Abstract
Background
Tetramethyl pyrazine (TMP) is a typical biologically active alkaloid isolated from the Chinese herb Ligusticum walliichi. It has been reported that TMP shows neuroprotective and stroke injury reductive properties in cerebral ischemia/reperfusion (I/R) animal models. In the present study we sought to investigate the effect and potential intervention mechanism of TMP in anoxia/reoxygenation (A/R) rat hippocampal neurons.
Material/Methods
After being cultured for 7 days, primary hippocampal neurons were randomly assigned into a normal control group (N), a TMP group (C: 0 ug/ml, L: 60 ug/ml, M: 200ug/ml and H: 800 ug/ml), and a JNK inhibitor group (S: SP600125, 10 μmol/L). A hypoxia/reoxygenation model were prepared 1 h after incubation. Hippocampal neurons were incubated in 90% N2 and 10% CO2 for 2 h, and then reoxygenated for 24 h in an incubator with 5%CO2 at the temperature of 37°C. The apoptosis rate, MKK4 and MKK7 mRNA and JNK kinase protein levels (C-fos, c-jun, and P-JNK) of hippocampal neurons were detected.
Results
The apoptosis rates of hippocampal neurons induced by A/R showed significant reduction after being pre-treated with JNK inhibitor, TMP 60 μg/ml, 200 μg/ml, and 800 μg/ml. The JNK kinase MKK4mRNA and MKK7mRNA levels, as well as the expressions of C-fos, C-jun, and P-JNK protein levels, were also be reduced.
Conclusions
TMP may produce a protective effect in anoxia/reoxygenation-induced primary hippocampal neuronal injury by inhibiting the apoptosis of the hippocampal neurons; the possible mechanism may be inhibition of the JNK signal pathway.
MeSH Keywords: Anoxia, MAP Kinase Signaling System, Signal Transduction
Background
Stroke is one of the leading causes of death and disability worldwide [1]. Nowadays, there were remarkably decreased functional deficits through the application of the intravascular techniques and thrombolytic agents [2]. Despite the advances in the therapy, the prevention of stroke-related brain damage is still unsatisfactory. Thus, effective prevention and cure of neurons anoxia/reoxygenation (A/R) injury can be to have the great clinical value. Many neuroprotective treatments for stroke rely on this mechanism. Traditional Chinese herbal medicine has been described in medicine systems as a neuroprotective treatment associated with A/R injury.
Tetramethyl pyrazine (TMP), a biologically active alkaloid extracted from the rhizome of the traditional herbal medicine Ligusticum walliichi, has been used routinely in China for the treatment of stroke and other vascular diseases [3]. TMP protects the neurons in animal ischemic stroke models through scavenging free radicals, inhibiting Ca2+ influx, increasing the transcription of thioredoxin and suppressing the inflammatory response. A previous study showed that TMP exhibited neuroprotective and anti-inflammatory effects in rats subjected to permanent cerebral ischemia [4]. Morphological studies have indicated that TMP has a protective effect on ischemic neuronal damage in hippocampus by regulating free radicals and free calcium [5]. However, the effects of TMP in the anoxia/reoxygenation induced by hippocampal neurons injury have not been well explored. Therefore, the present study of us focused on TMP’s protective effect to primary hippocampal neurons against anoxia/reoxygenation injury and the potential mechanisms with the aim of identifying possible novel targets for the treatment of neurological diseases and stroke.
Material and Methods
Animal and main reagents
SPF Sprague-Dawley rats with pregnancy of 18 days were obtained from the Experimental Animal Center of Guangzhou University of Chinese Medicine. All rats were given ad libitum normal chow diet. All experimental procedures involving animals were approved by the Animal Care and Use Ethics committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine.
Tetramethyl pyrazine Hydrochloride Injection was purchased from Shanghai Modern Hassan Pharmaceutical Co., Ltd. (11072711), FBS, NEUROBASAL™ Medium; trypsin-EDTA for cell culture was purchased from GIBCO, immunofluorescent antibody from Cell Signaling and Trizol, buffer, etc. PCR detection reagents from Invitrogen.
