Summary
Synaptic transmission is mediated by ionotropic and metabotropic receptors that together regulate the rate and pattern of action potential firing. Metabotropic receptors can activate ion channels and modulate other receptors and channels. The present paper examines the interaction between group 1 mGluR-mediated calcium release from stores, and GABAB/D2 mediated GIRK currents in rat dopamine neurons of the Substantia Nigra. Transient activation of mGluRs decreased the GIRK current evoked by GABAB and D2 receptors, though less efficaciously for D2. The mGluR-induced inhibition of GIRK current peaked in 1 second and recovered to baseline after 5 seconds. The inhibition was dependent on release of calcium from stores, was larger for transient than for tonic currents, and was unaffected by inhibitors of PLC, PKC, PLA2, or calmodulin. This inhibition of GABAB IPSCs through release of calcium from stores is a postsynaptic mechanism that may broadly reduce GIRK-dependent inhibition of many central neurons.
Keywords: calcium, GIRK inhibition, group I mGluR, GABAB, D2, IP3, dopamine neurons
Graphical Abstract
eTOC
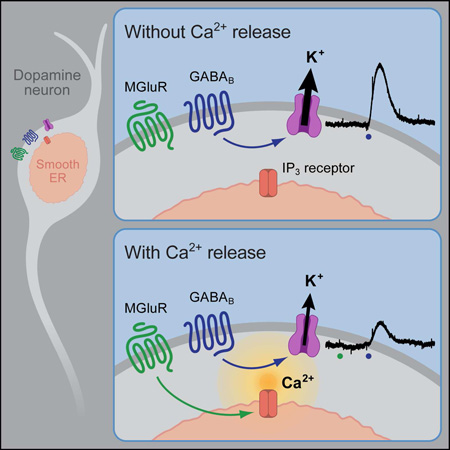
Kramer and Williams show that Gq-mediated calcium release from stores functionally inhibits Gi-coupled GIRK currents. This process occurs within milliseconds and lasts seconds, and is not mediated through classical Gq modulators of GIRK such as PKC, PLC, or PLA2.
Introduction
G protein-coupled receptors (GPCRs) can be separated into three major subclasses defined by their alpha G protein subunit: Gi/o, Gq/11, or Gs. These divisions confer the receptors with discrete signaling pathways. Dopamine neurons express a variety of GPCRs that activate different ion channels. For example, Gi/o GABAB receptors (GABABR) and dopamine D2 receptors (D2R) increase a G protein-gated inwardly rectifying potassium (GIRK) conductance (Beckstead et al., 2004; Arora et al., 2010). By contrast, Gq group I metabotropic glutamate receptors (mGluR1 and mGluR5) activate the small conductance calcium gated potassium channel SK (Fiorillo and Williams, 1998; Kramer and Williams, 2015). Thus, two signaling pathways activate distinct potassium conductances that hyperpolarize and pause action potential generation in dopamine neurons.
These signaling pathways do not always work in isolation. Gq receptor activation can inhibit GIRK currents generated by Gi receptors, although there are conflicting reports as to the mechanism underlying this interaction. The most commonly proposed mechanism(s) involve protein kinase C (PKC) (Mao et al., 2004). PKC-dependent inhibition has been reported to be either calcium-independent (Stevens et al., 1999; Leaney et al., 2001) or calcium-dependent (Hill and Peralta, 2001; Xia et al., 2010). The inhibition of GIRK by phospholipase C (PLC) has also been shown to be directly (Kobrinsky et al., 2000) and indirectly involved (Keselman et al., 2007). Finally, the activation of phospholipase A2 (PLA2), a calcium and G protein activated phospholipase has also been shown to inhibit GIRK (Sohn et al., 2007). Hence there are a variety of mechanisms underlying the Gq dependent inhibition of GIRK currents, depending on experimental conditions such as cell type, the particular GPCRs being studied, and agonist application protocols.
To date, the study of inhibition of GIRK by Gq signaling has relied on steady state application of agonists for both Gi and Gq receptors. It is known, however, that group I mGluRs signal differently depending on the method of activation. Steady state application of a selective agonist (DHPG) produced a tonic inward current, whereas transient activation via iontophoresis of aspartate or synaptic release of glutamate produced an outward current (Morikawa et al., 2003; Kramer and Williams, 2015). Thus distinct intracellular signaling pathways underlie the two methods of activation, and the question of how physiologically relevant receptor activation of group I mGluRs affects GIRK currents remains unexplored.
The present work shows that increases in cytosolic calcium transiently decreased a GIRK conductance that was activated by GABABR, D2R, or a non-hydrolyzable GTP analogue. Increases in cytosolic calcium were generated by transient mGluR activation, or by photolysis of caged-IP3 or caged-Ca2+ loaded into the neuron. The inhibition of GIRK was not affected by inhibition of PLC, PKC, PLA2, or calmodulin. In addition, the inhibition of transient GIRK currents evoked by synaptic release or iontophoretic application of GABA was greater than steady state currents. The results demonstrate a dynamic role for calcium in the inhibition of GIRK currents in dopamine neurons on the timescale of synaptic transmission, a potential mechanism for inhibition of GIRK currents in other brain areas.
Results
GABA receptor-dependent decrease in SK current
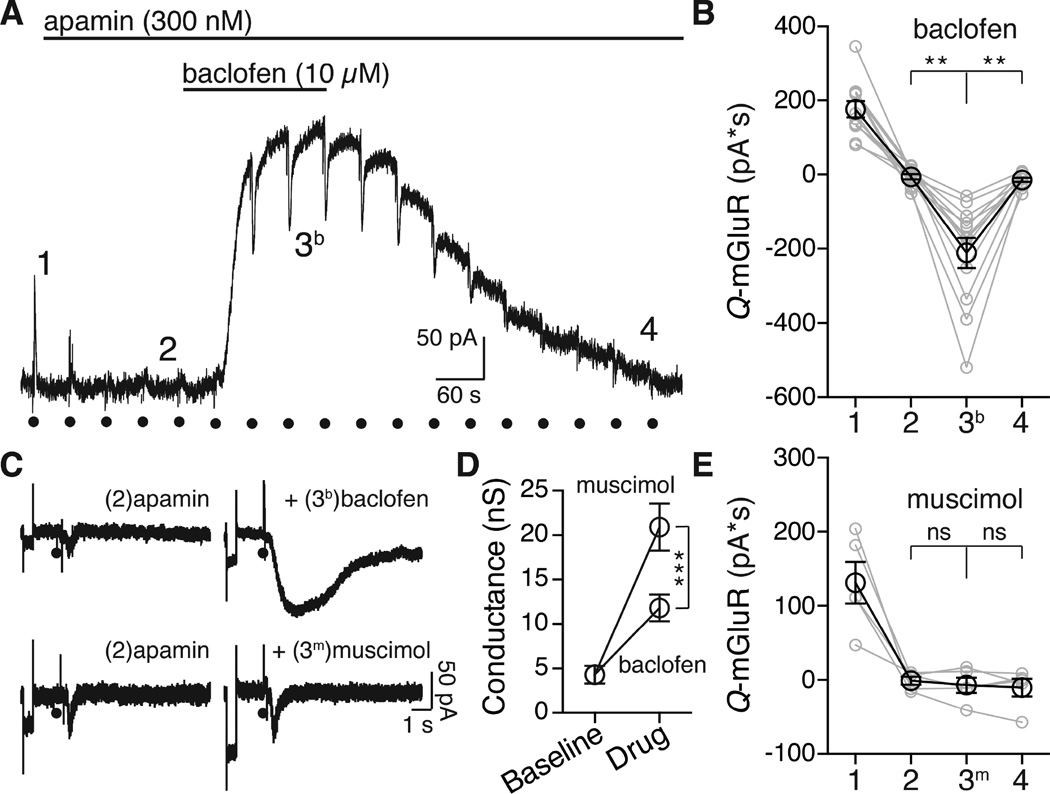
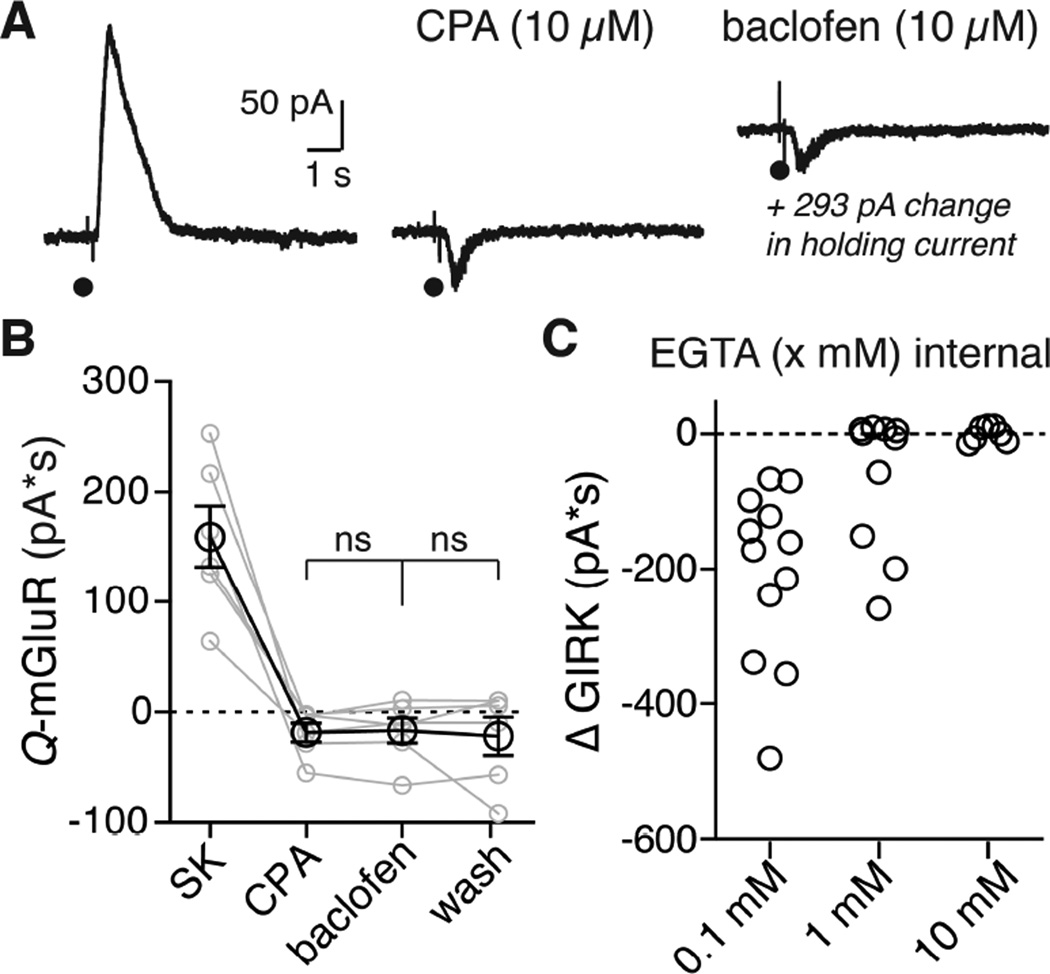
To examine the mGluR-induced inhibition of GIRK current, voltage-clamp recordings were made from adult rat Substantia Nigra pars compacta dopamine neurons. Iontophoretic application of aspartate at 45s intervals activated an SK current mediated by mGlu receptors (mGluR1 and mGluR5 (Kramer and Williams, 2015)). All experiments were carried out in the presence of ionotropic glutamate antagonists (see methods). Apamin (100–300 nM) was used to block SK (control 176 ± 21.4 pA*S, in apamin −5.97 ± 6.86 pA*S) in order to isolate the effect of the mGluR activation on the baclofen (10 µM) induced GIRK current. The small inward current that was induced by aspartate in the presence of apamin resembled the activation of a previously reported non-selective cation conductance (Figure S5E (Kim et al., 2003; Tozzi et al., 2003)).
