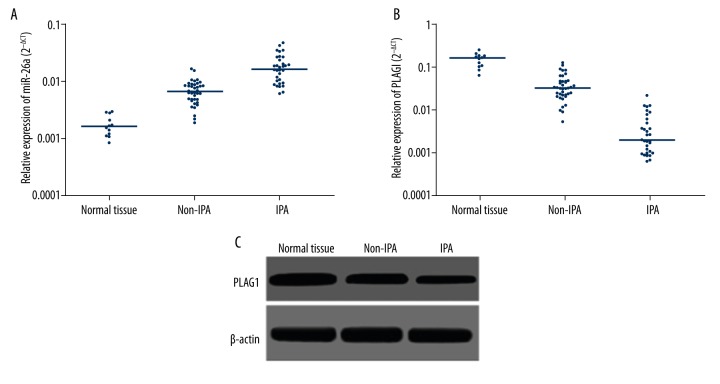
qRT-PCR results showed that, compared to normal pituitary tissues, miR-26a was significantly upregulated in pituitary tumor tissues (Figure 1A), while PLAG1 mRNA level was remarkably decreased (Figure 1B). IPA tissues had significantly higher miR-26a and lower PLAG1 mRNA compared to non-invasive pituitary tumor tissues. Western blotting obtained consistent results, as normal pituitary tissues had higher PLAG1 protein levels compared to those in non-invasive pituitary adenoma tissues, which again had higher PLAG1 protein compared to IPA tissues (Figure 1C). Further correlation analysis revealed a significant positive correlation between PLAG1 mRNA and protein expression levels (r=0.635, p=0.019) and a significant negative correlation between miR-26a and PLAG1 mRNA expression level (r=−0.791, p=0.021), supporting the targeted regulation of miR-26a on PLAG1.
Figure 1.
miR-26a and PLAG1 expression. (A) qRT-PCR for miR-26a expression in pituitary tissues. (B) qRT-PCR for PLAG1 mRNA expression in pituitary tissues. (C) Western blotting for PLAG1 protein expression in pituitary tissues.
Correlation between mir-26a, PLAG1 expression, and clinical features of patients
Using median values of mir-26a or PLAG1 mRNA expression levels of all patients as boundary lines, we further divided all patients into high-/low-expression subgroups. There was a significant difference in miR-26a or PLAG1 expression levels between non-IPA and IPA patients (p=0.004 and 0.006, respectively). The ratio of miR-26a high-expression in IPA patients was significantly higher than that in non-IPA patients (p=0.004), and higher PLAG1 low-expression population ratios (p=0.006). We did not observe a significant relationship between miR-26a or PLAG1 expression levels and patient age, sex, tumor size, or adenoma subtypes (p>0.05 in all cases, Table 1).
Table 1.
Relationship between miR-26a, PLAG1 and clinical features of patients.
| Clinical features | N | miR-26a expression | χ2 | P | PLAG1 expression | χ2 | P | |||
|---|---|---|---|---|---|---|---|---|---|---|
| High | Low | High | Low | |||||||
| Age (year) | ≤50 | 33 | 15 | 18 | 0.516 | 0.473 | 20 | 13 | 0.368 | 0.544 |
| >50 | 37 | 20 | 17 | 25 | 12 | |||||
| Sex | Male | 50 | 22 | 28 | 2.520 | 0.112 | 24 | 26 | 0.824 | 0.364 |
| Female | 20 | 13 | 7 | 12 | 8 | |||||