Culture of hippocampal neurons
SPF Sprague-Dawley rats with pregnancy of 18 days (Certificate No.: 0107721) were sacrificed by performing cervical spinal cord transaction (in order to avoid the influence of anesthetics and hypoxia on the experiment) and laparotomy to take out the fetus. Then, the skull cap of the fetus was opened with the brainstem pinched-off and the cerebrum was removed out and transferred into a drug-free culture medium in an ice bath. The blood vessels and meninges were removed under microscope after hippocampus was isolated. 4ml of 0.125% Trypsin-EDTA was added and digested in an incubator. The digestion was completed after 25–30 min. The cells were gently blown and knocked for 20 times with a pipette tip. After standing still for 3 mins, the upper monoplast suspension was filtered through 200 mesh stainless steel sieve, inoculated on a 6-well coated culture plate with 5×105~7×105/cm2 and cultured at 37°C in 5% CO2, with no shaking within 6–8 h and then was changed into serum-free medium (2%B27+NEUROBASAL™ Medium) 6–8 h later. Half volume of medium was changed every 3 days and neurons purity was detected by NSE ELISA after being cultured for 6–8 days.
Grouping and establishment of A/R model
The study object Fetal rat hippocampal neurons being cultured for 7 days were randomly divided into 6 groups: group N (normal group without anoxia), group C (blank control group 0 ug/ml), group S (10 μmol/L of JNK inhibitor SP600125), group L (a low concentration of 60 ug/ml TMP), group M (a medium concentration of 200 ug/ml TMP) and group H (a high concentration of 800 ug/ml TMP). Added the corresponding concentration of drugs to the experimental groups respectively and 1 h after drug effect delivered 90% N2 and 10% CO2 (flow rate 0.2 L/min) continuously to induce anoxia for 2 h. Transferred them into the incubator to reoxygenate for 2 h in 5% CO2 and detect.
Apoptosis proportion of hippocampal neurons detected by flow cytometry
Added 0.25% trypsin in digestion for 5 min and then added serum culture solution with repetition of blowing and beating after retraction of cytoplasm. Collected single cell suspension with per sample containing (1~5)×106 cells/ml, centrifuged for 5 min at a speed of 500~1000r/min., washed with pre-cooled PBS for 2 times, re-suspended cells with 250 μl binding buffer solution and adjusted cell concentration to 1×106 cells/ml. Then, measured 100 μl of cell suspension, added 5 μl AnnexinV/FITC and 10 μl of 20 μg/ml propidium iodide. Incubated for 15 min under protection from light, added 400 μl of PBS and analyze with flow cytometer (Beckman Coulter, USA) under excitation wavelength 488 nm. And FITC fluorescence was detected by band-pass filter under wavelength 515 nm and PI by another filter under wavelength more than 560 nm.
Expression of protein c-fos, c-jun, and p-jnk in A/R hippocampal neurons after pre-administration of TMP determined by immunofluorescence method
We discarded the culture solution and washed cells with PBS 3 times. Cells were then fixed in cold acetone for 10 min, washed with PBS 3 times, and dried naturally for 10–15 min. We added penetrant containing 0.5% of Triton PBS and incubated it for 30 min at room temperature. We dried the slide, added dropwise 300 μl of blocking solution and incubated it for 60 min at room temperature. After discarding the blocking solution, we added 300–500 ul (dilution 1:70; C-fos dilution 1:100) of primary antibody (C-fos, c-jun, and p-jnk) and incubated it at room temperature overnight at 4°C. Then it was washed with PBST 3 times, after which we added 300–500 μl (dilution 1:500) of secondary antibody, incubated at room temperature for 60 min under protection from light and washed with PBST 3 times. We then added 300 μl of Hoechst solution, incubated at room temperature for 10 min in the dark, washed with PBST 3 times, observed, and took photos (Zeiss laser confocal microscope LSM 700).
