Abstract
We showed previously that rat angiotensin-(1-12) [Ang-(1-12)] is metabolized by chymase and angiotensin converting enzyme (ACE) to generate Angiotensin II (Ang II). Here, we investigated the affinity of cardiac chymase and ACE enzymes for Ang-(1-12) and Angiotensin I (Ang I) substrates. Native plasma membranes (PMs) isolated from heart and lung tissues of adult spontaneously hypertensive rats (SHR) were incubated with radiolabeled 125I-Ang-(1-12) or 125I-Ang I, in the absence or presence of a chymase or ACE inhibitor (chymostatin and lisinopril, respectively). Products were quantitated by HPLC connected to an in-line flow-through gamma detector. The rate of 125I-Ang II formation from 125I-Ang-(1-12) by chymase was significantly higher (heart: 7.0 ± 0.6 fmol/min/mg; lung: 33 ± 1.2 fmol/min/mg, P < 0.001) when compared to 125I-Ang I substrate (heart: 0.8 ± 0.1 fmol/min/mg; lung: 2.1 ± 0.1 fmol/min/mg). Substrate affinity of 125I-Ang-(1-12) for rat cardiac chymase was also confirmed using excess unlabeled Ang-(1-12) or Ang I (0–250 µM). The rate of 125I-Ang II formation was significantly lower using unlabeled Ang-(1-12) compared to unlabeled Ang I substrate. Kinetic data showed that rat chymase has a lower Km (64 ± 6.3 µM vs 142 ± 17 µM), higher Vmax (13.2 ± 1.3 µM/min/mg vs 1.9 ± 0.2 µM/min/mg) and more than 15-fold higher catalytic efficiency (ratio of Vmax/Km) for Ang-(1-12) compared to Ang I substrate, respectively. We also investigated ACE mediated hydrolysis of 125I-Ang-(1-12) and 125I-Ang I in solubilized membrane fractions of the SHR heart and lung. Interestingly, no significant difference in 125I-Ang II formation by ACE was detected using either substrate, 125I-Ang-(1-12) or 125I-Ang I, both in the heart (1.8 ± 0.2 fmol/min/mg and 1.8 ± 0.3 fmol/min/mg, respectively) and in the lungs (239 ± 25 fmol/min/mg and 248 ± 34 fmol/min/mg, respectively). Compared to chymase, ACE-mediated Ang-(1-12) metabolism in the heart was several fold lower. Overall our findings suggest that Ang-(1-12), not Ang I, is the better substrate for Ang II formation by chymase in adult rats. In addition, this confirms our previous observation that chymase (rather than ACE) is the main hydrolyzing enzyme responsible for Ang II generation from Ang-(1-12) in the adult rat heart.
Keywords: Rat cardiac chymase, Angiotensin-converting enzyme, Angiotensin-(1-12), Angiotensin I, Angiotensin II, Renin-angiotensin system
1. Introduction
The renin-angiotensin system (RAS), as a major mechanism contributing to the regulation of blood pressure, body fluid, and tissue remodeling, has a critical role in the maintenance of tissue perfusion and homeostasis [1–3]. Seminal studies demonstrating the existence of an inhibitory arm of the RAS through the expression of angiotensin-(1-7) and angiotensin converting enzyme 2 (ACE2) [4] are now expanded with the discovery of an extended form of angiotensin I (Ang I) [5] which our laboratory characterized as an intracrine tissue precursor for direct angiotensin II (Ang II) production [6–8]. The ubiquitous distribution of the dodecapeptide angiotensin-(1-12) [Ang-(1-12)] in rodent tissue [5] was shown by us to be augmented in the heart of spontaneously hypertensive rats (SHR) [9] and also in the left atrial appendage tissue of patients with left-sided heart disease [10].
In pursuing the hypothesis that Ang-(1-12) serves as an intracrine substrate for direct Ang II formation by chymase in the heart [11,12], the question was raised as to whether ACE plays any role in the hydrolysis of Ang-(1-12) into Ang II either directly or through the intermediate production of Ang I. Previous studies demonstrating that both ACE and chymase could be involved in degrading Ang-(1-12) into Ang I and Ang II [6,13,14] prompted us to study the comparative specific enzymatic activities and affinities of ACE and chymase for Ang II formation. Although chymase, as an Ang II-forming enzyme from Ang I, has a long-standing history [15–19], the clinical importance of this alternate pathway continues to remain unappreciated. Recent research [20], however, clearly suggests that the documented relative therapeutic efficacy of ACE inhibitors and Ang II receptor blockers (ARBs) may be due to activation of an alternate pathway for Ang II formation, the inability of these drugs to access the intracellular sites at which Ang II is generated, or both mechanisms. While the existence of an intrinsic mechanism for the intracellular generation of Ang II in cardiac myocytes has been demonstrated by others [21–27], no data exist as to whether Ang-(1-12) or Ang I is the preferred substrate for cellular Ang II formation via chymase or ACE.
2. Materials and methods
2.1. Reagents
Rat angiotensin-(1-12) (>99% purity) was purchased from GenScript USA Inc. (Piscataway, NJ). MLN-4760 (ACE2 inhibitor) was obtained from Millennium Pharmaceuticals (Cambridge, MA). Lisinopril (ACE inhibitor), SCH39370 (neprilysin inhibitor), chymostatin (chymase inhibitor), amastatin, bestatin and benzyl succinate and 4-Chloromercuribenzoic acid (PCMB) were purchased from Sigma-Aldrich Co. (St. Louis, MO). Radioactive 125I was purchased from PerkinElmer Life and Analytical Sciences, Inc. (Waltham, Massachusetts). All other chemicals used in this study were of analytical grade and were obtained from Sigma (St. Louis, MO) and Fisher Scientific (Atlanta, GA).