| Tumor size | Micro | 48 | 26 | 22 | 1.914 | 0.167 | 23 | 25 | 2.496 | 0.114 |
| Mega | 22 | 8 | 14 | 15 | 7 | |||||
| Invasiveness | Non-IPA | 39 | 14 | 25 | 8.504 | 0.004 | 23 | 16 | 7.701 | 0.006 |
| IPA | 31 | 22 | 9 | 8 | 23 | |||||
| Adenoma type | ACTH | 14 | 5 | 9 | 2.238 | 0.525 | 8 | 6 | 3.441 | 0.328 |
| Prolactin | 16 | 9 | 7 | 11 | 5 | |||||
| GH | 15 | 8 | 7 | 6 | 9 | |||||
| Unfunction | 25 | 15 | 10 | 11 | 14 | |||||
Differential diagnosis of pituitary adenoma by miR-26a and PLAG1
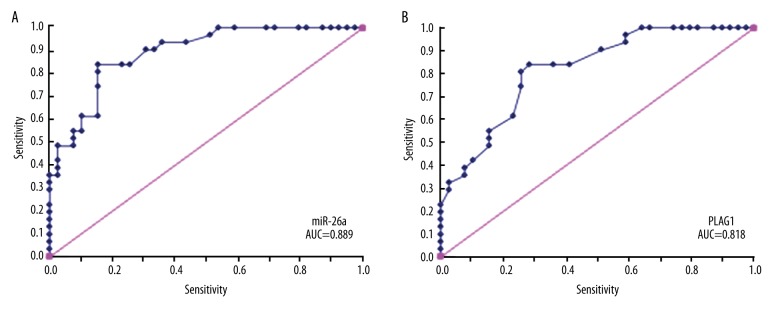
Using Hardy classification as the criterion standard, we divided pituitary adenomas into invasive and non-invasive types. By constructing an ROC, we calculated the classification accuracy of miR-26a/PLAG1 in differentiating invasive from non-invasive pituitary adenomas. The results showed certain differential diagnostic values of those 2 genes, as miR-26a had AUC values of 0.889 for differential diagnosis between invasive and non-invasive pituitary adenoma, higher than that of PLAG1 (AUC=0.818, Figure 2).
Figure 2.
ROC analysis for differential diagnostic values of miR-26a (A) and PLAG1 (B) genes on invasive/non-invasive pituitary adenoma. Blue curve, ROC for diagnostic trial. Pink curve, opportunistic line.
Prognosis of miR-26a/PLAG1 expression patients
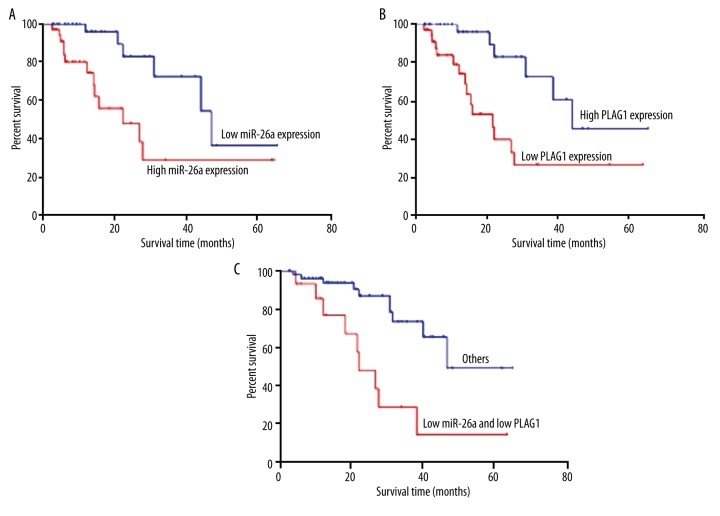
Those patients with miR-26a high-expression had a significantly sharper survival curve than those with lower miR-26a expressions. In contrast, PLAG1 low-expression patients had a significantly sharper survival curve than those with higher PLAG1 expression (Figure 3A, 3B). Log-rank test results showed significantly lower survival rate of miR-26a high-expression patients than those with lower miR-26a expression (χ2=7.393, p=0.007), while patients with lower PLAG1 expression had significantly lower survival rates than those with higher PLAG1 expression (χ2=9.475, p=0.002). For patients with both miR-26a high-expression and PLAG1 low-expression, the prognosis was remarkably worse than in other patients (χ2=11.400, p<0.001, Figure 3C).
Figure 3.
Relationship between miR-26a/PLAG1 expression and patient prognosis. (A) Survival curves of patients with miR-26a high/low expression; (B) Survival curves of patients with high/low expression of PLAG1; (C) Survival curves of patients with high mir-26a and low PLAG1 expressions and others.