Expression level of MKK4 and MKK7 mRNA in rats detected by fluorescence quantitative PCR (probe)
Design and synthesis
-
Sequence Name: R-MAP2K4 (MKK4) (amplified fragment length 113 bp)
Forward Primer: 5′-CTCGTGGACTCTATTGCCAAGA-3′
Reverse Primer: 5′-CAGACATCAGAGCGGACATCA-3′
Probe: 5′-FAM-AGAGATGCTGGCTGTAGGCCGTATATGGC-TAMRA-3′
-
Sequence Name: R-MAP2K7 (MKK7) (amplified fragment length 105 bp)
Forward Primer: 5′-AGCGAATCCTTGGCAAGATG-3′
Reverse Primer: 5′-GCAGGATGTTGGAGGGTTTG-3′
Probe: 5′-FAM-CCGTGGCGATTGTGAAAGCAT-TAMRA-3′
-
Sequence Name: R-GAPDH (amplified fragment length 134 bp)
Forward Primer: 5′-TGGTCTACATGTTCCAGTATGACT-3′
Reverse Primer: 5′-CCATTTGATGTTAGCGGGATCTC-3′
Probe: 5′-FAM-CCACGGCAAGTTCAACGGCACAGT-TAMRA-3′
Extraction of total RNA
We added 1 ml of TRIzol to the cell pellet in 1.5-ml Eppendorf tube, added 0.2 ml of chloroform, incubated it at 4°C for 2–3 min and centrifuged it for 15 min. To the supernatant, we added an equal volume of isopropanol, incubated it at 15–30°C for 10 min, and centrifuged it for 15 min at a speed of 12 000 rpm. The supernatant was discarded, and the precipitate was washed with 800 ul of 75% ethanol (containing DEPC water) and centrifuged at 4°C for 5 min at a speed 7500 rpm. We discarded the ethanol with air or vacuum, then dried for 5–10 min and dissolved the RNA with DEPC water.
Reverse transcription
The condition of reverse transcription was as follows: 5×4 μl of RNA template, BIO-RAD qualitative PCR (USA), reaction system: 5×reverse transcription buffer 4 μl, 0.5 μl of reverse primer (10 pmol/μl), 0.5 μl of dNTPs (10 mM), 0.5 μl of MMLV (200 U/μl), and 10.5 μl of DEPC water. Reaction conditions were 37°C for 1 h and then at 95°C for 3 min.
Fluorescent quantitative PCR
For preparation of the standard, we isolated the positive product of PCR amplification in the pre-test through 2% low-melting agarose gel electrophoresis, cut the target strips under the long-wave ultraviolet, recovered and purified it by use of the QIA Quick Gel Extraction Kit. Purity was qualified if OD 260/280 >1.8. We converted the OD260 values and fragment length to the concentration (copy/μl) and sterilized the negative quality control standard with double-distilled water.
For preparation of the positive standard gradient, we took 5 μl of positive standard diluted 10-fold serially to the 4 positive standard gradients.
The reaction system of sample and positive standards was prepared using 5×10 μl of quantitative PCR buffer, 1 μl of forward primer F (10 pmol/μl), 1 μl of reverse primer R (10 pmol/μl), 1 μl of probe (5 pmol/μl), 1 μl of dNTPs (10 mM), 1 μl of Taq polymerase (3 U/μl), 5 μl of cDNA, 30 μl of ddH2O, with 50 μl in total. Reaction conditions were: 93°C for 2 min, 93°C for 15 s, 55°C for 25 s, and 72°C for 25 s, for a total of 40 cycles.
Results were analyzed according to quantity copy number, expressed as B (B=copy number/μl cDNA). Taking into account the difference in the concentration of total RNA from each sample, the sample expression was calculated by the formula: A=B1 (target genes)/B2 (reference genes), and A value was analyzed statistically.
Statistical methods
This was a completely randomized study. All the data were analyzed using statistical software SPSS15.0 and all the measurement data were expressed as mean ± standard deviation (±S). Analysis of variance was carried out using the one-way ANOVA test, then Welch’s test for heterogeneity of variance and LSD and Dunnett’sT3 test for multiple comparisons. A difference was significant at P<0.05.
Results
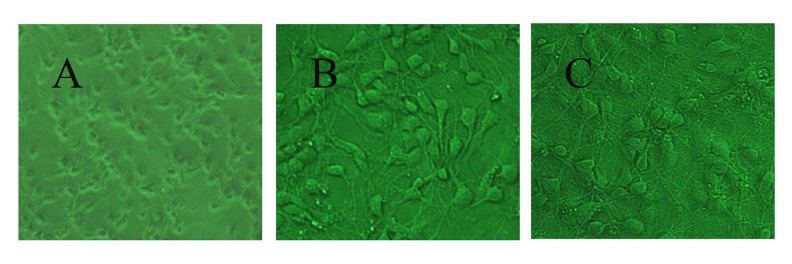
Morphological changes of primary rat hippocampal neuron observed under a phase-contrast microscope
Most of the hippocampal neurons were adherent and formed synapses when cultured for 4~6 h (Figure 1A). Neural networks developed between adjacent nerve cell synapses 3 days and 7 days later (Figure 1B, 1C). These cells were pyramidal, with soma stretching out completely. The neurites were of high purity and interwove into a network.
