Abstract
Background
Previous research showed that granulized Fu-Zheng-Xiao-Liu has a significant effect on breast cancer. However, it remains unclear whether HER-2 plays a role in this anti-cancer effect.
Material/Methods
Serum of male SD rats administered Fu-Zheng-Xiao-Liu granules (SF) was prepared and used to treat HER-2 positive breast cancer cell line SKBR-3. PBS and herceptin were used as negative and positive controls, respectively. MTT was used to detect the proliferation of SKBR-3 cells. Flow cytometry was used to measure the apoptosis of SKBR-3 cells. Western blot and immunofluorescence were used to measure the expression change of HER-2.
Results
Serum of male SD rats administered Fu-Zheng-Xiao-Liu granules had significantly reduced HER-2 expression at both mRNA level and protein level, significantly inhibited proliferation of SKBR-3 cells, and significantly increased apoptosis of SKBR-3 cells, compared to that of the blank control group or serum control group.
Conclusions
Fu-Zheng-Xiao-Liu granules affect proliferation and apoptosis through inhibition of HER-2 transcription and translation, providing an experimental basis for further study of the mechanism by which Fu-Zheng-Xiao-Liu granules affect breast cancer.
MeSH Keywords: Apoptosis Inducing Factor; Breast Neoplasms, Male; Interleukin-4; Nuclear Receptor Coactivator 3
Background
The incidence of breast cancer increases yearly, and has become one of the leading threats to women’s health. It has been shown that 30% of breast cancer patients showed HER-2 overexpression, indicating that HER-2 gene and the occurrence, development, treatment, and prognosis of breast cancer are closely related [1,2]. Compared to HER-2-negative breast cancer, HER-2-positive breast cancer is more aggressive and malignant; is not sensitive to radiotherapy, chemotherapy, or hormonal therapy; and has higher chance of recurrence and metastasis, worse prognosis, and lower survival rate [3–7]. Studies have shown that HER-2 can effectively activate the PI3K-ATK signalling pathway and lead to p53-mediated apoptosis in in vitro experiments, which needs to be investigated in breast cancer. Our previous study showed that Fu-Zheng-Xiao-Liu granules have a significant effect on breast cancer. In that study, we showed that Fu-Zheng-Xiao-Liu granules improve breast cancer patients’ immune function; relieve clinical symptoms after surgery, radiotherapy, and chemotherapy; improve the quality of life; and prevent tumor recurrence or metastasis, However, it remains unclear whether HER-2 plays a role in this anti-cancer effect [22]. In this study, the HER-2-positive breast cancer cell line SKBR-3 was used to study the effect of serum of male SD rats administered Fu-Zheng-Xiao-Liu granules (SFG) on the expression of HER-2 and the proliferation and apoptosis of SKBR-3; to explore the molecular mechanisms of the anti-cancer effect of Fu-Zheng-Xiao-Liu granule; and thereby provide a new pharmacological basis and therapeutic strategies for HER-2-positive breast cancer patients.
Material and Methods
Material
We used Fu-Zheng-Xiao-Liu granules (Shenzhen Second People’s Hospital), Herceptin (Roche), male SPF SD rats (Guangdong Medical Laboratory Animal Center, Guangzhou), SKBR-3 cells (breast adenocarcinoma cell line) (Fu-Xiang Bio, Shanghai), DMEM (Sigma,D1152), RPMI-1640 (Sigma, R4130), FBS (Hyclone, SV30087.02), trypsin (Gibco,25200072); MTT (Sigma, V900888-1G), apoptosis kit (Life, V13241), TRIzol (life, 15596-026); SYBR Green/Fluorescein qPCR Master Mix (2X) (Fermentas, #K0242), Ex TaqTM (Takara,DRR100A); RIPA Buffer (PIERCE, 89900), BCA protein concentration kit (Sigma, BCA1-1KT); rabbit anti-ErbB 2 antibody (Abcam, ab108371), mouse anti-beta-Actin antibody (Abcam, ab6276), goat anti-mouse IgG H&L (HRP) (Abcam, ab6789), goat anti-rabbit IgG H&L (FITC) (Abcam, ab6717), Bio-Rad Gel Imager (Bio-Rad); PCR amplifier 9700 (ABI), and real-time PCR (ABI, ViiA7).