Independent risk factors affecting patient survival by Cox regression analysis
We performed a Cox regression analysis including age, sex, tumor size, invasiveness, adenoma type, miR-26a expression level, and PLAG1 expression level. Using the maximal likelihood estimation biased forward selection method, we designated tumor invasiveness, miR-26a expression level, and PLAG1 expression level as independent risk factors affecting the survival of pituitary adenoma patients (Table 2). Invasiveness increased the death risk of pituitary adenoma patients by 0.899-fold (p=0.009). miR-26a high-expression increased the death risk by 1.833-fold compared to that in low-expression patients (p=0.022). On the contrary, elevated PLAG1 expression protected patients, reducing death risk by 39.7% (p=0.018).
Table 2.
Risk factor analysis of the survival of pituitary adenoma patients.
| Factor | Regression coefficient (B) | Standard error (S.E) | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age | 1.130 | 0.303 | 1.174 | 0.151 | 1.295 | 0.454–2.089 |
| Sex | −0.856 | 0.321 | 1.411 | 0.207 | 0.645 | 0.541–2.285 |
| Tumor size | 1.113 | 0.668 | 1.142 | 0.089 | 1.404 | 0.788–3.171 |
| Invasiveness | 1.951 | 0.201 | 3.056 | 0.009 | 1.899 | 1.346–4.537 |
| Adenoma type | 1.120 | 0.867 | 2.851 | 0.141 | 1.302 | 0.658–3.044 |
| miR-26a | 1.867 | 0.119 | 3.673 | 0.022 | 1.833 | 1.475–6.242 |
| PLAG1 | −1.914 | 0.112 | 5.442 | 0.018 | 0.603 | 0.310–0.801 |
Discussion
Pituitary tumors are monoclonal tumors originating from residual epithelial cells of anterior/posterior pituitary and cranial-pharyngeal tissues, and account for about 10~15% of all intracranial tumors. A previous study has confirmed the benign nature of most pituitary tumors. Clinically, however, about one-third of all pituitary tumors showed invasive growth, which is a biological behavioral of malignant tumors. Such pituitary adenomas often show aggressive growth toward peripheral tissues/nerves, and thus are termed invasive pituitary tumors. Invasive pituitary tumors have rapid progression, are difficult to completely removal during surgery, and have higher short-term recurrence rate after surgery, making clinical treatment a major challenge. The invasive growth of pituitary tumor is one important factor causing the recurrence of disease, further affecting clinical treatment efficacy and prognosis. Recent studies have focused on the mechanism directing pituitary tumor proliferation, activation of oncogenes, and inactivation of tumor suppression genes, but the biological mechanism affecting tumor invasion/migration is still not fully understood. For instance, CCND1 gene, a proto-oncogene, plays a role in pituitary tumorigenesis and invasiveness and its polymorphism in patients with different types of sporadic pituitary adenomas were determined [21]. Hypoxia can increase the expression of DDR1, a newly discovered kind of tyrosine kinase receptor on the cell surface, which in turn promotes pituitary adenoma cell proliferation and invasion [22]. In particular, a recent finding showed that the expression of Cold-Inducible RNA-Binding Protein (CIRP) in pituitary adenomas is closely related with tumor proliferation and invasion, and its significantly elevated expression level indicates post-operative recurrence [23]. Therefore, understanding the invasion/migration mechanism and identifying the specific molecular marker for acquiring invasion/migration property are of critical importance for early diagnosis of pituitary tumors, broadening clinical treatment strategy, improving treatment efficacy, and guiding individualized treatment.