2.2. Plasma membrane preparation
Native and soluble plasma membranes (PMs) were prepared from the heart and lungs of adult male SHR rats at 4 °C as described previously [7,8,28]. Briefly, frozen cardiac left ventricular or lung tissues (50–100 mg) were homogenized in 1 mL reaction buffer containing 25 mM HEPES, 125 mM NaCl and 10 µM ZnCl2 (pH 7.4) using a Qiagen Tissue Lyzer (Valencia, CA) for 60 s at 20 Hz. The homogenates were centrifuged at low spin (200 g) for 1 min to remove the connective tissues and cell debris. The supernatants were transferred into clean tubes and centrifuged at 28,000 g for 20 min to collect the native PMs. The resultant pellets (native PMs) were washed twice by re-suspending them in the reaction buffer, centrifuged, and stored at −80 °C until their use for evaluation of angiotensin peptide metabolism and measure of chymase and ACE enzyme activities.
ACE activity was measured in the solubilized PMs. For this, the native PMs were solubilized in a reaction buffer containing 0.5% triton X-100 on ice overnight. The soluble portion of the native PMs was separated from insoluble material by centrifugation at 28,000 g for 20 min. The protein concentrations (mg protein/mL) of PMs (native and soluble) were measured by BCA protein assay kit (Pierce, Thermo Scientific, Rockford, IL).
2.3. HPLC analysis of 125I-Ang metabolic products
Metabolic products of 125I-Ang-(1-12) and 125I-Ang I by PMs were analyzed by reverse-phase high-performance liquid chromatography (HPLC) under different combinations of RAS inhibitor cocktails as described in Table 1. Briefly, the PMs (50–100 µg per reaction mixture) were pre-incubated with various combinations of RAS and peptidase inhibitors (50 µM each) for 15 min, then a highly purified 125I-Ang-(1-12) or 125I-Ang I (1 nmol/l) substrate was added to the reaction mixtures and incubated for an additional 60 min (for chymase) or 120 min (for ACE) at 37° C. The enzymatic reactions were stopped by adding an equal volume of 1% phosphoric acid, mixing well and centrifuging at 28,000 g for 20 min to remove the PMs. The clear supernatants were filtered and 125I-Ang products were separated by HPLC on a C-18 column. A linear gradient from 10% to 50% mobile phase B at a flow rate of 0.35 mL/min at 32° C was used for the HPLC analyses. The solvent system consisted of 0.1% phosphoric acid (mobile phase A) and 80% acetonitrile/0.1% phosphoric acid (mobile phase B). The eluted 125IAng products were monitored by an in-line flow-through gamma detector (BioScan Inc., Washington, DC). Products were identified by comparison of retention times of synthetic [125I] standard Ang peptides and the data were analyzed with Shimadzu LCSolution (Kyoto, Japan) acquisition software.
Table 1.
Outline of enzyme inhibitors used in this study.
| Group | Inhibitors added (50 µM each) |
|---|---|
| All Inhibitors | RAS inhibitors (chymostatin for chymase; lisinopril for ACE; MLN-4760 for ACE2; and SCH39370 for neprilysin), aminopeptidase inhibitors (amastatin and bestatin), carboxypeptidase inhibitor (benzyl succinate) and PCMB. |
| No chymostatin | All above inhibitors except chymostatin. |
| No lisinopril | All above inhibitors except lisinopril. |
2.4. 125I-Ang-(1-12) and 125I-Ang I metabolism by chymase and ACE
The affinities of cardiac and lung chymase and ACE to hydrolyze the 125I-Ang-(1-12) or 125I-Ang I substrate were analyzed by measuring the amount of 125I-Ang products in the presence of a cocktail containing all RAS inhibitors and after subtracting from the cocktail either chymostatin or lisinopril. Supernatants were collected after exposing the 125I-Ang-(1-12) or 125I-Ang I substrate to native PMs for 60 min (cardiac chymase) and soluble PMs for 120 min (cardiac ACE) at 37 °C; the generated products were analyzed by HPLC. For lung chymase and ACE, the PMs were incubated for only 30 min at 37° C. The enzyme activities were calculated based on the amount of 125I-Ang products generated from 125I-Ang-(1-12) or 125I-Ang I by chymase and ACE. Enzyme activities were reported as fmoles of 125I-Ang II formation from parent substrate [125I-Ang-(1-12) or 125I-Ang I] per min per mg protein (fmol/ min/mg protein).
The affinities of chymase for Ang-(1-12) or Ang I substrates were investigated by measuring the amount of radiolabeled 125I-Ang II generated from 125I-Ang-(1-12) substrate in the presence of increasing concentrations of non-radiolabeled Ang-(1-12) or Ang I. Briefly, native PMs (50–100 µg) were incubated with radiolabeled 125I-Ang-(1-12) with or without increasing concentrations of non-radiolabeled Ang-(1-12) or Ang I substrate (range: 0–250 µM Ang peptides) added into the reaction mixture in the presence of all RAS inhibitors cocktail or absence of chymostatin only for 120 min at 37 °C. At the end of the incubation time, the reaction mixtures were stopped by adding an equal volume of 1% phosphoric acid and the amount of 125I-Ang II formation from 125I-Ang-(1-12) was analyzed by HPLC as described above.
2.5. Km and Vmax of cardiac chymase for Ang-(1-12) and Ang I substrates
To determine the Km and Vmax of cardiac chymase, native PMs (100 µg) were incubated with increasing concentrations (5–300 µM) of either Ang-(1-12) or Ang I substrate in the presence of lisinopril (200 µM; ACE inhibitor) at 37 °C for 30 min. The Ang II product formation from Ang-(1-12) or Ang I substrate was separated by HPLC and fraction elution was monitored as the absorbance at 215 nm. The concentration of Ang II was determined using a standard curve of Ang II synthetic peptide. The Km and Vmax of cardiac chymase for Ang-(1-12) and Ang I substrates were calculated using a Michaelis-Menten equation.