Application of aspartate in the presence of baclofen and apamin resulted in a separate inward current (−212 ± 40 pA*S, Figure 1A) that was present only during the application of baclofen. That inward current declined as the outward current induced by baclofen reversed upon washing (−14.2 ± 5.3 pA*S, Figure 1A,B). The inward current induced by mGluR activation during GIRK activation could result from a breakdown in voltage control secondary to an increase in cell conductance. This mechanism accounted for an inhibition of the hyperpolarization-activated cation current Ih by the activation of GABAB receptors (Watts et al., 1996). One test of this mechanism was to increase the conductance of the cell with the GABAA agonist muscimol (10 µM). In the presence of muscimol (10 µM) aspartate iontophoresis did not induce a separate inward current (in apamin −0.99 ± 5.3 pA*S, +muscimol −6.97 ± 10.2 pA*S, Figure 1C, 2E). Thus the aspartate induced inward current seen in the presence of baclofen (referred to as ΔGIRK) was not the result of an increase in cell conductance.
Figure 1. mGluR activation induced an inward current during GABABR but not GABAAR currents.
A. Representative trace of a voltage clamp whole cell recording showing the block of the SK current by application of apamin (300 nM), followed by application of baclofen (10 µM). The numbers (1, 2, 3, and 4) correspond to time points plotted in B. Black circles represent the activation of mGluRs with aspartate iontophoresis.
B. Quantification across cells of the total charge transfer for the mGluR-activated current at baseline (1), after apamin (2) during baclofen (3b) after washout (4). In the presence of baclofen aspartate resulted in a ΔGIRK inward current (one-way repeated measures ANOVA followed by Tukey test, n = 12 cells).
C. Representative episodic traces showing application of aspartate (indicated by the black circle), in apamin (left) showing the small non-selective cation conductance and during either baclofen (black, top) or muscimol (grey, bottom) treatment. Each episode begins with a 3 mV step to assay whole cell conductance (Figure 1C).
D. Quantification of the change in cell conductance. Both baclofen and muscimol caused a significant increase in the conductance (two-way repeated measure ANOVA followed by Bonferroni, n = 7 for muscimol, 11 for baclofen). The increase was significantly larger for muscimol than for baclofen (two-way repeated measure ANOVA followed by Bonferroni).
E. Comparison between cells of the aspartate induced current on baclofen or muscimol application. Muscimol and baclofen were not significantly different at baseline (1, p = .94), after apamin (2, p > 0.99), or after washout (4, p > 0.99). In muscimol the aspartate-induced current was not different from apamin or washout (p > 0.99). All statistics were conducted with a two-way repeated measures ANOVA followed by a Bonferroni. n = 5 (muscimol), n = 12 (baclofen), **p < 0.01, ***p < 0.001, bars and summary data points represent means ± s.e.m. in B. each dot indicates a single cell.
See also Figure S3
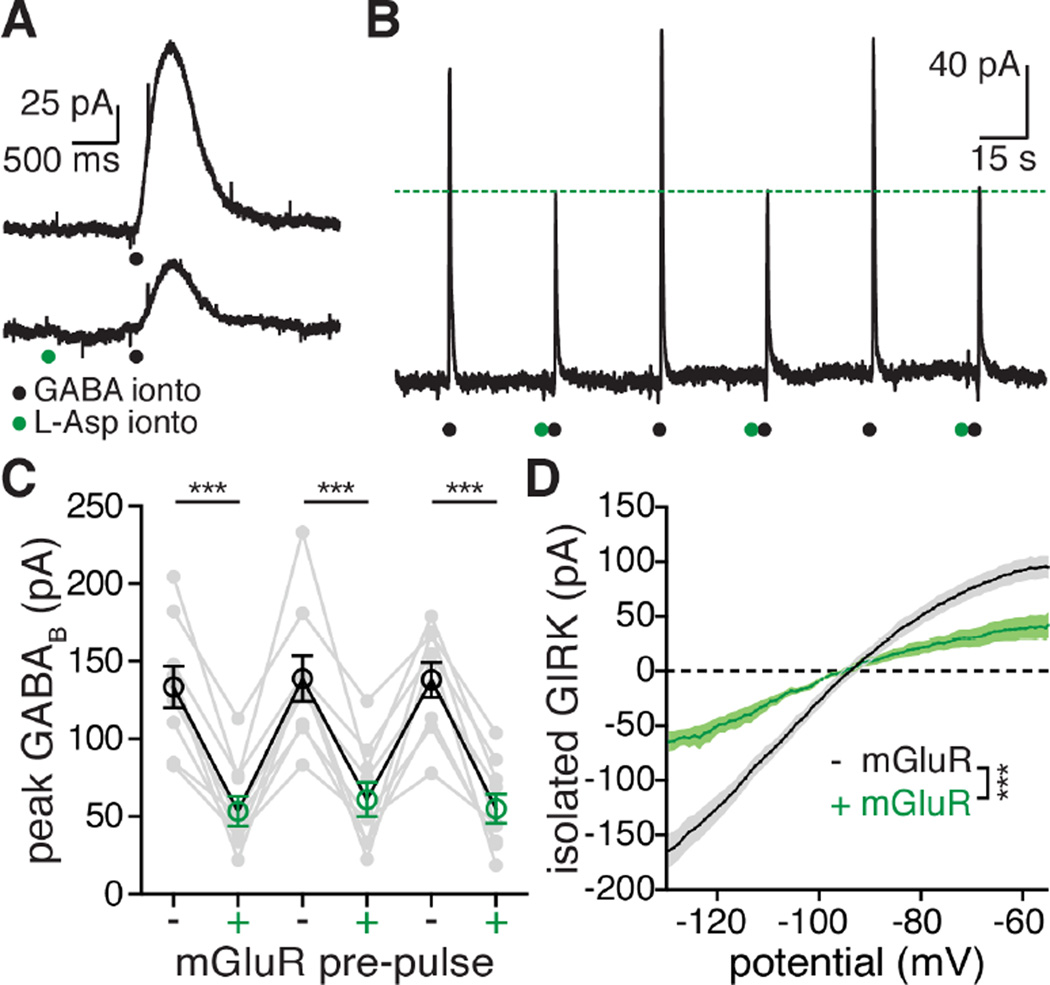
Figure 2. mGluR activation decreases GABABR GIRK currents by closing GIRK channels.
A. Representative traces (average of three raw traces for each condition) showing the effect of an mGluR pre-pulse (green dots) on the GIRK current mediated by GABAB receptor activation (black dots).
B. Representative chart record from a whole-cell recording showing iontophoresis of GABA (black circles) every 45 seconds. Aspartate (green circle) was applied by iontophoresis one second before every other application of GABA. These experiments were done in the presence of apamin (100–300 nM), as well as GABAA and AMPA receptor blockers (see methods).
C. Grouped data across cells showing the effect of an mGluR pre-pulse (green) one second before GABA on the peak GABABR GIRK current. In black are grouped traces where there was no mGluR pre-pulse. Each grey dot represents an individual cell, black and green circles are means ± s.e.m. Statistics were performed with a one-way repeated measures ANOVA followed by a Bonferroni test, n = 8 cells, ***p ≤ 0.001.
D. I–V plots of the GABAB mediated GIRK current grouped across cells with (green) and without (black) pre-application of aspartate. Each dot on the I–V plot represents the mean, shaded area around the curves represents s.e.m. Statistics were performed with a one-way repeated measures ANOVA, n = 6 cells, ***p < 0.001 (interaction between voltage and mGluR pre-pulse).