With the advancement of molecular biology, the pathogenesis mechanism of IPA has been illustrated from the molecular level by some scholars. Many signal molecules have been discovered during the modulation of cell proliferation, cycle, apoptosis, and differentiation, especially the critical role during tumor cell occurrence and invasion/migration, thus providing new evidence depicting the tumor invasion mechanism and advancing targeted treatment. MicroRNA has been found to participate in occurrence, progression, invasion, and metastasis of pituitary adenoma via degrading target gene mRNA or inhibiting their translation. Some researchers utilized high-throughput microarray to reveal differential miR expressions between invasive/non-invasive pituitary adenoma. Moreover, such differential expression of miR has been found to be closely correlated with invasive growth of pituitary adenomas. Bottoni et al. utilized microarray analysis to screen the differential expression of miR between pituitary adenoma and normal pituitary tissues, and found the overexpression of miR-26a in pituitary adenoma, indicating its possible involvement in occurrence of pituitary adenoma. Pleomorphic adenoma gene 1 (PLAG1) is located in human chromosome 8q12, with 7313bp full-length cDNA. PLAG1 encodes 1 nuclear protein, which belongs to the zinc-finger protein family. With transcription factor activity, PLAG1 exerts its biological activity via regulating downstream target genes. Abnormal expression of PLAG1 is correlated with the occurrence of multiple tumors. A study has confirmed PLAG1 as a target gene for miR-26a, which regulates PLAG1 gene expression. Under physiological conditions, PLAG1 is abundantly expressed in pituitary tissues, but is significantly downregulated in pituitary adenoma. The correlation between miR-26a/PLAG1 expression or function abnormality and the occurrence of pituitary adenoma is still unknown, as its relationship with the invasiveness of pituitary adenoma.
The results of the present study show that, compared to normal pituitary tissues, pituitary adenoma tissues have significantly elevated miR-26a expression, indicating its potential involvement with pituitary adenoma occurrence. Gentilin et al. found significantly elevated miR-26a expression in the ACTH-type pituitary adenoma cell line AtT20/D16v-F2. Such upregulated miR-26a directly suppressed the expression of PRKCD (protein kinase Cδ), and facilitated the expression of cell cycle modulatory protein cyclin E and cyclin A, thus shortening the G1 phase and accelerating the cell cycle, indicating the facilitating role of miR-26a in pituitary adenoma, consistent with our observations. They also revealed more abundantly expressed miR-26a in IPA tissues compared to non-IPA tissues, suggesting the correlation between miR-26a and invasiveness of pituitary adenoma, in addition to tumor occurrence. The study revealed lower expression level of PLAG1 in pituitary adenoma tissues compared to that in normal pituitary tissues, especially in IPA tissues, suggesting the correlation of PLAG1 low-expression in occurrence and invasiveness of pituitary adenoma. However, no statistical significance was found in the proportion of high and low expressions of miR-26a and plag1 within different functional types of adenomas. Pagotto et al. found the physiological expression of PLAG1 protein in pituitary tissues and several other brain regions. The knockout of endogenous PLAG1 gene in pituitary tumor cell line AtT-20 or TtT/GF significantly enhanced cell proliferation ability, suggesting the role of PLAG1 in anti-pituitary tumor proliferation. Moreover, PLAG1 functions as a tumor suppressor gene in pituitary adenoma via facilitating cell apoptosis, inducing G1 phase arrest. Ki-67 is a critical marker for differential evaluation of mitotic index between non-invasive and invasive pituitary adenoma, and is also an important marker determining the invasiveness of pituitary adenoma. Thapar et al. found significantly higher mitotic index of IPA cells than those in non-invasive pituitary adenoma, suggesting that more active mitosis and uncontrolled tumor cell growth are critical factors for acquiring invasiveness by pituitary adenoma. Therefore, the study showed that elevated miR-26a expression can affect the invasiveness of pituitary adenoma via targeted inhibition on PLAG1 expression, and antagonizing the tumor suppression effect of PLAG1 against pituitary adenoma cells. Moreover, they analyzed the correlation between miR-26a/PLAG1 expression and clinical features of patients, and found the relationship between expression levels and tumor invasiveness. The study found higher diagnostic values of miR-26a and PLAG1 in differentiating invasive and non-invasive pituitary adenoma, indicating that the utilization of miR-26a and PLAG1 expression levels could effectively differentiate and evaluate the invasiveness of pituitary adenoma. Compared to classical pre-operative imaging examination, endoscopy during the surgery, and post-operative pathological examination, the early clarification of tumor invasiveness by measuring miR-26a and PLAG1 expression levels is critical importance for early diagnosis and treatment of disease. The study found that miR-26a and PLAG1 expression levels are independent risk factors governing patient prognosis, and significantly affect patient’s survival and prognosis.