2.6. Statistical analysis
Experiments were repeated three or more times. All values are reported as mean ± SE. The Student's t-test and repeated-measures ANOVA followed by a Tukey's post hoc test for multiple comparisons were used to determine the significant differences at a probability of 0.05 using GraphPad Prism 5.0 software (San Diego, CA).
3. Results
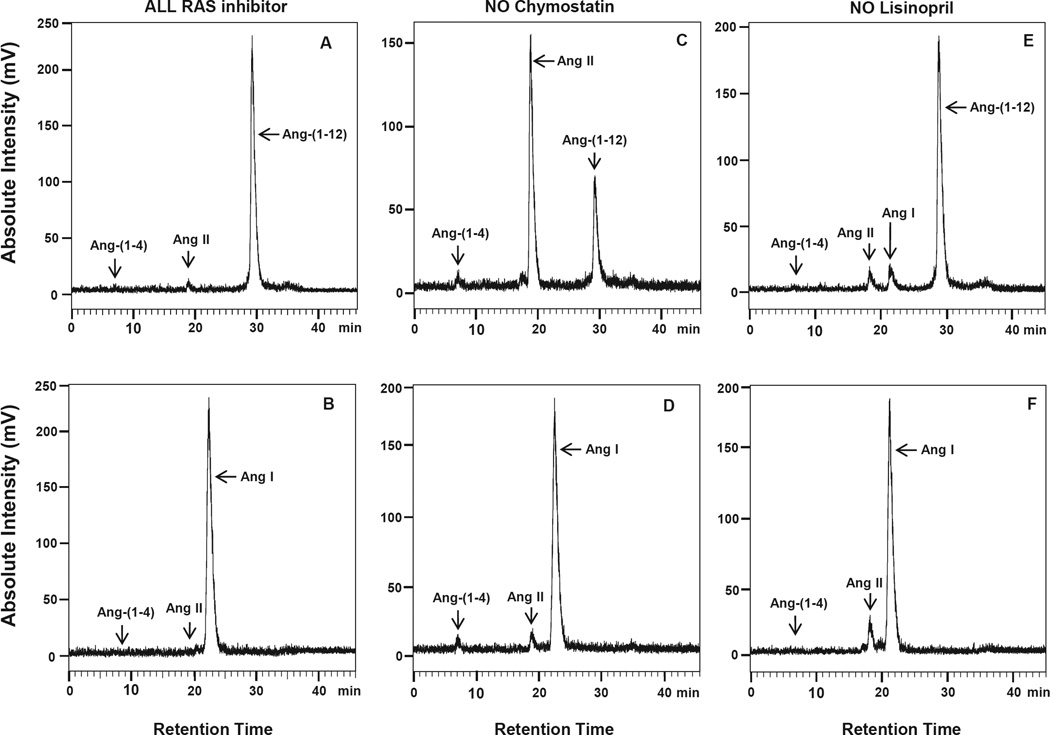
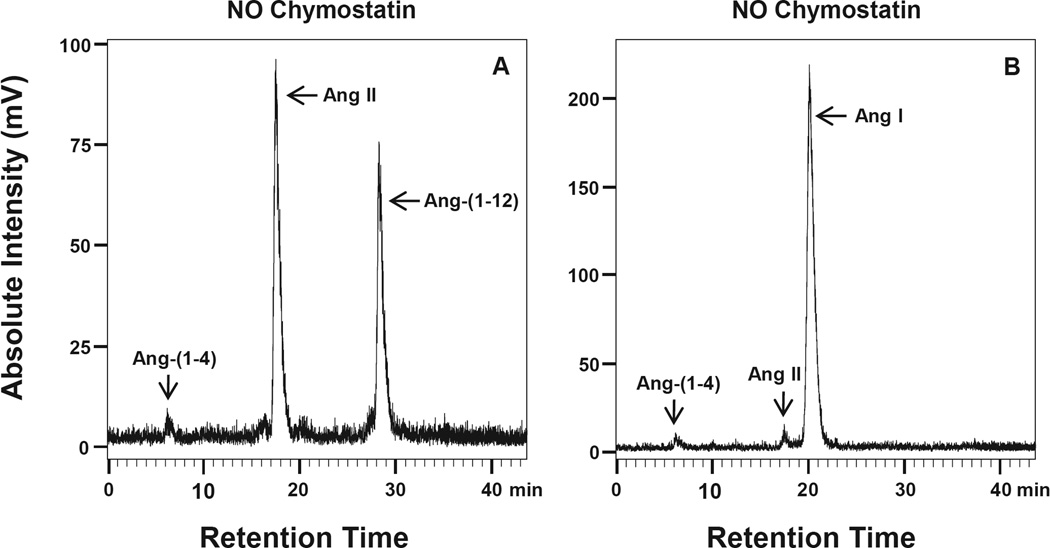
Fig. 1(A–F) illustrates the HPLC chromatograms of peptides generated during incubation of 125I-Ang-(1-12) and 125I-Ang I when either chymostatin (Panels 1C – 1D) or lisinopril (Panels 1E – 1F) were subtracted from the inhibitor cocktail (panels 1A – 1B) under the conditions described in the Methods. As shown in panels A and B of Fig. 1, no 125I-Ang II metabolic product formation was detected in the presence of all inhibitors either, from 125I-Ang-(1-12) or 125IAng I. In the absence of chymostatin only, a large peak of 125I-Ang II product formation by chymase was detected from the 125I-Ang-(1-12) parent substrate (Fig. 1C). Using 125I-Ang I as a substrate, however, only a small peak of 125I-Ang II was detected in the absence of chymostatin (Fig. 1D). On the other hand, a small 125IAng II peak was detected from either 125I-Ang-(1-12) or 125I-Ang I substrate in the absence of lisinopril only (Fig. 1E and F). Further analysis revealed that lung chymase (native PMs) also generates a large peak of 125I-Ang II product from 125I-Ang-(1-12), but not from the 125I-Ang I parent substrate (Fig. 2A and B).