Preparation of serum of male SD rats administered Fu-Zheng-Xiao-Liu granule (SFG)
Fifty 1-month-old male SPF SD rats with average body weight of 20±2 g were included in this study. The rats were randomly divided into 3 groups: control group (blank, n=10), positive control group (positive, n=10), and experimental group (E, n=30). The 3 doses used in the E group were 5.67 g/kg (1 dose), 11.34 g/kg (double dose), and 17.01 g/kg body weight (triple dose). The Fu-Zheng-Xiao-Liu granules were dissolved in water and delivered by oral gavage twice a day. Blood was collected after 3 days of drug administration via the inferior vena cava and serum was collected. D-PBS and herceptin were used in the C group and PC group.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of Shenzhen Second People’s Hospital.
SKBR-3 cell viability assay with MTT
SKBR-3 cells were cultured in RPMI-1640 with 10% FBS (penicillin and streptomycin, 100 u/ml of each). Cells were cultured in 6-well plates at concentration of 1×106/ml, serum from blank, positive, and experimental groups were added to the medium of cells 24 h later and the cells were cultured for another 72 h. We added 100 ul of MTT (5 mg/ml) to the medium and incubated it for 4 h before 200 ul of DMSO was added. OD550 were measured for each well. Each experiment was repeated 3 times.
SKBR-3 apoptosis measurement by flow cytometry
SKBR-3 cells were cultured in RPMI-1640 with 10% FBS (penicillin and streptomycin, 100 u/ml of each). Cells were cultured in 6-well plates at a concentration of 1×106/ml. Serum from blank, positive, and experimental groups were added to the medium of cells 24 h later and the cells were cultured for another 48 h. Cells were then trypsinized, collected, and washed once with PBS. PI was added, followed by incubated for 30 min in the dark. Fluorescence of DNA-PI was measured by flow cytometry.
Real-time PCR
SKBR-3 cells were cultured in RPMI-1640 with 10% FBS (penicillin and streptomycin, 100 u/ml of each). Cells were cultured in 6-well plates at a concentration of 1×106/ml. Serum from blank, positive, and experimental groups were added to the medium of cells 24 h later and the cells were cultured for another 24, 48, or 72 h. Cells were collected. RNA was extracted with TRIzol and reverse transcribed into cDNA. HER-2 gene expression was measured and normalized to β-actin. Primers used in this study were: HER-2-F: 5′-GGAGACCCGC TGAACAATACC-3; HER-2-R: 5′-GGGTTCCGCTGGATCAAGAC-3′; β-actin-F: 5′-CACGATGGAGGGGCCGGACTCATC-3′; β-actin-R: 5′-TAAAGACCTCTATGCCAACACAGT-3′. The quantitative PCR reaction system was as follows: 2 μl of cDNA, 0.5 μl of each primer (20 μM), 9 μl of ddH2O, 0.5 μl of ROX, and 12.5 μl of 2× SYBR Premix EX Taq. The PCR conditions were: 95°C for 130 s (pre-denature) followed by 40 cycles of 95°C for 5 s and 60°C for 31 s. The final extension cycle was 60°C 1 min and 95°C for 15 s.
Western blot analysis
Cells were collected 48 h after drug administration, and protein was extracted. Aliquots containing 50 μg proteins were loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE). After electrophoresis, proteins were transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was probed with antibodies against HER-2 and β-Actin. The membrane was then incubated with HRP-conjugated goat anti-rabbit IgG or goat anti-mouse IgG antibody. The protein bands were visualized by an enhanced chemiluminescence detection system (GE Healthcare, Piscataway, NJ) and quantified by densitometry analysis.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde at room temperature for 30 min at 24, 48, and 72 h after drug treatment. Cells were permeabilized with 0.05% Triton X-100 and blocked with 1% bovine serum albumin for 30 min. Subsequently, cells were incubated with primary antibody (1:50) overnight followed by incubation with secondary antibody. Fluorescence images were captured using the Leica TCS SP5 confocal system (Leica, Wetzlar, Germany).
Statistical analysis
Results are shown as mean ±SD. If the distribution was normal or its variances were equal, then the differences between various treatments were analyzed by one-way ANOVA. Otherwise, the differences between various treatments are analyzed by rank-sum test. P value < 0.05 was considered significant.