See also Figure S5
When the GABABR dependent current was reduced by the application of BaCl2 (100 µM), the ΔGIRK current induced by aspartate also decreased (−206 ± 37 pA*S without BaCl2 and −57.2 ± 5.1 pA*S with BaCl2, Figure S3). Finally, when mGluR1 and mGluR5 were reversibly inhibited by the low affinity non-selective antagonist, MCPG (1 mM), there was a reduction in the inhibition of GIRK (baseline: 64.8% inhibition, in MCPG: 34.5%, Figure S3D). The high affinity negative allosteric modulators JNJ-16259685 (1 µM, mGluR1) and MPEP (1 µM, mGluR5) abolished the inhibition of GIRK (inhibition in control: 65.4%, in JNJ/MPEP: 0.9%, Figure S3D). These results suggest that the baclofen induced GIRK current is reduced by transient activation of mGluRs.
mGluR-dependent inhibition of GIRK conductance
The interaction between GABAB and mGlu receptors was next examined with iontophoretic co-application of GABA and aspartate. This technique allowed activation of GABAB and mGlu receptors that resembled synaptically stimulated transmitter release. All experiments were carried out in the presence of apamin (300 nM). When aspartate was applied one second before GABA, the peak GABAB current was reduced to 43.3 ± 6.7% of control (from 137 ± 12.6 pA to 56.4 ± 9.9 pA; Figure 2C). The current induced by transient activation of GABAB receptors was inhibited significantly more than the steady state current induced by baclofen as measured from the peak of the baclofen current to the peak of the ΔGIRK current elicited by aspartate iontophoresis (inhibition of transient GIRK: 58.5 ± 5.8%, n = 9; inhibition of steady-state GIRK: 25.6 ± 3.0%, n = 30, p < 0.001, One-way ANOVA followed by Tukey test).
The voltage dependence of the decrease in the GABABR-induced current was examined using a voltage ramp protocol (Figure 2D). A hyperpolarizing ramp (−55 to −130 mV, 200 ms) was applied just before and at the peak of the GABABR outward current (see Figure S1 for detailed protocol). The I-V plot for the GABABR current without an mGluR pre-pulse had the characteristic inward rectification of a GIRK conductance and reversed near the potassium reversal potential (−93.7 ± 1.4 mV, calculated Ek = −103 mV, not corrected for junction potential; Figure 2D). When aspartate was applied one second prior to GABA the amplitude of the outward current was reduced but the rectification and reversal potential were unchanged (Figure 2D, reversal = −94.7 ± 1.2 mV, p = 0.11). The inhibition of GIRK was not voltage dependent; the scaled I-V plots with and without the mGluR pre-pulse were not different (Figure S5A–B). Similar results were obtained in experiments using steady-state application of baclofen (Figure S5C–E).
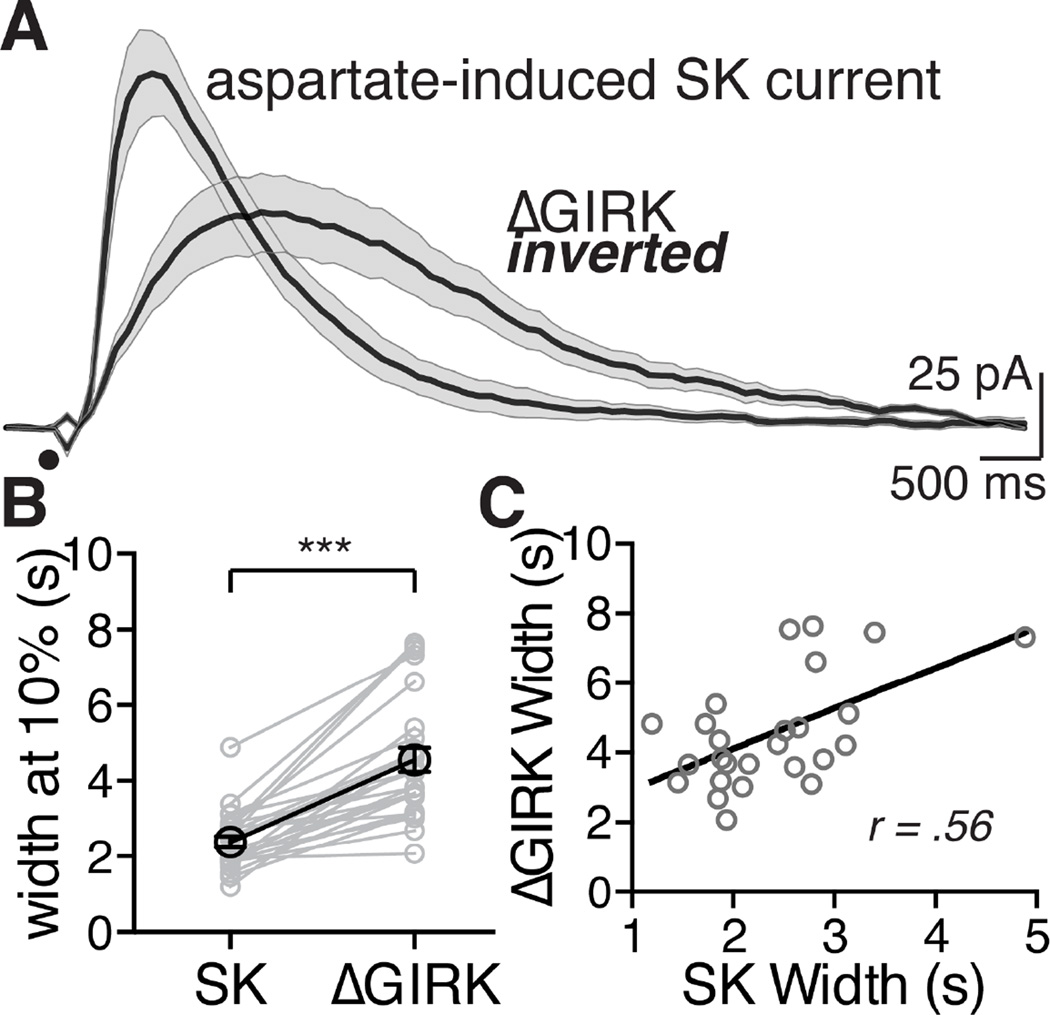
Taken together, mGluR activation during, or immediately preceding, a GABABR-mediated GIRK current decreased the GIRK conductance. Both the SK current and the ΔGIRK current were mediated by mGluRs, however the kinetics of the two currents were different. The duration of the SK current was about half that of the inhibition of the GIRK conductance measured at 10% of the peak (SK: 2.38 ± 0.15 s; ΔGIRK: 4.56 ± 0.31 s; Figure 3A and B). But when the duration of each was compared within cells there was a significant positive correlation suggesting an association between the two signaling pathways (Figure 3C).
Figure 3. The mGluR-mediated SK current and ΔGIRK inward current have different kinetics and are correlated.
A. Averaged traces of currents (SK and ΔGIRK) following iontophoresis of aspartate (black circle). Each trace is an average of 12 cells, the shaded area around the mean value represents s.e.m. ΔGIRK current is inverted to compare the timescale to SK.
B. Within-cell comparison of the width at 10% of the peak for the SK current and the corresponding ΔGIRK current for that cell. ΔGIRK was significantly longer at 10% of the peak than SK. ***p < 0.001, statistics were performed with a two-tail paired t-test.
C. Comparing within-cell the width of the SK versus the width (both at 10%) of the ΔGIRK reveals a significant positive correlation, analyzed with a Pearson correlation test, p < 0.01, r = 0.56, n = 26 cells for B and C.
Calcium release from stores inhibits GIRK
The mGluR dependent SK current is activated by release of calcium from stores, and depletion of stores by inhibition of the smooth endoplasmic reticulum calcium pump with cyclopiazonic acid (CPA, 10 µM) blocks this current (Morikawa et al., 2000). CPA was therefore tested on the mGluR inhibition of the GIRK conductance. CPA eliminated the SK current and blocked the inhibition of GIRK (aspartate evoked SK in control: 160 ± 27.7 pA*S, after CPA: −18.7 ± 8.49 pA*S, in baclofen: −16.9 ± 11.3 pA*S, wash baclofen: −22 ± 17.4 pA*S; Figure 4A, 5B). Strong calcium buffering also blocked the inhibition of GIRK. The control internal solution contained EGTA (100 µM) that minimally buffered calcium. The activation of mGluRs with this internal solution always resulted in the activation of SK and an inhibition GIRK (Figure 4C). When the concentration of EGTA was increased to 1 mM the SK current was inhibited in six of ten cells, and in those six cells the mGluR inhibition of GIRK was prevented. In the remaining four cells where the SK current persisted, so did the mGluR induced GIRK inhibition (Figure S6). Increasing the concentration of EGTA to 10 mM blocked both the SK current and inhibition of GIRK after mGluR activation in every cell tested (Figure 4C, Figure S5A and S5C).
Figure 4. Inhibition of GIRK requires calcium release from stores.
A. Representative traces showing the currents elicited by aspartate iontophoresis at baseline (left), after CPA (10 µM, middle), and during baclofen (10 µM, right). In this example, baclofen elicited a standing outward current of 293 pA (not pictured to scale).
B. Grouped data showing the aspartate induced current at baseline, after CPA, during baclofen and after washout. Treatment with CPA blocked the aspartate induced a ΔGIRK inward current during baclofen. Each grey dot represents a single cell, lines connect treatments within a cell. Black circles are means ± s.e.m. Statistics were performed with a repeated measures two-way ANOVA followed by a Bonferroni test, ns = not significant (p > 0.99), n = 6 cells.