Fig. 1. Rat 125I-Ang-(1-2) and 125I-Ang I substrate hydrolysis by PMs isolated from SHR heart.
Chromatograms show the 125I-Ang II product generation from 125I-Ang-(1-12) and 125I-Ang I by PMs isolated from the SHR heart. Panel A and B: In the presence of all RAS inhibitors (including chymostatin and lisinopril). Panel C and D: In the absence of chymostatin (chymase inhibitor) only. Panel E and F: In the absence of lisinopril (ACE inhibitor) only. Results are representative of three or more separate metabolism experiments for each group.
Fig. 2. 125I-Ang-(1-12) and 125I-Ang I substrate hydrolysis by PMs isolated from SHR lung.
Chromatograms show the 125I-Ang II product generation in the absence of chymostatin (chymase inhibitor) only from (A) 125I-Ang-(1-12), and (B) 125I-Ang I substrate. Results are representative of three or more separate metabolism experiments for each group.
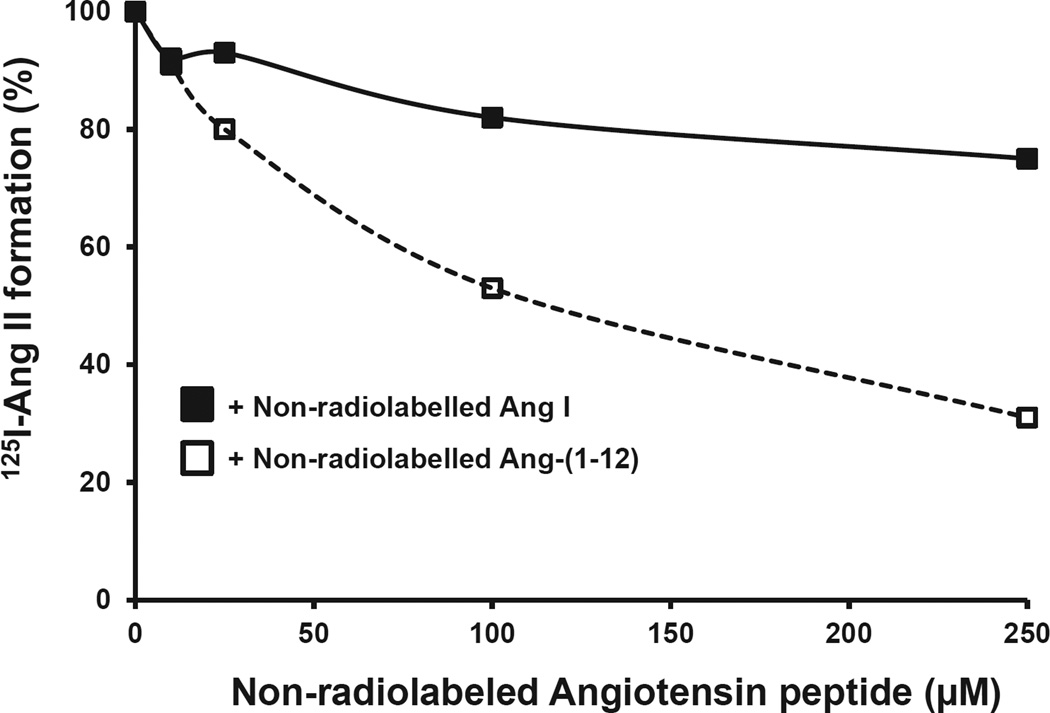
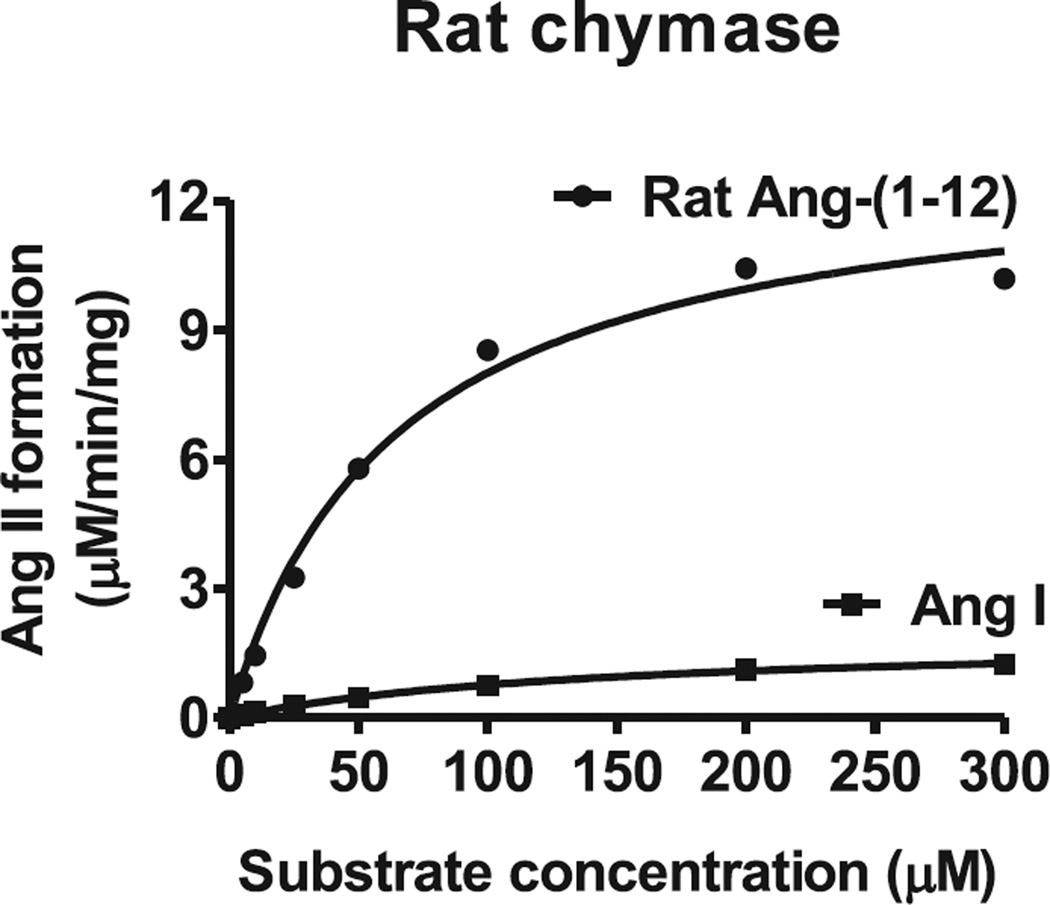
The substrate specificity of chymase for 125I-Ang-(1-12) was further confirmed using increasing concentrations of non-radiolabeled Ang-(1-12) or Ang I. This analysis indicated that radiolabeled 125I-Ang II formation from 125I-Ang-(1-12) substrate by cardiac chymase was significantly reduced with increasing concentrations of non-radiolabeled Ang-(1-12) compared to non-radiolabeled Ang I (Fig. 3). Kinetic analysis (Km, Vmax and catalytic efficiency) of cardiac chymase was also determined for Ang-(1-12) and Ang I substrates. A representative curve showing Ang II formation by cardiac chymase with increasing concentrations of Ang-(1-12) and Ang I substrates is shown in (Fig. 4). The Km and Vmax were 64 ± 6.3 µM and 13.2 ± 1.3 µM/min/mg of chymase/Ang-(1-12) and 142 ± 17 µM and 1.9 ± 0.2 µM/min/mg of chymase/Ang I reactions, respectively. The catalytic efficiency (the ratio of Vmax/Km ratio) was more than 15-fold higher for chymase/Ang-(1-12) compared to chymase/Ang I. These findings further support the previous demonstration that cardiac chymase has much higher substrate specificity and catalytic efficiency for Ang-(1-12) compared to Ang I substrates.
Fig. 3. Affinity of rat cardiac chymase for Ang-(1-12) and Ang I substrates.
The decrease in 125I-Ang product formation (%) from rat 125I-Ang-(1-12) substrate by PMs incubated in the absence of chymostatin only (chymase inhibitor) with increasing concentrations (0–250 µM) of non-radiolabeled substrates (□) Ang-(1-12) or (■) Ang I. Results are representative of three or more separate experiments for each group.