Results
Effect of Fu-Zheng-Xiao-Liu granules on SKBR-3 cell viability
Effect of Fu-Zheng-Xiao-Liu granules on the viability of breast cancer cell line SKBR-3 was measured by MTT. Results showed that when compared to blank serum controls, SFG from the double-dose group treated for 72 h had significantly decreased cell viability of SKBR-3 (p<0.01) (Figure 1).
Figure 1.
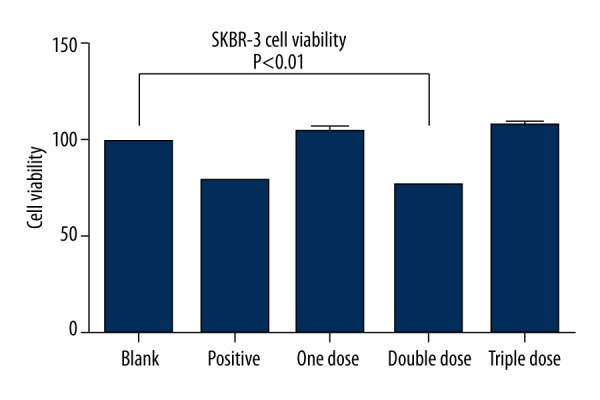
Effect of Fu-Zheng-Xiao-Liu granules on SKBR-3’s cell viability.
Effect of Fu-Zheng-Xiao-Liu granules on SKBR-3’s apoptosis
Compared to blank serum controls, SFG treatments increased the apoptosis of SKBR-3. In particular, SFG from the double-dose group treated for 48 h had significantly increased apoptosis of SKBR-3 (p<0.05) (Figure 2).
Figure 2.
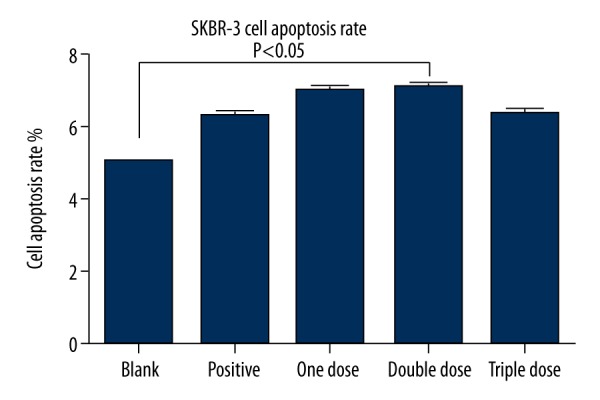
Effect of Fu-Zheng-Xiao-Liu granules on SKBR-3’s apoptosis.
Effect of Fu-Zheng-Xiao-Liu granule on the expression of HER-2 gene at mRNA level
The expression of HER-2 gene at the mRNA level was measured by real-time PCR. Results showed that positive serum significantly suppressed the expression of HER-2 gene. SFG treatment for 48 h also significantly suppressed the expression of HER-2. Among SFG treatments, 1 dose showed the best effect on suppression of HER-2 gene expression (p<0.05) (Figure 3).
Figure 3.
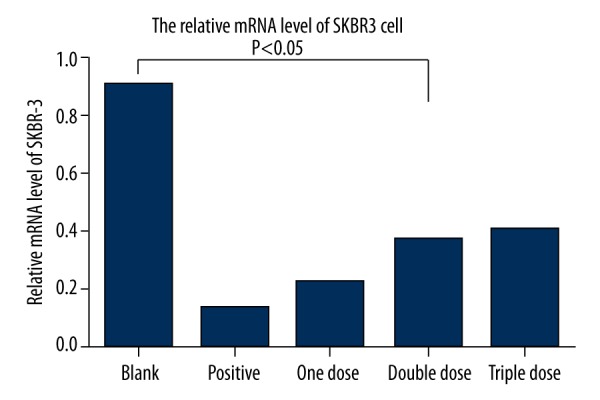
Effect of Fu-Zheng-Xiao-Liu granules on HER-2 gene expression at mRNA level.