C. Comparison of the ΔGIRK charge during baclofen (10 µM) with different concentration of EGTA in the internal solution. In EGTA (0.1 mM) there is always a ΔGIRK current (12/12 cells). In 1 mM EGTA there is a ΔGIRK current in 4/10 cells. In 10 mM EGTA the ΔGIRK (0/7 cells) was completely blocked.
Figure 5. Receptor activation is not required for GIRK inhibition.
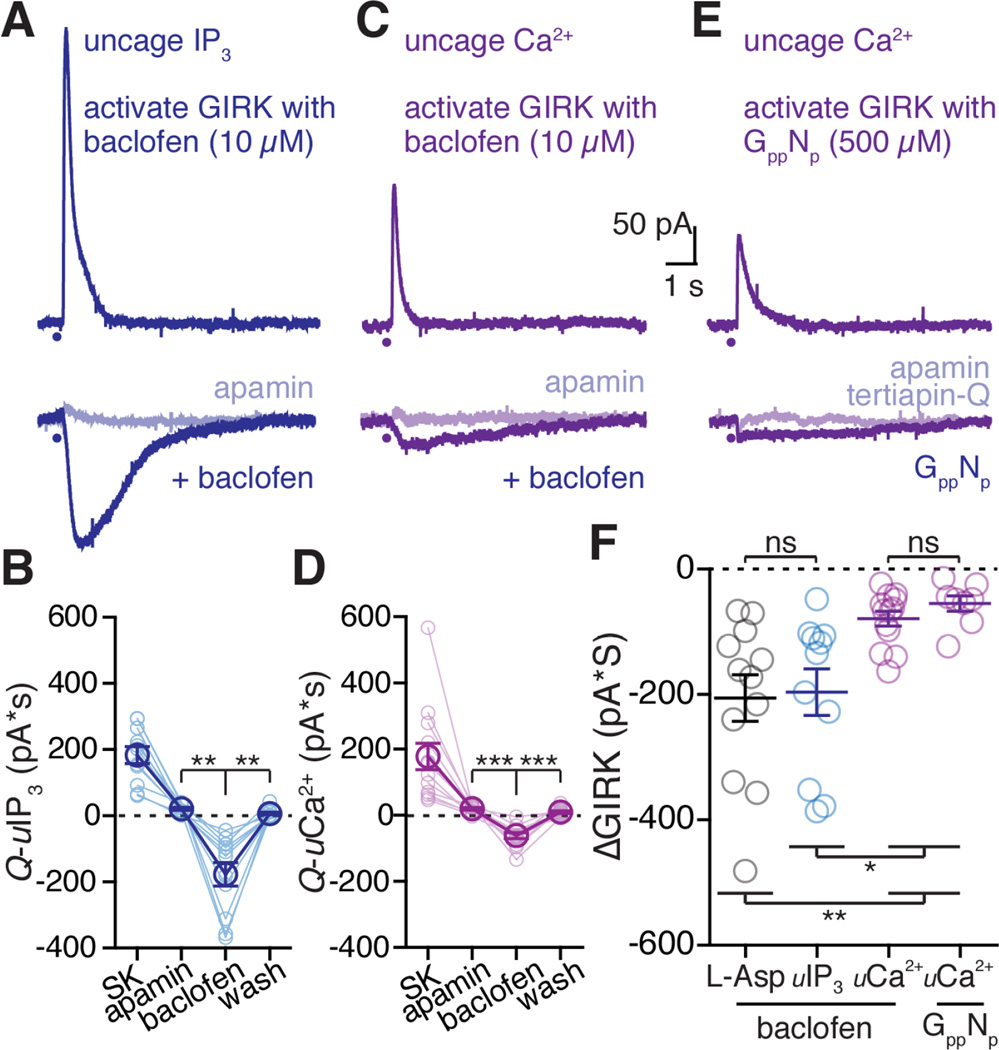
A. Representative traces from a whole cell voltage clamp recording using photolytic release (405 nM LED pulse) of caged IP3 (100 µM) in control (top trace), after application of apamin (bottom light blue trace) and following superfusion of baclofen (10 µM, blue trace).
B. Quantified data across cells of all caged-IP3 flash photolysis experiments. Light blue traces represent data from a single cells, dark blue represents mean data ± s.e.m. Statistics performed with a one-way repeated measures ANVOA followed by a Bonferroni, **p < 0.01, n = 11 cells.
C. Representative traces from a whole cell voltage clamp recording using photolytic release (365 nM LED pulse) of calcium with DMNPE-4 (1 mM). Top trace shows direct activation of SK, bottom trace (light purple) after apamin, and bottom trace (purple) following superfusion of baclofen (10 µM).
D. Quantified data across all caged-Ca2+ photolysis experiments. Light purple connected circles represent a data from a single cell, dark purple represents mean averaged data ± s.e.m. Statistics performed with a one-way repeated measures ANOVA followed by a Bonferroni test, ***p < 0.001, n = 13.
E. Representative traces from a whole cell voltage clamp recording using photolytic release of calcium with DMNPE-4. The internal solution also contained GppNHp (GppNp, 500 µM) that activated GIRK. Top trace is the SK current induced by photolysis of DMNPE-4. Bottom trace (light purple) is following treatment with apamin. Bottom trace is the decrease in GIRK induced by calcium at the peak of the outward current induced by GppNHp.
F. Comparing the ΔGIRK charge transfer (baseline subtracted) for GIRK currents activated with baclofen (10 µM) and calcium released via aspartate (black), photolysis of caged-IP3 (blue) or photolysis of DMNPE-4 (purple, left), and for GIRK currents activated with GppNHp and calcium released by photolysis of DMNPE-4 (purple, right). Each circle represents a single cell, bars are the mean value ± s.e.m. ns = not significant. Statistics performed with a one-way ANOVA followed by a Tukey test. *p<0.05, **p<0.01, n = 12 (ionto), n = 11 (IP3), n = 13 (DMNPE-4, baclofen), n = 8 (DMNPE-4, GppNHp) cells.
IP3-mediated calcium release from stores inhibits GIRK
To test if bypassing G protein activation and directly releasing calcium from internal stores could inhibit GIRK, neurons were loaded with caged-IP3 (100 µM) that was photolysed by blue light (100 ms, 405 nm). Photolytic release of IP3 produced an SK current (183 ± 25.7 pA*S, time to 5% of peak: 81.5 ± 10.1 ms, 10–90% rise time: 49.4 ± 3.6 ms, width at 20% of peak: 677 ± 68.3 ms, Figure 5A), which was blocked by apamin (300 nM, 21.0 pA*S ± 4.3 pA*S; Figure 5B). When baclofen (10 µM, average peak: 425 ± 42.6 pA) was superfused, the photolytic release of IP3 caused a ΔGIRK current (−177 ± 35.7 pA*S, time to 5% of peak: 143 ± 22.6 ms, 10–90% rise time: 324 ± 23.9 ms, width at 20% of peak: 2432 ± 181 ms, Figure 5A). The size of the ΔGIRK current induced by the release of IP3 (−196 ± 37, Figure 5F) was similar to that induced by aspartate (−206 ± 37 pA*S). The IP3-dependent inhibition of GIRK was eliminated by depleting calcium stores using CPA (inhibition after CPA: 14.8 ± 5.6 pA*S; in baclofen: −4.3 ± 1.2 pA*S; p = 0.23, n = 4 cells, one-way repeated measures ANOVA followed by a Tukey test).
Photolytic release of caged-Ca2+ inhibits GIRK
To test if the photolytic release of calcium alone is sufficient to inhibit GIRK, neurons were dialyzed with a calcium cage (DMNPE-4, see methods) loaded 90% with CaCl2. Under these conditions, the upper limit of resting calcium levels in the cell was calculated to be 406 nM and the lower limit 115 nM (see methods). Calcium was released with UV light (10–100 ms, 365 nm) resulting in the activation of an apamin sensitive SK current (Figure 5C). Baclofen (10 µM, 305 ± 28.7 pA) was applied following treatment with apamin, and photolysis of caged-Ca2+ produced a ΔGIRK current during baclofen superfusion (calcium current at baseline: 178 ± 40 pA*S; in apamin: 19.7 ± 3.8 pA*S; in baclofen: −61 ± 8.9 pA*S; after wash: 9.4 ± 3.5 pA*S; Figure 5D). Thus, photolytic release of calcium alone results in the inhibition of the GIRK conductance. To test if calcium could inhibit GIRK independently of receptor activation, neurons were loaded with a combination of DMNPE-4 caged-Ca2+ and GppNHp (500 µM), a non-hydrolyzable GTP analogue. Apamin (300 nM) was superfused immediately after the beginning of the recording, and experiments were performed in blockers of GABAB, mGlu, and D2 receptors. GppNHp produced an outward current that was partially reversed with the GIRK channel inhibitor tertiapin-Q (TPN-Q, 300 nM). The GppNHp evoked GIRK current (230 ± 17.7 pA) was obtained by subtracting the TPN-Q sensitive current from the peak outward current. Calcium was photolytically released at the beginning of the recording, at the peak of the GppNHp current, and again after TPN-Q. There was an inward current observed at the peak of the GppNHp current that was absent after TPN-Q treatment, representing a ΔGIRK current (Figure 5E). GIRK currents activated by GppNHp or by baclofen were similarly inhibited by photolytic release of caged-Ca2+ (Figure 5F). Thus, the calcium-mediated inhibition of GIRK does not depend on Gi-coupled receptor activation.