Fig. 4. Kinetics (Km and Vmax) of rat cardiac chymase for Ang-(1-12) and Ang I substrates.
PMs (100 µg) were incubated with increasing concentrations (5–300 µM) of Ang-(1-12) or Ang I substrate in the presence of lisinopril (200 µM, ACE inhibitor) at 37° C for 30 min. The Ang II generated was analyzed by HPLC connected to UV-detector at 215 nm. The Km and Vmax of cardiac chymase for Ang-(1-12) and Ang I substrates were calculated using the Michaelis-Menten equation.
The measurements of cardiac and lung chymase and ACE activities based on the amounts of 125I-Ang II production from either 125I-Ang-(1-12) or 125I-Ang I substrate are illustrated in Table 2. The results indicate that the chymase activity in heart (7.0 ± 0.6 fmol/ min/mg, P < 0.001) and lung (33 ± 1.2 fmol/min/mg, P < 0.001) PMs are several fold higher (8.75 and 15.7-fold, respectively) using 125IAng-(1-12) substrate compared to that obtained using 125I-Ang I substrate (0.8 ± 0.1 fmol/min/mg and 2.1 ± 0.1 fmol/min/mg, respectively). On the other hand, no significant differences in ACE activity were found in heart (1.8 ± 0.2 fmol/min/mg and 1.8 ± 0.3 fmol/min/mg) and lung (239 ± 25 fmol/min/mg and 248 ± 34 fmol/min/mg) using either 125I-Ang-(1-12) or 125I-Ang I substrates, respectively.
Table 2.
Metabolism of 125I-Ang-(1-12) and 125I-Ang I by chymase and ACE enzymes in SHR heart and lung (PMs).
| Tissue/Substrate | Chymase activity | ACE activity |
|---|---|---|
| Heart (PMs) | ||
| 125I-Ang-(1-12) substrate | 7.0 ± 0.6a,b | 1.8 ± 0.2c,d |
| 125I-Ang I substrate | 0.8 ± 0.1b | 1.8 ± 0.3 |
| Lungs (PMs) | ||
| 125I-Ang-(1-12) substrate | 33 ± 1.2a,b | 239 ± 25c,d |
| 125I-Ang I substrate | 2.1 ± 0.1b | 248 ± 34 |
Chymase and ACE mediated generation of 125I-Ang II product from the metabolism of either 125I-rAng-(1-12) or 125I-Ang I substrate (1 nmol/L) were analyzed in SHR rat heart and lung native and soluble PMs preparations as described in the Methods. The contribution of chymase and ACE activity (fmol Ang II formation/min/mg) was calculated based on 125I-Ang II product analysis by HPLC in each sample incubated with or without specific chymase or ACE inhibitor as described in Table 1. Data are expressed as mean ± SEM. Each experiment was done at least three times.
Significantly different (P < 0.001) vs. corresponding 125I-Ang I substrate.