Regression analysis of risk factors of SKBR-3 viability, apoptosis, and HER-2 expression
Regression analysis results showed that treatment with herceptin and Fu-Zheng-Xiao-Liu granules increased the risk of SKBR-3 viability, apoptosis, and HER-2 gene expression (P<0.05) (Tables 1, 2).
Table 1.
Risk factors of SKBR-3 viability and apoptosis.
| Independent viable | Correlation coefficient | Standard error | t | P | 95% CI |
|---|---|---|---|---|---|
| D-PBS | 0.317 | −5.022 | 4.003 | <0.01 | −0.146~−1.208 |
| Herceptin | 0.201 | 0.094 | 3.742 | <0.01 | 0.026~0.199 |
| Fu-Zheng-Xiao-Liu granules | 0.647 | 4.083 | 5.209 | <0.01 | 2.501~4.744 |
Table 2.
Risk factors of HER-2 gene expression.
| Independent viable | Correlation coefficient | Standard error | t | P | 95% CI |
|---|---|---|---|---|---|
| D-PBS | 0.000 | 5.049 | 0.000 | 0.000 | 0.000~0.000 |
| Herceptin | −0.496 | 2.304 | −4.210 | <0.01 | −2.126~−4.082 |
| Fu-Zheng-Xiao-Liu granules | −0.382 | 3.708 | −5.044 | <0.01 | −3.108~−4.299 |
Effect of Fu-Zheng-Xiao-Liu granules on the expression of HER-2 gene at protein level
The expression of HER-2 gene at the protein level was measured by Western blot. Results showed that SFG treatments for 48 h significantly suppressed the expression of HER-2. Among SFG treatments, 1 dose showed the best effect on suppression of HER-2 gene expression at the protein level (Figure 4).
Figure 4.
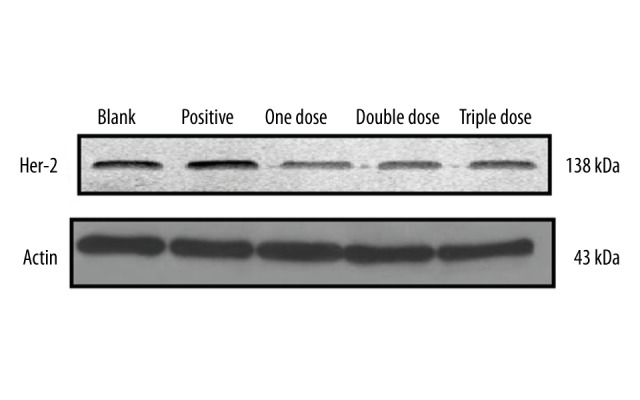
Effect of Fu-Zheng-Xiao-Liu granules on HER-2 gene expression at protein level.
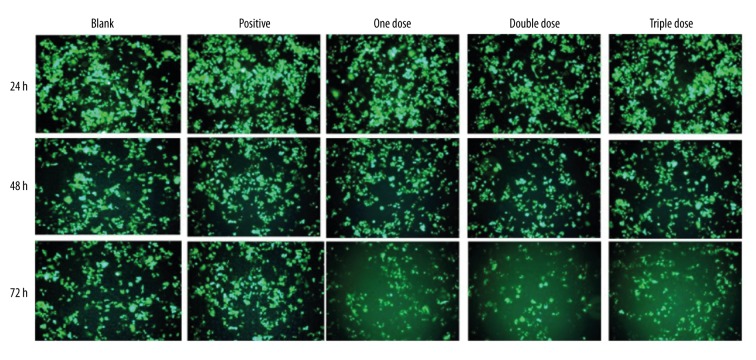
Effect of Fu-Zheng-Xiao-Liu granule on the expression of HER-2 in vitro
The expression of HER-2 protein in SKBR-3 was measured by immunofluorescence staining at 24, 48, 72 h after SFG treatments. Results showed that SFG treatments significantly suppressed the expression of HER-2. Among SFG treatments, the double dose showed the highest suppression of HER-2 gene expression (Figure 5).
Figure 5.
Effect of Fu-Zheng-Xiao-Liu granules on the expression of HER-2 in vitro.
Discussion
Breast cancer is characterized by high incidence, strong invasiveness, and slow progression [3]. In China, from 2001 to 2005, the incidence of breast cancer in women increased 38.5%, and the number of deaths from breast cancer increased by 37.1%. There is a tendency toward younger age at onset. Breast cancer has become the major threat to women’s lives. Therefore, it is urgent to explore practical strategies for controlling breast cancer recurrence and metastasis [8–12].