Inhibiting calcium-activated enzymes did not block the inhibition of GIRK
To test the possibility of downstream calcium-activated molecules being required for the inhibition of GIRK, known inhibitors of calcium-activated proteins were tested. Slices were incubated at least 10 minutes in staurosporine (1 µM) to inhibit PKC, but the mGluR inhibition of GIRK persisted (apamin: 11.6 ± 7.04 pA*s; baclofen: −179 ± 54.1 pA*s; wash: −14.3 ± 6.69 pA*s; Figure S4A). The PKC inhibitor calphostin-C was also tested, but did not block mGluR-mediated GIRK inhibition (apamin: 13.3 ± 14.8 pA*s; baclofen: −505 ± 155 pA*s; wash: −8.12 ± 13.5 pA*s; Figure S4B). Slices were incubated in OBAA (3–10 µM) to inhibit PLA2, but the mGluR-mediated GIRK inhibition was not blocked (apamin: 6.35 ± 15.0 pA*s; baclofen: −397 ± 112 pA*s; wash: −46.9 ± 36.8 pA*s; Figure S4D). The PLC inhibitor U73122 (1 µM) was included in the pipette and allowed to dialyze into the neuron for at least 10 minutes, but the mGluR-mediated GIRK inhibition was not affected (peak I-GABA at 1 minute: - mGluR: 114 ± 19.8 pA, + mGluR: 36.1 ± 7.23 pA; at 7 min: - mGluR: 112 ± 18.7 pA, + mGluR: 31.5 ± 11.5 pA; Figure S4E). Finally, slices were incubated at least 10 minutes in the the calmodulin inhibitor, calmidazolium (10 µM), but this did not block the mGluR-mediated GIRK inhibition (apamin: −10.8 ± 32.6 pA*s; baclofen: −276 ± 25.9 pA*s; wash: −37.3 ± 32.4 pA*s; Figure S4C). The only consistent and robust block of the inhibition of GIRK by mGluR activation is through robust calcium buffering in the neuron, or prevention of calcium release from stores.
GABAB IPSCs are inhibited by Calcium
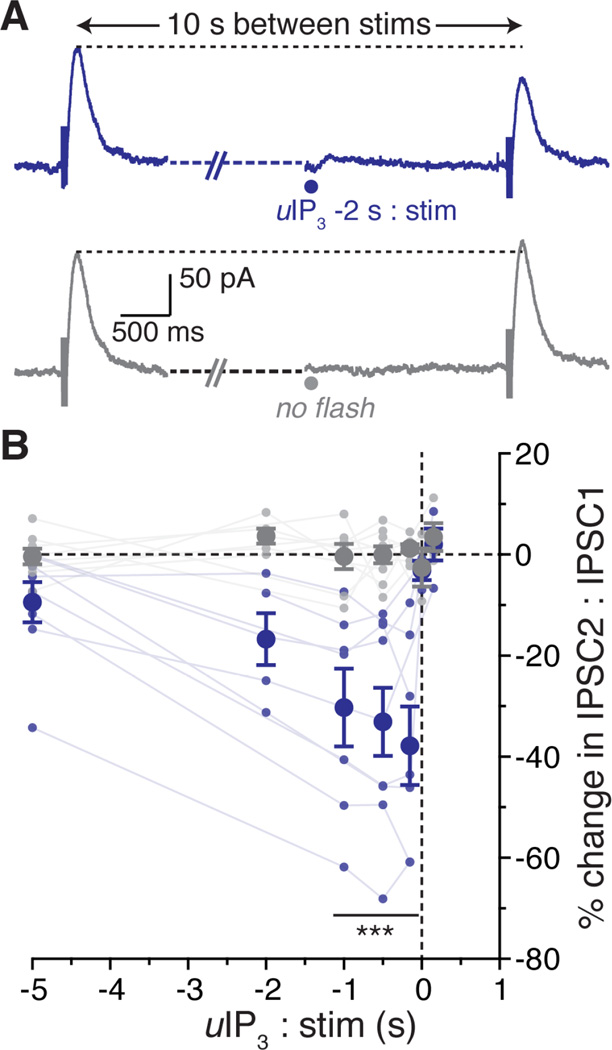
The synaptic release of GABA results in a GABABR-dependent inhibitory postsynaptic current (IPSC) mediated by GIRK. To test if calcium inhibits this synaptic current, paired electrically activated GABABR IPSCs (5 pulses at 100 Hz, 10 seconds apart) were evoked once per minute. Blockers of mGluR1 and mGluR5 were included in the solution to block synaptic activation of mGluRs by glutamate release. Calcium was released from intracellular stores using photolysis of caged-IP3 at various times before the second electrical stimulation (Figure 7A). The GABABR IPSC was maximally inhibited when calcium was released from stores 150 to 1000 ms prior to synaptic stimulation (inhibition after 1000 ms 30.3 ± 7.7%, after 500 ms 33.1 ± 6.8%, after 150 ms 37.8 ± 7.8 %; Figure 7B). Though, in three of five cells the IPSC was inhibited at last 10% by calcium release from stores two seconds prior to electrical stimulation, and in three of eight cells five seconds before stimulation. Thus a GABAB IPSC is sensitive to the release of calcium from stores.
Figure 7. mGluR-induced calcium release inhibits D2R mediated GIRK currents less than GABAB.
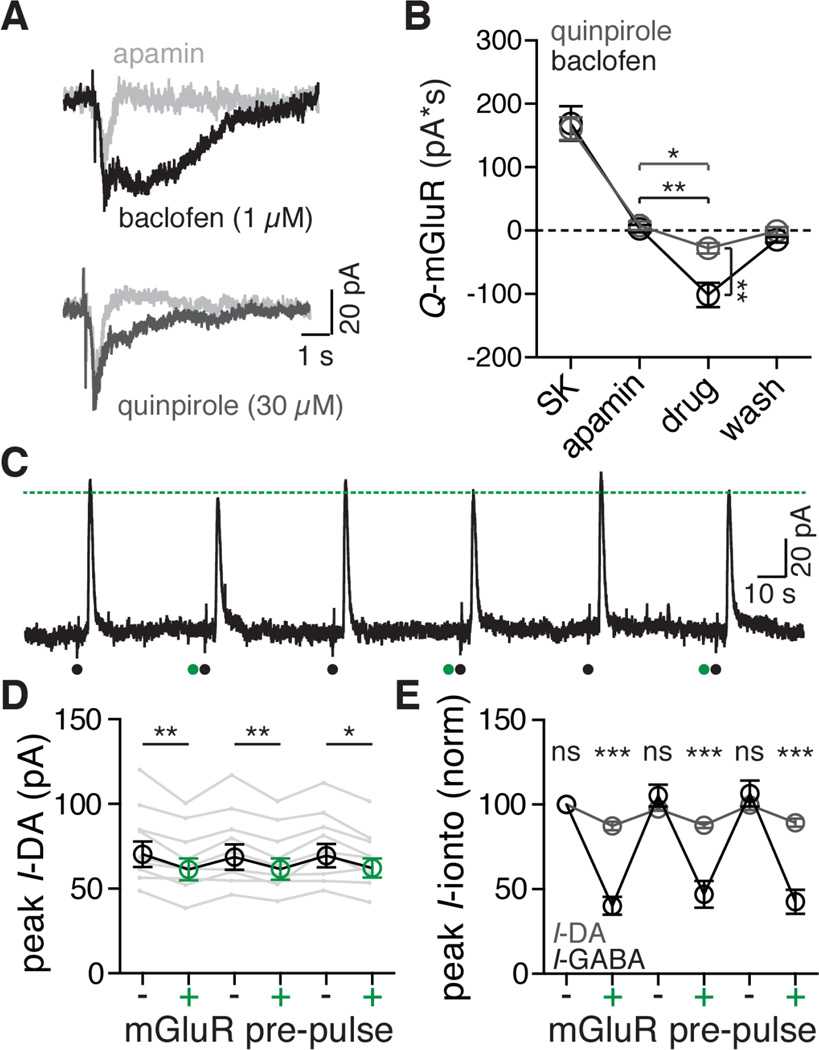
A. Representative traces of currents induced by iontophoresis of aspartate in the presence of apamin (300 nM, light gray), baclofen (1 µM, top trace, black), and quinpirole (30 µM, bottom trace, black).
B. Quantified data across cells comparing the aspartate induced mGluR currents at baseline (SK), after apamin (300 nM), in either quinpirole (30 µM, grey) or baclofen (1 µM, black), and upon washout or reversal of the agonist. Both quinpirole and baclofen resulted in a decrease in GIRK current relative to apamin. Statistics were performed with two one-way ANOVAs followed by a Bonferroni test. *p < 0.05, **p < 0.01, n = 8 (quinpirole), n = 7 (baclofen), points represent means ± s.e.m.
C. Representative trace from a dual iontophoresis protocol with dopamine (black dot) and aspartate (green dot) in the presence of apamin (300 nM). Dotted green line indicates the peak of the GIRK current induced by iontophoretic application of dopamine following application of aspartate. Aspartate iontophoresis caused a consistent decrease the D2R–mediated current.
D. Grouped data showing the peak amplitude of the dopamine D2 receptor-mediated GIRK current with (green) and without (black) iontophoretic application of aspartate one second before dopamine iontophoresis in the presence of apamin (300 nM). Statistics were performed with a one-way repeated measures ANOVA followed by a Bonferroni test, *p < 0.05, **p < 0.01, n = 9 cells. Grey traces indicate within-cell data for single neurons, circles (black and green) ± s.e.m.
E. Grouped data for all dopamine (grey) and GABA (black) dual iontophoresis experiments, normalized to the peak amplitude of the first pulse. Data points indicate means ± s.e.m. Statistics were performed with a two-way repeated measures ANOVA followed by a Bonferroni test, ***p < 0.001, ns = not significant (p > 0.99), n = 9 cells for both groups.