Significantly different (P < 0.001) vs. corresponding ACE activity.
NS = Not significantly different vs. corresponding 125I-Ang I substrate
NS = Not significantly different vs. corresponding ACE activity.
4. Discussion
Although an ACE-independent pathway for Ang II production from Ang I is well established [20], we now show for the first time that the hydrolytic activity of the chymase-mediated alternate pathway for Ang-(1-12) is at least 8–15-fold higher than the comparative activity for the Ang I substrate in the heart and lung tissue of SHR. Furthermore, our results demonstrate that with increasing concentrations of non-radiolabeled substrate, the 125IAng II formation from 125I-Ang-(1-12) by chymase was significantly lower in the presence of unlabeled Ang-(1-12) than unlabeled Ang I. Cardiac chymase kinetics also showed a 2-fold lower Km (64 ± 6.3 vs 142 ± 17 µM), a 7-fold higher Vmax (13.2 ± 1.3 vs 1.9 ± 0.2 µM/ min/mg), and more than a 15-fold higher catalytic efficiency (the ratio of Vmax/Km) for Ang-(1-12) compared to Ang I substrate. These data document for the first time that Ang-(1-12) rather than Ang I is a much better substrate for rat chymase.
Chymases are a family of mast cell serine proteases involved in angiotensin peptide hormone processing [29–32]. In non-rodent mammals only the α-form of the chymase gene selectively hydrolyzes the Phe8-His9 bond of Ang I to generate Ang II. Rodents have several isoforms of β-chymase in addition to the α-chymase [29,33]. One of the forms of the β-chymase (called rat mast cell protease-1; RMCP1) shows a strong preference to hydrolyze the Tyr4-Ile5 bond of angiotensin peptides, thus preventing Ang II formation from Ang I [31]. Although abundant expression of the RMCP1 form is found in connective tissue mast cells [34], the relative expressions of these chymase isoforms in the rodent heart are not well documented. Our findings suggest that a very high content of Ang II-forming chymase was present in the rat heart tissue compared to Ang II-destroying β-chymase (RMCP1). A very small peak corresponding to the 125I-Ang-(1-4) fragment [Asp1-Arg2-Val3-Tyr4-product of RMCP1] was detected from the Ang-(1-12) hydrolysis in cardiac membrane preparations. This finding suggests that the expression of the Ang II-destroying β-chymase isoform (RMCP1) was very low in the SHR heart. On the other hand, our findings do not exclude the possibility that RMCP1 may have a much higher specificity to cleave the Tyr4-Ile5 bond for bigger angiotensin peptides (Ang-12 and Ang I) rather than the smaller Ang II sequence. While our data clearly show that cardiac chymase rapidly hydrolyzes 125I-Ang-(1-12) into 125I-Ang II, further studies are necessary to determine the hydrolytic ability of β-chymase isoforms for Ang II, Ang I and Ang-(1-12) peptides to demonstrate the substrate preference between Ang II-forming and destroying isoforms of cardiac chymases.
Ang-(1-12) was first identified by Nagata and colleagues [5] in tissue and plasma of a Japanese strain of Wistar rats. Our later studies in neonatal WKY and SHR cardiac myocytes [6], anephric normotensive WKY rats [35], SHR [9], and human atrial tissues [7] demonstrated that both Ang-(1-12) and chymase were predominantly expressed intracellularly in cardiac tissue. Our recent translational studies in human and rodent cardiac tissue showed that Ang-(1-12) was the preferred substrate for intracellular Ang II formation via chymase rather than ACE [7,8]. As summarized in recent publications from our laboratory [12,20], these findings are in keeping with the limited efficacy of ACE inhibitors and ARBs to suppress the pathological actions of Ang II in cardiovascular disease [36] since these drugs act on the cell surface rather than in the intracellular sites where the biotransformation of angiotensinogen or Ang-(1-12) [Ang-(1-12)] occurs.
While ACE plays a major role in the circulation to generate Ang II, it has been convincingly shown that chymase plays a major role in rodent and human heart [7,8,10,11,37]. Since chymase activity is only detected in tissues and not in the circulation, the biochemical mechanisms forming Ang II within the cellular microenvironment are accounted for by alternative enzymatic pathways that are different than those in the circulation and that can't be blocked by either ACE inhibitors or ARBs [36,38]. The inability to block Ang II formation through these approaches suggests that these agents are not cell permeable, they are inhibited intracellularly, or they fail to reach the intracellular compartment containing Ang-(1-12) or Ang I. The intracellular formation of Ang II via the chymase pathway is independent of and unaffected by inhibition of Ang II production by ACE inhibitors or AT1R blockade [39,40]. A number of factors suggest that the Ang II formation pathway is mediated primarily via chymase and Ang-(1-12). First, a robust literature documents a primary role for chymase in Ang II formation [6,8,10,11]. Wei et al. [41] showed that chronic ACE inhibition did not change the concentration of Ang II in the interstitial fluid of the left ventricle of conscious mice. Ferrario et al. [36] found no changes in cardiac content of Ang II in Lewis rats that were medicated for a fortnight with either lisinopril or losartan alone or in combination. Second, the catalytic activity of chymase is 20-fold higher than ACE [32], and renin is not involved in Ang-(1-12) metabolism [35,42]. And finally, our present findings demonstrate that rat chymase metabolizes Ang-(1-12) in cardiac tissue with an affinity many fold higher than for Ang I.
Our present findings clearly demonstrate that Ang-(1-12) is a much better substrate in cardiac tissue to generate biologically active Ang II peptide via chymase. Compared to Ang I, high levels of Ang-(1-12) were found in several tissues including the heart [5]. We have shown increased expression of Ang-(1-12) and chymase in neonatal cultured cardiac myocytes of SHR compared to WKY which may serve as the preferred pathway to generate Ang II in the heart [6].