Conclusions
In summary, miR-26a plays a critical role during the occurrence and progression of pituitary adenoma, and may facilitate tumor occurrence and the acquisition of invasiveness, probably via inhibiting PLAG1 expression. Our study provides new concepts for illustrating the mechanism of the invasiveness of pituitary adenoma, and provides novel methods and approaches for biological therapy against pituitary adenoma, justifying further exploration.
Footnotes
Source of support: Departmental sources
References
- 1.Asa SL, Ezzat S. The pathogenesis of pituitary tumours. Nat Rev Cancer. 2002;2(11):836–49. doi: 10.1038/nrc926. [DOI] [PubMed] [Google Scholar]
- 2.Vasiljevic A, Jouanneau E, Trouillas J, Raverot G, et al. Clinicopathological prognostic and theranostic markers in pituitary tumours. Minerva Endocrinol. 2016 [Epub ahead of print] [PubMed] [Google Scholar]
- 3.Farrell WE. Epigenetics of pituitary tumours: An update. Curr Opin Endocrinol Diabetes Obes. 2014;21(4):299–305. doi: 10.1097/MED.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 4.Kontogeorgos G. Classification and pathology of pituitary tumors. Endocrine. 2005;28(1):27–35. doi: 10.1385/ENDO:28:1:027. [DOI] [PubMed] [Google Scholar]
- 5.Li-Ng M, Sharma M. Invasive pituitary adenoma. J Clin Endocrinol Metab. 2008;93(9):3284–85. doi: 10.1210/jc.2008-1026. [DOI] [PubMed] [Google Scholar]
- 6.Hansen TM, Batra S, Lim M, et al. Invasive adenoma and pituitary carcinoma: A SEER database analysis. Neurosurg Rev. 2014;37(2):279–85. doi: 10.1007/s10143-014-0525-y. discussion 285–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oruçkaptan HH, Senmevsim O, Ozcan OE, Ozgen T. Pituitary adenomas: Results of 684 surgically treated patients and review of the literature. Surg Neurol. 2000;53(3):211–19. doi: 10.1016/s0090-3019(00)00171-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhong CH, Tao B, Wu Y, et al. [The role of cancer-associated fibroblasts in invasive behavior of pituitary adenoma]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2015;46(5):673–78. [in Chinese] [PubMed] [Google Scholar]
- 9.Gong X, Zhou W, Chai Y, et al. MicroRNA-induced cascaded and catalytic self-assembly of DNA nanostructures for enzyme-free and sensitive fluorescence detection of microRNA from tumor cells. Chem Commun (Camb) 2016;52(12):2501–4. doi: 10.1039/c5cc08861e. [DOI] [PubMed] [Google Scholar]
- 10.Kuninty PR, Schnittert J, Storm G, Prakash J, et al. MicroRNA targeting to modulate tumor microenvironment. Front Oncol. 2016;6:3. doi: 10.3389/fonc.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivapragasam M, Rotondo F, Lloyd RV, et al. MicroRNAs in the human pituitary. Endocr Pathol. 2011;22(3):134–43. doi: 10.1007/s12022-011-9167-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen PS, Su JL, Cha ST, et al. miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J Clin Invest. 2011;121(9):3442–55. doi: 10.1172/JCI45390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentilin E, Tagliati F, Filieri C, et al. miR-26a plays an important role in cell cycle regulation in ACTH-secreting pituitary adenomas by modulating protein kinase Cdelta. Endocrinology. 2013;154(5):1690–700. doi: 10.1210/en.2012-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103(7):2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spengler D, Villalba M, Hoffmann A, et al. Regulation of apoptosis and cell cycle arrest by Zac1, a novel zinc finger protein expressed in the pituitary gland and the brain. Embo J. 1997;16(10):2814–25. doi: 10.1093/emboj/16.10.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pagotto U, Arzberger T, Theodoropoulou M, et al. The expression of the antiproliferative gene ZAC is lost or highly reduced in nonfunctioning pituitary adenomas. Cancer Res. 2000;60(24):6794–99. [PubMed] [Google Scholar]