Figure 1.
Morphology of hippocampal neurons cultured. (A) 6 h (×100), (B) 3 days (×200), (C) 7 days (×200).
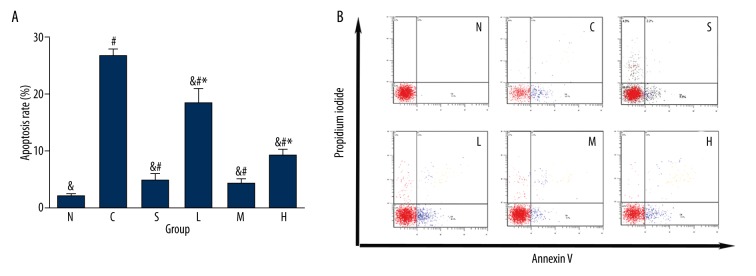
Effect of pre-administration with different concentrations of tetramethyl pyrazine on apoptosis rate of A/R rat hippocampal neurons
The apoptosis rate of hippocampal neurons was increased to different extents in each model group compared with the normal group (P<0.05). However, due to pre-administration in group S (inhibitor SP600125), TMP 60 μg/ml, 200 μg/ml or 800 μg/ml, the apoptosis rate of hippocampal neurons was decreased in different extents compared with the A/R treatment-only group (P<0.01). In addition, the apoptosis rate in group M (200 μg/ml) was significantly lower than in the 60 μg/ml and 800 μg/ml groups (P<0.01), but was not significantly different from that in group S (Table 1, Figures 2A, 2B, 3).
Table 1.
Effect of pre-administration with different concentrations of Tetramethylpyrazine and SP600125 for 1h on apoptosis rate of A/R rat hippocampal neurons (%, χ̄±S, n=6).
| Group | N | Apoptosis (%) |
|---|---|---|
| N | 6 | 1.93±0.41& |
| C | 6 | 26.67±1.28# |
| S | 6 | 4.72±1.25#& |
| L | 6 | 18.37±2.69#&* |
| M | 6 | 4.13±0.97#& |
| H | 6 | 9.13±1.19#&* |
| F | – | 261.997 |
| P | – | 0.000 |
P<0.05 vs. group N;
P<0.01 vs. group C;
P<0.01 vs. group M.
Figure 2.
(A) Effect of pre-administration with different concentrations of tetramethyl pyrazine and SP600125 for 1 h on apoptosis rate of A/R rat hippocampal neurons (%, ±S, n=6) (# P<0.05 vs. group N, & P<0.01 vs. group C, * P<0.01 vs. group M). (B). Effect of pre-administration with different concentrations of tetramethyl pyrazine and SP600125 for 1 h on apoptosis rate of A/R rat hippocampal neurons (%).
Figure 3.
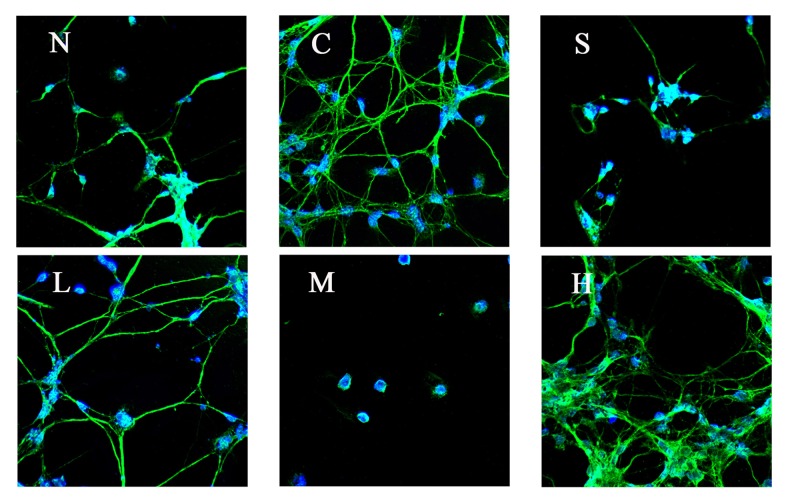
Effect of pre-administration with SP600125 or tetramethyl pyrazine for 1 h on the expression of protein p-JNK in A/R rat hippocampal neurons (N – normal, C – control, S – SP600125, L – 60 μg/ml, M – 200 μg/ml, H – 800 μg/ml).