Recent studies have found that about 30% of breast cancer patients showed overexpression of HER-2 gene, and breast cancer patients in China showed a higher HER-2 overexpression ratio of 39.5%. HER-2 overexpression has become an important marker of poor prognosis in breast cancer patients [9–16]. Our group previously found that the Fu-Zheng-Xiao-Liu granules could inhibit the proliferation of drug-resistant MCF-7 cells and enhance the sensitivity of drug-resistant MCF-7 cells to a combination of chemotherapy drugs. The inhibitory effect of Fu-Zheng-Xiao-Liu granules on cancer cell proliferation was dependent on the down-regulation of multidrug resistance 1 (MDR1) and P-gp expression [17–20].
Conclusions
In this study, we found that SFG treatment of SKBR-3 breast cancer cells significantly inhibited the proliferation and promoted the apoptosis of SKBR-3, indicating that Fu-Zheng-Xiao-Liu granules can regulate the proliferation and apoptosis of breast cancer cell line SKBR-3. We also found that the expression of HER-2 was suppressed at both mRNA and protein levels; therefore, it is reasonable to speculate that Fu-Zheng-Xiao-Liu granules affect the proliferation and apoptosis of SKBR-3 through suppression of HER-2 expression. Moreover, regression analysis showed that herceptin and Fu-Zheng-Xiao-Liu granules affect SKBR-3 viability, apoptosis, and HER-2 gene expression. All these results suggest that Fu-Zheng-Xiao-Liu granules significantly affect SKBR-3 viability and HER-2 gene expression. This study also showed that Fu-Zheng-Xiao-Liu granules inhibited proliferation and increased apoptosis of SKBR-3 through suppression of HER-2 gene expression. Although we showed the suppression of HER-2 expression by 3 different methods (real-time PCR, Western blot, and immunofluorescence), the underlying functional mechanism of the Fu-Zheng-Xiao-Liu granules needs to be further elucidated because the dose-dependent effects were not clear in this study. Taken together, our current results lay a scientific foundation for the study of the detailed mechanisms of the anti-breast cancer effect of Fu-Zheng-Xiao-Liu granules.
Acknowledgments
We thank the Shenzhen Science and Technology Innovation Committee (No. 990354, “Effect of tumor-suppression granules on breast cancer SKBR-3 the expression and regulation mechanism in the effect on cell proliferation and apoptosis”).
Footnotes
Disclosure of conflict of interest
The authors declare no competing financial or commercial interests in this manuscript.
Source of support: This project was supported by Shenzhen Science and Technology Innovation Committee (No. JCYJ20140414170821153)
References
- 1.Black JD, Lopez S, Cocco E, et al. PIK3CA oncogenic mutations represent a major mechanism of resistance to trastuzumab in HER2/neu overexpressing uterine serous carcinomas. Br J Cancer. 2015;113:1020–26. doi: 10.1038/bjc.2015.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boero S, Morabito A, Banelli B, et al. Analysis of in vitro ADCC and clinical response to trastuzumab: Possible relevance of FcgammaRIIIA/FcgammaRIIA gene polymorphisms and HER-2 expression levels on breast cancer cell lines. J Transl Med. 2015;13(1):324. doi: 10.1186/s12967-015-0680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.East EG, Pang JC, Kidwell KM, Jorns JM, et al. Utility of estrogen receptor, progesterone receptor, and HER-2/neu analysis of multiple foci in multifocal ipsilateral invasive breast carcinoma. Am J Clin Pathol. 2015;144(6):952–59. doi: 10.1309/AJCPFWXP54OLILMU. [DOI] [PubMed] [Google Scholar]
- 4.Trashkov AP, Panchenko AV, Korablev RV, et al. [Age dynamics of angiogenesis markers in transgenic HER-2/neu (FBV/N) mammary adenocarcinoma-prone mice]. Vopr Onkol. 2015;61(4):642–46. [in Russian] [PubMed] [Google Scholar]