- 17.Daly AF, Burlacu MC, Livadariu E, Beckers A. The epidemiology and management of pituitary incidentalomas. Horm Res. 2007;68(Suppl 5):195–98. doi: 10.1159/000110624. [DOI] [PubMed] [Google Scholar]
- 18.Minniti G, Scaringi C, Poggi M, et al. Fractionated stereotactic radiotherapy for large and invasive non-functioning pituitary adenomas: Long-term clinical outcomes and volumetric MRI assessment of tumor response. Eur J Endocrinol. 2015;172(4):433–41. doi: 10.1530/EJE-14-0872. [DOI] [PubMed] [Google Scholar]
- 19.Yu SY, Hong LC, Feng J, et al. Integrative proteomics and transcriptomics identify novel invasive-related biomarkers of non-functioning pituitary adenomas. Tumour Biol. 2016 doi: 10.1007/s13277-015-4767-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Ezzat S, Asa SL. Mechanisms of disease: The pathogenesis of pituitary tumors. Nat Clin Pract Endocrinol Metab. 2006;2(4):220–30. doi: 10.1038/ncpendmet0159. [DOI] [PubMed] [Google Scholar]
- 21.Gazioglu NM, Erensoy N, Kadioglu P, et al. Altered cyclin D1 genotype distribution in human sporadic pituitary adenomas. Med Sci Monit. 2007;13(10):CR457–63. [PubMed] [Google Scholar]
- 22.Li S, Zhang Z, Xue J, et al. Effect of hypoxia on DDR1 expression in pituitary adenomas. Med Sci Monit. 2015;21:2433–38. doi: 10.12659/MSM.894205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, Zhang H, Heng X, et al. Expression of cold-inducible RNA-binding protein (CIRP) in pituitary adenoma and its relationships with tumor recurrence. Med Sci Monit. 2015;21:1256–60. doi: 10.12659/MSM.893128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi X, Tao B, He H, et al. MicroRNAs-based network: A novel therapeutic agent in pituitary adenoma. Med Hypotheses. 2012;78(3):380–84. doi: 10.1016/j.mehy.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Bottoni A, Zatelli MC, Ferracin M, et al. Identification of differentially expressed microRNAs by microarray: A possible role for microRNA genes in pituitary adenomas. J Cell Physiol. 2007;210(2):370–77. doi: 10.1002/jcp.20832. [DOI] [PubMed] [Google Scholar]
- 26.Shinkai T, Masumoto K, Ono K, et al. A case of unusual histology of infantile lipoblastoma confirmed by PLAG1 rearrangement. Surg Case Rep. 2015;1(1):42. doi: 10.1186/s40792-015-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida H, Miyachi M, Ouchi K, et al. Identification of COL3A1 and RAB2A as novel translocation partner genes of PLAG1 in lipoblastoma. Genes Chromosomes Cancer. 2014;53(7):606–11. doi: 10.1002/gcc.22170. [DOI] [PubMed] [Google Scholar]
- 28.Katabi N, Ghossein R, Ho A, et al. Consistent PLAG1 and HMGA2 abnormalities distinguish carcinoma ex-pleomorphic adenoma from its de novo counterparts. Hum Pathol. 2015;46(1):26–33. doi: 10.1016/j.humpath.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotellini M, Palomba A, Baroni G, Franchi A. Diagnostic utility of PLAG1 immunohistochemical determination in salivary gland tumors. Appl Immunohistochem Mol Morphol. 2014;22(5):390–94. doi: 10.1097/PAI.0b013e3182936ea7. [DOI] [PubMed] [Google Scholar]
- 30.Pagotto U, Arzberger T, Ciani E, et al. Inhibition of Zac1, a new gene differentially expressed in the anterior pituitary, increases cell proliferation. Endocrinology. 1999;140(2):987–96. doi: 10.1210/endo.140.2.6532. [DOI] [PubMed] [Google Scholar]
- 31.Scheithauer BW, Kurtkaya-Yapicier O, Kovacs KT, et al. Pituitary carcinoma: A clinicopathological review. Neurosurgery. 2005;56(5):1066–74. discussion 1066–74. [PubMed] [Google Scholar]
- 32.Thapar K, Kovacs K, Scheithauer BW, et al. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: An analysis using the MIB-1 antibody. Neurosurgery. 1996;38(1):99–106. doi: 10.1097/00006123-199601000-00024. discussion 106–7. [DOI] [PubMed] [Google Scholar]