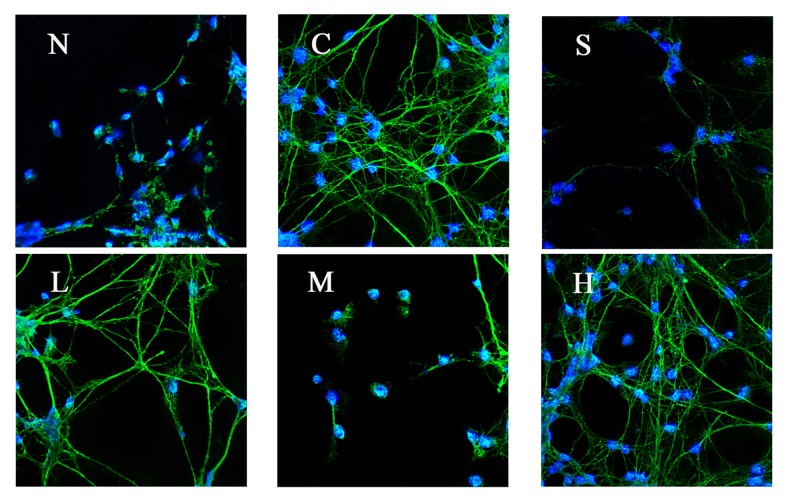
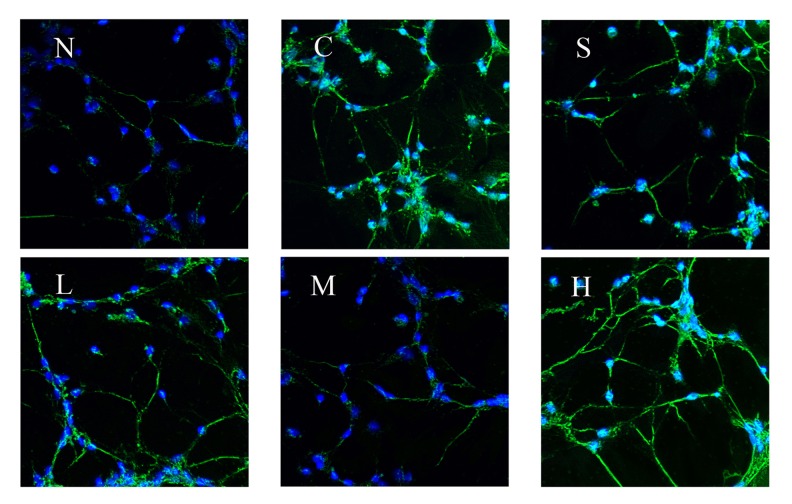
Effect of pre-administration with different concentrations of tetramethyl pyrazine on the expression of protein C-fos, c-jun, and p-JNK in A/R rat hippocampal neurons
Proteins C-fos, c-jun, and p-JNK in hippocampal neurons had no expression in the normal group (group N), but the expression was significantly increased after A/R. Pretreatment with inhibitor SP600125 (group S), TMP 60 μg/ml, 200 μg/ml, or 800 μg/ml reduced the expression of proteins C-fos, c-jun, and p-jnk in different extents compared with the A/R treatment-only group (P<0.01). The expression in TMP in the 200 μg/ml group (group M) showed significant differences when compared with 60 μg/ml (group L) and 800 μg/ml group (group H) (P <0.01), and the reduction of the protein expression in TMP 200 μg/ml group was the most significant. (Table 2, Figures 3–5).
Table 2.
Impact of the pre-administration of SP600125 and tetramethylpyrazine 1 h of anoxia – reoxygenation damage neurons in the C-fos, c-jun, p-JNK protein expression.
| Group | N | C-FOS | C-JUN | P-JNK |
|---|---|---|---|---|
| N | 6 | 0.40±0.08& | 0.43±0.06& | 0.46±0.06& |
| C | 6 | 1.67±0.10# | 2.02±0.22# | 2.38±0.33# |
| S | 6 | 0.79±0.04#& | 0.89±0.08#& | 0.93±0.05#& |
| L | 6 | 1.03±0.11#&* | 1.23±0.07#&* | 1.37±0.07#&* |
| M | 6 | 0.65±0.09#& | 0.70±0.13#& | 0.73±0.06#& |
| H | 6 | 0.87±0.05#&* | 0.91±0.06#&* | 1.08±0.10#&* |
| F | – | 163.997 | 128.252 | 123.235 |
| P | – | 0.000 | 0.000 | 0.000 |
P<0.01 vs. group N;
P<0.01 vs. group C;
P<0.01 vs. group M.