See also Figure S7
Preferential inhibition of GABAB induced GIRK
Dopamine neurons also express the dopamine D2 receptor (D2R) that activates a GIRK conductance (Beckstead et al., 2004). The inhibition of D2R GIRK current by mGluR activation was examined next. The amplitude of the D2R GIRK currents was smaller than GABAB GIRK currents. A saturating concentration of quinpirole induced a current that was about the same as that induced by sub-saturating baclofen (baclofen, 1 µM: 109 ± 17.4 pA; quinpirole, 30 µM: 138 ± 12.6 pA). Using baclofen (1 µM) and quinpirole (30 µM), mGluR activation produced a significantly larger inhibition of the baclofen GIRK current than the quinpirole GIRK current (baclofen control: 2.89 ± 5.78 pA*S, baclofen: −101 ± 19.2 pA*S; quinporole control: 7.85±6.72 pA*S, quinpirole: −27.9 ± 8.36 pA*S; Figure 7A,B)
This experiment was also carried out using iontophoretic application of dopamine, GABA and aspartate. The application of aspartate one second before dopamine significantly and repeatedly reduced the peak dopamine current (from 69.5 ± 7.3 pA to 61.7 ± 6.2 pA; Figure 8D). The percent inhibition of dopamine-mediated GIRK currents by mGluR activation (11.8 ± 1.3%) was significantly less than the inhibition of GABA-mediated GIRK currents (56.7 ± 3.8%; Figure 7E).
With the exception of experiments illustrated in Figure 1, all experiments were conducted in the presence of apamin to enable the characterization of the selective modulation of the GIRK conductance in the absence of the SK conductance. The interaction of currents induced by aspartate and dopamine or GABA applied using iontophoresis in the absence of apamin was examined next. The currents induced by aspartate, dopamine, or GABA applied alone were measured. Aspartate was then applied one second before either dopamine or GABA (Figure S7A B). The linear summation of the aspartate plus GABA or dopamine was compared with the observed current induced by iontophoretic application (GABA: supplemental figure 7A, C; dopamine: Figure S7B, D). The linear summation of the individual currents evoked by aspartate + GABA or dopamine was always larger than the observed co-application of agonists (GABA summated: 176 ± 27.9 pA, observed: 119 ± 23.7 pA; dopamine summated: 137 ± 20.3 pA, observed: 108 ± 18.2 pA). Furthermore, the inhibition of the peak GABABR current was always significantly greater than for the D2R mediated GIRK current (Figure S7E).
Discussion
Transient mGluR activation evoked an IP3-mediated increase in cytosolic calcium and a decrease GIRK conductance. The inhibition peaked in 1 s and lasted 2–5 s. The activation of mGluRs also inhibited GABABR IPSCs. The calcium-mediated inhibition of GIRK was not prevented by pharmacological inhibition of PLC, PKC, PLA2 or calmodulin, enzymes that had been previously implicated in the Gq-mediated inhibition of GIRK. The only manipulations that blocked the mGluR inhibition of GIRK were depletion of calcium stores and strong cytosolic calcium buffering. Furthermore, direct release of calcium from stores mimicked the GIRK inhibition produced by mGluR activation. While the precise mechanism remains unclear the results indicate that there is a functionally significant inhibition of GIRK by calcium released from stores.
Mechanism of GIRK inhibition by calcium release from stores
The inhibition of GIRK by mGluR activation and photolytic activation of IP3-mediated calcium release from stores was indistinguishable, but photolytic release of calcium produced a significantly smaller inhibition. This could be because the GIRK was already partially inhibited by the resting level of calcium (between ~115 and ~406 nM), as has been reported (Gantz et al., 2015b). On average, GIRK currents achieved with the same concentration of baclofen were ~30% smaller with caged-Ca2+ in the pipette than with caged-IP3, likely occluding further calcium inhibition and minimizing the apparent effect of photolytic release of calcium. However, despite this constraint, photolytic release of calcium was still able to inhibit GIRK currents whether they were mediated by GABABR activation or from inclusion of GppNHp in the pipette.
The most direct interpretation of the present study is that calcium directly inhibits the GIRK channel. It has been reported that external calcium, as well as other divalent cations, can block IRK (including Kir 3.1 and 3.4) channels (Owen et al., 1999). However there is no complete characterization of any direct inhibition of GIRK by intracellularly positioned calcium ions.
Given what is known about GIRK channel function, there are three potential sites for modulation by calcium. Calcium could act at the Gβγ site to inhibit binding, dissociate PIP2 from the channel, or competitively bind to the sodium site. GIRK is an AND gate, meaning Gβγ and PIP2 are both necessary for channel activation, so negative modulation of either of them would transiently close the channel until calcium was fully sequestered. Sodium, by contrast, enhances the activity of the channel but is not required for current flow, making it a less likely but still possible site of action of calcium (Whorton and MacKinnon, 2011; Wang et al., 2014).
It is possible the inhibition of GIRK currents by calcium is not a direct action of calcium on the channel itself. While pharmacological inhibitors of PLC, PKC, PLA2, and calmodulin failed in affect the calcium-mediated inhibition of GIRK, there could be some other unexpected calcium-activated protein responsible for the inhibition of GIRK currents.
IP3-mediated SK currents and GIRK inhibition have different kinetics
It is of interest to note that, when photolytically releasing caged-IP3, the SK current onset at 5% of peak was almost twice as fast as the inhibition of GIRK (82 vs 143 ms), the SK rise time from 10% to 90% of peak was about 6.5 times faster (49 ms vs 324 ms), and the SK width at 20% of peak was about 3.5 times shorter (677 ms vs 2432 ms). Both processes are calcium dependent, but SK channel activity has been better established and is known to depend solely on intracellular calcium levels and does not desensitize, making it a faithful sensor of intracellular calcium activity (Lancaster et al., 1991). Therefore, the differing kinetics of current onset, time to peak, and width indicate a difference in underlying mechanisms. Without knowing the precise mechanism of calcium-mediated GIRK inhibition, only speculations can be offered to account for the differences in kinetics. For example, the difference in rise time could result from an inherit difference in SK versus GIRK activation or deactivation rates. It also remains possible that an intermediary step is responsible for the inhibition of GIRK.
Preferential GABAB GIRK inhibition by calcium release from stores
In the present study D2R GIRK currents were decreased by calcium release from stores to a significantly smaller degree than the inhibition of GABABR GIRK currents. Previous work has shown that GABAB and D2 receptors have differential sensitivity to calcium. Resting cytosolic calcium levels play a central role in the desensitization and long-term depression of the dopamine D2 receptor, but not the GABAB receptor (Beckstead and Williams, 2007; Gantz et al., 2015b). Increasing cytosolic calcium decreased peak GABABR-, but not peak D2R-, mediated GIRK currents (Gantz et al., 2015b).
It is known that D2 receptors associate with the neuronal calcium sensor-1 (NCS-1), which alters desensitization of the receptor (Kabbani et al., 2002). D2 receptors are also localized in close proximity to the calcium channel Cav1.3 (Dragicevic et al., 2014). Furthermore, depleting calcium stores or inhibiting calcium entry through the L-type voltage gated calcium channel increased the amplitude of a D2R-medaited GIRK current in wild type mice (Gantz et al., 2015b). Given the results of the present study in light of these results, it is possible that D2 receptor mediated GIRK currents at baseline are nearly maximally inhibited by calcium entry through Cav1.3, IP3 receptors, and/or resting calcium levels. Indeed, D2R-mediated currents in the present study were consistently smaller than GABABR-mediated currents. A second interpretation of the present results is that calcium release from stores was preferentially affecting GABABR GIRK currents due to the close proximity of IP3 receptors with the GABAB signaling complex, and separated compartmentalization of the D2R complex.
A general mechanism to decrease GIRK dependent synaptic inhibition
Glutamate release resulting in an mGluR-mediated IPSC could have an initial inhibitory SK-mediated component followed by a period lasting as long as 5 seconds during which GIRK-mediated inhibition could be decreased. Given that some VTA neurons have very little SK-mediated current (Wolfart et al., 2001), the activation of mGluRs would disinhibit cells through the calcium-mediated inhibition of GIRK-dependent synaptic currents. Furthermore, many neurons have functional GIRK currents and IP3 receptors, such as hippocampal pyramidal cells (Nakamura et al., 2000; Degro et al., 2015) and 5-HT neurons of the dorsal raphe (Gantz et al., 2015a; Pan et al., 1994). Thus the interaction between calcium release from stores and GIRK channel currents described here may not be a dopamine neuron-specific effect.
Finally, calcium buffering and manipulations of the resting level of free calcium modulates the GIRK conductance activated by both D2 and GABAB receptors in that strong calcium buffering with BAPTA (10 mM) results in a larger GIRK currents (Beckstead and Williams, 2007; Gantz et al., 2015b). In addition the regulation of GIRK conductance by calcium is modulated following treatment of animals with psychostimulants. The calcium-dependent inhibition of GIRK is augmented following treatment of animals with cocaine (Arora et al., 2011; Gantz et al., 2015b) and animals that self-administered methamphetamine (Sharpe et al., 2014).The mechanisms underlying these observation may differ in that there was no receptor dependence in animals that self-administered methamphetamine (Sharpe et al., 2014) whereas the present results indicate that the GABAB-induced currents are more susceptible to the calcium dependent regulation.
To close, GIRK channels are well known mediators of neuronal inhibition, and the modulation of this conductance has been studied extensively. Typically modulation has been studied on the order of minutes (Xia et al., 2010) to days (Arora et al., 2011). The data presented here show that release of calcium from stores inhibits GIRK currents in milliseconds and reverses in seconds, a dynamic process that can modulate on the timescale of GIRK-dependent synaptic transmission. This demonstration of the direct effect of calcium on GIRK currents has broad implications for inhibition mediated by GIRK channels.