In summary, the results of the present study clearly suggest that rat chymase has very high substrate specificity and catalytic efficiency for Ang-(1-12) and that it is mainly responsible for generating cellular Ang II. Although renin shows high specificity for the cleavage of the Leu10-Leu11 bond at the N-terminus of rat angiotensinogen, our studies in the isolated heart preparations and anephric rats show that Ang II formation from Ang-(1-12) was not mediated by renin [35,42]. This working paradigm may resolve previous observations that Ang II formation in tissues is not prevented by ACE inhibitors and Ang II receptor blockers (ARBs) [24–26,36,38,40]. Our studies suggest that selective chymase inhibitors may provide greater benefit in the management of adverse cardiac remodeling than the previous therapeutic approaches.
Acknowledgments
This work was supported by grant from the National Heart, Blood, Lung Institute of the NIH (P01 HL-051952 to CMF).
Footnotes
Conflicts of interest
All authors have no potential conflicts of interest relevant to this article.
Disclosure
No potential conflicts of interest relevant to this article.
References
- 1.Ferrario CM, Chappell MC, Dean RH, Iyer SN. Novel angiotensin peptides regulate blood pressure, endothelial function, and natriuresis. J. Am. Soc. Nephrol. 1998;9:1716–1722. doi: 10.1681/ASN.V991716. [DOI] [PubMed] [Google Scholar]
- 2.Mendoza-Torres E, Oyarzun A, Mondaca-Ruff D, Azocar A, Cartro PF, Jalil JE, Chiong M, Lavandero S, Ocaranza MP. ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther. Adv. Cardiovasc. Dis. 2015;9:217–237. doi: 10.1177/1753944715597623. [DOI] [PubMed] [Google Scholar]
- 3.Cao T, Feng Y. The (pro)renin receptor and body fluid homeostatis. Am. J. Physiol. Integr. Com. Physiolol. 2013;305:R104–R106. doi: 10.1152/ajpregu.00209.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am. J. Physiol. Heart Cric. Physiol. 2005;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem. Biophys. Res. Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM. Uptake and metabolism of the novel peptide angiotensin-(1-12) by neonatal cardiac myocytes. PLoS ONE. 2011;6:e15759. doi: 10.1371/journal.pone.0015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of Angiotensin II from Angiotensin-(1-12) in human atrial tissue. PLoS ONE. 2011;6:e28501. doi: 10.1371/journal.pone.0028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad S, Wei C-C, Tallaj J, Dell'Italia LJ, Moniwa N, Varagic J, Ferrario CM. Chymase mediates angiotensin-(1-12) metabolism in normal human heart. J. Am. Soc. Hypertens. 2013;7:128–136. doi: 10.1016/j.jash.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the novel angiotensin peptide, angiotensin-(1-12) in heart and kidney of hypertensive and normotensive rats. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2614–H2618. doi: 10.1152/ajpheart.91521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata S, Varagic J, Kon ND, Wang H, Groban L, Simington SW, Ahmad S, Dell'Italia LJ, VonCannon JL, Deal D, Ferrario CM. Differential expression of the angiotensin-(1-12)/chymase axis in human atrial tissue. Ther. Adv. Cardiovasc. Dis. 2015;9:168–180. doi: 10.1177/1753944715589717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Ferrario CM, Ahmad S, Nagata S, Simington SW, Varagic J, Kon N, Dell'Italia LJ. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin. Sci. 2014;126:461–469. doi: 10.1042/CS20130400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrario CM, VonCannon J, Jiao Y, Ahmad S, Bader M, Dell'Italia LJ, Groban L, Varagic J. Cardiac angiotensin-(1-12) expression and systemic hypertension in rats expressing the human angiotensinogen gene. Am. J. Physiol. Heart Cric. Physiol. 2016;310:H995–H1005. doi: 10.1152/ajpheart.00833.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc. Res. 2009;82:40–50. doi: 10.1093/cvr/cvp003. [DOI] [PubMed] [Google Scholar]
- 14.Nagata S, Kato J, Kuwasako K, Asami M, Kitamura K. Plasma and tissue concentrations of proangiotensin-12 in rats treated with inhibitors of the renin-angiotensin system. Hypertens. Res. 2012;35:234–238. doi: 10.1038/hr.2011.165. [DOI] [PubMed] [Google Scholar]
- 15.Lorenz JN. Chymase: the other ACE? Am. J. Physiol. Ren. Physiol. 2010;298:F35–F36. doi: 10.1152/ajprenal.00641.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dell'Italia LJ, Meng QC, Balcells E, Wei C-C, Palmer R, Hageman GR, Durand J, Hankes GH, Oparil S. Compartmentalization of angiotensin II generation in the dog heart. J. Clin. Invest. 1997;100:253–258. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urata H, Boehm KD, Philip A, Kinoshita A, Gabrovsek J, Bumpus FM, Husain A. Cellular localization and reginal distribution of an angiotensin II-forming chymase in the heart. J. Clin. Invest. 1993;91:1269–1281. doi: 10.1172/JCI116325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urata H. Pathological involvement of chymase-dependent angiotensin II formation in the development of cardiovascular disease. J. Renin Angiotensin Aldosterone Syst. 2000;1:S35–S37. doi: 10.3317/jraas.2000.054. [DOI] [PubMed] [Google Scholar]
- 19.Kumar R, Boim MA. Diversity of pathways for intracellular angiotensin II synthesis. Curr. Opin. Nephrol. Hypertens. 2009;18:33–39. doi: 10.1097/MNH.0b013e32831a9e20. [DOI] [PubMed] [Google Scholar]