Figure 4.
Effect of pre-administration with SP600125 or tetramethyl pyrazine for 1 h on the expression of protein C-fos in A/R rat hippocampal neurons (N – normal, C – control, S – SP600125, L – 60 μg/ml, M – 200 μg/ml, H – 800 μg/ml).
Figure 5.
Effect of pre-administration with SP600125 or tetramethyl pyrazine for 1 h on the expression of protein c-jun in A/R rat hippocampal neurons (N – normal, C – 3 control, S – SP600125, L – 60 μg/ml, M – 200 μg/ml, H – 800 μg/ml).
Effect of pre-administration with different concentrations of TMP on the level of MKK4 and MKK7 mRNA in A/R rat hippocampal neurons
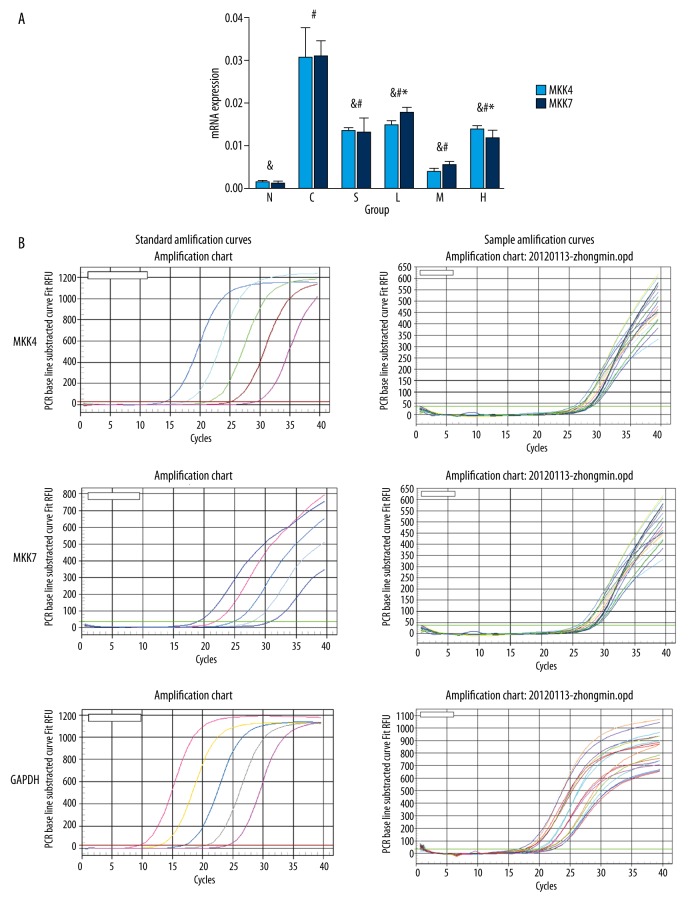
The content of MKK4 and MKK7 mRNA increased to various extents in each model group compared with the normal group. Pretreatment with inhibitor SP600125 (group S), TMP 60 μg/ml, 200 μg/ml, or 800 μg/ml reduced the content of MKK4 or MKK7 mRNA to various extents compared with that of the A/R treatment-only group. The content of MKK4 or MKK7 mRNA in the TMP 200 μg/ml group showed significant differences when compared with the 60 μg/ml and 800 μg/ml groups (P<0.01), with the greatest reduction in the TMP 200 μg/ml group (Table 3, Figure 6A, 6B).
Table 3.
Effect of pre-administration with SP600125 or TMP on the expression of MKK4 or MKK7 mRNA in A/R rat hippocampal neurons (%, χ̄±S, n=6).
| Groups | N | MKK4 | MKK7 |
|---|---|---|---|
| N | 6 | 0.0014±0.0006& | 0.0011±0.0007& |
| C | 6 | 0.0305±0.0072# | 0.0308±0.0039# |
| S | 6 | 0.0134±0.0009#& | 0.0131±0.0035#& |
| L | 6 | 0.0148±0.0012#&* | 0.0177±0.0014#&* |
| M | 6 | 0.0039±0.0009#& | 0.0055±0.0009#& |
| H | 6 | 60.0139±0.0009#&* | 0.0117±0.0021#&* |
| F | – | 68.022 | 111.223 |
| P | – | 0.000 | 0.000 |
P<0.05 vs. group N;
P<0.01 vs. group C;
P<0.01 vs. group M.