Experimental Procedures
Animals
All animal experiments were performed in accordance with the National Institutes of Health guidelines and with approval from the Institutional Animal Care and Use Committee of the Oregon Health & Science University (Portland, OR). Adult (6–10 week), male and female, Sprague-Dawley rats were used for all experiments.
Slices and solutions
Rats were anesthetized in a vapor chamber (isoflurane, OHSU pharmacy) and killed. Brains were extracted and blocked, removing the cerebellum and frontal cortex rostral to the optic chiasm, the dorsal surface of the brain was fixed onto the vibratome stage (krazy glue) and slices (230 µm) containing the Substantia Nigra were prepared. Slices were cut using a vibratome (Leica) in warm (30°C) physiological ACSF containing (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 11 glucose, 21.4 NaHCO3, 5 MK801, saturated with 95% O2 and 5% CO2, pH 7.4, 300 mOsm/kg, and then incubated in the same solution warmed to 34°C for 30–60 minutes. After incubation slices were transferred into the physiological ACSF lacking MK801. Slices were placed in a recording chamber and superfused with warmed (35°C) physiological ACSF at 1.5 to 2 ml/min.
Whole-cell recording
All recordings were performed from dopamine neurons of the Substantia Nigra pars compacta, defined as a region lateral of the Medial Terminal Nucleus of the Accessory Optic Tract (MT). Dopamine neurons were identified by their large cell bodies, the characteristic pacemaker-like firing (1–5 Hz) recorded in the cell-attached mode, and the presence of a large Ih current. Whole-cell pipettes had resistances of 1.3–1.8 MΩ. Unless otherwise noted, pipette solutions used for whole-cell recordings contained (in mM): 115 K-methanesulfonate, 20 NaCl, 1.5 MgCl2, 10 HEPES, 0.1 EGTA, 2 Mg-ATP, 0.25 Na2-GTP, and 10 Na2phosphocreatine, pH 7.42, 283 mOsm/kg. Voltage-clamp recordings were made (holding potential −55 mV) using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). Series resistance was monitored using a 3 mV test pulse (collected at 50 kHz and filtered at 10 kHz). Recordings were discarded if the series resistance exceeded 12 MΩ. Episodic data were obtained at 5 kHz and filtered at 2 or 5 kHz using AxoGraph X (Axon Instruments). Continuous recording at 200 Hz was obtained using Chart (version 5.5.6, AD Instruments, Colorado Springs, CO).
Iontophoretic application
Transient application of agonists (dopamine, GABA, or aspartate) using iontophoresis produced outward currents that mimicked synaptically evoked IPSCs. Iontophoresis was performed with an Axoclamp 2A amplifier (Axon Instruments) using pipettes containing L-aspartate (1 M, pH 7.4, 40–70 MΩ, −80–130 nA ejection and +1.5–3 nA backing current), dopamine (1 M, 40–60 MΩ, +30–100 nA ejection and −1.5–3 nA backing current), or GABA (500 mM, pH 7.3, 30–50 MΩ, +100–190 nA ejection and −0.2–3 nA backing current). Iontophoretic pipettes were placed within 5–10 µm of the soma. mGluR currents were isolated after pretreatment with (5S,10R)-(+)-5-methyl-10,11-dihydro-5H–dibenzo[a,d]cyclohepten-5,10-imine (MK-801, 50–100 µM) and in the presence NBQX (300 nM). Iontophoretic experiments with GABA included picrotoxin (100 µM) and SR-95531 (300 nM) to block GABAA receptors. Iontophoretic experiments with dopamine, included prazosin (100 nM) in the superfusion solution to block α1-adrenoceptors (Paladini et al., 2001). Apamin (100–300 nM) was used to block SK-mediated outward currents while testing the block of GIRK by mGluR activation. The protocol illustrated in Figure S1 was used to test the effect of aspartate iontophoresis on the current elicited by GABA or dopamine.
Synaptic stimulation
GABAB mediated IPSCs were evoked with a train (5 pulses at 100 Hz) using a monopolar electrode (filled with physiological ACSF, 2–2.5 MΩ) placed ~70–100 µm lateral and ~40–60 µm caudal from the patched neuron. Stimulation intensity was adjusted to obtain IPSCs (>20 pA) that did not evoke a direct action current in the recorded neuron. GABAB IPSCs were isolated by including NBQX (300 nM), picrotoxin (100 µM), sulpiride (300 nM), JNJ-16259685 (1 µM), and MPEP (300 nM), to block AMPA, GABAA, D2, mGluR1 and mGluR5 receptors respectively. Slices had been pre-incubated in MK801 (50–100 µM) to block NMDA receptors. In most experiments CGP-55845 (300 nM) was applied at the end of the experiment to verify that the IPSC resulted from GABAB receptors. Photolysis experiments of caged-IP3 in combination with the GABABR IPSC included at least three trials per time point that were averaged. IPSCs were evoked at 1 min intervals and interleaved with experiments using the combination of photolysis and electrical stimulation.
Construction of current-voltage plots
Current/voltage (I/V) plots were constructed using ramp protocols. Agonist-induced currents were isolated by subtracting the evoked current from its respective baseline. Averages of at least two ramps were combined before subtraction. The ramp protocol was used in combination of agonists applied by superfusion (at steady state, Figure S2) and iontophoresis (transient, Figure S1B).
Caged compound photolysis
Photolysis of caged-IP3 (Walker et al., 1989) and caged-Ca2+ (Ellis-Davies and Barsotti, 2005) was performed on a separate experimental set-up. Whole-cell recordings were made with an Axopatch 1D amplifier (Molecular Devices, Sunnyvale, CA). Episodic currents were recorded at 10 kHz for 1 minute using AxoGraphX (1.4.3; AxoGraphX, Berkeley, CA).
A stock solution of caged-IP3 (0.1 mM) was added to normal internal. Photolysis of caged-Ca2+ was done with a modified internal solution where DMNPE-4 (1 mM) and CaCl2 (0.9 mM) were added. All experiments were carried out in the dark.
All photolysis experiments were carried out with full-field illumination (365 or 405 nm LED, Thorlabs, Sterling, VA) coupled through a 60x objective (Olympus, 0.9 na). The IP3 generated SK currents were of similar size with 365 or 405 nm LED photolysis. The 365 nm LED was used for photolysis of caged-Ca2+.
Calculating resting calcium levels
The Chelator web application, version 1.3 (maxchelator.stanford.edu), was used to calculate resting calcium levels using the values given for whole-cell voltage clamp experiments, and 1.1 mM buffer with 900 µM added CaCl2. Because there are two buffers in the internal that have slightly different affinities for calcium at pH = 7.4 (DMNPE-4: 19 nM and EGTA: 67 nM) a range for the possible resting calcium concentration was calculated. The upper estimate was based on the EGTA affinity, whereas the lower limit was based on DMNPE-4.
Drugs
Caged-IP3 was a generous gift from Hitoshi Morikawa, PhD (University of Texas, Austin, TX), who obtained it from Kamran Khodakhah, PhD (Albert Einstein School of Medicine, Bronx, NY). DMNPE-4 caged-Ca2+ was a generous gift from Graham Ellis-Davies, PhD (GM053395, Ichan School of Medicine at Mount Sinai, NY, NY). CGP-55845, JNJ-16259685, and MPEP were obtained from Tocris (Minneapolis, MN). Apamin was obtained from Calbiochem (San Diego, CA). NBQX and MK801 were obtained from Abcam (Cambridge, MA). SR-95531 was obtained from Hello Bio (Princeton, NJ). The remaining compounds were obtained from Sigma-Aldrich (St. Louis, MO).
Data analysis
To determine the total charge transfer induced by mGluR activation, and to account for the duration of GIRK inhibition, the area under the curve (AUC) was measured from the start of the current until the end and expressed as pA*S. For most experiments apamin was used to isolate inward currents. When the peak of the evoked current was measured, 30 ms of data were averaged around a center point. Data are expressed as mean ± standard error of the mean (s.e.m.). For data in Supplemental Figure 9-1, currents were summated using AxoGraph X. Statistical significance was determined with Student’s t-test or one-way ANOVA followed by the Tukey’s post-hoc test or Bonferroni test. The difference was considered significant at p < 0.05. When necessary a two-way ANOVA was performed followed by Bonferroni’s post-hoc test. Data were graphed and analyzed using Prism 6 (GraphPad, La Jolla, CA). Figures were prepared using Illustrator CS5.1 (Adobe, San Jose, CA). At least three biological replicates were tested per condition.
Supplementary Material
Figure 6. Calcium release from stores inhibits GABAB IPSCs.
A. Representative trace showing GABABR IPSCs evoked with a monopolar stimulating electrode (100 Hz, 5 pulses) in pairs with a 10 second inter-stimulus interval. Photolysis of caged-IP3 (blue dot, 405 nm, 30–100 ms) was applied at differing times between the pair of stimulations. Experiments were interleaved with traces where IP3 was not applied (lower trace, grey).
B. Each small dot represents a data point from a single cell (average of three repetitions), connected by a line to show a within-cell data set. Points were averaged across cells (large dots). Pairs where IP3 was released before the second stimulation are in blue, traces with no flash are in grey. Error bars represent s.e.m. Statistics were performed with a two-way repeated measures ANOVA followed by a Bonferroni test. ***p < 0.001, n = 3 – 9 cells per averaged data point.