- 5.Li Z, Yang SS, Yin PH, et al. Activated estrogen receptor-mitogen-activated protein kinases cross talk confer acquired resistance to lapatinib. Thorac Cancer. 2015;6(6):695–703. doi: 10.1111/1759-7714.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khelwatty SA, Essapen S, Seddon AM, et al. Acquired resistance to anti-EGFR mAb ICR62 in cancer cells is accompanied by an increased EGFR expression, HER-2/HER-3 signalling and sensitivity to pan HER blockers. Br J Cancer. 2015;113:1010–19. doi: 10.1038/bjc.2015.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yong CS, Sharkey J, Duscio B, et al. Embryonic lethality in homozygous human Her-2 transgenic mice due to disruption of the Pds5b gene. Plos One. 2015;10(9):e0136817. doi: 10.1371/journal.pone.0136817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Q, Wang XZ, Mu DB, et al. Correlation analysis of hormone receptors and the expressions of HER-2 and Ki-67 in breast cancer. Eur J Gynaecol Oncol. 2015;36(1):78–83. [PubMed] [Google Scholar]
- 9.Bailur JK, Derhovanessian E, Gueckel B, Pawelec G. Prognostic impact of circulating Her-2-reactive T-cells producing pro- and/or anti-inflammatory cytokines in elderly breast cancer patients. J Immunother Cancer. 2015;3:45. doi: 10.1186/s40425-015-0090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SB, Lee JW, Yu JH, et al. Preoperative serum HER2 extracellular domain levels in primary invasive breast cancer. BMC Cancer. 2014;14:929. doi: 10.1186/1471-2407-14-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakha EA, Pinder SE, Bartlett JM, et al. Updated UK recommendations for HER2 assessment in breast cancer. J Clin Pathol. 2015;68(2):93–99. doi: 10.1136/jclinpath-2014-202571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chmielecki J, Ross JS, Wang K, et al. Oncogenic alterations in ERBB2/HER2 represent potential therapeutic targets across tumors from diverse anatomic sites of origin. Oncologist. 2015;20(1):7–12. doi: 10.1634/theoncologist.2014-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 14.Twombly R. Hints of future progress for HER-2 breast cancer. J Natl Cancer Inst. 2011;103(7):535–37. doi: 10.1093/jnci/djr122. [DOI] [PubMed] [Google Scholar]
- 15.Cánepa M, Denninghoff V, Perazzo F, et al. [Her-2/neu oncogen amplification in breast cancer]. Medicina (B Aires) 2012;72(1):88–89. [in Spanish] [PubMed] [Google Scholar]
- 16.Ligthart ST, Bidard FC, Decraene C, et al. Unbiased quantitative assessment of Her-2 expression of circulating tumor cells in patients with metastatic and non-metastatic breast cancer. Ann Oncol. 2013;24(5):1231–38. doi: 10.1093/annonc/mds625. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Zhonghai Z, Ruiqing Z, et al. [Effect of the Law of Fuzheng Xiaoliu on immunity function in patients with breast cancer postopreation]. Guiding Journal of Traditional Chinese Medicine and Pharmacy. 2011;7(17):19–21. [in Chinese] [Google Scholar]
- 18.Zhang R, Zhang ZH, Li H, et al. [Inhibit effect of TCM to enhance resistance for slaking neoplasm(herb serum)on drug-resistant cells in breast cancer]. Guiding Journal of Traditional Chinese Medicine and Pharmacy. 2011;4(17):95–97. [in Chinese] [Google Scholar]
- 19.Rakovich TY, Mahfoud OK, Mohamed BM, et al. Highly sensitive single domain antibody-quantum dot conjugates for detection of HER2 biomarker in lung andbreast cancer cells. ACS Nano. 2014;8(6):5682–95. doi: 10.1021/nn500212h. [DOI] [PubMed] [Google Scholar]
- 20.Masuda N, Higaki K, Takano T, et al. A phase II study of metronomic paclitaxel/cyclophosphamide/capecitabine followed by 5-fluorouracil/epirubicin/cyclophosphamide as preoperative chemotherapy for triple-negative or low hormonereceptor expressing/HER2-negative primary breast cancer. Cancer Chemother Pharmacol. 2014;74(2):229–38. doi: 10.1007/s00280-014-2492-y. [DOI] [PubMed] [Google Scholar]