Figure 6.
(A) Effect of pre-administration with SP600125 or TMP on the expression of MKK4 or MKK7 mRNA in A/R rat hippocampal neurons (%, ±S, n=6) (# P<0.05 vs. group N, & P<0.01 vs. group C, * P<0.01 vs. group M). (B). Standard amplification curves and sample amplification curves of MKK4, MKK7, and GAPDH.
Discussion
Hippocampal neuron apoptosis is an important pathological basis for cerebral ischemia-reperfusion injury. In recent years, many studies have shown that the JNK transduction pathway is an important signaling pathway, which plays an essential role in cell differentiation, apoptosis, stress response, and the occurrence and development of a variety of human diseases [6–8]. Anthrapyrazolone SP600125 [9], an effective and selective inhibitor of JNK-1/2/3, can competitive binding with ATP reversibly to inhibit JNK and phosphorylation of c-jun in a dose-related manner.
Recent studies suggest that cerebral ischemic injury is closely related to the mitogen-activated protein kinase (MAPK) cascade pathway [10]. N-terminal protein kinase (JNK) C-Jun is an important member of the MAPK family and it has been proven that the JNK transduction pathway is a signal pathway leading to apoptosis. The study of the JNK signal transduction pathway-associated protein is important and significant in the prevention of cerebral hypoxia-ischemia-reperfusion injury and the exploration of the mechanisms of neural cell apoptosis [11–15].
Our results suggest that SP600125, an effective and selective inhibitor of JNK-1/2/3, can significantly reduce the apoptosis rate of A/R hippocampal neurons and reduce the cascade reaction of apoptosis through inhibiting JNK activity, which is important for apoptosis. Pre-administration with TMP 60 μg/ml, 200 μg/ml or 800 μg/ml can reduce the apoptosis and necrosis of hippocampal neurons induced by anoxia to various extents. Interestingly, the most dramatic reduction of neuron apoptosis was caused by pre-administration of 200 μg/ml TMP.
The proto-oncogene C-fos is a fast and transient expression gene that can induce the transcription of C-fos and synthesis of mRNA in the cytoplasm and translate mRNA into proto-oncogene protein C-fos. C-fos enters into the nucleus and binds with the target gene to activate the target gene expression to respond to stimuli when the central neurons are stimulated. Consequently, the expression level of C-fos protein to some extent reflects the repair capability of hippocampal neurons and the potential of cell-induced secondary brain injury. Research using semi-quantitative RT-PCR method to detect the level of C-fos mRNA levels in hippocampus and cortex of neonatal rat has confirmed that TMP can significantly reduce the obviously increased C-fos mRNA level in hypoxic-ischemic brain injury in brain tissue. Studies have also shown that TMP can significantly reduce the infarct volume after cerebral ischemia, and can down-regulate C-fos expression in cortex and basal ganglia following cerebral ischemic reperfusion [16].
A representative immediate early gene (IEG), c-jun can induce rapid and transient expression of c-JUN following cerebral ischemic reperfusion in brain tissue. c-JUN is involved in the transcription of effector enzymes in signal transmission systems and regulates the expression of various genes and the synthesis of target proteins. Moreover, JNK can cause the phosphorylation and overexpression of c-jun, in which the phosphorylation will greatly increase the activity of c-jun activation gene transcription, while the overexpression of c-jun and the activation of JNK will lead to neuronal apoptosis [17].
MKK4 (SEK1) and MKK7 (SEK2) are parts of the MAPK signal transduction pathway and direct upstream kinases of JNK, which activate JNK through the phosphorylation of Thr-183 and Tyr-185 in region JNK VIII, transduce extracellular stimulation signal into the cell and nucleus, and cause a series of biological reaction in cells [18]. The inactivation of MKK4 and MKK7 can block the JNK signal transduction pathway, which is an ideal method to control neuronal hypoxia injury.