Highlights.
mGluR-mediated calcium release from stores inhibits GIRK currents
GIRK inhibition does not require Gq or Gi receptor activation
GABABR IPSCs are inhibited when calcium is released 1–5 s before stimulation
Inhibitors of PKC, PLC, PLA2, and calmodulin had no effect on GIRK inhibition
Acknowledgments
This work was supported by NIH DA04523 and the OHSU Brain Institute Neurobiology of Disease Lacroute Fellowship. We thank Dr. Hitoshi Morikawa for gifting us caged-IP3 originally produced by Dr. Kamran Khodakhah, and Dr. Graham Ellis-Davies (GM053395) for creating and supplying the DMNPE-4 calcium cage. We also thank members of the Williams lab for comments on the work and manuscript.
Financial disclosure: No competing financial interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, P.F.K. and J.T.W.; Methodology, P.F.K. and J.T.W.; Investigation, P.F.K.; Formal Analysis, P.F.K.; Resources, P.F.K and J.T.W.; Writing – Original Draft, P.F.K.; Writing – Review & Editing, P.F.K. and J.T.W.; Visualization, P.F.K.; Supervision, J.T.W. Funding Acquisition, P.F.K. and J.T.W.
References
- Arora D, Haluk DM, Kourrich S, Pravetoni M, Fernández-Alacid L, Nicolau JC, Luján R, Wickman K. Altered neurotransmission in the mesolimbic reward system of Girk−/− mice. Journal of Neurochemistry. 2010;114:1487–1497. doi: 10.1111/j.1471-4159.2010.06864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora D, Lujan R, Hearing M, Haluk DM, Mirkovic K, Fajardo-Serrano A, Wessendorf MW, Watanabe M, Wickman K. Acute Cocaine Exposure Weakens GABAB Receptor-Dependent G-Protein-Gated Inwardly Rectifying K+ Signaling in Dopamine Neurons of the Ventral Tegmental Area. Journal of Neuroscience. 2011;31:12251–12257. doi: 10.1523/JNEUROSCI.0494-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Williams JT. Long-term depression of a dopamine IPSC. Journal of Neuroscience. 2007;27:2074–2080. doi: 10.1523/JNEUROSCI.3251-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degro CE, Kulik A, Booker SA, Vida I. Compartmental distribution of GABAB receptor-mediated currents along the somatodendritic axis of hippocampal principal cells. Frontiers in Synaptic Neuroscience. 2015;7:1–15. doi: 10.3389/fnsyn.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic E, Poetschke C, Duda J, Schlaudraff F, Lammel S, Schiemann J, Fauler M, Hetzel A, Watanabe M, Luján R, Malenka RC, Striessnig J, Liss B. Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons. Brain. 2014;137:2287–2302. doi: 10.1093/brain/awu131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies GCR, Barsotti RJ. Tuning caged calcium: Photolabile analogues of EGTA with improved optical and chelation properties. Cell Calcium. 2005;39:75–83. doi: 10.1016/j.ceca.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- Gantz SC, Levitt ES, Llamosas N, Neve KA, Williams JT. Depression of Serotonin Synaptic Transmission by the Dopamine Precursor L-DOPA. Cell Reports. 2015a;12:944–954. doi: 10.1016/j.celrep.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz SC, Robinson BG, Buck DC, Bunzow JR, Neve RL, Williams JT, Neve KA. Distinct regulation of dopamine D2S and D2L autoreceptor signaling by calcium. eLife 2015–4. 2015b:09358. doi: 10.7554/eLife.09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JJ, Peralta EG. Inhibition of a Gi-activated potassium channel (GIRK1/4) by the Gq-coupled m1 muscarinic acetylcholine receptor. The Journal of Biological Chemistry. 2001;276:5505–5510. doi: 10.1074/jbc.M008213200. [DOI] [PubMed] [Google Scholar]
- Kabbani N, Negyessy L, Lin R, Goldman-Rakic P, Levenson R. Interaction with neuronal calcium sensor NCS-1 mediates desensitization of the D2 dopamine receptor. Journal of Neuroscience. 2002;22:8476–8486. doi: 10.1523/JNEUROSCI.22-19-08476.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keselman I, Fribourg M, Felsenfeld DP, Logothetis DE. Mechanism of PLC-mediated Kir3 current inhibition. Channels. 2007;1:113–123. doi: 10.4161/chan.4321. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Kobrinsky E, Mirshahi T, Zhang H, Jin T, Logothetis DE. Receptor-mediated hydrolysis of plasma membrane messenger PIP2 leads to K+-current desensitization. Nature Cell Biology. 2000;2:507–514. doi: 10.1038/35019544. [DOI] [PubMed] [Google Scholar]
- Kramer PF, Williams JT. Cocaine Decreases Metabotropic Glutamate Receptor mGluR1 Currents in Dopamine Neurons by Activating mGluR5. Neuropsychopharmacology. 2015;40:2418–2424. doi: 10.1038/npp.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA, Perkel DJ. Calcium activates two types of potassium channels in rat hippocampal neurons in culture. Journal of Neuroscience. 1991;11:23–30. doi: 10.1523/JNEUROSCI.11-01-00023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaney JL, Dekker LV, Tinker A. Regulation of a G protein-gated inwardly rectifying K+ channel by a Ca2+ -independent protein kinase C. Journal of Physiology. 2001;534:367–379. doi: 10.1111/j.1469-7793.2001.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Wang X, Chen F, Wang R, Rojas A, Shi Y, Piao H, Jiang C. Molecular basis for the inhibition of G protein-coupled inward rectifier K(+) channels by protein kinase C. PNAS. 2004;101:1087–1092. doi: 10.1073/pnas.0304827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Imani F, Khodakhah K, Williams JT. Inositol 1,4,5-triphosphate-evoked responses in midbrain dopamine neurons. Journal of Neuroscience. 2000;20:RC103. doi: 10.1523/JNEUROSCI.20-20-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Khodakhah K, Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. Journal of Neuroscience. 2003;23:149–157. doi: 10.1523/JNEUROSCI.23-01-00149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Nakamura K, Lasser-Ross N, Barbara JG, Sandler VM, Ross WN. Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release evoked by metabotropic agonists and backpropagating action potentials in hippocampal CA1 pyramidal neurons. Journal of Neuroscience. 2000;20:8365–8376. doi: 10.1523/JNEUROSCI.20-22-08365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JM, Quinn CC, Leach R, Findlay JBC, Boyett MR. Effect of extracellular cations on the inward rectifying K+ channels Kir2.1 and Kir3.1/Kir3.4. Experimental Physiology. 1999;84:471–488. [PubMed] [Google Scholar]
- Paladini CA, Fiorillo CD, Morikawa H, Williams JT. Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nature Neuroscience. 2001;4:275–281. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Grudt TJ, Williams JT. Alpha 1-adrenoceptors in rat dorsal raphe neurons: regulation of two potassium conductances. Journal of Physiology. 1994;478:437–447. doi: 10.1113/jphysiol.1994.sp020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AL, Varela E, Bettinger L, Beckstead MJ. Methamphetamine self-administration in mice decreases GIRK channel-mediated currents in midbrain dopamine neurons. International Journal of Neuropsychopharmacology. 2014;18:pyu073–pyu073. doi: 10.1093/ijnp/pyu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J-W, Lee D, Cho H, Lim W, Shin H-S, Lee S-H, Ho W-K. Receptor-specific inhibition of GABAB-activated K+ currents by muscarinic and metabotropic glutamate receptors in immature rat hippocampus. Journal of Physiology. 2007;580:411–422. doi: 10.1113/jphysiol.2006.125914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens EB, Shah BS, Pinnock RD, Lee K. Bombesin receptors inhibit G protein-coupled inwardly rectifying K+ channels expressed in Xenopus oocytes through a protein kinase C-dependent pathway. Molecular Pharmacology. 1999;55:1020–1027. [PubMed] [Google Scholar]
- Tozzi A, Bengtson CP, Longone P, Carignani C, Fusco FR, Bernardi G, Mercuri NB. Involvement of transient receptor potential-like channels in responses to mGluR-I activation in midbrain dopamine neurons. The European Journal of Neuroscience. 2003;18:2133–2145. doi: 10.1046/j.1460-9568.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- Walker JW, Feeney J, Trentham DR. Photolabile precursors of inositol phosphates. Preparation and properties of 1-(2-nitrophenyl)ethyl esters of myo-inositol 1,4,5-trisphosphate. Biochemistry. 1989;28:3272–3280. doi: 10.1021/bi00434a023. [DOI] [PubMed] [Google Scholar]
- Wang W, Whorton MR, MacKinnon R. Quantitative analysis of mammalian GIRK2 channel regulation by G proteins, the signaling lipid PIP2 and Na+ in a reconstituted system. eLife 2014–3. 2014:e03671. doi: 10.7554/eLife.03671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AE, Williams JT, Henderson G. Baclofen inhibition of the hyperpolarization-activated cation current, Ih, in rat substantia nigra zona compacta neurons may be secondary to potassium current activation. Journal of Neurophysiology. 1996;76:2262–2270. doi: 10.1152/jn.1996.76.4.2262. [DOI] [PubMed] [Google Scholar]
- Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell. 2011;147:199–208. doi: 10.1016/j.cell.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J, Neuhoff H, Franz O, Roeper J. Differential Expression of the Small-Conductance, Calcium-Activated Potassium Channel SK3 Is Critical for Pacemaker Control in Dopaminergic Midbrain Neurons. Journal of Neuroscience. 2001;21:3443–3456. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y-F, Margolis EB, Hjelmstad GO. Substance P inhibits GABAB receptor signalling in the ventral tegmental area. Journal of Physiology. 2010;588:1541–1549. doi: 10.1113/jphysiol.2010.188367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.