- 20.Ferrario CM, Ahmad S, Varagic J, Cheng CP, Groban L, Wang H, Collawn JF, Dell'Italia LJ. Intracrine Angiotensin II Functions Originate from Non-canonical Pathways in the Human Heart. [May 27, 2016];Am. J. Physiol. Heart Cric. Physiol. 2016 311 doi: 10.1152/ajpheart.00219.2016. http://dx.doi.org/10.1152/ajpheart.00219.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: a new paradigm. Trends. Endocrinol. Metab. 2007;18:208–214. doi: 10.1016/j.tem.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: implications in cardiovascular remodeling. Curr. Opin. Nephrol. Hypertens. 2008;17:168–173. doi: 10.1097/MNH.0b013e3282f521a8. [DOI] [PubMed] [Google Scholar]
- 23.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system in the heart. Curr. Hypertens. Rep. 2009;11:104–110. doi: 10.1007/s11906-009-0020-y. [DOI] [PubMed] [Google Scholar]
- 24.Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul. Pept. 2004;120:5–13. doi: 10.1016/j.regpep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H939–H948. doi: 10.1152/ajpheart.00391.2007. [DOI] [PubMed] [Google Scholar]
- 26.Baker KM, Kumar R. Intracellular angiotensin II induces cell proliferation independent of AT1 receptor. Am. J. Physiol. Cell. Physiol. 2006;291:C995–C1001. doi: 10.1152/ajpcell.00238.2006. [DOI] [PubMed] [Google Scholar]
- 27.Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am. J. Physiol. Heart. Circ. Physiol. 2008;294:H1675–H1684. doi: 10.1152/ajpheart.91493.2007. [DOI] [PubMed] [Google Scholar]
- 28.Wei C-C, Chen Y, Powell LC, Zheng J, Shi K, Bradley WE, Powell PC, Ahmad S, Ferrario CM, Dell'Italia LJ. Cardiac kallikrein-kinin system is upregulated in chronic volume overload and mediates an inflammatory induced collagen loss. PLoS ONE. 2012;7:e40110. doi: 10.1371/journal.pone.0040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caughey GH, Leidig F, Viro NF, Nadel JA. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymae. J. Pharmacol. Exp. Ther. 1988;244:133–137. [PubMed] [Google Scholar]
- 30.Caughey GH, Raymond WW, Wolters PJ. Angiotensin II generation by mast cell a- and b-chymases. Biochem. Biophys. Acta. 2000;1480:245–257. doi: 10.1016/s0167-4838(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 31.Chandrasekharan UM, Sanker S, Glynias MJ, Karnik SS, Husain A. Angiotensin II-forming activity in a reconstructed ancestral chymase. Science. 1996;271:502–505. doi: 10.1126/science.271.5248.502. [DOI] [PubMed] [Google Scholar]
- 32.Shankar S, Chandrasekharan UM, Wilk D, Glynias MJ, Karnik SS, Husain A. Distinct multisite synergistic interactions determine substrate specificities of human chymase and rat chymase-1 for angiotensin II forming and degradation. J. Biol. Chem. 1997;272:2963–2968. doi: 10.1074/jbc.272.5.2963. [DOI] [PubMed] [Google Scholar]
- 33.Lutzelschwab C, Pejler G, Aveskogh M, Hellman L. Secretory granule proteases in rat mast cells. Cloning of 10 different serine proteases and carboxypeptidase A from various rat mast cell populations. J. Exp. Med. 1997;185:13–29. doi: 10.1084/jem.185.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson S, Miller HR. Mast cell subsets in the rat distinguished immunohistochemically by their content of serine proteinases. Immunology. 1986;58:101–104. [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR. Differential regulation of angiotensin-(1-12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1184–H1192. doi: 10.1152/ajpheart.01114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrario CM, Jessup JA, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz D, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 37.Moniwa N, Varagic J, Simington SW, Ahmad S, Nagata S, VonCannon JL, Ferrario CM. Primacy of Angiotensin Converting Enzyme in Angiotensin-(1-12) Metabolism. Am. J. Physiol. Heart Cric. Physiol. 2013;305:H644–H650. doi: 10.1152/ajpheart.00210.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varagic J, Ahmad S, VonCannon JL, Moniwa N, Brosnihan KB, Wysocki J, Batlle D, Ferrario CM. Predominance of AT(1) blockade over mas-mediated angiotensin-(1-7) mechanisms in the regulation of blood pressure and renin-angiotensin system in mRen2.Lewis rats. Am. J. Hypertens. 2013;26:583–590. doi: 10.1093/ajh/hps090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moniwa N, Varagic J, Ahmad S, VonCannon JL, Simington SW, Wang H, Groban L, Brosnihan KB, Nagata S, Kato J, Kitamura K, Gomez RA, Lopez MLS, Ferrario CM. Hemodynamic and hormonal changes to dual renin-angiotensin system inhibition in experimental hypertension. Hypertension. 2013;61:417–424. doi: 10.1161/HYPERTENSIONAHA.112.201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moniwa N, Varagic J, Ahmad S, VonCannon JL, Ferrario CM. Restoration of the blood pressure circadian rhythm by direct renin inhibition and blockade of angiotensin II receptors in mRen2Lewis hypertensive rats. Ther. Adv. Cardiovasc. Dis. 2012;6:15–29. doi: 10.1177/1753944711434039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei C-C, Hase N, Inoue Y, Bradley EW, Yahiro E, Li M, Naqvi N, Powell PC, Shi K, Takahashi Y, Saku K, Urata H, Dell'Italia LJ, Husain A. Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents. J. Clin. Invest. 2010;120:1229–1239. doi: 10.1172/JCI39345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1-12) is an alternate substrate for angiotensin peptide production in the heart. Am. J. Physiol. Heart Cric Physiol. 2008;294:H2242–H2247. doi: 10.1152/ajpheart.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]