In the present study we showed that TMP can inhibit the expression of the JNK pathway-associated proteins c-jun, C-fos, and P-JNK and significantly reduce the upstream content of mRNA in the JNK pathway, which is associated with kinases MKK4 and MKK7. This inhibition was dose-related with high, medium, or low concentrations of TMP, among which the medium concentration had the most significant protective effect.
Conclusions
TMP has a protective effect on A/R rat hippocampal neurons. The mechanism may be through inhibiting the JNK transduction pathway-associated proteins and kinases, and then blocking the cell apoptosis pathway.
Footnotes
Conflicts of interest
None.
Source of support: This project was supported by Guangdong Natural Science Foundation (No. 10151040701000024)
References
- 1.George PM, Steinberg GK. Novel stroke therapeutics: Unraveling stroke pathophysiology and its impact on clinical treatments. Neuron. 2015;87(2):297–309. doi: 10.1016/j.neuron.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: A systematic review. JAMA. 2015;313(14):1451–62. doi: 10.1001/jama.2015.3058. [DOI] [PubMed] [Google Scholar]
- 3.Shang YH, Tian JF, Hou M, Xu XY. Progress on the protective effect of compounds from natural medicines on cerebral ischemia. Chin J Nat Med. 2013;11(6):588–95. doi: 10.1016/S1875-5364(13)60068-0. [DOI] [PubMed] [Google Scholar]
- 4.Kao TK, Chang CY, Ou YC, et al. Tetramethyl pyrazine reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. Exp Neurol. 2013;247:188–201. doi: 10.1016/j.expneurol.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, Sun AY. Oxidative mechanisms involved in kainate-induced cytotoxicity in cortical neurons. Neurochem Res. 1994;19:1557–64. doi: 10.1007/BF00969006. [DOI] [PubMed] [Google Scholar]
- 6.Ovize M, Baxter GF, Di Lisa F, et al. Post conditioning and protection from reperfusion injury: Where do we stand? Cardiovasc Res. 2010;87(5):406–23. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 7.Okano S, Saito A, Hayashi T, Chan PH. The c-jun N-terminal protein kinase signaling pathway mediates Bax activation and suhsequent neuronal apoptosis through interaction with bim after transient focal cerebral ischemia. Neurosci. 2004;24(36):7879–87. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung JE, Kim GS, Chen H, et al. Reperfusion and neurovascular dysfunction in stroke: From basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41(2):172–79. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loadman PM, Calabrese CR. Separation methods for anthraquinone related anti-cancer drugs. J Chromatogr B Biomed Sci Appl. 2001;764(1–2):193–206. doi: 10.1016/s0378-4347(01)00281-x. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Fang Y, Chen T, et al. WIN55, 212-2 promotes differentiation of oligodendrocyte precursor cells and improve remyelination through regulation of the phosphorylation level of the ERK 1/2 via cannabinoid receptor 1 after stroke-induced demyelination. Brain Res. 2013;1491:225–35. doi: 10.1016/j.brainres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Ikegaya Y, Itsukaichi-Nishida Y, Ishihara M, et al. Distance of target search of isolated rat hippocampal neuron is about 150 microm. Neuroscience. 2000;97(2):215–17. doi: 10.1016/s0306-4522(00)00098-1. [DOI] [PubMed] [Google Scholar]
- 12.Liss TVP, Collingride GA. A synaptic mode of memory: Long-termpotentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 13.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79(1):143–80. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 14.Ichijo H, Nishda E, Irie K, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275(5296):90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Chong ZZ, Maiese K. Vital elements of the Wnt-Frizzled signaling pathway in the nervous system. Curr Neurovasc Res. 2005;2(4):331–40. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macaya A, Munell F, Reventos J, Ferrer I. Molecular factors of cerebral hypoxia-ischemia. Rev Neurol. 1996;24(131):855–64. [PubMed] [Google Scholar]
- 17.Paquet C, Dumurgier J, Hugon J. Pro-apoptotic kinase levels in cerebrospinal fluid as potential future biomarkers in Alzheimer’s disease. Front Neurol. 2015;6:168. doi: 10.3389/fneur.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haeusgen W, Herdegen T, Waetzig V. The bottleneck of JNK signaling: Molecular and functional characteristics of MKK4 and MKK7. Eur J Cell Biol. 2011;90(6–7):536–44. doi: 10.1016/j.ejcb.2010.11.008. [DOI] [PubMed] [Google Scholar]